Extraction of Rare Earth Elements from Idaho-Sourced Soil Through Phytomining: A Case Study in Central Idaho, USA
Abstract
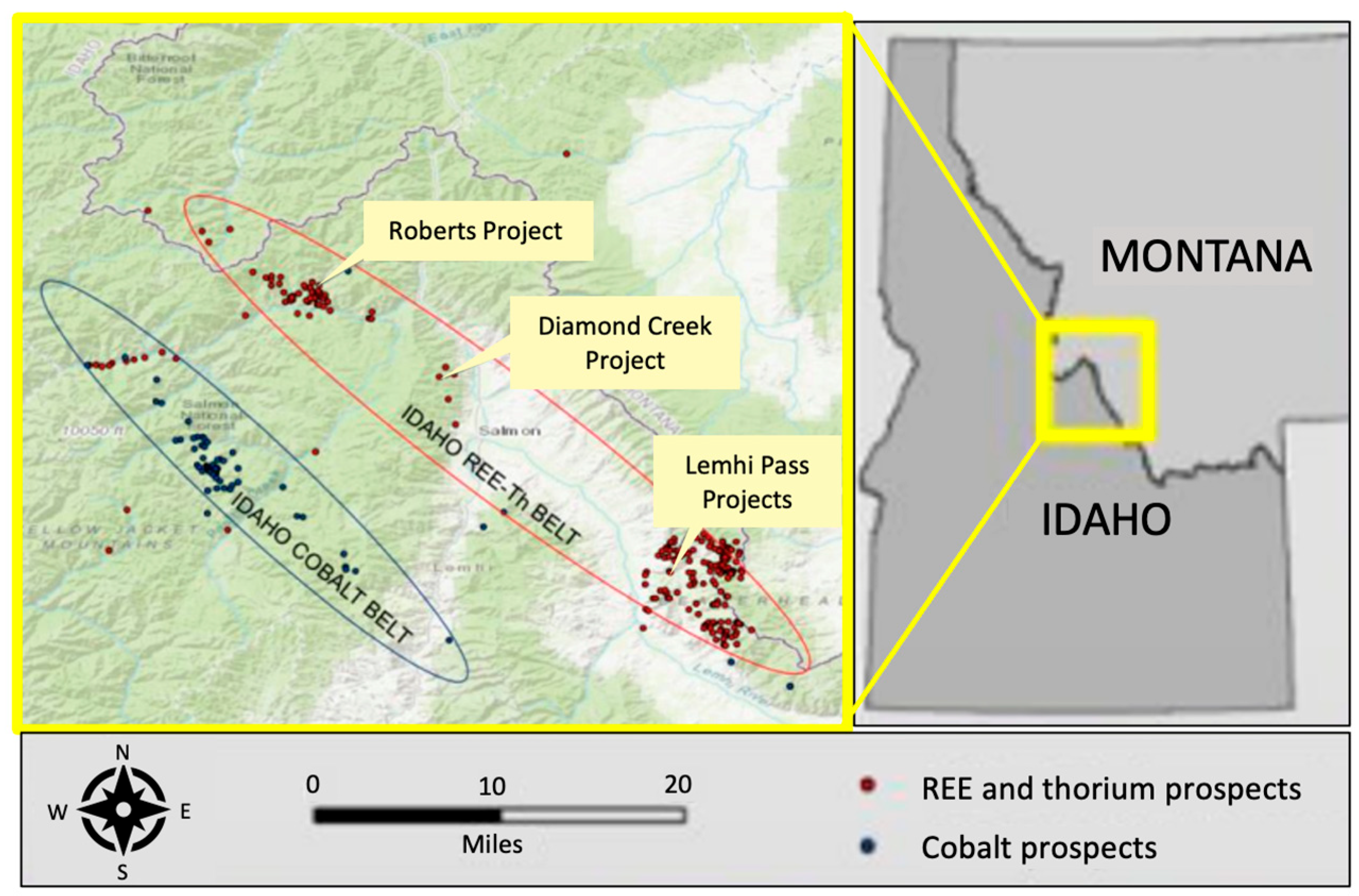
1. Introduction
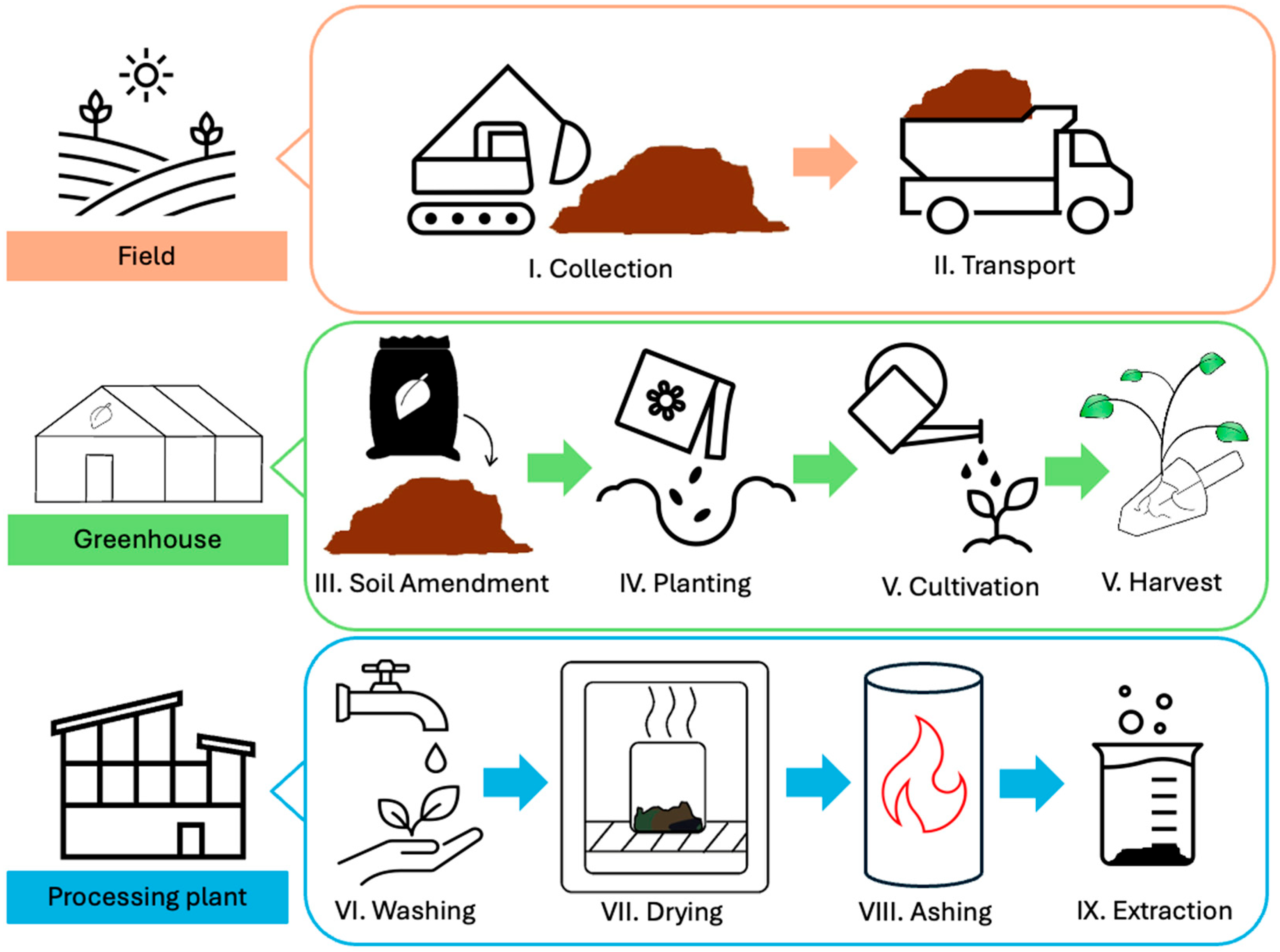
2. Materials and Methods
2.1. Soil Preparation
2.2. Seed Preparation
2.3. Experimental Design, Growing, and Treatments
2.4. Plant Harvesting
2.5. Pyrolysis and Elemental Analysis
2.6. Statistical Analysis
2.7. Life Cycle Assessment
2.7.1. Goal and Scope Definition
2.7.2. Life Cycle Inventory
2.7.3. Life Cycle Impact Assessment
- The greenhouse climate control system and grow lights used 24,200 kWh and 64,500 kWh, respectively, for a total of 88,700 kWh of electricity consumed during controlled-environment cultivation.
- A diesel tractor is used to fertilize, sow seeds, and harvest P. arundinaceae biomass in the field study.
- A growth period of six weeks is enough for P. arundinacea to hyperaccumulate REEs optimally.
- P. arundinacea biomass is 90% water by weight and oven drying it for 24 h at 95 °C removes all possible moisture content.
- Biomass is pyrolyzed directly after drying (no grinding).
- Dried biomass weight is reduced by 50% when converted to bio-ore.
- Nitrogen gas pumped through the pyrolysis reaction chamber remains unchanged after it is emitted into the atmosphere.
- Emissions from pyrolysis of biomass at 350 °C have the following chemical contents: 69% CO2, 25% CO, 2.5% H2, 1% CH4, and 2.5% other mixed hydrocarbon gases [38].
- All GHG emissions produced are emitted directly into the atmosphere.
- All electricity used was supplied by Western Electricity Coordinating Council (WECC) Northwest. Emission factors from this provider are as follows: 602.1 lb/MWh CO2, 0.056 lb/MWh CH4, and 0.008 lb/MWh N2O [39].
- Energy used to power the biomass drying and pyrolysis processes was considered, but energy used to power the facility itself (e.g., lighting, heating) was not considered.
- Transport between the field and biomass processing and pyrolysis facilities was not considered.
2.7.4. Interpretation
2.8. Techno-Economic Assessment
3. Results
3.1. Growing Success and Bio-Ore Mass Production
3.2. Hyperaccumulation Ability
3.3. Life Cycle Assessment and Techno-Economic Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.H. The US Should Get Serious about Mining Critical Minerals for Clean Energy. Nature 2023, 615, 563. [Google Scholar] [CrossRef]
- Gillerman, V.S. Rare Earth Elements and Other Critical Metals in Idaho: Geological Survey of Idaho. GeoNote 2011, 44, 4. [Google Scholar]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements—Critical Resources for High Technology | USGS Fact Sheet 087-02. Available online: https://pubs.usgs.gov/fs/2002/fs087-02/ (accessed on 23 March 2025).
- The Global Fight for Critical Minerals Is Costly and Damaging. Nature 2023, 619, 436. [CrossRef]
- Zou, B.; Poncin, S.; Bertinelli, L. The US-China Supply Competition for Rare Earth Elements: A Dynamic Game View. Environ. Model. Assess. 2022, 27, 883–900. [Google Scholar] [CrossRef]
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to Build a Circular Economy for Rare-Earth Elements. Nature 2023, 619, 248–251. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, J.; Liang, X.; Ling, B.; Xu, J.; Yang, Y.; Kang, S.; Tan, W.; Xu, Y.; Zou, X.; et al. Industrial-Scale Sustainable Rare Earth Mining Enabled by Electrokinetics. Nat. Sustain. 2025, 8, 182–189. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, H.; Zhao, Y.; Shen, L.; Gu, G.; Qiu, G. Effective Recovery of Rare Earth from (Bio)Leaching Solution through Precipitation of Rare Earth-Citrate Complex. Water Res. 2023, 233, 119752. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.S.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective; U.S. Geological Survey: Reston, VA, USA, 2010.
- Cao, Y.-W.; Liu, X.-M.; Wang, C.; Bai, E.; Wu, N. Rare Earth Element Geochemistry in Soils along Arid and Semiarid Grasslands in Northern China. Ecol. Process. 2022, 11, 29. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Austin, J.C.; Thompson, A.; Richter, D.D. Mineralogical and Elemental Trends in Regolith on Historically Managed Sites in the Southeastern United States Piedmont. Clays Clay Miner. 2022, 70, 539–554. [Google Scholar] [CrossRef]
- Rugh, C.L.; Senecoff, J.F.; Meagher, R.B.; Merkle, S.A. Development of Transgenic Yellow Poplar for Mercury Phytoremediation. Nat. Biotechnol. 1998, 16, 925–928. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.; Echevarria, G.; Simonnot, M.-O.; Morel, J.-L. Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030589035. [Google Scholar]
- Wang, H.; Chen, Z.; Feng, L.; Chen, Z.; Owens, G.; Chen, Z. Uptake and Transport Mechanisms of Rare Earth Hyperaccumulators: A Review. J. Environ. Manag. 2024, 351, 119998. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of Rare Earth Elements—A Review. Chemosphere 2022, 297, 134259. [Google Scholar] [CrossRef]
- Brown, R.M.; Struhs, E.; Mirkouei, A.; Raja, K.; Reed, D. Mixed Rare Earth Metals Production from Surface Soil in Idaho, USA: Techno-Economic Analysis and Greenhouse Gas Emission Assessment. Sci. Total Environ. 2024, 944, 173945. [Google Scholar] [CrossRef]
- Swamy, K.M.; Narayana, K.L.; Misra, V.N. Bioleaching with Ultrasound. Ultrason. Sonochem. 2005, 12, 301–306. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Ok, Y.S.; Shen, C.; Xue, S. Biochar Provides a Safe and Value-Added Solution for Hyperaccumulating Plant Disposal: A Case Study of Phytolacca Acinosa Roxb. (Phytolaccaceae). Chemosphere 2017, 178, 59–64. [Google Scholar] [CrossRef]
- Shan, X.; Wang, H.; Zhang, S.; Zhou, H.; Zheng, Y.; Yu, H.; Wen, B. Accumulation and Uptake of Light Rare Earth Elements in a Hyperaccumulator Dicropteris Dichotoma. Plant Sci. 2003, 165, 1343–1353. [Google Scholar] [CrossRef]
- Branquinho, C.; Serrano, H.C.; Pinto, M.J.; Martins-Loução, M.A. Revisiting the Plant Hyperaccumulation Criteria to Rare Plants and Earth Abundant Elements. Environ. Pollut. 2007, 146, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczak, P.; Borowiak, K.; Niedzielski, P. Phytoextraction of Rare Earth Elements in Herbaceous Plant Species Growing Close to Roads. Environ. Sci. Pollut. Res. 2017, 24, 14091–14103. [Google Scholar] [CrossRef]
- Jalali, J.; Gaudin, P.; Ammar, E.; Lebeau, T. Bioaugmentation Coupled with Phytoextraction for the Treatment of Cd and Sr, and Reuse Opportunities for Phosphogypsum Rare Earth Elements. J. Hazard. Mater. 2020, 399, 122821. [Google Scholar] [CrossRef]
- Zhiqiang, C.; Zhibiao, C. Clipping Strategy to Assist Phytoremediation by Hyperaccumulator Dicranopteris Dichotoma at Rare Earth Mines. Int. J. Phytoremediat. 2020, 22, 1038–1047. [Google Scholar] [CrossRef]
- Liu, W.-S.; Chen, Y.-Y.; Huot, H.; Liu, C.; Guo, M.-N.; Qiu, R.-L.; Morel, J.L.; Tang, Y.-T. Phytoextraction of Rare Earth Elements from Ion-Adsorption Mine Tailings by Phytolacca americana: Effects of Organic Material and Biochar Amendment. J. Clean. Prod. 2020, 275, 122959. [Google Scholar] [CrossRef]
- Mganga, N.; Manoko, M.; Rulangaranga, Z. Classification of Plants According to Their Heavy Metal Content around North Mara Gold Mine, Tanzania: Implication for Phytoremediation. Tanzan. J. Sci. 2011, 37, 109–119. [Google Scholar]
- Grosjean, N.; Le Jean, M.; Berthelot, C.; Chalot, M.; Gross, E.M.; Blaudez, D. Accumulation and Fractionation of Rare Earth Elements Are Conserved Traits in the Phytolacca Genus. Sci. Rep. 2019, 9, 18458. [Google Scholar] [CrossRef]
- Mohsin, M.; Salam, M.M.A.; Nawrot, N.; Kaipiainen, E.; Lane, D.; Wojciechowska, E.; Kinnunen, N.; Heimonen, M.; Tervahauta, A.; Peräniemi, S.; et al. Phytoextraction and Recovery of Rare Earth Elements Using Willow (Salix spp.). Sci. Total Environ. 2021, 809, 152209. [Google Scholar] [CrossRef]
- Dhiman, S.; Zhao, X.; Li, J.; Kim, D.; Kalia, V.; Kim, I.-W.; Kim, J.; Lee, J.-K. Metal Accumulation by Sunflower (Helianthus annuus L.) and the Efficacy of Its Biomass in Enzymatic Saccharification. PLoS ONE 2017, 12, e0175845. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Yusoff, I.; Abu Bakar, N.K.; Abu Bakar, A.F.; Alias, Y.; Mispan, M. Accumulation, Uptake and Bioavailability of Rare Earth Elements (REEs) in Soil Grown Plants from Ex-Mining Area in Perak, Malaysia. Appl. Ecol. Environ. Res. 2017, 15, 117–133. [Google Scholar] [CrossRef]
- Tabasi, S.; Hassani, H.; Azadmehr, A.R. Field Study on Re and Heavy Metal Phytoextraction and Phytomining Potentials by Native Plant Species Growing at Sarcheshmeh Copper Mine Tailings, SE Iran. J. Min. Environ. 2018, 9, 183–194. [Google Scholar] [CrossRef]
- Turra, C.; De Nadai Fernandes, E.A.; Bacchi, M.A.; Sarries, G.A.; Lai Reyes, A.E. Uptake of Rare Earth Elements by Citrus Plants from Phosphate Fertilizers. Plant Soil 2019, 437, 291–299. [Google Scholar] [CrossRef]
- Okoroafor, P.U.; Kunisch, N.; Epede, M.N.; Ogunkunle, C.O.; Heilmeier, H.; Wiche, O. Phytoextraction of Rare Earth Elements, Germanium and Other Trace Elements as Affected by Fertilization and Liming. Environ. Technol. Innov. 2022, 28, 102607. [Google Scholar] [CrossRef]
- Wei, S.H.; Zhou, Q.X.; Wang, X.; Zhang, K.S.; Guo, G.L.; Ma, Q.Y.L. A Newly-Discovered Cd-Hyperaccumulator Solanum nigrum L. Chin. Sci. Bull. 2005, 50, 33–38. [Google Scholar] [CrossRef]
- Wiche, O.; Heilmeier, H. Germanium (Ge) and Rare Earth Element (REE) Accumulation in Selected Energy Crops Cultivated on Two Different Soils. Miner. Eng. 2016, 92, 208–215. [Google Scholar] [CrossRef]
- Bare, W.F.R.; Struhs, E.; Mirkouei, A.; Overturf, K.; Chacón-Patiño, M.L.; McKenna, A.M.; Chen, H.; Raja, K.S. Controlling Eutrophication of Aquaculture Production Water Using Biochar: Correlation of Molecular Composition with Adsorption Characteristics as Revealed by FT-ICR Mass Spectrometry. Processes 2023, 11, 2883. [Google Scholar] [CrossRef]
- EPA Method 3050B No. SW-846; Test Methods for Evaluating Solid Waste, Physical/Chemical Methods: Acid Digestion of Sediments, Sludges, and Soils. Environmental Protection Agency (EPA): Washington, DC, USA, 1996.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Hong, Z.; Zhong, F.; Niu, W.; Zhang, K.; Su, J.; Liu, J.; Li, L.; Wu, F. Effects of Temperature and Particle Size on the Compositions, Energy Conversions and Structural Characteristics of Pyrolysis Products from Different Crop Residues. Energy 2020, 190, 116413. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Emission Factors for Greenhouse Gas Inventories; United States Environmental Protection Agency: Washington, DC, USA, 2024.
- Zheng, C.; Wang, X.; Liu, J.; Ji, X.; Huang, B. Biochar-Assisted Phytoextraction of Arsenic in Soil Using Pteris vittata L. Environ. Sci. Pollut. Res. 2019, 26, 36688–36697. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, J.; Tlustos, P.; Szakova, J. Comparison of Willow and Sunflower for Uranium Phytoextraction Induced by Citric Acid. J. Radioanal. Nucl. Chem. 2010, 285, 279–285. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; van Balgooy, M.M.J.; Tjoa, A. Ultramafic Nickel Laterites in Indonesia (Sulawesi, Halmahera): Mining, Nickel Hyperaccumulators and Opportunities for Phytomining. J. Geochem. Explor. 2013, 128, 72–79. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, M.-N.; Liu, W.-S.; Liu, C.; van der Ent, A.; Morel, J.L.; Huot, H.; Zhao, W.-Y.; Wei, X.-G.; Qiu, R.-L.; et al. The Accumulation and Fractionation of Rare Earth Elements in Hydroponically Grown Phytolacca americana L. Plant Soil 2017, 421, 67–82. [Google Scholar] [CrossRef]
- Wiche, O.; Zertani, V.; Hentschel, W.; Achtziger, R.; Midula, P. Germanium and Rare Earth Elements in Topsoil and Soil-Grown Plants on Different Land Use Types in the Mining Area of Freiberg (Germany). J. Geochem. Explor. 2017, 175, 120–129. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad, M.N.V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D.; et al. Role of Rare Earth Elements in Plants. Plant Mol. Biol. Report. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Gibson, M.J. Understanding Soil pH. Available online: https://extension.psu.edu/understanding-soil-ph (accessed on 23 March 2025).
- Gavlak, R.G.; Horneck, D.A.; Miller, R.O. Plant, Soil, and Water Reference Methods for the Western Region; Western Rural Development Center: Logan, UT, USA, 1994. [Google Scholar]
- Cunniff, P.; Washington, D. Official methods of analysis of AOAC International. J. AOAC Int. 1997, 80, 127A. [Google Scholar]


| Study | Objective | Elements | Plant Species | Substrate Type | Treatment | |
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| [19] | ✗ | ✓ | La, Ce, Pr, Nd | Dicropteris dichotoma | Soil from REE ore deposit | Histidine, citric acid, malic acid |
| [20] | ✗ | ✓ | Al, Fe, K, Ca, Mg, Mn Ni, Zn, Cr, Pb, Co, Cu, Cd | Plantago almogravensis | Podzol | - |
| [21] | ✗ | ✓ | Lanthanides (Atomic # 57-71) | Achillea millefolium, Artemisia vulgaris, Papaver rhoeas, Taraxacum officinale, Tripleurospermum inodorum | Roadside-sourced soil | - |
| [22] | ✗ | ✓ | Cd, Ce, La, Nd, Sr, Y | Trifolium pratense, Helianthus annuus | Phosphogypsum compost mix | Bacillus cereus |
| [23] | ✗ | ✓ | Lanthanides | Dicranopteris dichotoma | Soil from rare earth mines | - |
| [24] | ✗ | ✓ | Lanthanides | Phytolacca americana | Soil with REE mine tailings | Manure and sawdust mixture, biochar |
| [25] | ✗ | ✓ | Cu, Pb, Cr, Zn, Cd, Ni | Ludwigia stolonifera, Sphaeranthus gomphrenoides, Leersia hexandra, Commelina benghalensis, Sphaeranthus kirkii, Typha capensis, Cyperus articulantus, Fuirena umbellate, Agave sisalana, Cyperus exaltatus, Crinum papilosum, Hoslundia opposita, Pluchea dioscoridis, Hygrophylla auricultata, Ipomoea batata | Heavy metal contaminated soil | - |
| [26] | ✗ | ✓ | Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | Phytolacca americana, P. acinose, P. clavigera, P. bogotensis, P. isosandra | Murashige and Skoog medium, quarter-strength Hoagland nutrient solution | H2SO4, REE tri-chloride salt |
| [27] | ✗ | ✓ | La, Y, Nd, Dy, Ce, Tb | Salix myrsinfolia, S. shwerinii | Hydroponically grown | REE-enriched tap water |
| [28] | ✓ | ✓ | As, Cd, Cu, Ni, Pb, Zn | Helianthus annuus | Soil with various heavy metal concentrations | none |
| [29] | ✗ | ✓ | Sc, Y, La, Ce, Pr, Nd, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu | Dicranopterus dichotoma, D. linearis, Melastoma malabathricum, Cyperus difformis, C. kyllingia. C. distans, C. rotundus | Soil from a former mining area | - |
| [30] | ✓ | ✓ | As, Cu, Mo, Ni, Zn, Re | Arundo donax, Tamarix ramosissima, Salsola kali, Cynodon dactylon, Chenopodium album, Atriplex leucoclada, Zygophyllum fabago | Soil with mine tailings | - |
| [31] | ✗ | ✓ | La, Ce, Nd, Sm, Eu, Tb, Yb, Lu, Sc | Citrus limonia | Commercial substrate | Superphosphate fertilizer |
| [32] | ✓ | ✓ | Lanthanides, Ge | Lupinus albus, Brassica napus, Zea mays | Soil containing trace REEs and Ge | Fertilizer, lime |
| This study | ✓ | ✓ | Ce, La, Nd, Y, Pr | P. arundinaceae, S. nigrum, P. americana, B. juncea | REE-rich Idaho-sourced soil | Fertilizer, biochar, citric acid |
| Objective | Evaluate REE Uptake Across Plant Species and Soil/Water Treatments |
|---|---|
| Species | Phalaris arundinacea, Solanum nigrum, Phytolacca americana, Brassica juncea |
| Experimental units | 72 pots total, 18 per species, 3 replications of each species–soil–water combination |
| Soil treatment | Non-treated vs. fertilizer vs. biochar |
| Water treatment | Non-treated vs. citric acid |
| Greenhouse conditions | 18–32 °C controlled temperature, 16:8 h light:dark cycle with minimum 308 µmol/m2/s light intensity |
| Duration | 6 weeks from germination to harvest |
| Sample processing | Biomass washed, dried, pyrolyzed, and acid-digested |
| Sample analysis | Via ICP-MS |
| Elements analyzed | REEs including Ce, La, Nd, Pr, Y |
| Postanalysis evaluation | Life cycle analysis and techno-economic analysis |
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| pH | 7.6 | Calcium (meq/100 g) | 12.4 |
| Sand % | 47.3 | Magnesium (meq/100 g) | 3.4 |
| Silt % | 39.5 | Sulfate-S (µg/g) | 3.0 |
| Clay % | 13.2 | Zinc (µg/g) | 1.3 |
| Salts (mmhos/cm) | 0.6 | Iron (µg/g) | 9.2 |
| Chlorides (µg/g) | 7.0 | Manganese (µg/g) | 1.5 |
| Sodium (meq/100 g) | 0.2 | Copper (µg/g) | 0.1 |
| CEC (meq/100 g) | 16.3 | Boron (µg/g) | 0.2 |
| Excess lime (%) | 3.2 | Cerium (µg/g) | 2889 |
| Organic matter (%) | 2.8 | Lanthanum (µg/g) | 2071 |
| Organic N (lb/Acre) | 55.0 | Neodymium (µg/g) | 1690 |
| Ammonium-N (µg/g) | 2.6 | Praseodymium (µg/g) | 435 |
| Nitrate-N (µg/g) | 5.0 | Yttrium (µg/g) | 808 |
| Phosphorus (µg/g) | 5.0 | Total mixed target REEs (µg/g) | 7893 |
| Potassium (µg/g) | 97.0 |
| Plant Species | Success Rate (out of 18) | Treatment # | Soil Treatment | Water Treatment | Avg Bio-ore Mass (g) | Ce | La | Nd | Pr | Y | Total Target REE | Total Mixed REE | Target REE BF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phalaris arundinacea (reed canary grass) | 22% (4) | 1 | Non-treated | Non-treated | 0.3 | 2820 | 74 | 1567 | 420 | 1895 | 6676 | 9121 | 0.79 |
| 2 | 1% CA | - | - | - | - | - | - | - | - | - | |||
| 3 | Fertilizer | Non-treated | 4.5 | 14,930 | 392 | 7236 | 1858 | 11,623 | 36,041 | 47,830 | 4.72 | ||
| 4 | 1% CA | 4.2 | 15,977 | 391 | 7800 | 2009 | 13,726 | 39,904 | 52,910 | 4.77 | |||
| 5 | Biochar | Non-treated | 1.6 | 11,170 | 245 | 6460 | 1564 | 9100 | 29,138 | 41,513 | 4.40 | ||
| Average | 2.7 | 11,224 | 276 | 5766 | 1463 | 9086 | 27,940 | 37,844 | 3.67 | ||||
| Solanum nigrum (black nightshade) | 61% (11) | 1 | Non-treated | Non-treated | 0.5 | 11,950 | 282 | 5245 | 1428 | 6020 | 24,924 | 32,309 | 2.6 |
| 2 | 1% CA | - | - | - | - | - | - | - | - | - | |||
| 3 | Fertilizer | Non-treated | 3.6 | 2653 | 84 | 1390 | 367 | 2890 | 7384 | 9349 | 1.00 | ||
| 4 | 1% CA | 6.0 | 3362 | 78 | 1569 | 419 | 2521 | 7948 | 10,204 | 1.32 | |||
| 5 | Biochar | Non-treated | 6.4 | 5017 | 104 | 2577 | 668 | 3322 | 11,690 | 16,072 | 1.20 | ||
| Average | 4.1 | 5746 | 137 | 2695 | 721 | 3688 | 12,987 | 16,984 | 1.53 | ||||
| Phytolacca americana (pokeweed) | 50% (9) | 1 | Non-treated | Non-treated | 0.4 | 1731 | 30 | 1060 | 276 | 1467 | 4562 | 6165 | 0.53 |
| 2 | 1% CA | 7.4 | 1702 | 68 | 994 | 251 | 1579 | 4594 | 6163 | 0.61 | |||
| 3 | Fertilizer | Non-treated | 1.1 | 507 | 0 | 277 | 76 | 769 | 1625 | 2072 | 0.19 | ||
| 4 | 1% CA | 7.1 | 1523 | 540 | 738 | 210 | 1487 | 4500 | 5429 | 0.60 | |||
| 5 | Biochar | Non-treated | 0.8 | 1644 | 70 | 922 | 235 | 1091 | 3962 | 5382 | 0.57 | ||
| Average | 3.4 | 1421 | 142 | 798 | 210 | 1279 | 3849 | 5042 | 0.5 | ||||
| Brassica juncea (brown mustard) | 61% (11) | 1 | Non-treated | Non-treated | 3.5 | 266 | 6 | 142 | 37 | 270 | 720 | 950 | 0.08 |
| 2 | 1% CA | - | - | - | - | - | - | - | - | - | |||
| 3 | Fertilizer | Non-treated | 8.9 | 566 | 28 | 318 | 76 | 675 | 1666 | 2200 | 0.29 | ||
| 4 | 1% CA | 10.9 | 1099 | 28 | 553 | 147 | 980 | 2808 | 3629 | 0.51 | |||
| 5 | Biochar | Non-treated | 3.7 | 288 | 64 | 163 | 41 | 284 | 840 | 1108 | 0.16 | ||
| Average | 6.8 | 555 | 32 | 294 | 75 | 552 | 1508 | 1972 | 0.26 | ||||
| Impact Category | Unit | Greenhouse Study | Field Study |
|---|---|---|---|
| Global warming | kg CO2 eq. | 69.11 | 14.28 × 102 |
| Smog | kg O3 eq. | 1.41 × 10−2 | 6.48 |
| Respiratory effects | kg PM2.5 eq. | 8.89 × 10−5 | 4.10 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, K.; Mirkouei, A.; Duellman, K.; Aylward, A.; Zirker, D.; Schwarz, E.; Sun, Y. Extraction of Rare Earth Elements from Idaho-Sourced Soil Through Phytomining: A Case Study in Central Idaho, USA. Sustainability 2025, 17, 5118. https://doi.org/10.3390/su17115118
Richardson K, Mirkouei A, Duellman K, Aylward A, Zirker D, Schwarz E, Sun Y. Extraction of Rare Earth Elements from Idaho-Sourced Soil Through Phytomining: A Case Study in Central Idaho, USA. Sustainability. 2025; 17(11):5118. https://doi.org/10.3390/su17115118
Chicago/Turabian StyleRichardson, Kathryn, Amin Mirkouei, Kasia Duellman, Anthony Aylward, David Zirker, Eliezer Schwarz, and Ying Sun. 2025. "Extraction of Rare Earth Elements from Idaho-Sourced Soil Through Phytomining: A Case Study in Central Idaho, USA" Sustainability 17, no. 11: 5118. https://doi.org/10.3390/su17115118
APA StyleRichardson, K., Mirkouei, A., Duellman, K., Aylward, A., Zirker, D., Schwarz, E., & Sun, Y. (2025). Extraction of Rare Earth Elements from Idaho-Sourced Soil Through Phytomining: A Case Study in Central Idaho, USA. Sustainability, 17(11), 5118. https://doi.org/10.3390/su17115118







