Abstract
Given the growing interest of the rubber industry in seeking alternatives that contribute to environmental sustainability, this work aims to present a study of the mechanical, thermal, and structural properties of natural rubber composites using tannin extracted from Acacia mearnsii as an antioxidant agent. Tannin is a natural and biodegradable product, rich in polyphenols and known for its antioxidant properties. The analyses assessed the effectiveness of incorporating tannins (0, 1, 1.5, and 2 parts per hundred rubber) into sulfur-crosslinked natural rubber composites using a binary accelerator system across three distinct vulcanization schemes: conventional, semi-efficient, and efficient. Initially, tannin characterization tests were conducted, revealing characteristic polyphenol bands of proanthocyanidin catechins, a high total phenolic content, and a substantial reduction in antioxidant activity. These findings highlight the significant antioxidant potential of tannins, particularly for industrial and biological applications. The analyses of the characteristics of natural rubber composites with tannin incorporation indicated that the type of vulcanization process directly affects the antioxidant action of the plant tannin, with the tannins being most effective in the efficient system due to the formation of monosulfidic and disulfidic bonds. Furthermore, the incorporation of tannin did not compromise the physical and chemical properties of the materials, highlighting it as a viable additive for the rubber industry.
1. Introduction
Natural rubber (NR) is a material widely used in various industrial applications due to its excellent elastic properties, abrasion resistance, and flexibility [1]. However, one of the significant challenges in using NR is its susceptibility to oxidative aging, which leads to the degradation of its physical and mechanical properties over time [2]. This aging process is triggered by factors that enhance additional reactions, such as exposure to oxygen, ozone, sunlight, and heat, which react with the unsaturated carbon-carbon double bonds present in the rubber’s chemical structure, resulting in the loss of elasticity and strength [3,4].
To mitigate the effects of oxidative aging, the rubber industry traditionally uses synthetic antioxidants, such as phenolics like 2,6-di-tert-butyl-4-methylphenol (Butylated Hydroxytoluene, BHT); 3,4,5-Trihydroxybenzoic acid (Gallic Acid); Tetrakis [methylene (3,5-di-tert-butyl-4-hydroxyhydrocinnamate)] methane (Irganox 1010); among others, and amine-based antioxidants like N-isopropyl-N′-phenyl-p-phenylenediamine (antioxidant RD); Benzenamine, 4-octyl-N-(4-octylphenyl) (antioxidant ODA); diaryl-p-phenylene diamine (antioxidant DAPD); among others [5,6,7]. These compounds are effective in preventing degradation but raise significant environmental concerns due to their production process and potential toxicity [8]. In response to these concerns, there has been a growing interest in the development and use of natural antioxidants, which are more sustainable and environmentally friendly [9].
Recent studies have shown that, in addition to cellulose and lignin, traditionally used as filler, oak bark (Quercus cortex) can be utilized as such in rubber composites due to its high content of tannins, flavonoids, and phenolic acids, which promote anti-aging action [10]. In this research conducted by Smejda-Krzewicka et al., both modified and unmodified oak bark were investigated, with the modification performed using n-octadecyltrimethoxysilane. It was observed that the composites containing bark exhibited a significant increase in crosslink density. The mechanical properties were excellent with the addition of bark, particularly the modified bark, which achieved superior tensile strength. The inclusion of bark resulted in improved aging resistance, while the modification increased the hydrophobicity of the samples. Thus, oak bark proved to be a promising eco-friendly filler option with anti-aging properties for elastomeric composites [10].
Öncel et al. [11] evaluated the performance of henna as a natural antioxidant in sulfur vulcanization systems, both efficient and conventional, analyzing rheological, mechanical, aging, and stress relaxation properties over a temperature sweep. The researchers concluded that henna could be a viable alternative to synthetic antioxidants, offering equivalent protection against aging, especially at moderate temperatures and for short to medium aging periods. Henna did not significantly alter the curing characteristics and maintained tensile strength retention comparable to traditional antioxidants.
Another similar study, conducted by Shuhaimi et al. [12], investigated the effect of different vulcanization systems on the thermal degradation of natural rubber (NR) compounds in the presence of natural antioxidants (NA) obtained from palm leaves. The researchers used efficient (EV), semi-efficient (SEV), and conventional (CV) vulcanization systems, comparing the performance of NA with the commercial antioxidant trimethyl quinoline (TMQ). The results showed that the SEV and EV systems exhibited only a slight reduction in mechanical properties after aging, while the CV system showed a significant reduction due to the breakdown of polysulfidic bonds. They concluded that NA is an effective substitute for commercial antioxidants in NR compounds, especially in the SEV system, providing better mechanical properties and moderate curing characteristics.
Given the above, it is evident that there is a need to conduct a more comprehensive study on the impact of antioxidants in different vulcanization systems. This work aims to evaluate the mechanical, thermal, and structural properties of natural rubber composites using tannin extracted from Acacia mearnsii as an antioxidant. Tannins, polyphenols present in various plants, are known for their antioxidant properties, with Acacia mearnsii being an abundant source of these compounds. In this context, this study aims to investigate the efficacy of tannins in three vulcanization systems—conventional, semi-efficient, and efficient—comparing their properties with each other.
The choice of Acacia mearnsii tannin as an antioxidant is supported by several reasons. First, tannins are natural and biodegradable, making them environmentally sustainable. Additionally, previous studies have demonstrated their antioxidant efficacy and ability to enhance oxidative stability and mechanical properties of various polymeric materials [13,14]. The incorporation of tannins into NR vulcanization systems can not only improve aging resistance but also contribute to environmental sustainability in the rubber industry [15].
2. Materials and Methodological Procedure
2.1. Materials
For this study, Vietnamese natural rubber (VRS CV60) with Mooney viscosity ML (1′ + 4′) 100 °C, supplied by Dakruco Ltd., Vietnam, was used. The antioxidant employed was plant tannin extracted from black acacia (Acacia mearnsii) in the form of anionic powder, beige-colored and hygroscopic, with an average pH of 5.0 (in 20% w/v aqueous solution), active matter content above 93.5%, and density of 1.47 g ml−1, supplied by Tanac S.A., Brazil. Other chemical reagents such as zinc oxide (99.8%, AS, neon, Suzano, SP, Brazil), stearic acid (95%, AS, Scientific Exotic, São Paulo, SP, Brazil), sulfur (99.5%, Scientific Exotic), vulcanization accelerators benzothiazole disulfide/MBTS (99%, Basile Química, São Paulo, SP, Brazil), and tetramethyl thiuram disulfide/TMTD (99%, Basile Química) were commercially sourced.
2.2. Formulation and Preparation of Composites
The antioxidant properties of plant tannin derived from black acacia were evaluated in natural rubber composites crosslinked by three different vulcanization systems: conventional, semi-efficient, and efficient. In each vulcanization type, the amount of antioxidants was varied. The formulation of each vulcanization system is detailed in Table 1. It is emphasized that the composition of the produced composites is expressed in parts per hundred rubbers (phr).

Table 1.
Formulation of NR/Tan composites.
The compounds were prepared using an open two-roll mill, following the guidelines of ASTM D3182-21a [16], with a friction ratio of 1:1.25. Natural rubber, reaction activators (zinc oxide and stearic acid), and the antioxidant tannin were added to the mill in proportions of 0, 1.0, 1.5, and 2.0 phr. After homogenizing the mixture for 20 min, it was allowed to rest for 24 h at an ambient temperature of 23 °C. Subsequently, the mixture was reintroduced into the mill for the addition of the crosslinking agent (sulfur) and vulcanization accelerators (MBTS and TMTD). After homogenizing for 15 min, the mixture was left to rest for 2 h at room temperature. Upon completing these steps, the composite was subjected to rheometric testing and then processed in a hot press to fabricate test specimens.
2.3. Characterization of Black Acacia Plant Tannin Antioxidant
Analysis by gel permeation chromatography (GPC) determined the molecular weight distribution of tannin, employing high-performance liquid chromatography (HPLC) using an Agilent Technologies 1260 Infinity II Quaternary System chromatograph equipped with a Refractive Index Detector (RID) model G7162A and a UV detector (256 nm). The GPC column used in the experiments was an Agilent Technologies pLgel MIXED B. Polystyrene (PS) standards ranging from 161 to 65.7 × 105 g mol−1 (Agilent—Polystyrene High EasiVials 4 mL, Santa Clara, CA, USA) were used to construct the calibration curve. Approximately 3 to 5 mg of tannin were dissolved in 10 mL of THF (mobile phase). For the analysis, 20 µL of the solution was injected at a flow rate of 1 mL min−1 and a pressure of 22 bar, with a temperature of 40 °C for the isocratic pump and 40 °C for the Refractive Index Detector. Additionally, Fourier transform infrared spectroscopy (FTIR) was performed to characterize the tannin samples. Total phenolic content (TPC) was determined by the Folin–Ciocalteu colorimetric method, as described by Swain et al. [17]. Antioxidant activity was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical assay, following the protocol of Brand-Williams et al. [18].
2.4. Characterization of Natural Rubber Composites
2.4.1. Rheometric Analysis
The rheometric parameters were obtained using a rotational disk rheometer from Team Equipamentos. Tests were conducted according to ASTM D2084-19a [19], where the composites underwent a 1° arc oscillation and 150 °C isotherms. With the rheometric parameters measured, the NR and tannin compounds were subjected to a compression molding process using the Mastermac Vulcan 400/20-1 press, with a maximum pressure of 210 kg/cm−2 and using a 1010/1020 steel mold (150 × 150 × 2 mm).
2.4.2. Determination of Specific Density of Composites
The determination of composite density was obtained according to ASTM D297-21 [20] using ethanol with a density of 0.85 g cm−3 and calculated by Equation (1):
where ρ is the density of the sample (g cm−3); ρL represents the density of ethanol at the analysis temperature (g cm−3); mA is the mass of the sample in air (g), and mB is the mass of the sample in liquid (g).
2.4.3. Determination of Crosslink Density by the Flory–Rehner Method
The crosslink density of the composites was calculated using the swelling technique. Specimens weighing approximately 0.25 ± 0.05 g were immersed in toluene for 5 days. They were then removed, dried to remove excess solvent, and weighed again. Subsequently, they were dried in an oven at 80 °C for 24 h and weighed. The resulting values of the dry specimen mass, swollen specimen mass with solvent, and specimen mass after swelling were recorded and used to estimate the volumetric fraction of rubber in the swollen specimen. The crosslink density was then calculated using Equation (2), developed by Flory and Rehner [21]. The values used for the molar volume of toluene (V0) and the Flory–Huggins interaction parameter (χ) for natural rubber and toluene were 106.3 cm3 mol−1 and 0.393, respectively.
where ν is the crosslink density (mol cm−3), ρB is the density of rubber (g cm−3), and VB is the volume fraction of rubber in the swollen form, determined from the weight increase due to swelling.
2.4.4. Determination of Crosslink Density by the Mooney–Rivlin Method
The crosslink densities were also estimated using the Mooney–Rivlin method [22] based on tensile strength tests. The semi-empirical Equation (3) [23] was used to plot the graph in the linear region and obtain the network parameters.
where F is the force required in the vulcanized material; A0 is the cross-sectional area (mm2); λ is the extension ratio (1 + ɛ) where ɛ is the strain; C1 e C2 are material constants, with C1 assumed to be the contribution from crosslinking units, and C2 is the Mooney–Rivlin elastic constant representing the contribution from trapped entanglements.
The material constant C1 can be used to calculate the crosslink densities through Equation (4) [24]:
where η is the crosslink density (mol cm−3), R is the universal gas constant, and T is the absolute temperature (K).
2.4.5. Fourier Transform Infrared Spectroscopy (FTIR) in Attenuated Total Reflection (ATR) Mode
The FTIR spectroscopy was performed using a Bruker Vector 22 spectrometer in Attenuated Total Reflection (ATR) mode over the range of 4000–400 cm−1 with a spectral resolution of 4 cm−1 e 32 scans.
2.4.6. Accelerated Aging Process in Photodegradation Chamber
The characterizations were performed for the complete set of samples (0 weeks) and after 1 and 2 weeks of exposure to UV-C light, aiming to highlight the changes occurring in the substrate after photoexposure. The effectiveness of the tannin antioxidant property in the polymer matrix was analyzed through photodegradation, where the films were exposed to high-intensity UV-C light. Germicidal fluorescent lamps of 15 W, emitting predominantly at a wavelength of 254 nm with an incident energy of 610 ± 10 μWcm−2, were used. This UV range was chosen for its high energy, accelerating sample aging within a shorter testing period. Samples were exposed to UV-C light using a lab-made photodegradation chamber. They were positioned inside the chamber, 20 cm away from the UV-C lamps, aligned parallel to the lamp lengths for uniform testing. Exposure intervals included 1 and 2 weeks (approximately 168 and 336 h).
2.4.7. Wetting Analysis
Wettability analyses were performed using the sessile drop technique on a Ramé Hart goniometer, model 100-00, with deionized water. The process involved depositing 3 drops of water at different points on the surface, with each resulting in 10 contact angle measurements. Results were averaged from 30 measurements.
2.4.8. Determining the Hardness of the Composites
The determination of surface hardness in the composites was obtained according to ASTM D2240-15 [25] on the Shore A scale, using a Digimess analog durometer, with a range from 0 to 100 and a 1 Shore A graduation.
2.4.9. Determination of Abrasion Resistance of Composites
The abrasion loss was calculated using Equation (5), according to ASTM D5963-22 [26], using MaqTest equipment with an abrasion path equivalent to 40 m and a pressure on the specimen over the cylinder of 5 N.
where PA represents the abrasion loss (mm3/40 m); Δm is the mass loss of the composite (mg); S0 is the theoretical wear index of the abrasive on the standard rubber (200 ± 20 mg); S is the actual wear index of the abrasive on the standard rubber (mg); and ρ is the density of the composite (mg mm−3).
2.4.10. Tensile Strength Test
The stress-strain tests were performed using a Biopdi Universal testing machine manufactured in Brazil, with a speed of 500 mm min−1, a 5 kN load cell, and an internal deformation transducer. For these tests, five type A specimens (straight and dumbbell-shaped) were used according to ASTM D412-16 [27].
2.4.11. Tensile Strength Test with Accelerated Thermal Aging Process
The aging process of the samples was conducted according to the specifications of ASTM D573-04 [28]. The specimen was placed in a circulating air oven for 70 h at a temperature of 70 °C. Following this process, the samples were subjected to a tensile strength test to compare with the strength achieved by the non-aged samples.
2.4.12. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis (TGA) tests were conducted using a Netzsch instrument, model 209, over a temperature range from ambient (~25 °C) to 900 °C, with a heating rate of 10 °C min−1, under a nitrogen atmosphere at a flow rate of 20 mL min−1. Approximately 5 mg of sample mass was used for the measurements.
3. Results and Discussion
3.1. Characterization of the Antioxidant from Black Acacia Vegetable Tannin
The results of gel permeation chromatography (GPC), antioxidant activity (DPPH), and total phenolic content (TPC) analyses for the black acacia vegetable tannin antioxidant used in this study are shown in Table 2. The table includes values for weight-average molecular weight (MW), number-average molecular weight (MN), polydispersity index (PD), DPPH antioxidant activity reduction, and total phenolic content (TPC).

Table 2.
Results of GPC, DPPH, and TPC assays for black wattle tannin.
The results of the molecular weight distribution of tannin revealed MW and MN values of 345 and 89 g mol−1, respectively, close to the flavonoid catechin family. The low molecular weight of the tannin suggests that this sample underwent industrial processes that reduced its molecular chains. The high polydispersity (3.876) also reflects the heterogeneity resulting from the extraction of black acacia vegetable tannin [29]. The high value of DPPH antioxidant activity reduction suggests strong antioxidant capacity in the extracts [30]. This implies that the tannin acts as a hydrogen donor in the antioxidant process, converting free radicals into stable forms. This correlation between high antioxidant capacity and TPC is common, as phenolic compounds are known for their antioxidant properties. Therefore, both results indicate that tannin has significant potential as an antioxidant agent, which could have important implications for industrial and biological applications.
Figure 1 shows the spectrum of black acacia vegetable tannin. FTIR analysis revealed characteristic polyphenol bands of proanthocyanidin catechins, including axial deformations of O-H and aromatic C-H, axial deformations of C=C, and axial deformations of aliphatic C-H groups. Symmetric axial deformations of C-O-C, axial deformations of O-H and C-O, and out-of-plane angular deformations of aromatic C-H were also identified. The high total phenolic content (775 μg GAE mg−1) determined by the Folin–Ciocalteu method was consistent with the presence of phenolic groups identified in FTIR [31,32]. Transmittance peaks around 1610 cm−1 and 1429 cm−1 in the FTIR spectrum were associated with stretching vibrations of C=C bonds and O-H bonds, respectively, indicative of phenolic group presence in the tannin structure. These results are consistent with findings from previous studies by Falcão et al. [33], Irman et al. [34], and Hameed et al. [35].
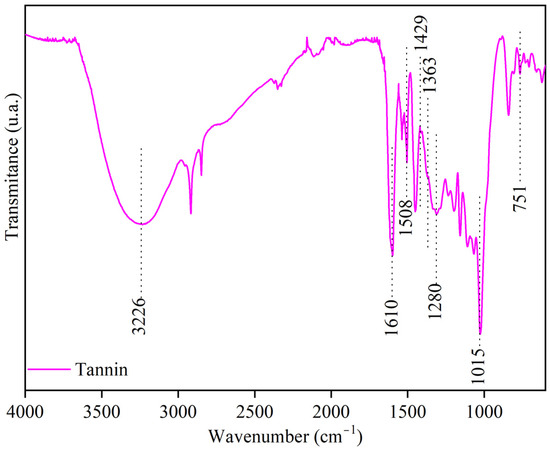
Figure 1.
Spectra of Black Acacia Vegetable Tannin.
3.2. Characterization Analysis of Natural Rubber Composites
3.2.1. Analysis of Rheometric Parameters
Figure 2 presents the values of the rheometric parameters obtained from the rheometry tests, such as the minimum torque (ML), maximum torque (MH), and torque variation (ΔM); the scorch time (tS1) and the optimum cure time (t90); as well as the cure rate index (CRI).
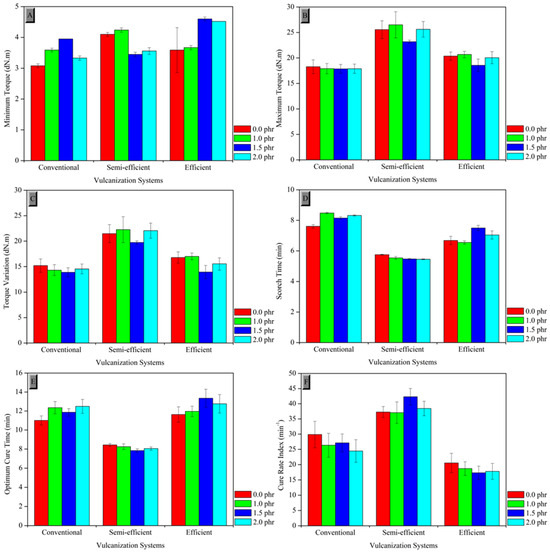
Figure 2.
Rheometric parameters of the composites in their respective curing systems: (A) minimum torque, (B) maximum torque, (C) torque variation, (D) scorch time, (E) optimum cure time, and (F) cure rate index.
The tannin extracted from black acacia acts as an antioxidant, preventing oxidative degradation of the composite; however, its proportions influence the viscoelastic properties and crosslinking structure. In the conventional vulcanization (CV) system, the minimum torque increased up to 1.5 phr of tannin, indicating greater initial strength, but decreased at 2 phr due to possible plasticization. The maximum torque remained stable at around 18 dNm, suggesting that tannin does not significantly affect the degree of crosslinking. Torque variation initially decreased, reflecting reduced stiffness, but increased at higher concentrations, indicating enhanced final mechanical strength. The scorch time increased up to 1 phr and then stabilized, while the optimum cure time (t90) increased continuously, demonstrating the retarding effect of tannin on curing. The cure rate index (CRI) decreased with higher tannin levels, promoting a more controlled and homogeneous curing process.
In the semi-efficient vulcanization (SEV) system, the minimum torque increased up to 1 phr of tannin, decreased at 1.5 phr, and rose again at 2 phr, indicating greater initial strength at lower concentrations and a plasticizing effect at intermediate levels. The maximum torque followed a similar trend, suggesting a more rigid network at 1 phr, less efficient crosslinking at 1.5 phr, and stability at 2 phr. Torque variation mirrored this pattern, highlighting a balance between stiffness and flexibility at low and intermediate concentrations. The scorch time (ts1) slightly decreased up to 1.5 phr and remained constant at 2 phr, indicating minimal impact of tannin on processing safety. The optimum cure time (t90) decreased up to 1.5 phr, promoting faster curing, and stabilized at 2 phr. The cure rate index (CRI) increased up to 1.5 phr, reaching 42.32, demonstrating faster curing, ideal for reducing processing times without compromising control.
In the efficient vulcanization (EV) system, the minimum torque increased up to 1.5 phr of tannin (4.60 dNm) and remained elevated at 2 phr (4.52 dNm), indicating greater initial strength and compatibility with tannin. The maximum torque showed a slight reduction at 1.5 phr, returning to initial values at 2 phr, demonstrating the system’s lower sensitivity to tannin concentration in forming the crosslinked network. Torque variation followed a pattern of increase up to 1 phr, reduction at 1.5 phr, and a subsequent rise at 2 phr, highlighting the antioxidant effect of tannin on structural strength. The scorch time (ts1) increased up to 1.5 phr, suggesting a retardation effect at moderate concentrations, with a slight decrease at 2 phr. The optimum cure time (t90) rose from 11.63 to 13.35 min at 1.5 phr and remained high at 2 phr, indicating slower curing due to tannin’s interaction with vulcanization agents. The cure rate index (CRI) decreased from 20.56 to 17.33, promoting more controlled crosslinking, ideal for achieving specific mechanical properties and greater homogeneity.
Therefore, rheometric analysis reveals that tannin exerts distinct influences on the CV, SEV, and EV vulcanization systems. The addition of 1 phr of tannin appears to be ideal in many aspects, providing greater initial strength and efficient crosslinking without compromising the flexibility of the composites. However, higher concentrations (such as 1.5 phr) may lead to slight plasticization, particularly in the CV and SEV systems, whereas the EV system demonstrates greater robustness against concentration variations. In conventional systems (CV), tannin acts as a cure retarder, increasing scorch and optimum cure times while reducing the cure rate. This effect can be advantageous for processes requiring slower and more controlled curing. In the SEV system, tannin initially accelerates the cure rate, particularly at intermediate concentrations, enabling faster and more efficient curing. In the EV system, tannin decreases the cure rate, promoting controlled crosslinking and preventing scorch at higher concentrations.
3.2.2. Analysis of Specific Density
Figure 3 presents the specific density values of rubber composites in different crosslinking systems. It is observed that the tannin content tends to cause a slight increase in specific density. The increase in specific density can be attributed to the higher density of tannin (1.47 g mL−1) compared to natural rubber (approximately 1.0 g mL−1). When tannin is incorporated into the same volume of material, it contributes to the overall increase in specific density. This effect, although subtle, reflects the higher concentration of dense molecules within a predominantly less dense elastic matrix.
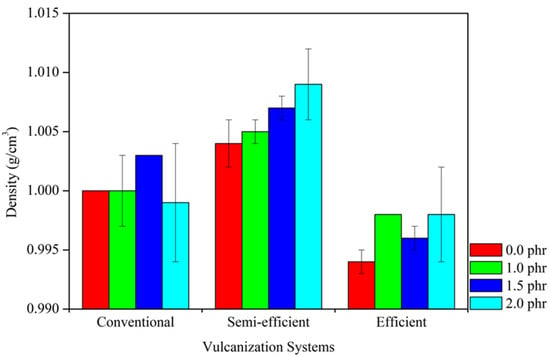
Figure 3.
Specific Density of Composites in Different Crosslinking Systems.
Additionally, among the different crosslinking systems, the semi-efficient system, followed by the conventional system, exhibited the highest specific density values, which is related to the small differences in the quantities of reagents added to the composite formulation. In the semi-efficient system, the formation of shorter disulfide and monosulfide bonds reduces matrix mobility and limits the permeation of organic solvents, therefore increasing the crosslink density. This more compact structure may result in a higher specific density compared to other systems.
3.2.3. Analysis of Crosslink Density by the Flory–Rehner Method and the Mooney–Rivlin Method
Table 3 presents the results of the crosslink densities obtained from the swelling tests in organic solvent (Flory–Rehner method) and the tensile strength tests (Mooney–Rivlin method). It is observed that, in both methodologies, the tannin content does not influence the crosslink density. However, among the different crosslinking systems, the semi-efficient system, followed by the efficient system, showed the highest crosslink density values in both methodologies. This may be related to the type of crosslinks formed in the SEV (semi-efficient) and EV (efficient) systems, which are predominantly monosulfidic and disulfidic linkages. These sulfur linkages between the polymer chains are shorter than the polysulfidic linkages found in the CV (conventional) system. The shortening of sulfur linkages tends to reduce the passage of organic solvent through the composite, which, according to the Flory–Rehner methodology, represents an increase in crosslink density. In the Mooney–Rivlin methodology, derived from the tensile strength tests, the mono- and disulfidic linkages tend to deform more compared to the polysulfidic ones. Thus, it can be inferred that in the SEV and EV systems, the shorter linkages tend to increase the crosslink density.

Table 3.
Crosslink density by the Flory–Rehner and Mooney–Rivlin methods.
3.2.4. Analysis of Fourier Transform Infrared Spectroscopy Before and After Exposure to UV-C Rays Through Accelerated Aging by Photodegradation
Figure 4 shows the spectra of NR/Tan composites at different tannin proportions and their respective crosslinking systems without exposure to UV-C rays. It was possible to observe structural similarity among the samples, with prominent peaks characteristic of natural rubber. The first functional group detected around 2918 cm−1 corresponds to symmetric axial deformation -C-H. Around 2847 cm−1, the peak corresponds to asymmetric deformation of C-H in CH3. The peak at 1539 cm−1 corresponds to symmetric axial deformation C=C. In the region of 1453 cm−1, the peak corresponds to angular deformation of CH2, and at 1375 cm−1, to symmetric angular deformation of CH3. Finally, near 820 cm−1, the peak refers to angular deformation C=CH. The mechanisms resulting from the vulcanization processes were also verified by the main bands in the spectra of Figure 3: stretching of O-H in the region of 3370 cm−1, angular deformation of C-H in the structure at 1140 cm−1, and S=O stretching in the region between 1069 and 1010 cm−1 [36]. There were no significant interferences in the spectra regarding the incorporation of vegetable tannin into the samples, possibly due to its low concentration relative to the total elastomeric mass.
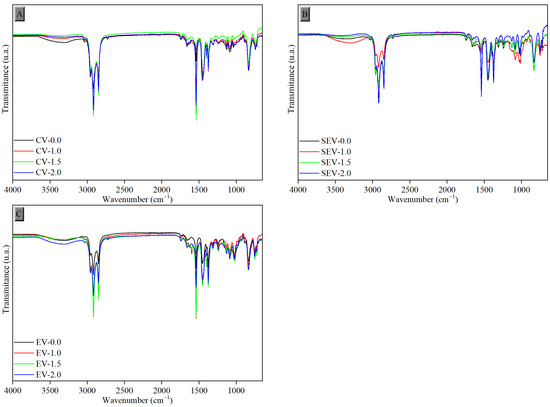
Figure 4.
FTIR-ATR spectra of the samples without exposure to UV-C radiation: (A) conventional system, (B) semi-efficient system, and (C) efficient system.
Figure 5, Figure 6 and Figure 7 present the FTIR results for monitoring the photodegradation process of the samples using FTIR-ATR spectroscopy over a period of exposure of up to two weeks under ultraviolet (UV-C) radiation in an oxidative environment (ambient air circulation). Figure 5 displays the spectra of the samples vulcanized by the conventional process (CV).
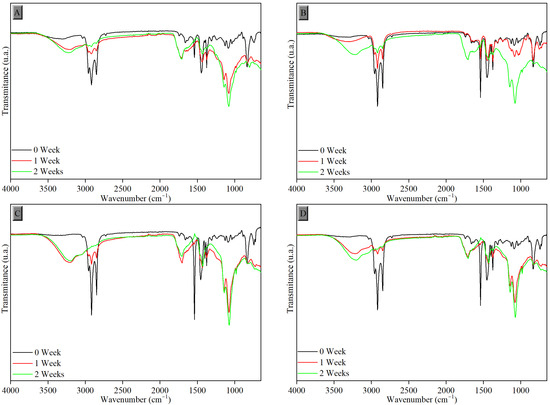
Figure 5.
FTIR-ATR spectra of samples vulcanized in the conventional system after exposure to UV-C radiation with tannin content of (A) 0.0 phr, (B) 1.0 phr, (C) 1.5 phr, and (D) 2.0 phr.
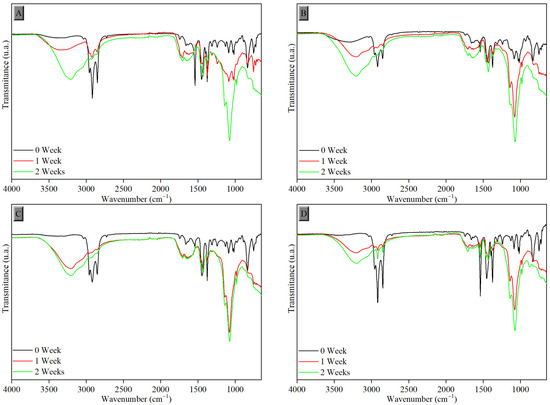
Figure 6.
FTIR-ATR spectra of samples vulcanized in the semi-efficient system after exposure to UV-C radiation with tannin content of (A) 0.0 phr, (B) 1.0 phr, (C) 1.5 phr, and (D) 2.0 phr.
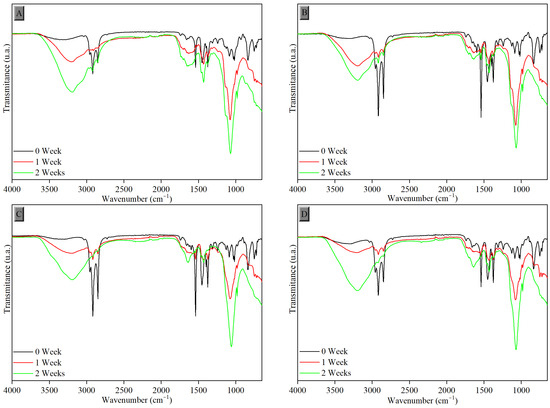
Figure 7.
FTIR-ATR spectra of samples vulcanized in the efficient system after exposure to UV-C radiation with tannin content of (A) 0.0 phr, (B) 1.0 phr, (C) 1.5 phr, and (D) 2.0 phr.
Through comparative observation, the results presented in all FTIR-ATR spectra of the samples in Figure 5 and Figure 6, before and after the degradation periods of 1 and 2 weeks, show the changes resulting from exposure to UV-C radiation, demonstrated by the emergence of some peaks.
The increased proportion of carbonyl groups, caused by the deformation of the carbonyl functional group (C=O) and stretching of epoxy groups (C-O-C) due to interaction with oxygen groups formed, can be observed in all samples after degradation in the regions around 1718 cm−1 and 1069 cm−1, respectively. The intensity of the broad peak in the region between 3500 and 3100 cm−1, attributed to the hydroxyl group (O-H), also progressively increased after the degradation process during weeks of photodegradation due to water adsorption. Peaks between 2918 and 2847 cm−1 and between 1539 and 1375 cm−1 were reduced, corresponding to C-H bonds of alkyl groups. All these changes confirm the degradation of the samples [37]. The same behavior was observed in samples vulcanized by the efficient process (EV), as shown in Figure 7.
The measurement of sample degradation degree was conducted through the study of the hydroxyl index. The formation of these groups was used to monitor structural changes in the polymeric samples during exposure to UV-C radiation, resulting from chain scission due to the degradation mechanism, as described below. The hydroxyl index (HI) was determined considering the bands corresponding to hydroxyl formation in the region between 3500 cm−1 and 3100 cm−1, and from this absorption, calculating the material oxidation level, as per Equation (6) [38]:
where A1 is the absorption area of the band between 3500 and 3100 cm−1, which corresponds to the peaks of hydroxyl groups, and A2 is the area around 3820 cm−1, as it remains unchanged and apparently is not affected by the effects of photodegradation. The result of the Hydroxyl Index (HI) in relation to the added tannin concentration is shown in Figure 8.
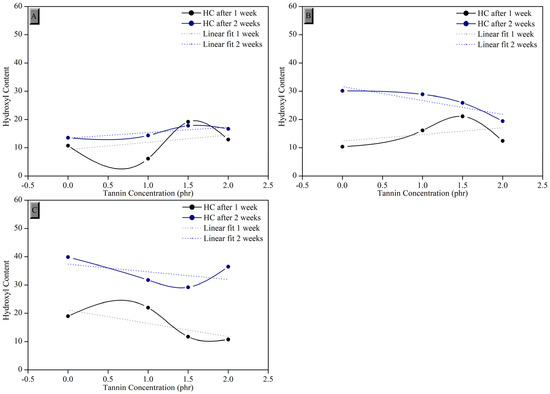
Figure 8.
Comparison of the hydroxyl index concerning the tannin concentration in the samples of composites crosslinked in the (A) conventional system, (B) semi-efficient system, and (C) efficient system.
Figure 8 presents the hydroxyl index (HI), a critical measure for evaluating the degree of oxidative degradation in natural rubber composites after exposure to UV-C radiation. This index reflects the formation of hydroxyl groups resulting from the oxidative cleavage of polymer chains. The HI analysis reveals that the antioxidant performance of tannin varies significantly among the vulcanization systems (CV, SEV, and EV) and with the incorporated tannin concentration.
In the conventional system (CV), a substantial increase in HI is observed over the exposure period, suggesting reduced antioxidant protection from tannin in this system. This behavior can be attributed to the predominance of polysulfidic bonds, which are more susceptible to thermal and photo-oxidative degradation. These long bonds fail to stabilize free radicals generated by UV interaction, allowing oxidation to propagate more intensively.
In the semi-efficient system (SEV), the HI shows an initial increase during the first week, followed by stabilization or a slight reduction in the second week, indicating moderate protection from tannin. This response may be linked to the combination of mono- and disulfidic bonds, which confer some chemical stability to the system while still allowing for localized oxidation.
Conversely, in the efficient system (EV), the lowest increase in HI is observed throughout the exposure period, demonstrating the superior antioxidant efficacy of tannin in this system. This outcome is related to the predominance of monosulfidic bonds, which are chemically more stable and less prone to degradation under UV radiation. The interaction between tannin and these shorter bonds creates a more effective antioxidant barrier, limiting free radical formation and propagation.
Furthermore, the efficacy of tannin as an antioxidant is directly related to its concentration. At lower levels (1.0 phr), tannin already exhibits a protective effect, but at higher concentrations (2.0 phr), the hydroxyl index demonstrates greater stability, especially in the EV system. This suggests that tannin effectively neutralizes free radicals and interacts with sulfhydryl groups, delaying oxidative degradation.
These findings underscore the importance of selecting the appropriate vulcanization system to maximize the efficacy of tannin as an antioxidant additive. While the CV system exhibits greater degradation due to less stable bonds, the EV system provides an optimal combination of structural stability and antioxidant protection. This analysis highlights the potential of tannin as a sustainable alternative to prolong the lifespan of rubber composites, particularly in applications exposed to adverse environmental conditions.
3.2.5. Analysis of Composite Wettability
Through contact angle analysis, it was possible to assess surface changes in hydrophobicity and/or hydrophilicity due to photodegradation. Figure 9 presents the water contact angle results for natural rubber composites before light exposure and after one week of accelerated aging. It is important to note that contact angle measurements could not be performed for samples exposed to light for two weeks, as the surface of these samples became highly hydrophilic, preventing measurements due to the rapid and complete spreading of the water drop on the sample surface.
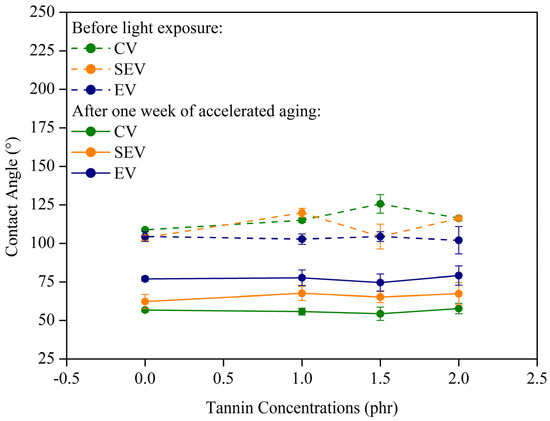
Figure 9.
Water contact angles of the surface of the composites before light exposure and after one week of accelerated aging.
Based on the data presented in Figure 9, it is evident that the contact angle of the samples underwent significant changes after light exposure. Samples that were initially hydrophobic became hydrophilic, a behavior attributed to the formation of polar groups on the sample surface, such as hydroxyl and carbonyl groups [39]. These chemical groups can decrease the surface energy, providing active sites for intermolecular interaction with water through hydrogen bonding [40]. It is also observed that the type of vulcanization process used directly interfered with the antioxidant action of tannin. The efficient process (EV) provided the least variation in contact angle values, indicating better overall photoprotection, with angles ranging from approximately 105° before light exposure to values close to 80°. In the semi-efficient process (SEV), values ranged from an average of 112° to 65°. The conventional process (CV) showed the greatest change in wettability, indicating more material photodegradation, with the angle ranging from 120° to 55°.
No clear trend was identified regarding wettability change based on the percentage of added tannin. Therefore, it is not possible to assert a direct relationship between the amount of tannin and the increase in photoprotective efficiency, which may be related to the low tannin amounts studied. A study conducted by Oliveira [41] highlighted an enhanced protective capacity when a content of 5 phr of black wattle tannin was incorporated into the composite. It is noteworthy that, in this work, the addition of tannin was limited to 2.0 phr considering experimental feasibility and the scope of the study, which initially focused on the analysis of natural rubber composites with 0.0, 1.0, 1.5, and 2.0 phr tannin.
3.2.6. Analysis of Composite Hardness
Figure 10 displays the Shore A hardness values of the composites at various tannin contents and in their respective crosslinking systems. It is observed that tannin content does not exert a significant influence on the hardness of the composites. However, when comparing different crosslinking systems, the semi-efficient system showed the highest hardness values. This may be attributed to the greater number of crosslinks formed during crosslinking, which tend to limit the mobility of polyisoprene molecular chains, imparting greater rigidity.
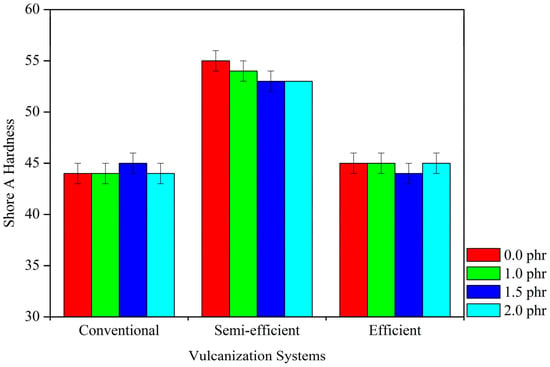
Figure 10.
Shore A Hardness tests in different vulcanization systems.
3.2.7. Analysis of the Abrasion Resistance of Composites
Figure 11 presents the abrasion resistance curves of NR/Tan composites in their respective crosslinking systems. The tannin content exerts different effects in each crosslinking system. In the conventional system, the inclusion of tannin tends to increase abrasion wear, whereas in the semi-efficient system, the addition of 1.5 phr of tannin tends to improve abrasion resistance. In the efficient system, the addition of 2 phr of tannin also tends to improve abrasion resistance. Among the vulcanization systems, the efficient system demonstrates the highest abrasion wear. The efficient system is characterized by the predominance of monosulfidic bonds, which, although more resistant to heat and oxidation, are less flexible and, therefore, more prone to the propagation of microcracks under abrasive stress [42].
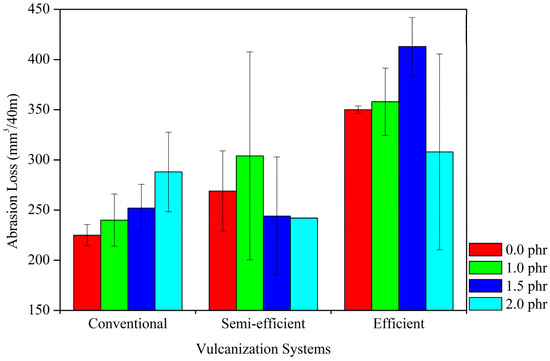
Figure 11.
Abrasion Resistance Tests in Different Vulcanization Systems.
3.2.8. Analysis of Tensile Strength
Table 4 shows the results of tensile strength at break tests for NR/Tan composites in their respective crosslinking systems before and after the accelerated thermal aging process.

Table 4.
Results of tensile strength tests on NR/Tan composites in their respective curing systems before and after thermal aging.
According to the results presented in Table 4, it is observed that the tannin content does not influence the tensile strength results of the composites in their respective crosslinking systems, both in the process without thermal aging and in the process with thermal aging. However, when comparing the crosslinking systems among themselves, the efficient system presented the highest tensile strength values, followed by the conventional system, while the semi-efficient system presented the lowest strength values. This phenomenon is evident both in the process without thermal aging and in the process with thermal aging. This fact may be related to the type of crosslinks formed during the vulcanization process. The efficient system presents monosulfidic bonds, which are chemical bonds formed by a single sulfur atom between the molecular chains of the rubber’s polyisoprene, and these short bonds are difficult to break. The conventional system presents polysulfidic bonds, which are chemical bonds formed by three or more sulfur atoms, and these bonds are easier to break. However, natural rubber has the ability to form stress-induced crystallization, meaning the polysulfidic bonds can break and form shorter bonds, such as mono- and disulfidic bonds [43,44].
In Table 4, it is observed that in the conventional crosslinking system, the properties of tensile strength at rupture and deformation seem to have been maintained even after the accelerated thermal aging process. Considering that polysulfide bonds predominate in this system, which is more sensitive to thermodegradation, the tensile strength at break and deformation being maintained after thermodegradation may suggest that tannin acted as an effective antioxidant. However, considering that in the conventional system, the properties of the composites that did not receive tannin were also maintained after thermodegradation, one cannot rule out the hypothesis that, in addition to the aid of tannin, this system also had the influence of the post-degradation phenomenon. This phenomenon may have occurred due to the greater proportion of sulfur in relation to the accelerator in the formulation of this system. In this specific case, the sulfur added in excess, which initially did not react with the carbon in the rubber’s molecular chains, may have reacted later when subjected to new conditions, such as increased temperature, thus completing the crosslinking of the rubber.
In the efficient system, although there was a significant decrease in mechanical properties after aging, a good preservation of tensile strength can still be noted. This observation may corroborate the idea that tannin was able to effectively protect the samples against thermodegradation.
In the semi-efficient system, in turn, there was a decrease in the tensile strength of the samples after the accelerated thermal aging process. This may suggest that in this vulcanization system there was not as pronounced protection as in the others.
3.2.9. Thermogravimetric Analysis (TGA)
To evaluate the mass loss of the composites as a function of temperature, thermogravimetric analysis was performed. Figure 12 shows the thermogravimetric (TGA) curve of natural rubber (NR) composites crosslinked with tannins (Tan) in different vulcanization systems.
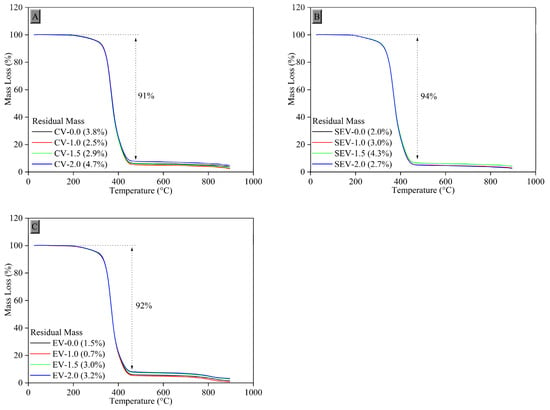
Figure 12.
Thermogravimetric curves of the crosslinked composites in the (A) conventional system, (B) semi-efficient system, and (C) efficient system.
The onset temperatures of thermolysis were obtained from the thermogravimetric curves by extrapolating the initial mass loss of the analyzed rubber composites. Figure 11 shows that, in the three vulcanization systems studied, the composites remained stable up to a temperature of approximately 200 °C. From this temperature onward, degradation of the composites began in a single stage, with a mass loss ranging from 91 to 94%.
To facilitate thermogravimetric studies, Figure 13 presents the first derivative thermogravimetric (DTG) curve of the rubber composites in their respective crosslinking systems. DTG curves can provide a better analysis as they exhibit peaks whose areas are proportional to the sample’s mass loss.

Figure 13.
DTG curve of the crosslinked composites in the (A) conventional system, (B) semi-efficient system, and (C) efficient system.
In Figure 13, it can be observed that for all samples in the studied vulcanization systems, the DTG curves’ behavior aligns with the analyses conducted using TGA curves, showing only a single, more intense event at a temperature of 370 °C. The extensive mass loss occurring in this temperature range is associated with the cleavage and degradation of the natural rubber polymer chain [45].
The results obtained indicate that, regardless of the vulcanization system used, the addition of black wattle vegetable tannin in the studied proportions did not cause significant changes in the thermolysis process of the samples.
4. Integrated Discussion of Results
The discussion of the properties of rubber composites with incorporated tannin can be enriched by correlating specific density, hardness, tensile strength, abrasion behavior, photodegradation, wettability, and thermogravimetry, highlighting the combined influence of vulcanization systems and tannin concentrations. These aspects reveal how composition and vulcanization processes simultaneously affect the properties, providing insights into the material’s performance.
The specific density of the composites showed a slight increase with the addition of tannin, which can be attributed to the higher density of tannin compared to the rubber matrix. However, this increase did not compromise the overall properties of the composites, indicating that the crosslinked structure of the different systems could accommodate tannin without significantly altering the material’s internal cohesion. Among the vulcanization systems, the semi-efficient system exhibited the highest specific density values due to the formation of disulfide and monosulfide bonds, which make the matrix more compact and reduce solvent permeability.
The hardness of the composites was influenced by the vulcanization system, being higher in the semi-efficient system. This increased hardness can be explained by the high crosslink density, which reduces the mobility of polymer chains, imparting greater rigidity to the material. However, this rigidity negatively affected tensile strength, which was lower in the semi-efficient system. The inverse relationship between hardness and tensile strength illustrates how crosslink density simultaneously impacts rigidity and the material’s ability to deform elastically under tension. This behavior is consistent with the formation of shorter, more rigid bonds typical of the semi-efficient system, which limits the flexibility of the polymeric matrix.
In terms of abrasion behavior, the efficient system exhibited the highest wear rates, reflecting its lower resistance to shear forces. This phenomenon can be associated with the predominant monosulfidic bonds in this system, which are chemically more stable but less flexible, favoring the propagation of microcracks under abrasive conditions. Nonetheless, this system showed superior thermal and oxidative degradation resistance, highlighting a trade-off between mechanical resistance and chemical stability.
The efficacy of tannin was more pronounced in the efficient system due to the short, stable monosulfidic bonds that create an environment conducive to tannin acting as an antioxidant. These bonds limit the propagation of free radicals generated by oxidation, allowing tannin to neutralize them more effectively. Furthermore, higher tannin concentrations (up to 2.0 phr) did not significantly compromise the physical or chemical properties of the composites. This can be attributed to tannin’s compatibility with the rubber matrix and its uniform distribution within the polymer network, preventing excessive localized concentrations and maintaining structural integrity.
Photodegradation tests showed that the efficient system (EV) exhibited the smallest increases in the hydroxyl index (HI), indicating superior antioxidant protection against UV-C radiation. This protection reflects the capacity of the predominant monosulfidic bonds in the EV system to limit free radical formation and propagation. Tannin, interacting with these short bonds, acts as an effective neutralizer, reducing oxidation and maintaining the material’s structural integrity.
Wettability tests corroborate these results, as the composites from the EV system showed the least variation in contact angle after UV exposure. This suggests that tannin, together with the stable bonds in the EV system, better protects the material’s surface against the formation of polar groups such as hydroxyls and carbonyls, which are responsible for increasing hydrophilicity. In the conventional system (CV), greater variation in contact angle was observed, indicating greater surface degradation due to the breakdown of polysulfidic bonds.
Thermogravimetric tests revealed that all vulcanization systems exhibited thermal stability up to approximately 200 °C, regardless of tannin concentration. Beyond this temperature, the composites began degrading in a single stage, with mass loss associated with the breakdown of polymer chains. The efficient system demonstrated greater thermal resistance due to the short, stable bonds that delay chain breakdown. The addition of tannin did not significantly alter the thermal degradation profiles, suggesting that its incorporation does not compromise the intrinsic thermal properties of the rubber.
When integrating the results, it is observed that the efficient system offers a superior balance between mechanical, thermal, and antioxidant properties. Specific density and hardness are directly related to crosslink density, which is higher in the semi-efficient system, while tensile strength is higher in the efficient system due to the greater stability of monosulfidic bonds. However, the efficient system also demonstrated higher abrasion wear, reflecting the relative fragility of its structure under shear stresses.
The incorporation of tannin, even at higher concentrations (2.0 phr), did not compromise the thermal, mechanical, or chemical properties of the composites. This stability can be attributed to the uniform distribution of tannin in the polymer matrix and its synergistic interaction with the short bonds in the efficient system. However, in the conventional system, the antioxidant effect of tannin was limited by the greater susceptibility of polysulfidic bonds to degradation.
5. Conclusions
In this study, the antioxidant properties of black wattle vegetable tannin were evaluated in vulcanized natural rubber composites using conventional, semi-efficient, and efficient vulcanization systems. The characterization test results of the tannin showed significant antioxidant potential, particularly suitable for industrial and biological applications.
The integrated analysis reveals that the performance of the composites is multifactorial, influenced by both the vulcanization system and the presence of tannin. The efficient system stands out as the most robust for applications requiring high thermal resistance and protection against photo-oxidative degradation, while the conventional and semi-efficient systems offer specific advantages in terms of hardness and mechanical strength. The use of tannin as a sustainable additive proves to be a promising solution for balancing properties and enhancing the durability of rubber composites under adverse conditions.
Author Contributions
M.d.S.F. (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing—original draft and Writing—review and editing); L.F.P. (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing); C.T.H. (Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing—original draft and Writing—review and editing); A.d.S.M.d.F. (Conceptualization, Data curation, Formal analysis, Resources, Validation, Visualization, Writing—original draft and Writing—review and editing); J.d.S.R. (Investigation, Methodology, Validation, Visualization, Writing—original draft and Writing—review and editing); A.L.d.O. (Conceptualization, Data curation, Methodology, Validation, Visualization, Writing—original draft and Writing—review and editing); M.F. (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft and Writing—review and editing); S.A.K. (Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing); D.d.S.S. (Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing); M.J.d.S. (Conceptualization, Data curation, Formal analysis, Resources, Validation, Visualization, Writing—original draft and Writing; E.A.d.S. (Conceptualization, Data curation, Formal analysis, Resources, Validation, Visualization, Writing—original draft and Writing; E.S.N. (Conceptualization, Data curation, Formal analysis, Resources, Validation, Visualization, Writing—original draft and Writing; F.C.C. (Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing); E.M.G. (Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing) and R.J.d.S. (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Resources, Supervision, Validation, Visualization, Writing—original draft and Writing—review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) through grants N°: 88887.704583/2022-00 (1 August 2022–28 February 2023) and no. 88887.817542/2023-00 (1 March 2023–31 July 2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw and processed data supporting the conclusions are available upon reasonable request to the corresponding author.
Acknowledgments
The Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support under grant N°: 2016/03208-0.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Sethulekshmi, A.S.; Saritha, A.; Joseph, K. A comprehensive review on the recent advancements in natural rubber nanocomposites. Int. J. Biol. Macromol. 2022, 194, 819–842. [Google Scholar] [CrossRef]
- Mathew, N.M.; De, S.K. Thermo-oxidative ageing and its effect on the network structure and fracture mode of natural rubber vulcanizates. Polymer 1983, 24, 1042–1054. [Google Scholar] [CrossRef]
- Ngudsuntear, K.; Limtrakul, S.; Vatanatham, T.; Arayapranee, W. Mechanical and aging properties of hydrogenated epoxidized natural rubber and its lifetime prediction. ACS Omega 2022, 7, 36448–36456. [Google Scholar] [CrossRef] [PubMed]
- Guzmán Sánchez, M.A.; Giraldo-Vásquez, D.H.; Moreno Sánchez, R. The effect of thermal ageing on the mechanical properties of natural rubber-based compounds used for rubber bearings. J. Eng. Technol. Sci. 2021, 53, 210310. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Zhong, B.; Zhu, L.; Liu, F. A synthesized multifunctional rubber additive and its improvements on the curing and antioxidative properties of styrene-butadiene rubber/silica composites. Polym. Degrad. Stab. 2019, 170, 108999. [Google Scholar] [CrossRef]
- Poompradub, S.; Luthikaviboon, T.; Linpoo, S.; Rojanathanes, R.; Prasassarakich, P. Improving oxidation stability and mechanical properties of natural rubber vulcanizates filled with calcium carbonate modified by gallic acid. Polym. Bull. 2011, 66, 965–977. [Google Scholar] [CrossRef]
- Nie, J.; Huang, X.; Xu, C.; Ding, J.; Chen, Y. Antioxidant effects on curing/processing and thermo-oxidative aging of filled nitrile rubber. Mater. Chem. Phys. 2020, 253, 123403. [Google Scholar] [CrossRef]
- Dong, J.; Migdal, C.A. 1 Antioxidants. In Lubricant Additives: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2009; Volume 1, ISBN 978-1-4200-5964-9. [Google Scholar]
- Zhao, W.; He, J.; Yu, P.; Jiang, X.; Zhang, L. Recent progress in the rubber antioxidants: A review. Polym. Degrad. Stab. 2023, 207, 110223. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Mrozowski, K.; Strzelec, K. Effect of ModifieEd and Unmodified Oak Bark (Quercus Cortex) on the Cross-Linking Process and Mechanical, Anti-Aging, and Hydrophobic Properties of Biocomposites Produced from Natural Rubber (NR). Materials 2024, 17, 1968. [Google Scholar] [CrossRef] [PubMed]
- Öncel, Ş.; Kurtoğlu, B.; Karaağaç, B. An alternative antioxidant for sulfur-vulcanized natural rubber: Henna. J. Elastomers Plast. 2019, 51, 440–456. [Google Scholar] [CrossRef]
- Shuhaimi, N.H.H.; Ishak, N.S.; Othman, N.; Ismail, H.; Sasidharan, S. Effect of different types of vulcanization systems on the mechanical properties of natural rubber vulcanizates in the presence of oil palm leaves-based antioxidant. J. Elastomers Plast. 2014, 46, 747–764. [Google Scholar] [CrossRef]
- Palanisamy, S.; Kalimuthu, M.; Nagarajan, R.; Marlet, J.M.F.; Santulli, C. Physical, chemical, and mechanical characterization of natural bark fibers (NBFs) reinforced polymer composites: A bibliographic review. Fibers 2023, 11, 13. [Google Scholar] [CrossRef]
- Taflick, T.; Maich, G.; Ferreira, L.D.; Bica, C.I.D.; Rodrigues, S.R.S.; Nachtigall, S.M.B. Acacia bark residues as filler in polypropylene composites. Polímeros 2015, 25, 289–295. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Xi, X.; Zhou, X. Polypropylene blend with polyphenols through dynamic vulcanization: Mechanical, rheological, crystalline, thermal, and UV protective property. Polymers 2019, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Astm D3182-21a; Standard Practice for Rubber—Materials, Equipment, and Procedures for Mixing Standard Compounds and Preparing Standard Vulcanized Sheets. ASTM International: West Conshohocken, PA, USA, 2021.
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Brand-Williams, W. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1999, 28, 1231–1237. [Google Scholar] [CrossRef]
- ASTM D2084-19a; Standard Test Method for Rubber Property—Vulcanization Using Oscillating Disk Cure Meter. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D297-21; Standard Test Methods for Rubber Products—Chemical Analysis. ASTM International: West Conshohocken, PA, USA, 2024.
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Mooney, M. A theory of large elastic deformation. J. Appl. Phys. 1940, 11, 582–592. [Google Scholar] [CrossRef]
- Gruendken, M.; Koda, D.; Dryzek, J.; Blume, A. Low molecular weight ‘liquid’polymer extended compounds, impact on free volume and crosslink density studied by positron lifetime spectroscopy and stress-strain analysis according to Mooney-Rivlin. Polym. Test. 2021, 100, 107239. [Google Scholar] [CrossRef]
- Sombatsompop, N. Practical use of the Mooney-Rivlin equation for determination of degree of crosslinking of swollen nr vulcanisates. J. Sci. Soc. Thail. 1998, 24, 199–204. [Google Scholar] [CrossRef]
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D5963-22; Standard Test Method for Rubber Property—Abrasion Resistance (Rotary Drum Abrader). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D412-16; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D573-04; Standard Test Method for Rubber—Deterioration in an Air Oven. ASTM International: West Conshohocken, PA, USA, 2024.
- de Cássia Oliveira Carneiro, A.; Vital, B.R.; Carvalho, A.M.M.L.; Oliveira, A.C.; Pereira, B.L.C.; de Andrade, B.G. Determinação da massa molar de taninos vegetais através da técnica da cromatografia de permeação em gel Tannins molar mass determination using gel permeation chromatography technique. Sci. For. 2010, 38, 419–429. [Google Scholar]
- Sekowski, S.; Veiko, A.; Olchowik-Grabarek, E.; Dubis, A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zavodnik, I.B.; Lapshina, E.; Dobrzynska, I.; Abdulladjanova, N.; et al. Hydrolysable tannins change physicochemical parameters of lipid nano-vesicles and reduce DPPH radical-Experimental studies and quantum chemical analysis. Biochim. Biophys. Acta (BBA)-Biomembr. 2022, 1864, 183778. [Google Scholar] [CrossRef] [PubMed]
- Schaumlöffel, L.S.; Fontoura, L.A.; Santos, S.J.; Pontes, L.F.; Gutterres, M. Vegetable tannins-based additive as antioxidant for biodiesel. Fuel 2021, 292, 120198. [Google Scholar] [CrossRef]
- Grasel, F.d.S.; Ferrão, M.F.; Wolf, C.R. Ultraviolet spectroscopy and chemometrics for the identification of vegetable tannins. Ind. Crops Prod. 2016, 91, 279–285. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J. Cult. Herit. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Irman, N.; Latif, N.H.A.; Brosse, N.; Gambier, F.; Syamani, F.A.; Hussin, M.H. Preparation and characterization of formaldehyde-free wood adhesive from mangrove bark tannin. Int. J. Adhes. Adhes. 2022, 114, 103094. [Google Scholar] [CrossRef]
- Hameed, Y.T.; Idris, A.; Hussain, S.A.; Abdullah, N. A tannin-based agent for coagulation and flocculation of municipal wastewater: Chemical composition, performance assessment compared to Polyaluminum chloride, and application in a pilot plant. J. Environ. Manag. 2016, 184, 494–503. [Google Scholar] [CrossRef]
- Galiani, P.D.; Malmonge, J.A.; dos Santos, D.P.; Malmonge, L.F. Compósitos de borracha natural com polianilina. Polímeros 2007, 17, 93–97. [Google Scholar] [CrossRef]
- Dos Santos, K.A.M.; Suarez, P.A.Z.; Rubim, J.C. Photo-degradation of synthetic and natural polyisoprenes at specific UV radiations. Polym. Degrad. Stab. 2005, 90, 34–43. [Google Scholar] [CrossRef]
- Mena, R.L.; Cacuro, T.A.; De Freitas, A.S.M.; Rangel, E.C.; Waldman, W.R. Polymer photodegradation followed by infrared: A tutorial. Rev. Virtual Química 2020, 12, 959–968. Available online: http://hdl.handle.net/11449/210531 (accessed on 3 July 2024).
- Cacuro, T.A.; Freitas, A.S.M.; Waldman, W.R. Demonstration of polymer photodegradation using a simple apparatus. J. Chem. Educ. 2018, 95, 2222–2226. [Google Scholar] [CrossRef]
- Blais, P.; Carlsson, D.J.; Wiles, D.M. Surface changes during polypropylene photo-oxidation: A study by infrared spectroscopy and electron microscopy. J. Polym. Sci. Part A-1 Polym. Chem. 1972, 10, 1077–1092. [Google Scholar] [CrossRef]
- Oliveira, A.L.d. Avaliação do Potencial Antioxidante do Tanino Vegetal da Acácia Negra em Matrizes Poliméricas de Poliestireno após Processo de Envelhecimento Acelerado. 2022. Available online: https://repositorio.ufscar.br/handle/ufscar/16173 (accessed on 1 July 2024).
- Rattanasom, N.; Poonsuk, A.; Makmoon, T. Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polym. Test. 2005, 24, 728–732. [Google Scholar] [CrossRef]
- Sotta, P.; Albouy, P.-A. Strain-induced crystallization in natural rubber: Flory’s theory revisited. Macromolecules 2020, 53, 3097–3109. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, L.; Wang, R.; Yu, H.; Zheng, T.; Lian, Y.; Luo, M.; Liao, S.; Liu, H.; Peng, Z. Research of strain induced crystallization and tensile properties of vulcanized natural rubber based on crosslink densities. Ind. Crops Prod. 2023, 202, 117070. [Google Scholar] [CrossRef]
- Varkey, J.T.; Augustine, S.; Thomas, S. Thermal degradation of natural rubber/styrene butadiene rubber latex blends by thermogravimetric method. Polym. -Plast. Technol. Eng. 2000, 39, 415–435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).