Effect of Compost and Vermicompost Amendments on Biochemical and Physiological Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown under Different Salinity Gradients
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Design, Treatments and Plant Material
2.2. Soil Physiochemical Analysis
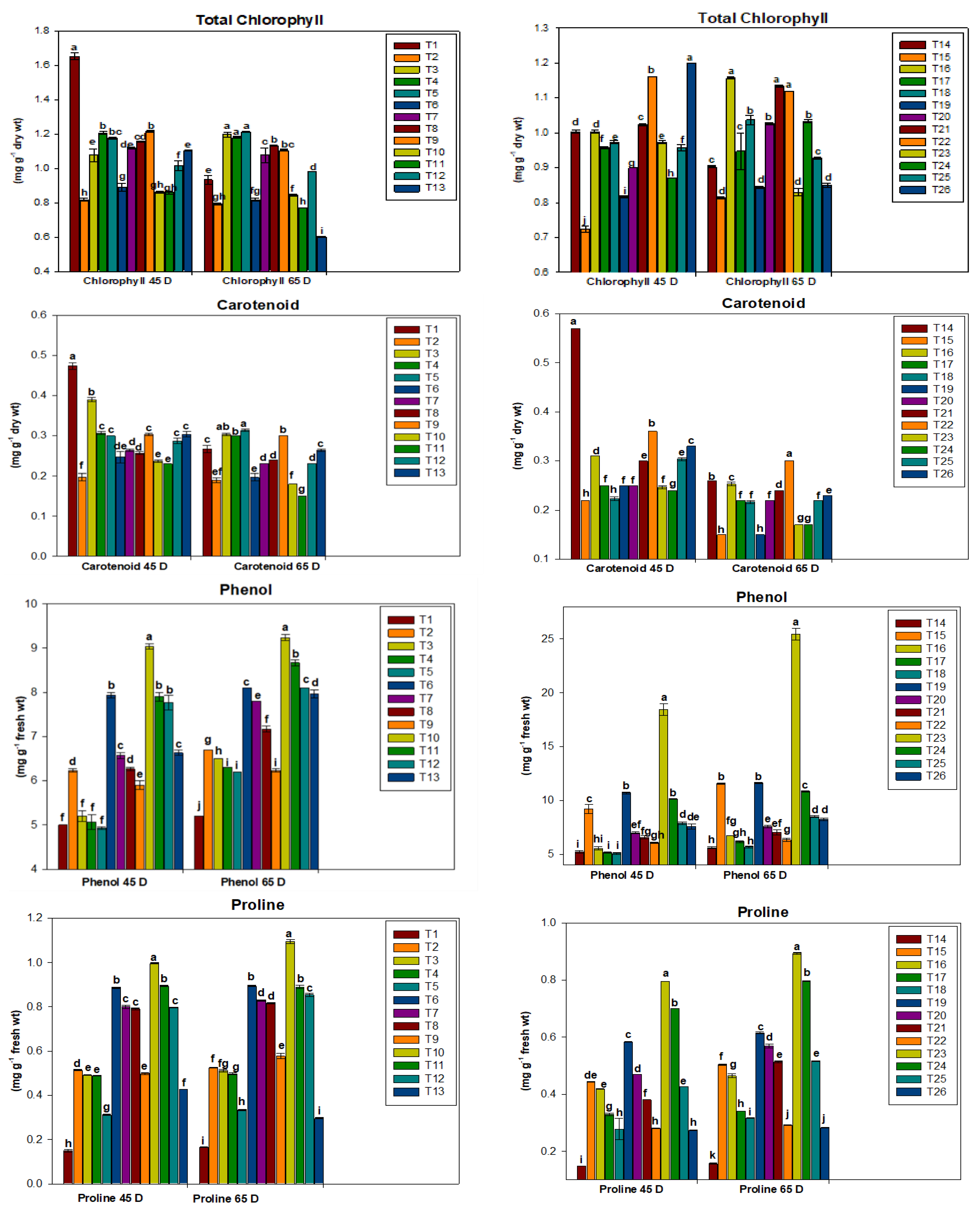
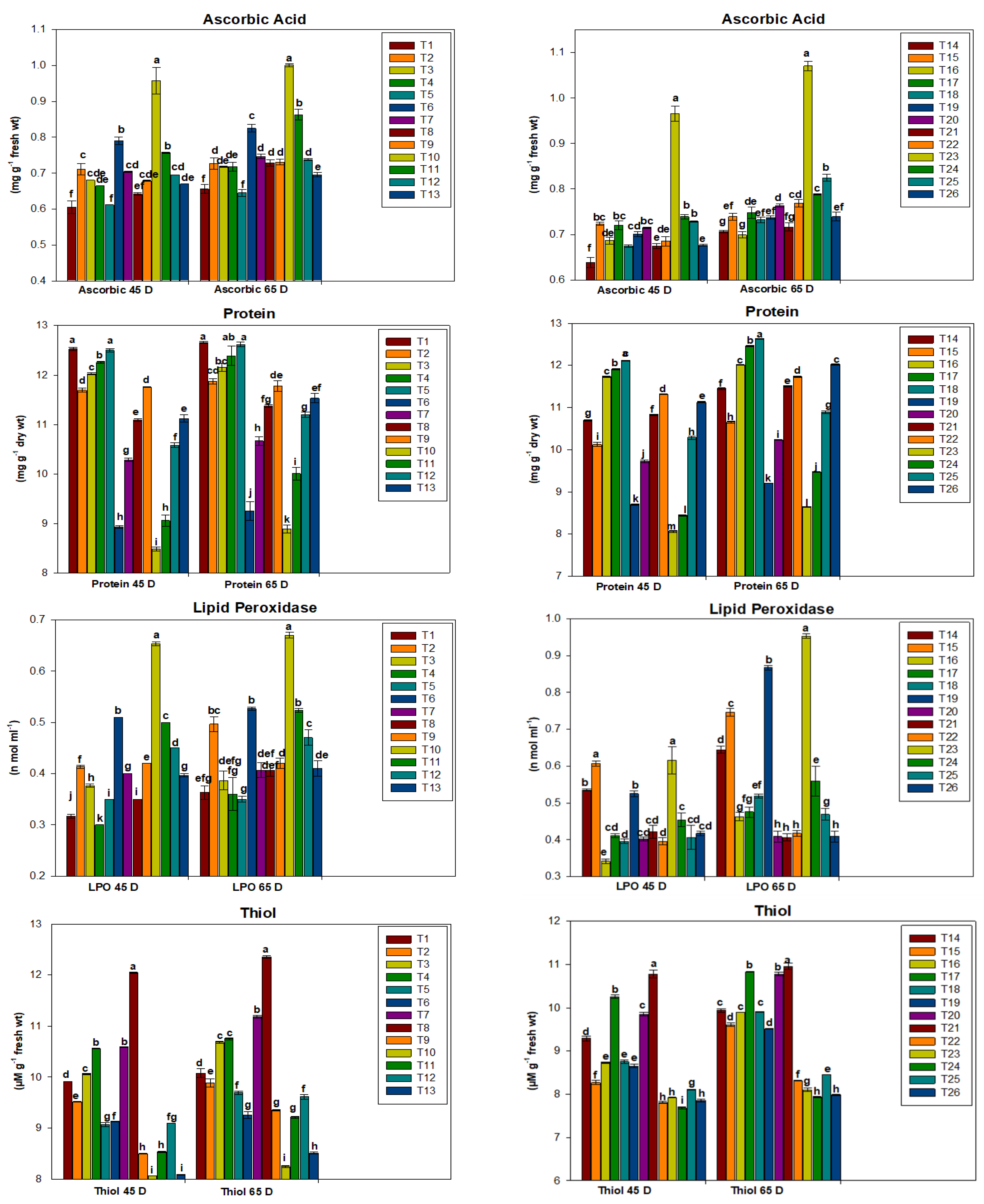
2.3. Biochemical Parameters of Plants Grown
2.4. Plant Growth Variables
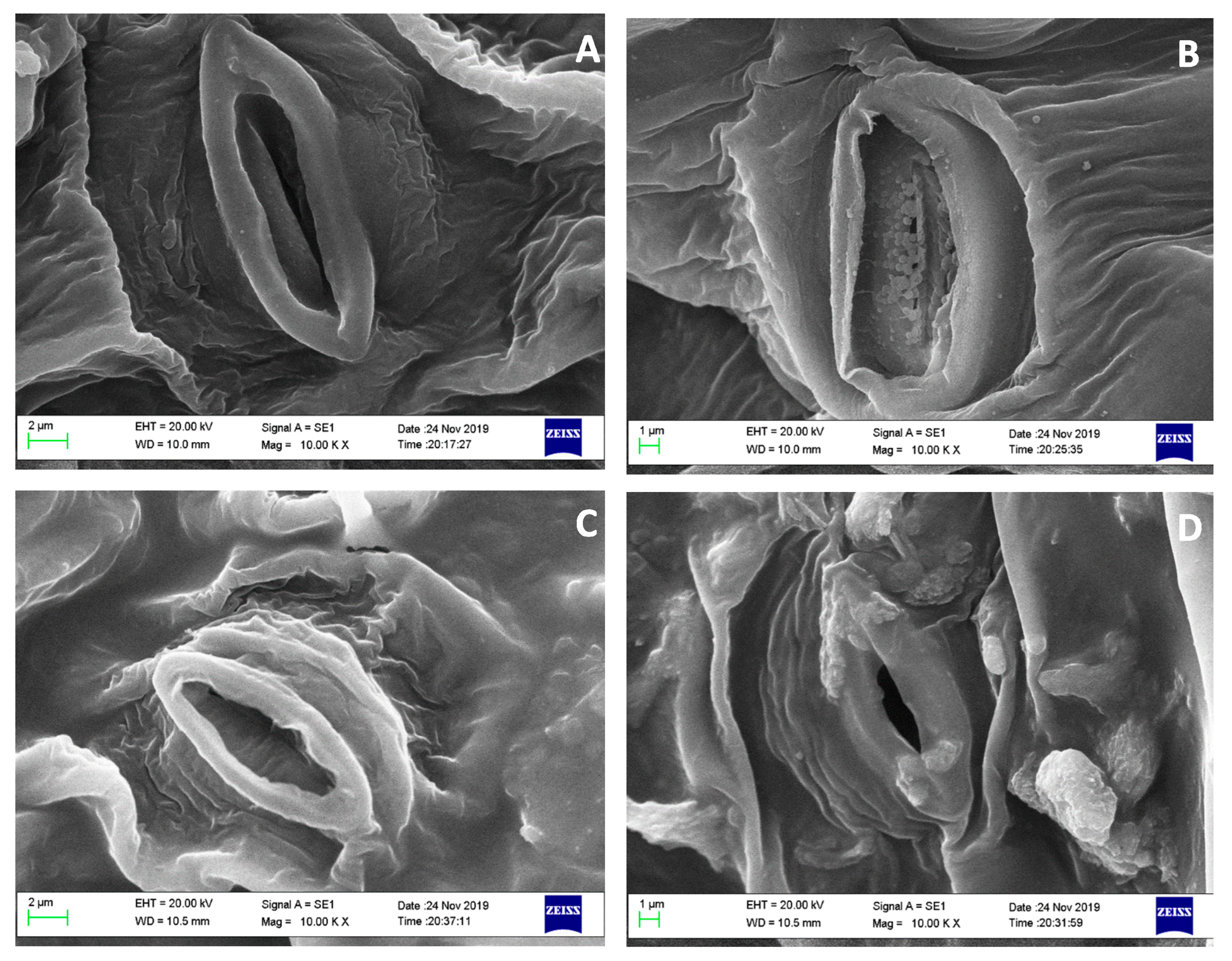
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Analysis at 0 and 45 Days
3.2. Biochemical Evaluation of Plants
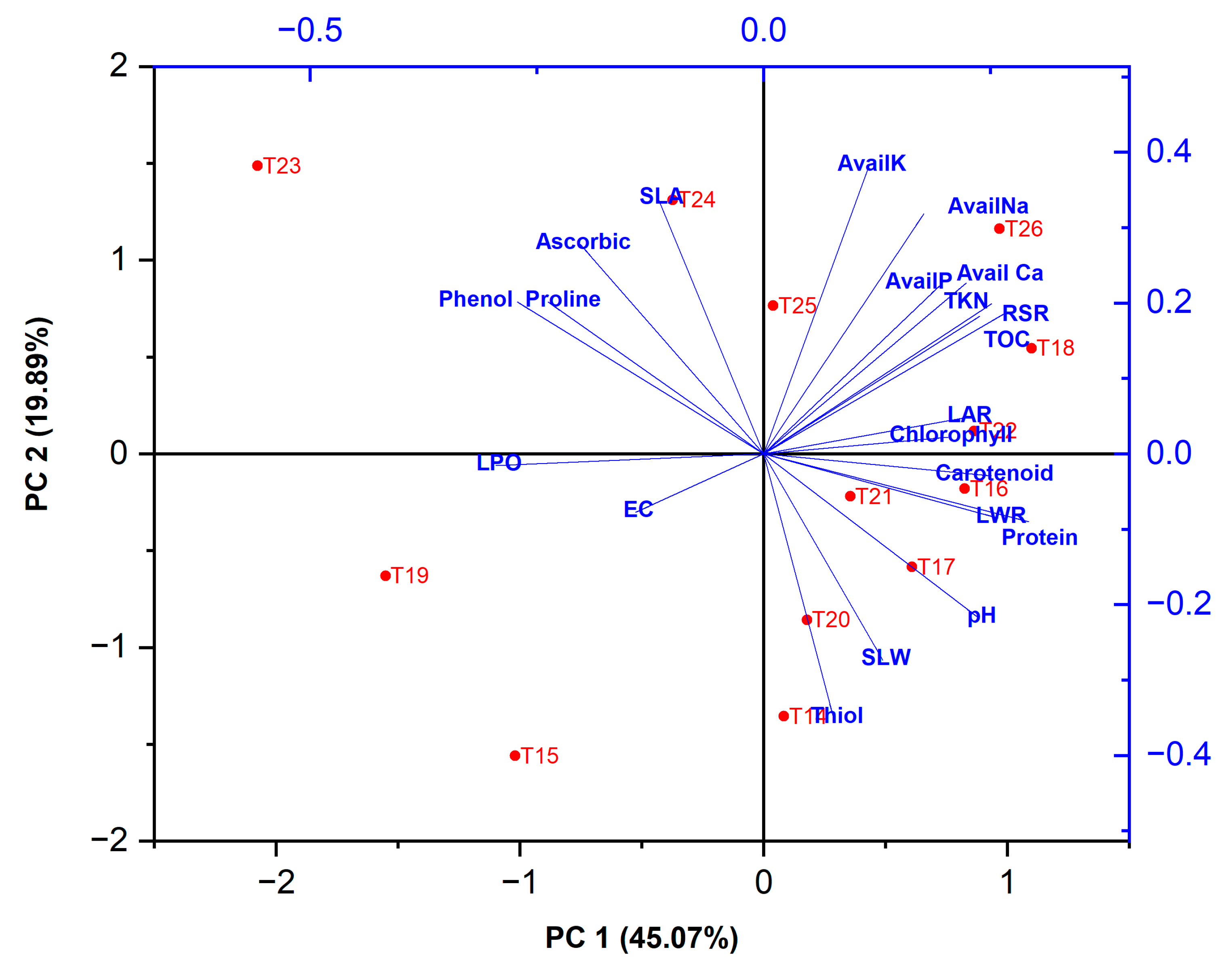
3.3. Plant Growth, SEM and PCA Analysis
4. Discussions
| Plants Examined | Irrigated with Saline Solution | Experiment Type | Country | Observations | References |
|---|---|---|---|---|---|
| Populus euphratica | 50, 100, 150, and 200 mM NaCl | Pot experiments | China | The POD activity increased with the increase in the severity of NaCl stress, but SOD activity was varied at different levels of salt. Results indicated that salt treatment reduced stomatal aperture and leaf photosynthetic capacity | [66] |
| Phaseolus vulgaris L. | 50, 100, and 150 mM NaCl | Tray experiment | Egypt | The treatment of bean plant with 100 mM of salt combined with different concentration of nano chitosan (0.1%, 0.2% and 0.3%) indicated that nano chitosan in all concentrations significantly promoted seed germination and radical length under salt stress. | [67] |
| Solanum lycopersicum L. | 0, 25, 50, 75, 100, 150 mM sodium chloride (NaCl) | Pot experiments | Southern France | The NaCl treatments of 75, 100 and 150 mM salt resulted in shorter plants, decreased stem width, a lower plant dry weight, fewer flowers, and smaller leaf area, while yield was reduced by treatment with concentrations of 50 mM NaCl and above. | [68] |
| Lavandula species | 0, 25, 50, 100 and 200 mM NaCl | Pot experiment | Greece | All lavender species showed low stomatal conductance values, suggesting the presence of a drought defense strategy. L. dentata var. candicans showed the lowest stomatal conducatance value, similar to those of L. dentata var. dentata and followed in ascending order by L. stoechas and L. angustifolia, with the greatest stomatal conductance. | [69] |
| Salvia officinalis L. | 0, 50, 100, 150, and 200 mM with different salt compounds (NaCl, KCl, MgSO4, MgCl2, Na2SO4, and CaCl2) | Pot experiment | Turkey | The study showed, α-pinene and camphor percentage increased under all salt stress. The percentage of camphene was also augmented under all stress types except CaCl2 treatment whereas β-thujone percentage increased except MgCl2 treatment. Moreover, NaCl and KCl treatments decreased the percentage of α-thujone while other treatments caused an increase in the percentage. | [70] |
| Corchorus olitorius L. | 50, 100, and 150 mM NaCl | Pot experiment | Bangladesh | Biochar and chitosan supplementation increased oxidative stress tolerance and improved the growth and physiology of salt-affected jute plants, while also significantly reducing Na+ accumulation and ionic toxicity and decreasing the Na+/K+ ratio. | [71] |
| Abelmoschus esculentus | 0, 25, 50 and 75 mM NaCl | Tray experiment | Pakistan | The results clearly indicated that seeds of all varieties can tolerate the lower concentration of salt (25 mM) and higher (50 mM) greatly reduced the seeds germination while at highest concentration (75 mM) no germination was recorded. | [72] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljerf, L. Data of thematic analysis of farmer’s use behavior of recycled industrial wastewater. Data Brief 2018, 21, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- Polash, M.A.S.; Sakil, M.A.; Hossain, M.A. Plants responses and their physiological and biochemical defense mechanisms against salinity: A review. Trop. Plant Res. 2019, 6, 250–274. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Naseer, S.; Ashraf, M.; Akram, N.A. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. J. Plant Interact. 2013, 8, 85–96. [Google Scholar] [CrossRef]
- Sahab, S.; Suhani, I.; Singh, R.P. Valuing each patch of land: Utilizing plant-microbe interactions for the betterment of agriculture. In Microbes in Land Use Change Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 471–507. [Google Scholar]
- Ha-Tran, D.M.; Nguyen TT, M.; Hung, S.H.; Huang, E.; Huang, C.C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Saberali, S.F.; Moradi, M. Effect of salinity on germination and seedling growth of Trigonella foenum-graecum, Dracocephalummoldavica, Satureja hortensis and Anethum graveolens. J. Saudi Soc. Agric. Sci. 2019, 18, 316–323. [Google Scholar]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Patel, A.; Khare, P.; Patra, D.D. Biochar mitigates salinity stress in plants. In Plant Adaptation Strategies in Changing Environment; Springer: Singapore, 2017; pp. 153–182. [Google Scholar]
- Ben Hamed, K.; Castagna, A.; Salem, E.; Ranieri, A.; Abdelly, C. Sea fennel (Crithmum maritimum L.) under salinity conditions: A comparison of leaf and root antioxidant responses. Plant Growth Regul. 2007, 53, 185–194. [Google Scholar] [CrossRef]
- Tartoura, K.A.; Youssef, S.A.; Tartoura, E.S.A. Compost alleviates the negative effects of salinity via up-regulation of antioxidants in Solanum lycopersicum L. plants. Plant Growth Regul. 2014, 74, 299–310. [Google Scholar] [CrossRef]
- Beykkhormizi, A.; Abrishamchi, P.; Ganjeali, A.; Parsa, M. Effect of vermicompost on some morphological, physiological and biochemical traits of bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Nutr. 2016, 39, 883–893. [Google Scholar] [CrossRef]
- Ahirwar, C.S.; Singh, A.P.; Nath, R.; Verty, P. Assessments Effect of Nitrogen and Phosphorus on the Phenological and Fruit Characters of Okra (Abelmoschus esculentus L.). Int. J. Curr. Microbiol. App. Sci. 2021, 10, 1918–1925. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Chang, S.C.; Jackson, M.L. Soil Phosphorus Fractions in some representative soils. J. Soil Sci. 1958, 9, 109–119. [Google Scholar] [CrossRef]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Duxbury, A.C.; Yentsch, C.S. Plankton Pigment Nomographs; Canadian Science Publishing: Ottawa, ON, Canada, 1956. [Google Scholar]
- Bray, H.G.; Thorpe, W. Analysis of phenolic compounds of interest in metabolism. Methods Biochem. Anal. 1954, 1, 27–52. [Google Scholar] [PubMed]
- Fahey, R.C.; Brown, W.C.; Adams, W.B.; Worsham, M.B. Occurrence of glutathione in bacteria. J. Bacteriol. 1978, 133, 1126–1129. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Keller, T.; Schwager, H. Air pollution and ascorbic acid. Eur. J. For. Pathol. 1977, 7, 338–350. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Use of sewage sludge as fertiliser supplement for Abelmoschus esculentus plants: Physiological, biochemical and growth responses. Int. J. Environ. Waste Manag. 2009, 3, 91–106. [Google Scholar] [CrossRef]
- Hunt, R. Plant growth curves. In The Functional Approach to Plant Growth Analysis; Edward Arnold Ltd.: London, UK, 1982. [Google Scholar]
- Parida, A.K.; Days, A.B.; Mittra, B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Golinejad, S.; Mirjalili, M.H. Fast and cost-effective preparation of plant cells for scanning electron microscopy (SEM) analysis. Anal. Biochem. 2020, 609, 113920. [Google Scholar] [CrossRef]
- Kwon, O.K.; Mekapogu, M.; Kim, K.S. Effect of salinity stress on photosynthesis and related physiological responses in carnation (Dianthus caryophyllus). Hortic. Environ. Biotechnol. 2019, 60, 831–839. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.Q.; Fujiyama, H. Difference in-response of rice and tomato subjected to sodium salinization to the addition of calcium. Soil Sci. Plant Nutr. 1996, 42, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Yasemin, S.; Koksal, N. Comparative Analysis of Morphological, Physiological, Anatomic and Biochemical Responses in Relatively Sensitive Zinnia elegans ‘Zinnita Scarlet’ and Relatively Tolerant Zinnia marylandica ‘Double Zahara Fire Improved’under Saline Conditions. Horticulturae 2023, 9, 247. [Google Scholar] [CrossRef]
- Rajabi Dehnavi, A.; Zahedi, M.; Ludwiczak, A.; Cardenas Perez, S.; Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Lakhdar, A.; Hafsi, C.; Rabhi, M.; Debez, A.; Montemurro, F.; Abdelly, C.; Jedidi, N.; Ouerghi, Z. Application of municipal solid waste compost reduces the negative effects of saline water in Hordeum maritimum L. Bioresour. Technol. 2008, 99, 7160–7167. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Fakhech, A.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Meddich, A. Compost as an eco-friendly alternative to mitigate salt-induced effects on growth, nutritional, physiological and biochemical responses of date palm. Int. J. Recycl. Org. Waste Agric. 2022, 11, 85–100. [Google Scholar]
- Singh, Y.P.; Arora, S.; Mishra, V.K.; Bhardwaj, A.K. Regaining the agricultural potential of sodic soils and improved smallholder food security through integration of gypsum, pressmud and salt tolerant varieties. Agroecol. Sustain. Food Syst. 2022, 46, 410–431. [Google Scholar] [CrossRef]
- Tammam, A.A.; Rabei Abdel Moez Shehata, M.; Pessarakli, M.; El-Aggan, W.H. Vermicompost and its role in alleviation of salt stress in plants–I. Impact of vermicompost on growth and nutrient uptake of salt-stressed plants. J. Plant Nutr. 2023, 46, 1446–1457. [Google Scholar] [CrossRef]
- Demir, Z. Alleviation of adverse effects of sodium on soil physicochemical properties by application of vermicompost. Compos. Sci. Util. 2020, 28, 100–116. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, O.A.; Hafez, E.M.; ElShamey, E.A.; Zhou, Z.; Wang, B.; Ge, Y.; Fahmy, A.E.; Seleiman, M.F. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J. Environ. Manag. 2021, 277, 111388. [Google Scholar] [CrossRef]
- Yassin, M.; El Sabagh, A.; Mekawy, A.M.M.; Islam, M.S.; Hossain, A.; Barutcular, C.; Saneoka, H. Comparative performance of two bread wheat (Triticum aestivum L.) genotypes under salinity stress. Appl. Ecol. Environ. Res. 2019, 17, 5029–5041. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Hu, Y.; Han, W.; Gong, H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol. Plant 2015, 37, 71. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J. Exp. Bot. 2021, 72, 5795–5806. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Waheed, A. Responses of some local/exotic accessions of lentil (Lens culinaris Medic.) to salt stress. J. Agron. Crop Sci. 1993, 170, 103–112. [Google Scholar] [CrossRef]
- Ashraf, M. Organic substances responsible for salt tolerance inEruca sativa. Biol. Plant 1994, 36, 255–259. [Google Scholar] [CrossRef]
- Ashraf, M.; Fatima, H. Responses of salt-tolerant and salt-sensitive lines of safflower [Carthamus tinctorius L.] to salt stress. Acta Physiol. Plantarum. 1995, 17, 61–71. [Google Scholar]
- Singh, R.P.; Agrawal, M. Biochemical and physiological responses of rice (Oryza sativa L.) grown on different sewage sludge amendments rates. Bull. Environ. Contam. Toxicol. 2010, 84, 606–612. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.; Hossain, R.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Burguieres, E.; McCue, P.; Kwon, Y.I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Attia, M.S.; Osman, M.S.; Mohamed, A.S.; Mahgoub, H.A.; Garada, M.O.; Abdelmouty, E.S.; Latef AA, H.A. Impact of foliar application of chitosan dissolved in different organic acids on isozymes, protein patterns and physio-biochemical characteristics of tomato grown under salinity stress. Plants 2021, 10, 388. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef]
- De Vos, C.R.; Schat, H.; Vooijs, R.; Ernst, W.H. Copper-induced damage to the permeability barrier in roots of Silene cucubalus. J. Plant Physiol. 1989, 135, 164–169. [Google Scholar] [CrossRef]
- Srivastava, V.; Gupta, S.K.; Singh, P.; Sharma, B.; Singh, R.P. Biochemical, physiological, and yield responses of lady’s finger (Abelmoschus esculentus L.) grown on varying ratios of municipal solid waste vermicompost. Int. J. Recycl. Org. Waste Agric. 2018, 7, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Sairam, R.K.; Srivastava, G.C. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 2002, 162, 897–904. [Google Scholar] [CrossRef]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Alamer, K.H.; Perveen, S.; Khaliq, A.; Zia Ul Haq, M.; Ibrahim, M.U.; Ijaz, B. Mitigation of salinity stress in maize seedlings by the application of vermicompost and sorghum water extracts. Plants 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wei, X.; Han, J.; Wang, J.; Mu, C.; Zhang, J. Changes in mass allocation play a more prominent role than morphology in resource acquisition of the rhizomatous Leymus chinensis under drought stress. Ann. Bot. 2023, mcad073. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Nagata, A.; Endo, R. Far-red light interacts with salinity stress in Cucumis sativus seedlings partly through changes in photosynthate allocation. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Cao, J.J.; Chen, J.; Yang, Q.P.; Xiong, Y.M.; Ren, W.Z.; Kong, D.L. Leaf hydraulics coordinated with leaf economics and leaf size in mangrove species along a salinity gradient. Plant Divers. 2023, 45, 309–314. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Niedojadło, K.; Mierek-Adamska, A.; Dąbrowska, G.B.; Piernik, A. Maternal salinity influences anatomical parameters, pectin content, biochemical and genetic modifications of two Salicornia europaea populations under salt stress. Sci. Rep. 2022, 12, 2968. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ge, J.; Li, R.; Zhang, R.; Zhang, Y.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. Improved physiological and morphological traits of root synergistically enhanced salinity tolerance in rice under appropriate nitrogen application rate. Front. Plant Sci. 2022, 13, 982637. [Google Scholar] [CrossRef]
- Rajput, V.D.; Chen, Y.; Ayup, M. Effects of high salinity on physiological and anatomical indices in the early stages of Populus euphratica growth. Russ. J. Plant Physiol. 2015, 62, 229–236. [Google Scholar] [CrossRef]
- Zayed, M.M.; Elkafafi, S.H.; Zedan, A.M.; Dawoud, S.F. Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Prod. 2017, 8, 577–585. [Google Scholar] [CrossRef] [Green Version]
- El-Mogy, M.M.; Garchery, C.; Stevens, R. Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 727–737. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.T.; Kontodaimon Karantzi, A.; Liakopoulos, G.; Londra, P.A.; Bertsouklis, K. The effect of salinity on the growth of lavender species. Water 2020, 12, 618. [Google Scholar] [CrossRef] [Green Version]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crop. Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef]
- Saima, S.; Ghaffar, F.; Yasin, G.; Nawaz, M.; Ahmad, K.M. Effect of salt stress on germination and early seedling growth in Okra (Abelmoschus esculentus). Sarhad J. Agric. 2022, 38, 388–397. [Google Scholar] [CrossRef]

| NaCl Solution (mM) | pH | EC (dS/m) |
|---|---|---|
| 0 | 6.65 | 0.3 |
| 50 | 6.82 | 5.2 |
| 100 | 6.74 | 10.4 |
| 150 | 6.64 | 14.8 |
| Treatments | Mixing Ratio | Irrigated with Saline Solution (mM) |
|---|---|---|
| For compost | ||
| T1 | 8 kg soil | 0 |
| T2 | 8 kg soil | 50 |
| T3 | 6 kg soil + 2 kg compost | 50 |
| T4 | 4 kg soil + 4 kg compost | 50 |
| T5 | 2 kg soil + 6 kg compost | 50 |
| T6 | 8 kg soil | 100 |
| T7 | 6 kg soil + 2 kg compost | 100 |
| T8 | 4 kg soil + 4 kg compost | 100 |
| T9 | 2 kg soil + 6 kg compost | 100 |
| T10 | 8 kg soil | 150 |
| T11 | 6 kg soil + 2 kg compost | 150 |
| T12 | 4 kg soil + 4 kg compost | 150 |
| T13 | 2 kg soil + 6 kg compost | 150 |
| For vermicompost | ||
| T14 | 8 kg soil | 0 |
| T15 | 8 kg soil | 50 |
| T16 | 6 kg soil + 2 kg vermicompost | 50 |
| T17 | 4 kg soil + 4 kg vermicompost | 50 |
| T18 | 2 kg soil + 6 kg vermicompost | 50 |
| T19 | 8 kg soil | 100 |
| T20 | 6 kg soil + 2 kg vermicompost | 100 |
| T21 | 4 kg soil + 4 kg vermicompost | 100 |
| T22 | 2 kg soil + 6 kg vermicompost | 100 |
| T23 | 8 kg soil | 150 |
| T24 | 6 kg soil + 2 kg vermicompost | 150 |
| T25 | 4 kg soil + 4 kg vermicompost | 150 |
| T26 | 2 kg soil + 6 kg vermicompost | 150 |
| Compost | Vermicompost | |
|---|---|---|
| pH | 7.21 ± 0.02 | 7.15 ± 0.12 |
| EC (dS/m) | 0.365 ± 0.01 | 0.451 ± 0.1 |
| TKN (%) | 1.5 ± 0.15 | 1.37 ± 0.12 |
| OC (%) | 31 ± 0.52 | 32 ± 0.25 |
| Avail. Na (mg kg−1) | 1561 ± 0.7 | 1477 ± 1.5 |
| Avail. P (mg kg−1) | 186 ± 0.8 | 150 ± 2.1 |
| Avail. K (mg kg−1) | 2035 ± 0.25 | 1178 ± 2.3 |
| Avail. Ca (mg kg−1) | 214 ± 0.18 | 170 ± 1.1 |
| C/N Ratio | 20.5 ± 0.5 | 24.3 ± 0.7 |
| Treatments | pH | EC (dS/m) | TKN (%) | OC (%) | Avail. Na (mg kg−1) | Avail. P (mg kg−1) | Avail. K (mg kg−1) | Avail. Ca (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| At 0 day | ||||||||
| T1 | 7.19 ± 0.4 bc | 0.543 ± 0.0 e | 0.66 ± 0.0 e | 19 ± 0.1 e | 224 ± 1.8 m | 55 ± 1.5 h | 146 ± 2.2 m | 52 ± 1.7 i |
| T2 | 7.44 ± 0.0 ab | 0.896 ± 0.0 a | 0.79 ± 0.0 d | 19 ± 0.1 e | 659 ± 1.17 l | 59 ± 1.3 h | 411 ± 1.7 i | 64 ± 2.3 h |
| T3 | 7.73 ± 0.0 a | 0.750 ± 0.0 bc | 0.98 ± 0.0 c | 26 ± 0.1 d | 784 ± 1.0 j | 129 ± 7.1 d | 794 ± 0.8 f | 119 ± 2.9 e |
| T4 | 7.78 ± 0.0 a | 0.420 ± 0.0 f | 1.09 ± 0.0 b | 27 ± 0.0 bc | 1031 ± 2.8 f | 101 ± 0.7 e | 1171 ± 4.0 d | 168 ± 3.6 c |
| T5 | 7.82 ± 0.1 a | 0.263 ± 0.0 g | 1.21 ± 0.0 a | 29 ± 0.0 a | 1430 ± 6.6 b | 180 ± 0.7 a | 2047 ± 5.7 a | 218 ± 3.0 a |
| T6 | 6.18 ± 0.0 d | 0.800 ± 0.0 b | 0.67 ± 0.0 e | 19 ± 0.1 e | 889 ± 3.2 i | 75 ± 0.7 g | 182 ± 0.5 k | 55 ± 0.2 i |
| T7 | 6.21 ± 0.0 d | 0.699 ± 0.0 cd | 0.79 ± 0.0 d | 27 ± 0.0 c | 928 ± 4.9 h | 135 ± 0.7 cd | 286 ± 0.8 j | 86 ± 1.5 f |
| T8 | 6.47 ± 0.0 d | 0.638 ± 0.0 de | 0.98 ± 0.0 c | 27 ± 0.0 b | 994 ± 10.8 g | 101 ± 0.7 e | 534 ± 0.8 h | 75 ± 2.2 g |
| T9 | 6.88 ± 0.0 c | 0.572 ± 0.0 e | 1.13 ± 0.0 ab | 29 ± 0.0 a | 1361 ± 0.8 c | 136 ± 3.6 cd | 824 ± 2.9 ee | 112 ± 1.9 e |
| T10 | 7.55 ± 0.0 ab | 0.832 ± 0.0 ab | 0.60 ± 0.0 e | 19 ± 0.1 e | 1103 ± 2.5 e | 87 ± 0.7 f | 161 ± 0.1 l | 49 ± 1.0 i |
| T11 | 7.73 ± 0.0 a | 0.615 ± 0.0 de | 0.81 ± 0.0 d | 26 ± 0.1 d | 735 ± 0.4 k | 139 ± 3.6 c | 738 ± 1.4 g | 75 ± 1.8 g |
| T12 | 7.73 ± 0.0 a | 0.574 ± 0.0 e | 0.95 ± 0.0 c | 27 ± 0.1 bc | 1251 ± 3.0 d | 131 ± 0.7 cd | 1192 ± 3.9 c | 130 ± 1.4 d |
| T13 | 7.82 ± 0.0 a | 0.548 ± 0.0 e | 1.19 ± 0.0 a | 29 ± 0.0 a | 1534 ± 1.7 a | 161 ± 0.7 b | 1667 ± 0.8 b | 191 ± 2.8 b |
| At 45 days | ||||||||
| T1 | 7.07 ± 0.0 bc | 0.381 ± 0.0 l | 0.54 ± 0.0 e | 19 ± 0.1 c | 176 ± 0.3 f | 34 ± 0.7 j | 55 ± 0.2 k | 51 ± 0.2 h |
| T2 | 6.76 ± 0.3 c | 0.908 ± 0.0 g | 0.72 ± 0.0 d | 19 ± 0.3 c | 458 ± 4.6 de | 41 ± 0.7 i | 282 ± 0.1 h | 74 ± 0.1 g |
| T3 | 6.78 ± 0.0 c | 0.783 ± 0.0 i | 0.89 ± 0.0 c | 24 ± 2.2 b | 384 ± 0.6 e | 85 ± 1.5 f | 364 ± 0.1 g | 81 ± 0.3 fg |
| T4 | 6.99 ± 0.0 bc | 0.694 ± 0.0 j | 1.00 ± 0.0 b | 27 ± 0.0 a | 414 ± 5.7 e | 84 ± 0.7 f | 776 ± 0.1 d | 101 ± 0.1 e |
| T5 | 7.12 ± 0.0 abc | 0.566 ± 0.0 k | 1.16 ± 0.0 a | 28 ± 0.0 a | 595 ± 1.4 b | 107 ± 5.5 d | 1145 ± 1.2 a | 141 ± 1.2 bc |
| T6 | 6.11 ± 0.0 d | 2.171 ± 0.0 a | 0.60 ± 0.0 e | 20 ± 0.2 c | 622 ± 2.8 b | 63 ± 0.7 g | 104 ± 1.6 j | 58 ± 1.2 h |
| T7 | 6.26 ± 0.0 d | 1.163 ± 0.0 c | 0.71 ± 0.0 d | 26 ± 0.0 a | 528 ± 0.8 bcd | 97 ± 0.7 e | 204 ± 3.7 i | 86 ± 2.8 f |
| T8 | 6.75 ± 0.0 c | 1.001 ± 0.0 d | 0.88 ± 0.0 c | 27 ± 0.0 a | 561 ± 1.3 bc | 88 ± 1.3 f | 374 ± 1.0 g | 145 ± 3.0 b |
| T9 | 6.82 ± 0.0 c | 0.373 ± 0.0 l | 0.98 ± 0.0 b | 28 ± 0.0 a | 790 ± 114.2 a | 113 ± 2.1 c | 726 ± 3.2 e | 165 ± 1.4 a |
| T10 | 7.05 ± 0.0 bc | 1.32 ± 0.0 b | 0.64 ± 0.0 e | 19 ± 0.0 c | 828 ± 2.2 a | 55 ± 2.3 h | 116 ± 0.5 j | 84 ± 3.9 f |
| T11 | 7.26 ± 0.0 ab | 0.957 ± 0.0 e | 0.72 ± 0.0 d | 27 ± 0.2 a | 468 ± 3.5 cde | 133 ± 1.5 b | 544 ± 2.1 f | 122 ± 1.7 d |
| T12 | 7.35 ± 0.0 ab | 0.938 ± 0.0 f | 0.89 ± 0.0 c | 27 ± 0.0 a | 592 ± 0.3 b | 85 ± 1.5 f | 950 ± 1.6 b | 136 ± 5.4 c |
| T13 | 7.45 ± 0.0 a | 0.851 ± 0.0 h | 1.00 ± 0.0 b | 28 ± 0.0 a | 766 ± 2.0 a | 155 ± 1.3 a | 857 ± 1.0 c | 138 ± 3.1 bc |
| Treatments | pH | EC (dS/m) | TKN (%) | OC (%) | Avail. Na (mg kg−1) | Avail. P (mg kg−1) | Avail. K (mg kg−1) | Avail. Ca (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| At 0 days | ||||||||
| T14 | 7.54 ± 0.1 a | 0.289 ± 0.0 j | 0.66 ± 0.0 e | 19 ± 0.1 g | 221 ± 0.8 l | 38.3 ± 1.3 h | 107 ± 0.5 i | 99 ± 2.6 g |
| T15 | 6.81 ± 0.0 cde | 1.591 ± 0.0 a | 0.66 ± 0.0 e | 20 ± 0.1 e | 596 ± 2.2 k | 55 ± 2.8 g | 377 ± 0.8 f | 102 ± 6.3 fg |
| T16 | 6.95 ± 0.0 bcd | 1.163 ± 0.0 d | 0.87 ± 0.0 d | 28 ± 0.3 cd | 982 ± 0.6 g | 118 ± 0.7 d | 551 ± 1.1 e | 129 ± 0.6 d |
| T17 | 7.11 ± 0.0 bc | 0.776 ± 0.0 f | 0.96 ± 0.0 c | 28 ± 0.0 c | 1236 ± 2.0 d | 107 ± 0.7 e | 1000 ± 0.8 d | 151 ± 2.3 b |
| T18 | 7.13 ± 0.0 bc | 0.446 ± 0.0 h | 1.33 ± 0.0 a | 29 ± 0.0 a | 1461 ± 1.3 b | 127 ± 1.5 c | 1163 ± 2.9 c | 169 ± 3.3 a |
| T19 | 6.57 ± 0.2 e | 1.318 ± 0.0 b | 0.62 ± 0.0 e | 19 ± 0.1 f | 792 ± 2.8 j | 67 ± 0 f | 127 ± 0.5 h | 109 ± 2.4 ef |
| T20 | 6.61 ± 0.1 de | 0.601 ± 0.0 g | 0.81 ± 0.0 d | 28 ± 0.0 d | 875 ± 1.9 h | 140 ± 2.8 b | 227 ± 1.1 g | 117 ± 3.2 e |
| T21 | 6.63 ± 0.1 de | 0.411 ± 0.0 h | 1.02 ± 0.0 c | 28 ± 0.0 b | 1044 ± 2.0 f | 115 ± 1.5 d | 377 ± 0.8 f | 118 ± 3.1 e |
| T22 | 7.17 ± 0.1 b | 0.364 ± 0.0 i | 1.35 ± 0.0 a | 29 ± 0.0 a | 1312 ± 2.8 c | 149 ± 2.1 a | 551 ± 1.1 e | 138 ± 2.9 c |
| T23 | 6.77 ± 0.1 cde | 1.556 ± 0.0 a | 0.62 ± 0.0 e | 19 ± 0.1 f | 841 ± 1.1 i | 55 ± 1.3 g | 1000 ± 0.8 d | 110 ± 1.7 ef |
| T24 | 6.83 ± 0.0 bcde | 1.243 ± 0.0 c | 0.85 ± 0.0 d | 28 ± 0.0 cd | 983 ± 2.9 g | 138 ± 1.5 b | 1163 ± 2.9 c | 141 ± 3.4 c |
| T25 | 7.03 ± 0.0 bc | 0.968 ± 0.0 e | 1.15 ± 0.0 b | 28 ± 0.0 b | 1128 ± 3.7 e | 120 ± 1.5 d | 1267 ± 3.1 a | 143 ± 1.2 bc |
| T26 | 7.04 ± 0.0 bc | 0.566 ± 0.0 g | 1.32 ± 0.0 a | 29 ± 0.0 a | 1535 ± 4.0 a | 135 ± 3.4 b | 1188 ± 7.6 b | 140 ± 1.6 c |
| At 45 days | ||||||||
| T14 | 7.54 ± 0.0 a | 0.425 ± 0.0 hi | 0.60 ± 0.0 g | 19 ± 0.1 g | 221 ± 0.7 l | 41 ± 2.1 i | 108 ± 0.2 k | 94 ± 2.8 ef |
| T15 | 7.1 ± 0.1 cde | 1.803 ± 0.0 a | 0.65 ± 0.0 h | 19 ± 0.1 f | 318 ± 0.5 k | 43 ± 1.5 hi | 228 ± 0.1 i | 83 ± 3.4 g |
| T16 | 7.14 ± 0.0 cd | 1.346 ± 0.0 c | 0.79 ± 0.0 ef | 28 ± 0.0 de | 984 ± 3.4 d | 107 ± 2.7 c | 365 ± 0.2 g | 110 ± 4.2 c |
| T17 | 7.21 ± 0.0 bc | 1.008 ± 0.0 e | 0.90 ± 0.0 def | 28 ± 0.0 cd | 882 ± 0.2 e | 90 ± 1.5 d | 794 ± 1.4 d | 99 ± 1.4 e |
| T18 | 7.33 ± 0.0 b | 0.561 ± 0.0 g | 1.28 ± 0.0 bc | 29 ± 0.0 a | 1017 ± 3.8 c | 200 ± 3.1 a | 1046 ± 1.2 a | 132 ± 0.4 a |
| T19 | 6.44 ± 0.0 h | 1.213 ± 0.0 d | 0.54 ± 0.0 h | 19 ± 0.0 g | 746 ± 4.5 i | 43 ± 2.3 hi | 111 ± 0.2 k | 54 ± 0.9 h |
| T20 | 6.85 ± 0.0 f | 0.852 ± 0.0 f | 0.67 ± 0.0 ef | 28 ± 0.0 de | 760 ± 5.7 h | 73 ± 0.7 e | 181 ± 1.8 j | 60 ± 1.9 h |
| T21 | 6.93 ± 0.0 ef | 0.979 ± 0.0 e | 0.95 ± 0.0 cd | 28 ± 0.0 b | 818 ± 2.4 g | 57 ± 2.7 g | 310 ± 2.9 h | 90 ± 2.7 fg |
| T22 | 7.33 ± 0.0 b | 0.380 ± 0.0 k | 1.20 ± 0.0 ab | 29 ± 0.0 a | 1077 ± 1.8 b | 65 ± 3.9 f | 533 ± 4.4 f | 124 ± 1.1 b |
| T23 | 6.37 ± 0.0 h | 0.932 ± 0.0 ef | 0.57 ± 0.0 h | 19± 0.0 f | 686 ± 5.5 j | 50 ± 2.3 gh | 643 ± 2.8 e | 88 ± 1.3 fg |
| T24 | 6.63 ± 0.0 g | 1.158 ± 0.0 d | 0.78 ± 0.0 f | 28 ± 0.0 e | 854 ± 1.4 f | 111 ± 3.4 c | 883 ± 2.1 c | 93 ± 1.3 ef |
| T25 | 6.87 ± 0.0 f | 1.621 ± 0.0 b | 0.99 ± 0.0 cde | 28 ± 0.0 bc | 981 ± 1.1 d | 87 ± 2.1 d | 928 ± 14.8 b | 104 ± 4.7 cd |
| T26 | 6.97 ± 0.0 def | 0.507 ± 0.0 gh | 1.20 ± 0.0 a | 29 ± 0.0 a | 1284 ± 1.1 a | 122 ± 1.3 b | 1055 ± 10.2 a | 133 ± 1.8 a |
| Treatments | LAR (cm2 g−1) | LWR (g g−1) | SLA (cm2 g−1) | RSR (g g−1) | SLW (g cm−2) |
|---|---|---|---|---|---|
| T1 | 6.62 ± 0.17 a | 0.044 ± 0.002 ab | 151.76 ± 3.70 a | 0.248 ± 0.019 c | 0.0065 ± 0.0001 |
| T2 | 4.73 ± 0.06 d | 0.037 ± 0.000 bcd | 128.90 ± 4.19 ab | 0.166 ± 0.019 d | 0.0077 ± 0.0002 b |
| T3 | 6.32 ± 0.36 ab | 0.044 ± 0.007 ab | 151.53 ± 19.86 a | 0.319 ± 0.027 a | 0.0068 ± 0.0008 b |
| T4 | 4.55 ± 0.21 d | 0.044 ± 0.002 ab | 104.32 ± 8.32 b | 0.286 ± 0.001 abc | 0.0097 ± 0.0008 b |
| T5 | 5.90 ± 0.79 abc | 0.041 ± 0.000 ab | 145.07 ± 21.30 a | 0.298 ± 0.028 abc | 0.0071 ± 0.0009 a |
| T6 | 4.33 ± 0.03 d | 0.03 ± 0.000 d | 142.85 ± 3.64 a | 0.180 ± 0.006 d | 0.0070 ± 0.0000 b |
| T7 | 6.06 ± 0.18 ab | 0.046 ± 0.001 a | 131.99 ± 4.51 ab | 0.315 ± 0.007 ab | 0.0075 ± 0.0002 b |
| T8 | 6.28 ± 0.23 ab | 0.039 ± 0.001 abc | 162.23 ± 8.77 a | 0.270 ± 0.015 abc | 0.0062 ± 0.0003 b |
| T9 | 5.10 ± 0.23 cd | 0.036 ± 0.001 bcd | 140.58 ± 8.96 a | 0.260 ± 0.003 bc | 0.0071 ± 0.0004 b |
| T10 | 4.73 ± 0.15 d | 0.032 ± 0.000 cd | 145.87 ± 4.17 a | 0.185 ± 0.004 d | 0.0068 ± 0.0001 b |
| T11 | 6.37 ± 0.08 ab | 0.04 ± 0.002 ab | 158.41 ± 7.84 a | 0.302 ± 0.021 abc | 0.0063 ± 0.0003 b |
| T12 | 5.75 ± 0.14 abc | 0.039 ± 0.002 abc | 148.30 ± 7.93 a | 0.294 ± 0.013 abc | 0.0067 ± 0.0003 b |
| T13 | 5.63 ± 0.12 bc | 0.043 ± 0.002 ab | 130.84 ± 7.65 ab | 0.282 ± 0.015 abc | 0.0076 ± 0.0004 b |
| T14 | 6.29 ± 0.07 ab | 0.043 ± 0.000 bc | 146.79 ± 1.83 a | 0.277 ± 0.007 c | 0.0068 ± 0.0000 a |
| T15 | 4.81 ± 0.05 cd | 0.036 ± 0.000 c | 133.19 ± 0.35 a | 0.149 ± 0.005 e | 0.0075 ±0.0000 a |
| T16 | 6.85 ± 0.05 a | 0.048 ± 0.002 a | 143.79 ± 5.37 a | 0.325 ± 0.024 b | 0.0069 ± 0.0002 a |
| T17 | 5.82 ± 0.25 abc | 0.043 ± 0.000 bc | 136.45 ± 4.99 a | 0.282 ± 0.006 c | 0.0073 ± 0.0002 a |
| T18 | 5.86 ± 1.10 abc | 0.04 ± 0.000 cde | 146.15 ± 28.43 a | 0.288 ± 0.022 e | 0.0073 ± 0.0014 a |
| T19 | 4.40 ± 0.15 d | 0.031 ± 0.001 f | 144.32 ± 3.88 a | 0.207 ± 0.000 d | 0.0069 ± 0.0001 a |
| T20 | 6.14 ± 0.14 abc | 0.045 ± 0.001 ab | 136.00 ± 4.97 a | 0.335 ± 0.015 b | 0.0073 ± 0.0002 a |
| T21 | 6.25 ± 0.27 abc | 0.042 ± 0.001 bcd | 149.95 ± 3.46 a | 0.276 ± 0.001 c | 0.0066 ± 0.0001 a |
| T22 | 5.23 ± 0.22 bcd | 0.038 ± 0.000 de | 138.52 ± 4.45 a | 0.284 ± 0.004 c | 0.0072 ± 0.0002 a |
| T23 | 5.08 ± 0.84 bcd | 0.031 ± 0.002 f | 159.52 ± 16.18 a | 0.206 ± 0.003 d | 0.0064 ± 0.0007 a |
| T24 | 6.07 ± 0.41 abc | 0.04 ± 0.002 cde | 154.67 ± 13.61 a | 0.323 ± 0.000 b | 0.0065 ± 0.0005 a |
| T25 | 5.77 ± 0.11 abcd | 0.039 ± 0.000 cde | 146.85 ± 3.98 a | 0.284 ± 0.003 c | 0.0068 ± 0.0001 a |
| T26 | 6.17 ± 0.03 abc | 0.043 ± 0.000 bc | 143.60 ± 3.31 a | 0.387 ± 0.010 a | 0.0069 ± 0.0001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhani, I.; Srivastava, V.; Megharaj, M.; Suthar, S.; Garg, V.K.; Singh, R.P. Effect of Compost and Vermicompost Amendments on Biochemical and Physiological Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown under Different Salinity Gradients. Sustainability 2023, 15, 11590. https://doi.org/10.3390/su151511590
Suhani I, Srivastava V, Megharaj M, Suthar S, Garg VK, Singh RP. Effect of Compost and Vermicompost Amendments on Biochemical and Physiological Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown under Different Salinity Gradients. Sustainability. 2023; 15(15):11590. https://doi.org/10.3390/su151511590
Chicago/Turabian StyleSuhani, Ibha, Vaibhav Srivastava, Mallavarapu Megharaj, Surindra Suthar, Vinod Kumar Garg, and Rajeev Pratap Singh. 2023. "Effect of Compost and Vermicompost Amendments on Biochemical and Physiological Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown under Different Salinity Gradients" Sustainability 15, no. 15: 11590. https://doi.org/10.3390/su151511590
APA StyleSuhani, I., Srivastava, V., Megharaj, M., Suthar, S., Garg, V. K., & Singh, R. P. (2023). Effect of Compost and Vermicompost Amendments on Biochemical and Physiological Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown under Different Salinity Gradients. Sustainability, 15(15), 11590. https://doi.org/10.3390/su151511590










