Response of Soil Microbial Community Diversity to Long-Term Cultivation of Rice (Oryza sativa L.)/Cherry Tomato (Lycopersicon esculentum Mill.) in Rotation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Soil Sample Collection and Analysis of Chemical Properties
2.3. Soil DNA Extraction, PCR Amplification, and Sequencing
2.4. Sequence Processing and Statistical Analyses
3. Results
3.1. Effects of Cultivation Years with the Rice/Cherry Tomato Rotation on Soil Physicochemical Properties
3.2. Effects of Cultivation Years with the Rice/cherry Tomato Rotation on Soil Microbial Community Composition
3.3. Effects of Cultivation Years with the Rice/Cherry Tomato Rotation on Soil Microbial Diversity
3.4. Effects of Soil Environmental Factors on Microbial Communities in Soil from Different Cultivation Years with the Rice/Cherry Tomato Rotation
4. Discussion
4.1. Response of Soil Microbial Community Diversity to Planting Years with the Rice/Cherry Tomato Rotation
4.2. Correlation between Soil Environmental Factors and Soil Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padalia, K.; Bargali, S.S.; Bargali, K.; Khulbe, K. Microbial biomass carbon and nitrogen in relation to cropping systems in Central Himalaya, India. Curr. Sci. 2018, 115, 1741–1750. [Google Scholar] [CrossRef]
- Padalia, K.; Bargali, S.S.; Bargali, K.; Manral, V. Soil microbial biomass phosphorus under different land use systems. Trop. Ecol. 2022, 63, 30–48. [Google Scholar] [CrossRef]
- Manral, V.; Bargali, K.; Bargali, S.S.; Karki, H.; Chaturvedi, R.K. Seasonal dynamics of soil microbial biomass C, N and P along an altitudinal gradient in Central Himalaya, India. Sustainability 2023, 15, 1651. [Google Scholar] [CrossRef]
- Pant, M.; Negi, G.C.S.; Kumar, P. Macrofauna contributes to organic matter decomposition and soil quality in Himalayan agroecosystems, India. Appl. Soil Ecol. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Bargali, S.S.; Padalia, K.; Bargali, K. Effects of tree fostering on soil health and microbial biomass under different land use systems in central Himalaya. Land Degrad. Dev. 2019, 30, 1984–1998. [Google Scholar] [CrossRef]
- Karki, H.; Bargali, K.; Bargali, S.S. Dynamics of fine root and soil nitrogen in Mangifera indica based agroforestry systems in Central Himalaya, India. Land Degrad. Dev. 2022, 33, 3523–3538. [Google Scholar] [CrossRef]
- Shahi, C.; Bargali, S.S.; Bargali, K.; Vibhuti. Dry matter dynamics and CO2 mitigation in the herb layer of Central Himalayan agroecosystems along an altitudinal gradient, India. Trop. Ecol. 2023, 64, 180–192. [Google Scholar] [CrossRef]
- Zhu, S.W.; Gao, T.P.; Liu, Z.; Ning, T.Y. Rotary and subsoiling tillage rotations influence soil carbon and nitrogen sequestration and crop yield. Plant Soil Environ. 2022, 68, 89–97. [Google Scholar] [CrossRef]
- Ma, Z.M.; Guan, Z.J.; Liu, Q.C.; Hu, Y.Y.; Liu, L.F.; Wang, B.Q.; Huang, L.F.; Li, H.F.; Yang, Y.F.; Han, M.K.; et al. Chapter Four—Obstacles in continuous cropping: Mechanisms and control measures. Adv. Agron. 2023, 179, 205–256. [Google Scholar]
- Ali, A.; Ghani, M.I.; Ding, H.; Iqbal, M.; Cheng, Z.; Cai, Z. Arbuscular mycorrhizal inoculum coupled with organic substrate induces synergistic effects for soil quality changes, and rhizosphere microbiome structure in long-term monocropped cucumber planted soil. Rhizosphere 2021, 20, 100428. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.Z.; Liu, Q.Z.; Zhang, Y.T.; Li, X.Y.; Li, H.Q.; Li, W.H. Phase changes of continuous cropping obstacles in strawberry (Fragaria × ananassa Duch.) production. Appl. Soil Ecol. 2020, 155, 103626. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Li, Z.; Shi, J.; Zhang, G.; Zhang, H.; Yang, L. Vermicompost improves microbial functions of soil with continuous tomato cropping in a greenhouse. J. Soil Sediment. 2020, 20, 380–391. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tian, L.; Zhang, J.F.; Ma, L.N.; Li, X.J.; Tian, C.J. Rhizospheric fungi and their link with the nitrogen-fixing Frankia harbored in host plant Hippophae rhamnoides L. J. Basic Microb. 2017, 57, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, J.; Zhou, X.; Han, Y.X.; Zhang, J.B.; Cai, Z.C. How green alternatives to chemical pesticides are environmentally friendly and more efficient. Eur. J. Soil Sci. 2019, 70, 518–529. [Google Scholar] [CrossRef]
- Liu, Z.X.; Liu, J.J.; Yu, Z.H.; Li, Y.S.; Hu, X.J.; Gu, H.D.; Li, L.J.; Jin, J.; Liu, X.B.; Wang, G.H. Archaeal communities perform an important role in maintaining microbial stability under long term continuous cropping systems. Sci. Total Environ. 2022, 838, 156413. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soil borne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017, 7, 44382. [Google Scholar] [CrossRef]
- Sui, J.; Wang, X.; Liu, Z.; Sa, R.; Hu, Y.; Wang, C. A plant growth promoting bacterium alters the microbial community of continuous cropping poplar trees’rhizosphere. J. Appl. Microbiol. 2019, 126, 1209–1220. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.Y.; Chu, G.X.; Hu, B.W.; Tao, R. Continuous cropping of cut chrysanthemum reduces rhizospheric soil bacterial community diversity and co-occurrence network complexity. Appl. Soil Ecol. 2023, 185, 104801. [Google Scholar] [CrossRef]
- Chongtham, I.R.; Bergkvist, G.; Watson, C.A.; Sandström, E.; Bengtsson, J.; Öborn, I. Factors influencing crop rotation strategies on organic farms with different time periods since conversion to organic production. Biol. Agric. Hortic. 2016, 33, 14–27. [Google Scholar] [CrossRef]
- Wright, P.J.; Falloon, R.E.; Hedderley, D. A long-term vegetable crop rotation study to determine effects on soil microbial communities and soil borne diseases of potato and onion. N. Z. J. Crop Hortic. Sci. 2017, 45, 29–54. [Google Scholar] [CrossRef]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Zhang, X.M.; Wei, M.T.; Wang, Y.; Liang, Z.S.; Xia, P.G. Appropriate crop rotation alleviates continuous cropping barriers by changing rhizosphere microorganisms in Panax notoginseng. Rhizosphere 2022, 23, 100568. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.W.; Jiang, X.S. Rotations improve the diversity of rhizosphere soil bacterial communities, enzyme activities and tomato yield. PLoS ONE 2023, 18, e0270944. [Google Scholar] [CrossRef]
- Vibhuti, C.S.; Bargali, K.; Bargali, S.S. Bargali Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian J. Agr. Sci. 2015, 85, 102–108. [Google Scholar]
- Dai, Y.Q.; Wang, Z.; Li, J.; Xu, Z.; Qian, C.; Xia, X.D.; Liu, Y.; Feng, Y.F. Tofu by-product soy whey substitutes urea: Reduced ammonia volatilization, enhanced soil fertility and improved fruit quality in cherry tomato production. Environ. Res. 2023, 226, 115662. [Google Scholar] [CrossRef]
- Zhu, Q.S. Grafting culture technology of cherry tomato in Lingshui County. China Agric. Technol. Ext. 2017, 33, 32–33. (In Chinese) [Google Scholar]
- Liu, H.F.; Han, H.W.; Wang, Q.; Zhuang, H.M.; Wang, H. Effect of vegetables-tomato rotation on soil microbial diversity, enzyme activity and physicochemical properties of vegetables in greenhouse. Acta Microbiol. Sinica 2021, 61, 167–182. (In Chinese) [Google Scholar]
- Lu, R.K. Analytical Methods of Soil and Agro-Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wu, X.Y.; Shan, Y.; Li, Y.; Wu, C.Y. The soil nutrient environment determines the strategy by which bacillus velezensis HN03 suppresses Fusarium wilt in banana plants. Front. Plant Sci. 2020, 11, 599904. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.T.; Wang, Y.N.; Cui, L. Effects of crop rotation and continuous cropping on the bacterial diversity of carrot rhizosphere and non-rhizosphere. Genomics Appl. Biol. 2019, 38, 2078–2085. (In Chinese) [Google Scholar]
- Jiao, X.L.; Zhang, X.S.; Lu, X.H.; Qin, R.; Gao, W.W. Effects of maize rotation on the physicochemical properties and microbial communities of American ginseng cultivated soil. Sci. Rep. 2019, 9, 9–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Zhang, X.Y.; Wang, J.G.; Gao, L.H. Soil microbial communities associated with the rhizosphere of cucumber under different summer cover crops and residue management: A 4-year field experiment. Sci. Hortic. 2013, 150, 100–109. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, W.; Xing, Y.Z.; Sun, Y.; Lin, X.J.; Dong, Y.P. Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Carteni, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant-soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef]
- Bini, D.; dos Santos, C.A.; Bouillet, J.P.; de Morais Goncalves, J.L.; Nogueira Cardoso, E.J.B. Eucalyptus grandis and Acacia mangium in monoculture and intercropped plantations: Evolution of soil and litter microbial and chemical attributes during early stages of plant development. Appl. Soil Ecol. 2013, 63, 57–66. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Mazzoleni, S.; Abd-ElGawad, A.M. Microbiota modulation of allelopathy depends on litter chemistry: Mitigation or exacerbation? Sci. Total Environ. 2021, 776, 145942. [Google Scholar] [CrossRef]
- Idbellaa, M.; De Filippis, F.; Zotti, M.; Sequino, G.; Abd-ElGawad, A.M.; Fechtali, T.; Mazzoleni, S.; Bonanomi, G. Specific microbiome signatures under the canopy of Mediterranean shrubs. Appl. Soil Ecol. 2022, 173, 104407. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.M.; Brookes, P.C.; Xu, J.M.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; De Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Shi, S.J.; Nuccio, E.E.; Shi, Z.J.; He, Z.L.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Truu, J.; Chatterjee, P.; Truu, M.; Kim, K.; Kim, S.; Seshadri, S.; Sa, T. Long-term silicate fertilization increases the abundance of Actinobacterial population in paddy soils. Biol. Fertil. Soils 2019, 55, 109–120. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L. Taxonomy, physiology and natural products of Actinobacteria. Microbiol. Mol. Biol. R. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Yousuf, B.; Keshri, J.; Mishra, A.; Jha, B. Application of targeted metagenomics to explore abundance and diversity of CO2-fixing bacterial community using cbbL gene from the rhizosphere of Arachis hypogaea. Gene 2012, 506, 18–24. [Google Scholar] [CrossRef]
- Shen, C.; Liang, W.; Shi, Y.; Lin, X.G.; Zhang, H.Y.; Wu, X.; Xie, G.; Chain, P.; Grogan, P.; Chu, H.Y. Contrasting elevational diversity patterns between eukaryotic soil microbes and plants. Ecology 2014, 95, 3190–3202. [Google Scholar] [CrossRef]
- Pachiadaki, M.G.; Sintes, E.; Bergauer, K.; Brown, J.M.; Record, N.R.; Swan, B.K.; Mathyer, M.E.; Hallam, S.J.; Lopaz-Garcla, P.; Takaki, Y.; et al. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science 2017, 358, 1046–1051. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Macdonald, L.M.; Rogers, S.L. Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol. Biochem. 2008, 40, 803–813. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Han, M.K.; Hu, Y.Y.; Li, Z.Q.; Ma, Z.M. Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef]
- Liu, Z.X.; Liu, J.J.; Yu, Z.H.; Yao, Q.; Li, Y.S.; Liang, A.Z.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.B.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Till. Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Feng, Z.H.; Han, Z.; Song, S.Q.; Lin, S.H.; Wu, A.B. First report of pepper fruit rot caused by Fusarium concentricum in China. Plant Dis. 2013, 97, 1657. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Y.; Guo, N.; Shi, J.; Zhang, H.J.; Liu, S.S.; Zhang, J.Q. Detection and analysis of fungi carried by maize grain in Huang-Huai-Hai summer maize region. Scientia Agric. Sinica. 2018, 51, 3508–3519. (In Chinese) [Google Scholar]
- Zapata-Sarmiento, D.H.; Palacios-Palaa, E.F.; Rodríguez-Hernándezb, A.A.; Melchora, D.L.M.; Rodríguez-Monroya, M.; Sepúlveda-Jiménez, M. Trichoderma asperellum, a potential biological control agent of Stemphylium vesicarium, on onion (Allium cepa L.). Biol. Control 2020, 140, 104105. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. Effects of inorganic and organic treatments on the microbial community of maize rhizosphere by a shotgun metagenomics approach. Ann. Microbiol. 2020, 70, 49. [Google Scholar] [CrossRef]
- Ding, L.J.; Su, J.Q.; Xu, H.J.; Jia, Z.J.; Zhu, Y.G. Long-term nitrogen fertilization of paddy soil shifts iron-reducing microbial community revealed by RNA-13C-acetate probing coupled with pyrosequencing. ISME J. 2015, 9, 721–734. [Google Scholar] [CrossRef]
- Muehe, E.M.; Weigold, P.; Adaktylou, I.J.; Planer-Friedrich, B.; Kraemer, U.; Kappler, A.; Behrens, S. Rhizosphere microbial community composition affects cadmium and zinc uptake by the metal-hyper accumulating plant Arabidopsis halleri. Appl. Environ. Microbiol. 2015, 81, 2173–2181. [Google Scholar] [CrossRef]
- Sheremet, A.; Jones, G.M.; Jarett, J.; Bowers, R.M.; Bedard, I.; Culham, C.; Eloe-Fadrosh, E.A.; Ivanova, N.; Malmstrom, R.R.; Grasby, S.E.; et al. Ecological and genomic analyses of candidate phylum WPS-2 bacteria in an unvegetated soil. Environ. Microbiol. 2020, 22, 3143–3157. [Google Scholar] [CrossRef]
- Xu, J.S.; Gao, W.; Zhao, B.Z.; Chen, M.Q.; Ma, L.; Jia, Z.J.; Zhang, J.B. Bacterial community composition and assembly along natural sodicity/salinity gradient in surface and subsurface soils. Appl. Soil Ecol. 2021, 157, 103731. [Google Scholar] [CrossRef]
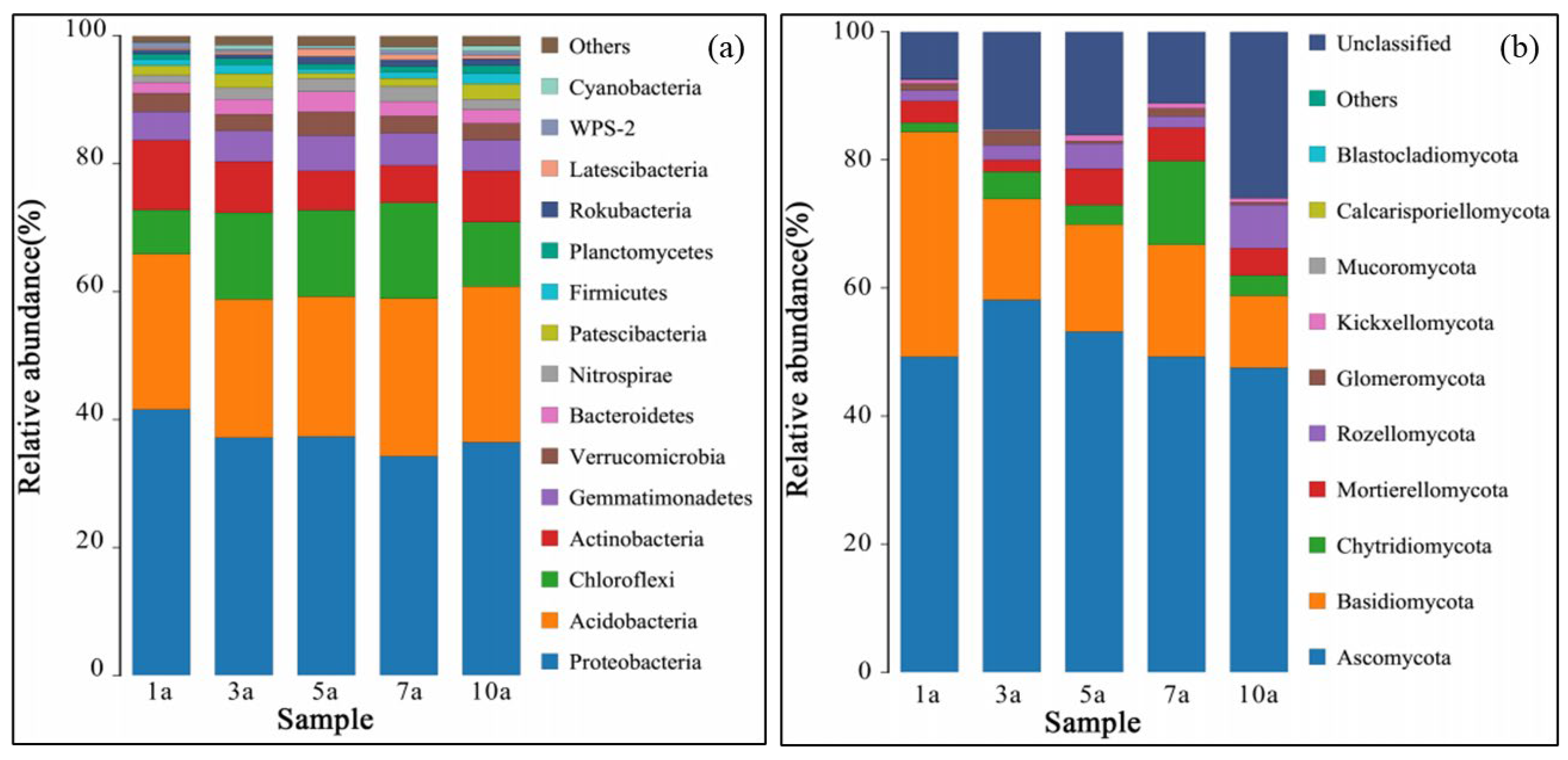
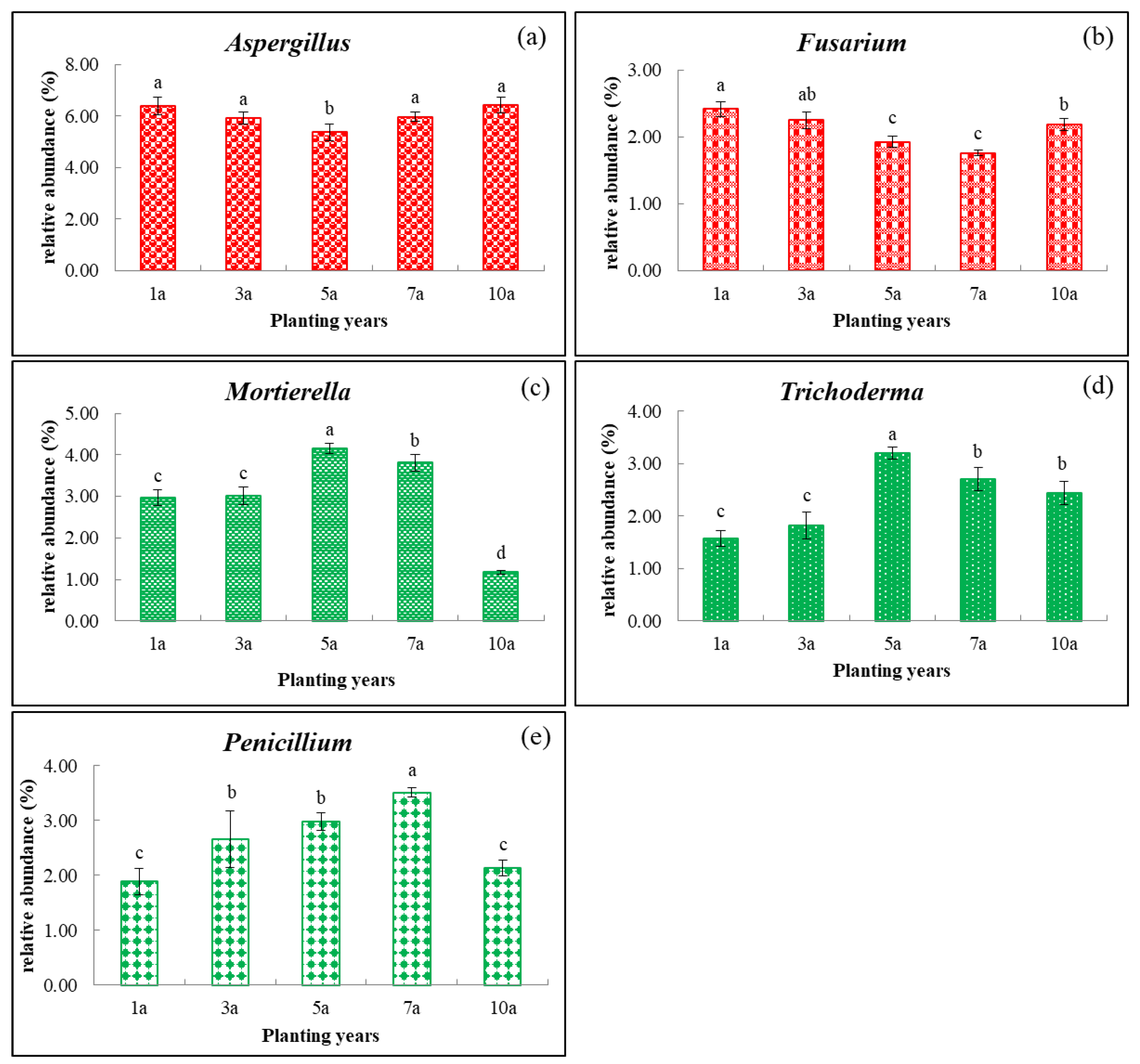
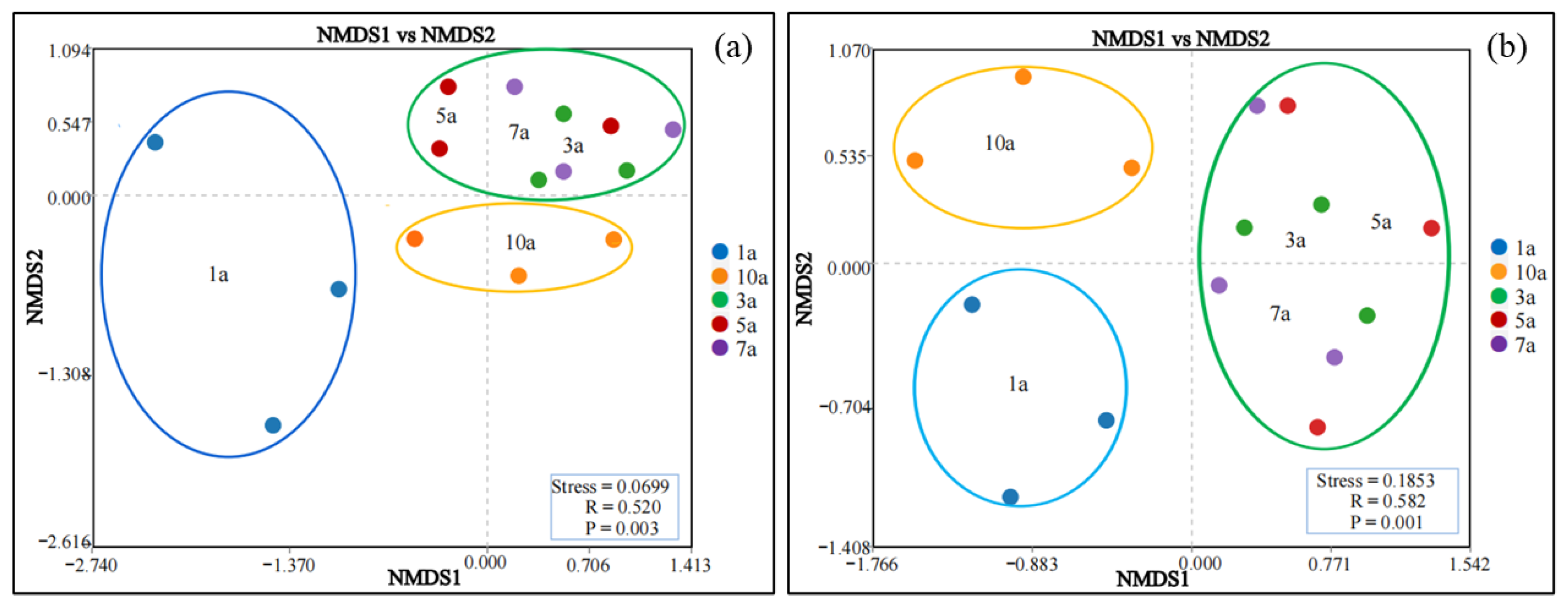
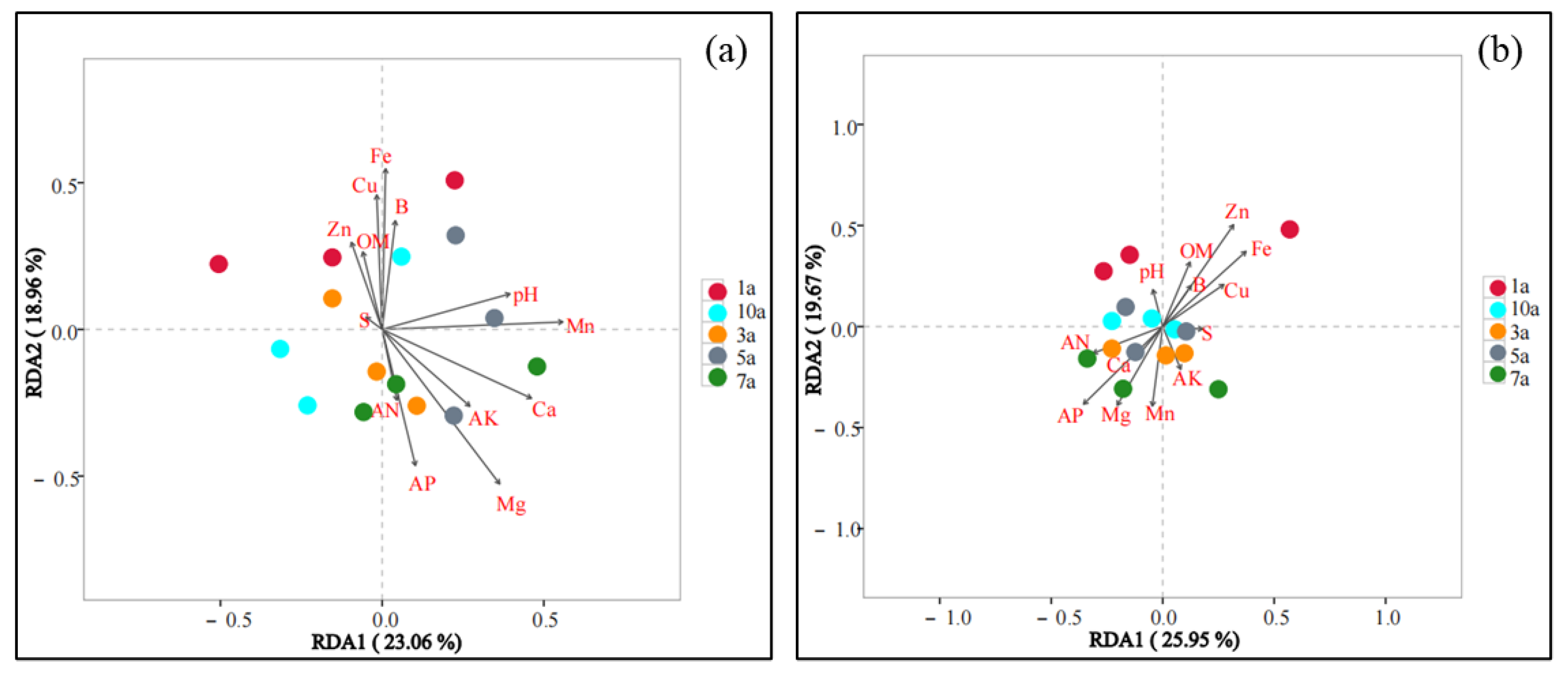
| Planting Years | pH | OM (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | Ca (mg·kg−1) | Mg (mg·kg−1) | S (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| 1a | 4.99 ± 0.14 b | 24.0 ± 1.35 a | 107 ± 13.3 c | 26.7 ± 0.79 c | 160 ± 9.32 b | 321 ± 38.5 d | 13.3 ± 2.67 b | 0.38 ± 0.05 b |
| 3a | 5.03 ± 0.05 b | 21.3 ± 1.03 b | 171 ± 5.15 a | 56.2 ± 4.44 b | 163 ± 20.4 b | 677 ± 71.6 a | 60.4 ± 9.97 a | 0.51 ± 0.04 a |
| 5a | 5.32 ± 0.07 a | 18.4 ± 0.81 c | 133 ± 14.5 b | 59.7 ± 4.50 b | 166 ± 8.42 b | 570 ± 41.7 b | 59.2 ± 5.41 a | 0.28 ± 0.05 c |
| 7a | 4.79 ± 0.16 c | 17.4 ± 1.90 c | 112 ± 5.76 c | 61.5 ± 4.73 b | 218 ± 14.7 a | 408 ± 36.1 c | 54.2 ± 6.16 a | 0.26 ± 0.04 c |
| 10a | 4.48 ± 0.16 d | 12.0 ± 1.17 d | 107 ± 11.4 c | 73.0 ± 4.11 a | 168 ± 21.3 b | 268 ± 22.7 d | 51.5 ± 4.44 a | 0.26 ± 0.05 c |
| Planting Years | Fe (mg·kg−1) | Mn (mg·kg−1) | Zn (mg·kg−1) | Cu (mg·kg−1) | B (mg·kg−1) |
|---|---|---|---|---|---|
| 1a | 19.8 ± 2.54 a | 5.88 ± 0.38 c | 0.67 ± 0.04 a | 0.20 ± 0.02 a | 0.05 ± 0.01 a |
| 3a | 16.4 ± 0.62 b | 6.95 ± 0.43 ab | 0.57 ± 0.04 b | 0.19 ± 0.05 a | 0.04 ± 0.00 a |
| 5a | 14.6 ± 1.20 b | 7.39 ± 0.51 a | 0.55 ± 0.08 b | 0.17 ± 0.02 ab | 0.05 ± 0.02 a |
| 7a | 11.4 ± 0.89 c | 6.31 ± 0.90 bc | 0.54 ± 0.06 b | 0.14 ± 0.02 ab | 0.04 ± 0.01 a |
| 10a | 9.26 ± 0.83 c | 5.31 ± 0.35 c | 0.51 ± 0.02 b | 0.12 ± 0.01 b | 0.06 ± 0.00 a |
| Planting Years | Bacterial α-Diversity Indices | Fungal α-Diversity Indices | ||
|---|---|---|---|---|
| Chao1 | Shannon | Chao1 | Shannon | |
| 1a | 1877.5 ± 64.3 b | 8.7179 ± 0.3770 b | 650.0 ± 36.7 c | 6.4210 ± 0.2702 d |
| 3a | 2071.3 ± 76.5 a | 9.2812 ± 0.1062 a | 701.7 ± 84.8 b | 6.8260 ± 0.0766 bc |
| 5a | 2078.9 ± 107 a | 9.4095 ± 0.1736 a | 767.2 ± 78.8 a | 7.2901 ± 0.3152 a |
| 7a | 2052.4 ± 65.2 a | 9.3541 ± 0.1964 a | 754.3 ± 23.8 a | 7.0088 ± 0.1273 ab |
| 10a | 2038.0 ± 101 a | 9.2806 ± 0.1781 a | 701.9 ± 41.0 b | 6.5542 ± 0.1958 cd |
| Environmental Factors | Bacterial Community Composition | Fungal Community Composition | ||
|---|---|---|---|---|
| r | p | r | p | |
| pH | −0.0301 | 0.555 | −0.1268 | 0.781 |
| OM | 0.0939 | 0.258 | 0.1872 | 0.178 |
| AN | −0.0104 | 0.472 | 0.0148 | 0.336 |
| AP | 0.3989 | 0.030 | 0.3359 | 0.042 |
| AK | −0.2302 | 0.956 | −0.0439 | 0.539 |
| Ca | −0.0694 | 0.702 | −0.1506 | 0.903 |
| Mg | 0.4889 | 0.01 | 0.1875 | 0.197 |
| S | −0.0105 | 0.454 | −0.0724 | 0.612 |
| Fe | 0.3761 | 0.011 | 0.4064 | 0.028 |
| Mn | 0.0065 | 0.428 | 0.0393 | 0.312 |
| Zn | 0.2708 | 0.043 | 0.4031 | 0.026 |
| Cu | 0.1478 | 0.135 | 0.1578 | 0.112 |
| B | −0.0248 | 0.500 | 0.0605 | 0.301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Yin, H.; Tan, H.; Li, Y.; Wu, C.; Su, J. Response of Soil Microbial Community Diversity to Long-Term Cultivation of Rice (Oryza sativa L.)/Cherry Tomato (Lycopersicon esculentum Mill.) in Rotation. Sustainability 2023, 15, 10148. https://doi.org/10.3390/su151310148
Deng X, Yin H, Tan H, Li Y, Wu C, Su J. Response of Soil Microbial Community Diversity to Long-Term Cultivation of Rice (Oryza sativa L.)/Cherry Tomato (Lycopersicon esculentum Mill.) in Rotation. Sustainability. 2023; 15(13):10148. https://doi.org/10.3390/su151310148
Chicago/Turabian StyleDeng, Xiao, Hao Yin, Huadong Tan, Yi Li, Chunyuan Wu, and Jiancheng Su. 2023. "Response of Soil Microbial Community Diversity to Long-Term Cultivation of Rice (Oryza sativa L.)/Cherry Tomato (Lycopersicon esculentum Mill.) in Rotation" Sustainability 15, no. 13: 10148. https://doi.org/10.3390/su151310148
APA StyleDeng, X., Yin, H., Tan, H., Li, Y., Wu, C., & Su, J. (2023). Response of Soil Microbial Community Diversity to Long-Term Cultivation of Rice (Oryza sativa L.)/Cherry Tomato (Lycopersicon esculentum Mill.) in Rotation. Sustainability, 15(13), 10148. https://doi.org/10.3390/su151310148





