Impact of Oyster (Crassostrea virginica) Reef Restoration on Benthic Invertebrates and Coastal Birds in a Subtropical Estuary
Abstract
:1. Introduction
2. Materials and Methods
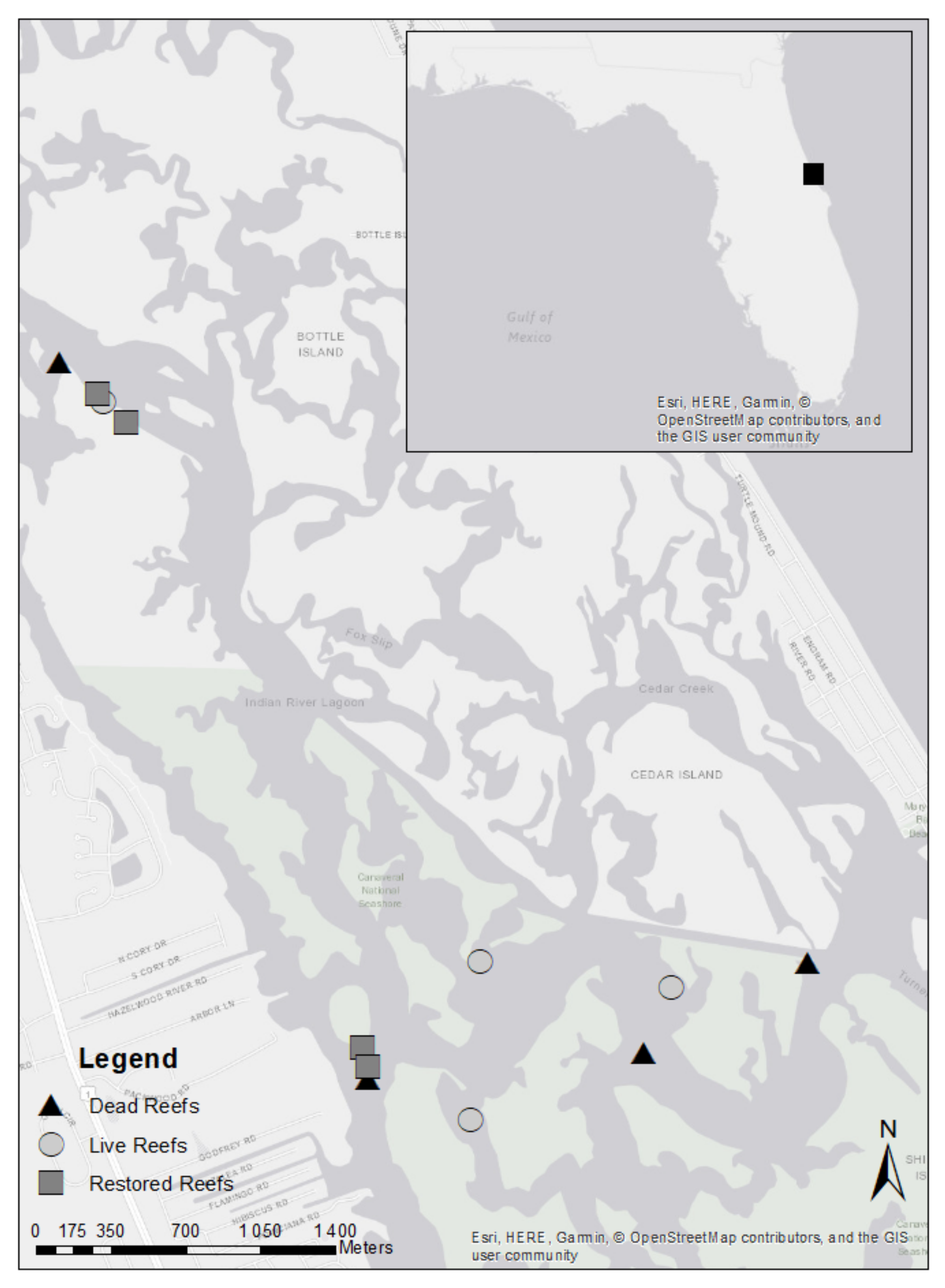
2.1. Study Site
2.2. Study Set-Up
2.3. Benthic Invertebrate Sampling and Sorting Methods
2.4. Bird Survey Methods
2.5. Statistical Methods
3. Results
3.1. Benthic Invertebrate Results
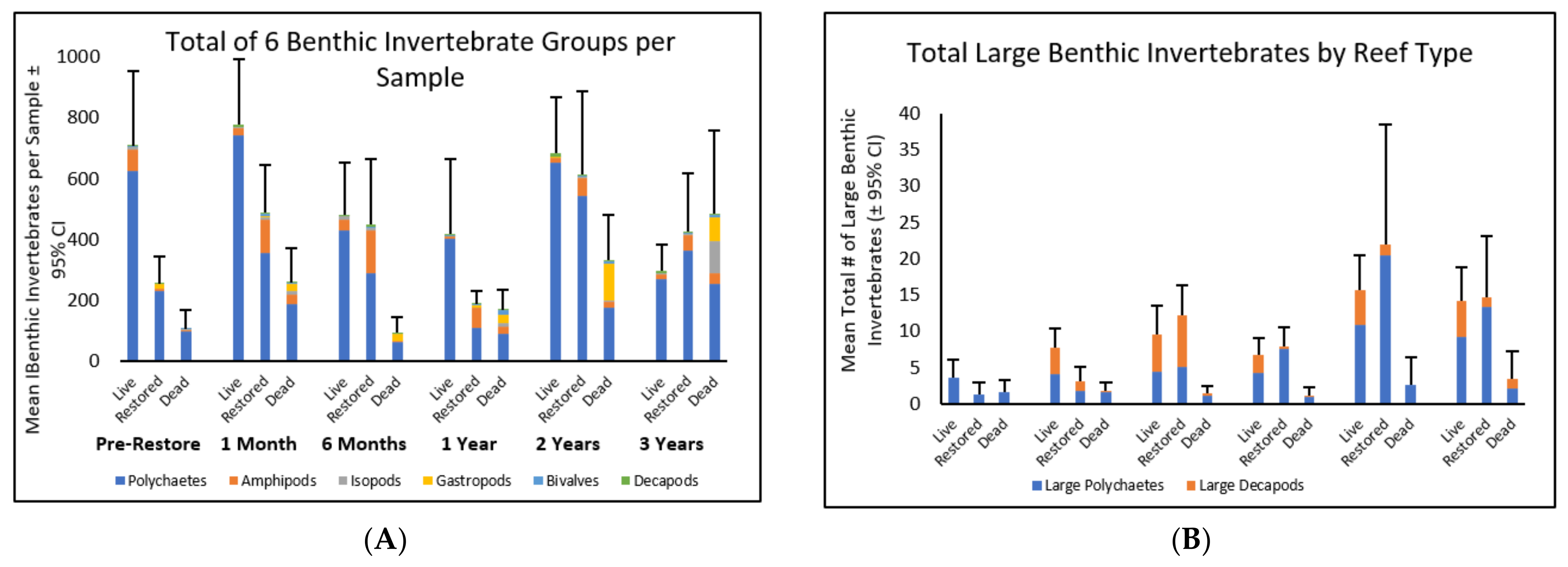
3.1.1. Benthic Invertebrate Abundance
3.1.2. Abundance of Large Benthic Invertebrates
3.1.3. Changes in Benthic Invertebrate Abundance over Time
3.1.4. Changes in Abundance of Different Taxa over Time
3.2. Bird Survey Results
3.2.1. Bird Survey Abiotic Data
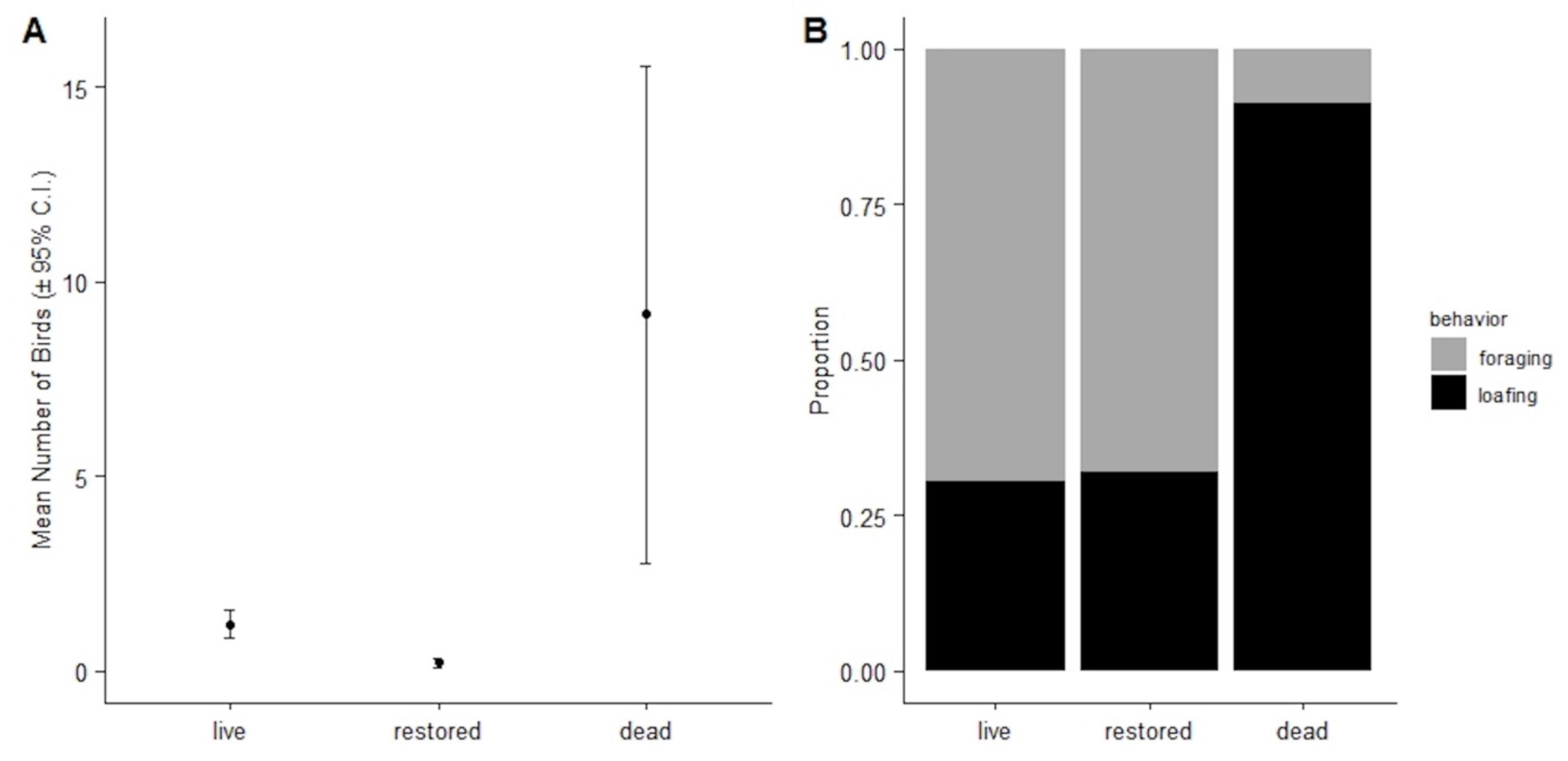
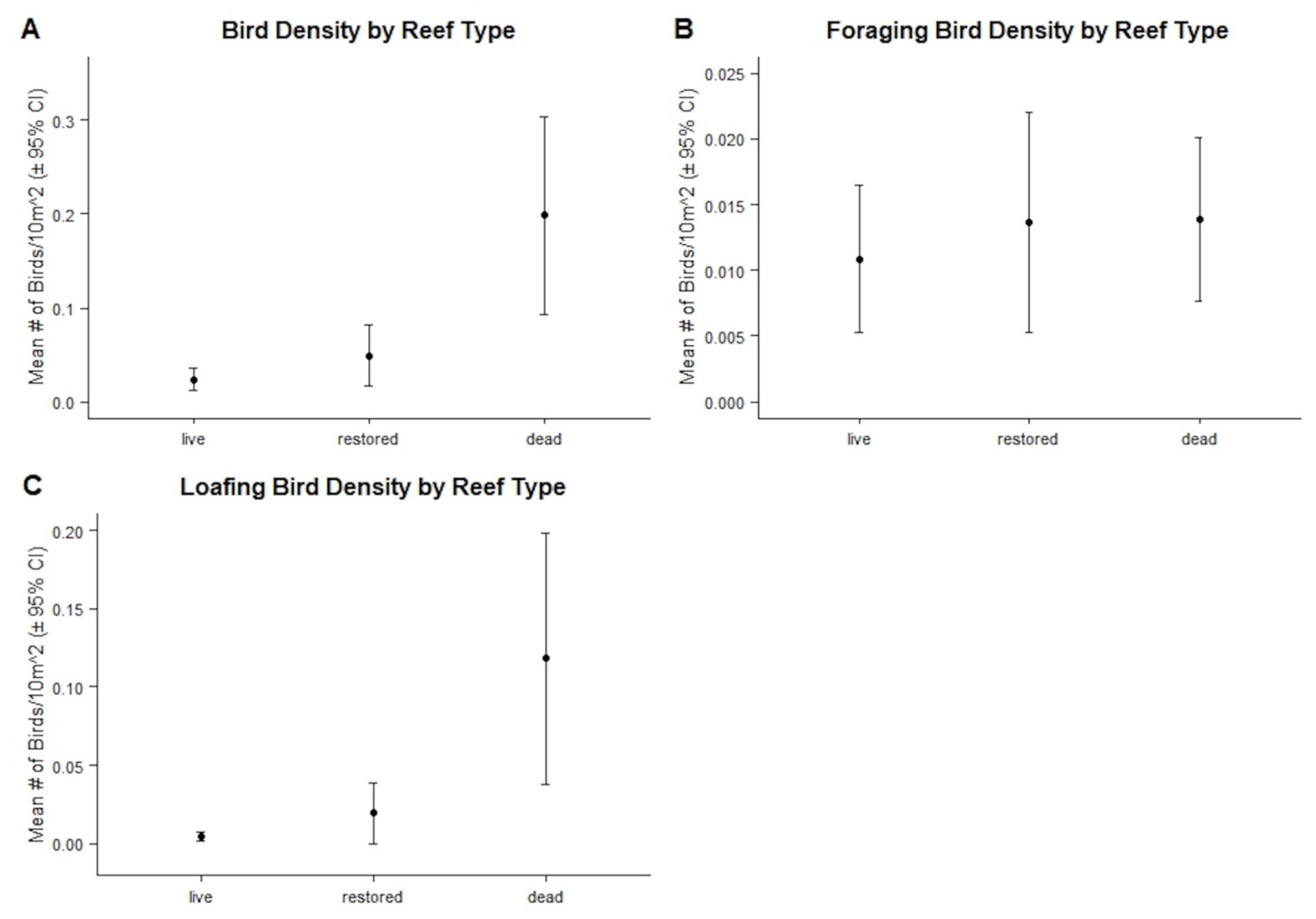
3.2.2. Bird Abundances, Densities, and Behaviors by Reef Type
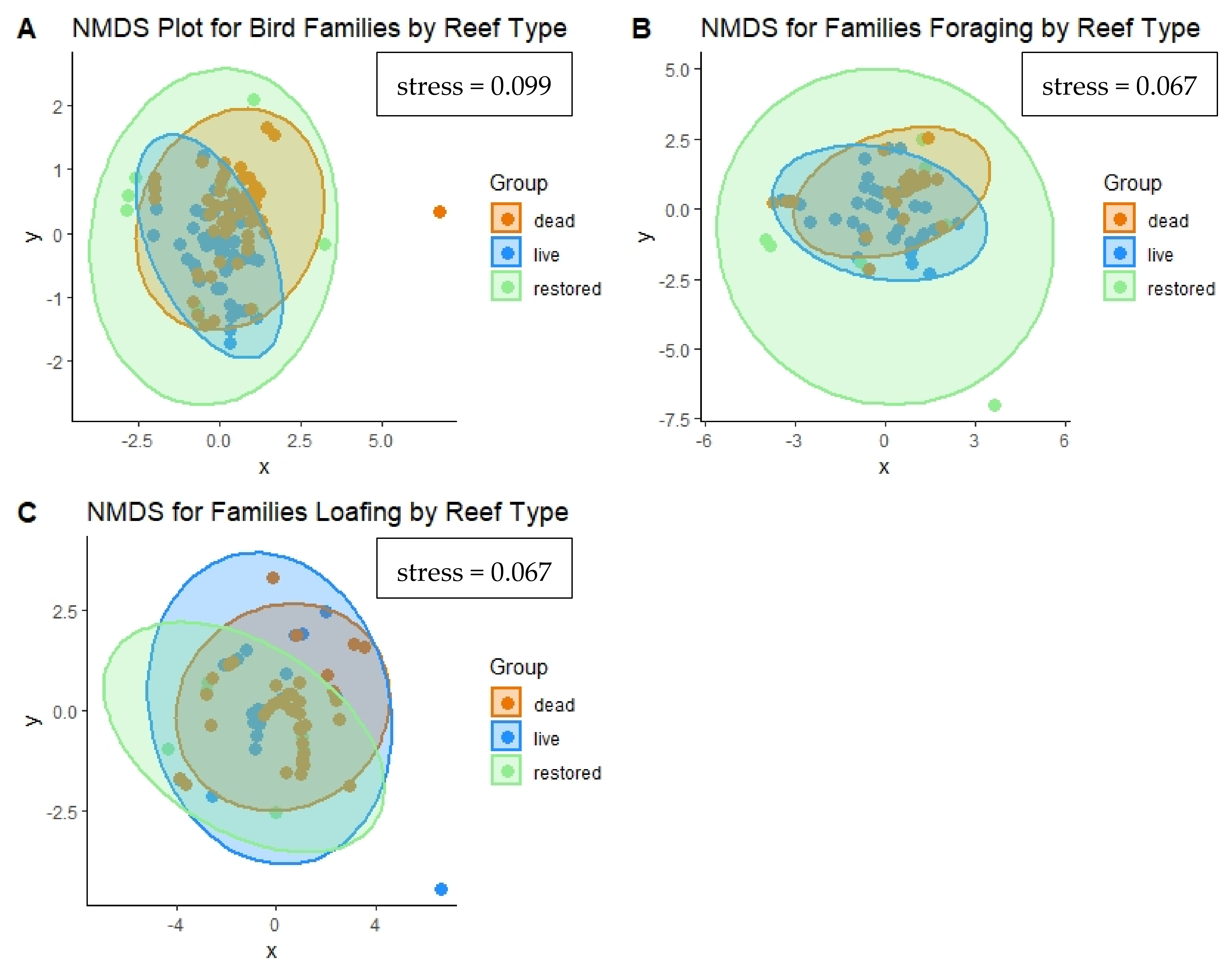
3.2.3. Community Assemblages
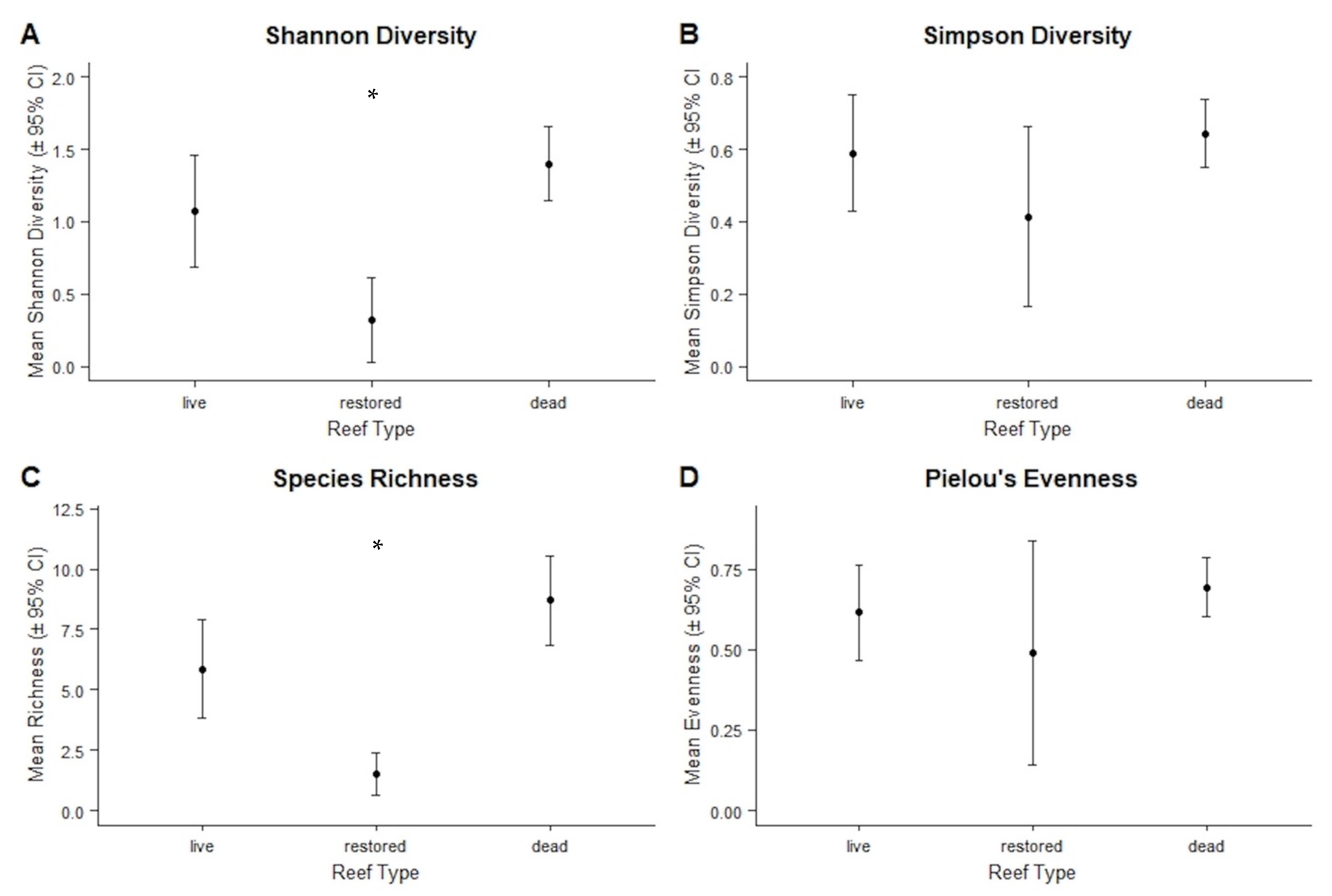
3.2.4. Diversity Indices and Representative Species
4. Discussion
4.1. Bird Abundance and Behaviors
4.2. Diversity and Community Assemblages
4.3. Unexpected Benthic Invertebrate Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reef | Latitude (DD) | Longitude (DD) |
|---|---|---|
| Live 1 | 28.968048 | −80.879798 |
| Live 2 | 28.944458 | −80.861368 |
| Live 3 | 28.937995 | −80.862099 |
| Live 4 | 28.943279 | −80.852491 |
| Dead 1 | 28.969679 | −80.881873 |
| Dead 2 | 28.939420 | −80.867132 |
| Dead 3 | 28.940506 | −80.853897 |
| Dead 4 | 28.944189 | −80.845954 |
| Restore 1 | 28.968420 | −80.880051 |
| Restore 2 | 28.940849 | −80.867377 |
| Restore 3 | 28.939989 | −80.867109 |
| Restore 4 | 28.967207 | −80.878734 |
| Taxa | Time Frame | Mean Abundance Live Reefs (±95% CI) | Mean Abundance Restored Reefs (±95% CI) | Mean Abundance Dead Reefs (±95% CI) |
|---|---|---|---|---|
| Polychaetes | Pre-Restoration | 626.67 ± 254.69 | 232.33 ± 82.20 | 174.83 ± 62.20 |
| 1 Month | 744.58 ± 210.23 | 358.33 ± 125.05 | 187.33 ± 105.94 | |
| 6 Months | 429.17 ± 161.88 | 291.75 ± 130.35 | 65.50 ± 34.43 | |
| 1 Year | 398.17 ± 243.04 | 104.17 ± 23.34 | 92.33 ± 61.57 | |
| 2 Years | 644.25 ± 184.89 | 525.25 ± 224.12 | 166.58 ±144.76 | |
| 3 Years | 260.42 ± 82.61 | 351.50 ± 154.49 | 251.58 ± 167.16 | |
| Amphipods | Pre-Restoration | 69.33 ± 69.60 | 6.67 ± 4.51 | 3.92 ± 2.41 |
| 1 Month | 22.00 ± 10.86 | 105.92 ± 41.17 | 32.00 ± 31.61 | |
| 6 Months | 35.08 ± 45.50 | 139.17 ± 97.92 | 2.50 ± 1.98 | |
| 1 Year | 9.58 ± 7.57 | 64.00 ± 47.93 | 22.67 ± 17.16 | |
| 2 Years | 13.75 ± 7.75 | 57.83 ± 57.19 | 21.58 ± 25.58 | |
| 3 Years | 16.67 ± 6.75 | 51.08 ± 61.29 | 35.17 ± 31.42 | |
| Isopods | Pre-Restoration | 6.67 ± 5.89 | 2.33 ± 2.53 | 4.67 ± 5.86 |
| 1 Month | 2.75 ± 1.33 | 10.17 ± 11.94 | 14.00 ± 17.67 | |
| 6 Months | 11.75 ± 9.57 | 8.67 ± 6.39 | 1.25 ± 0.82 | |
| 1 Year | 0.17 ± 0.24 | 0.91 ± 1.13 | 11.25 ± 14.51 | |
| 2 Years | 1.50 ± 1.45 | 1.67 ± 1.92 | 2.00 ± 2.48 | |
| 3 Years | 3.08 ± 3.64 | 1.33 ± 2.21 | 105.17 ± 110.28 | |
| Gastropods | Pre-Restoration | 1.00 ± 1.15 | 13.25 ± 11.54 | 2.75 ± 2.70 |
| 1 Month | 1.50 ± 1.10 | 3.33 ± 3.91 | 23.50 ± 30.75 | |
| 6 Months | 0.25 ± 0.29 | 0.00 ± 0.00 | 20.67 ± 30.87 | |
| 1 Year | 0.42 ± 0.33 | 9.25 ± 10.81 | 28.41 ± 25.50 | |
| 2 Years | 0.92 ± 1.10 | 0.83 ± 0.76 | 123.67 ± 80.75 | |
| 3 Years | 0.17 ± 0.25 | 0.17 ± 0.37 | 81.25 ± 128.54 | |
| Bivalves | Pre-Restoration | 2.42 ± 1.83 | 2.00 ± 2.03 | 1.00 ± 1.51 |
| 1 Month | 1.33 ± 0.95 | 6.33 ± 2.10 | 2.42 ± 2.04 | |
| 6 Months | 0.50 ± 0.64 | 1.25 ± 0.82 | 3.42 ± 2.72 | |
| 1 Year | 1.58 2.34 | 1.58 ± 1.72 | 12.17 ± 11.05 | |
| 2 Years | 1.50 ± 0.69 | 2.58 ± 1.39 | 7.58 ± 5.81 | |
| 3 Years | 1.00 ± 0.66 | 5.58 ± 5.40 | 7.25 ± 4.34 | |
| Decapods | Pre-Restoration | 1.08 ± 1.74 | 0.33 ± 0.73 | 0.00 ± 0.00 |
| 1 Month | 5.75 ± 2.97 | 4.17 ± 5.96 | 0.33 ± 0.73 | |
| 6 Months | 6.33 ± 4.97 | 10.67 ± 6.98 | 0.41 ± 0.42 | |
| 1 Year | 2.58 ± 2.19 | 0.33 ± 0.41 | 0.08 ± 0.18 | |
| 2 Years | 7.83 ± 7.67 | 2.92 ± 3.40 | 2.42 ± 2.85 | |
| 3 Years | 3.67 ± 3.73 | 1.42 ± 2.21 | 1.33 ± 1.72 |
| Species | Total Count | Percent of Observations | ||
|---|---|---|---|---|
| LOR | ROR | DOR | ||
| Actitis macularius (Spotted Sandpiper) | 4 | 1.21 | 2.94 | 0.08 |
| Ardea alba (Great Egret) | 4 | 1.82 | 0.00 | 0.08 |
| Ardea herodias (Great Blue Heron) | 11 | 1.82 | 5.88 | 0.45 |
| Arenaria interpres (Ruddy Turnstone) | 92 | 3.64 | 14.71 | 6.09 |
| Butorides virescens (Green Heron) | 1 | 0.00 | 2.94 | 0.00 |
| Calidris alba (Sanderling) | 4 | 1.21 | 0.00 | 0.15 |
| Calidris mauri (Western Sandpiper) | 1 | 0.00 | 2.94 | 0.00 |
| Cathartes aura (Turkey Vulture) | 3 | 1.82 | 0.00 | 0.00 |
| Charadrius vociferus (Killdeer) | 1 | 0.00 | 0.00 | 0.08 |
| Charadrius semipalmatus (Semi-palmated Plover) | 26 | 1.21 | 0.00 | 1.80 |
| Coragyps atratus (Black Vulture) | 2 | 1.21 | 0.00 | 0.00 |
| Egretta caerulea (Little Blue Heron) | 14 | 6.67 | 5.88 | 0.08 |
| Egretta rufescens (Reddish Egret) | 6 | 1.21 | 0.00 | 0.30 |
| Egretta thula (Snowy Egret) | 13 | 6.06 | 2.94 | 0.15 |
| Egretta tricolor (Tri-colored Heron) | 2 | 0.61 | 0.00 | 0.08 |
| Eudocimus albus (White Ibis) | 41 | 21.21 | 2.94 | 0.38 |
| Haematopus palliatus (American Oystercatcher) | 14 | 1.82 | 8.82 | 0.75 |
| Haliaeetus leucocephalus (Bald Eagle) | 2 | 0.00 | 5.88 | 0.00 |
| Hydroprogne caspia (Caspian Tern) | 9 | 0.00 | 0.00 | 0.68 |
| Larus argentatus (Herring Gull) | 16 | 0.00 | 0.00 | 1.20 |
| Larus delawarensis (Ring-billed Gull) | 21 | 4.85 | 0.00 | 0.98 |
| Leucophaeus atricilla (Laughing Gull) | 437 | 9.70 | 8.82 | 31.40 |
| Megaceryle alcyon (Belted Kingfisher) | 5 | 2.42 | 0.00 | 0.08 |
| Mergus serrator (Red-breasted Merganser) | 3 | 0.00 | 0.00 | 0.23 |
| Nyctanassa violacea (Yellow-crowned Night Heron) | 1 | 0.00 | 0.00 | 0.08 |
| Pandion haliaetus (Osprey) | 22 | 7.88 | 5.88 | 0.53 |
| Pelecanus occidentalis (Brown Pelican) | 27 | 0.00 | 0.00 | 2.03 |
| Phalacrocorax auritus (Double-crested Cormorant) | 43 | 0.00 | 5.88 | 3.09 |
| Pluvialis squatarola (Black-bellied Plover) | 29 | 7.88 | 0.00 | 1.20 |
| Quiscalus major (Boat-tailed Grackle) | 1 | 0.00 | 0.00 | 0.08 |
| Rynchops niger (Black Skimmer) | 1 | 0.00 | 0.00 | 0.08 |
| Sterna forsteri (Forster’s Tern) | 11 | 0.00 | 0.00 | 0.83 |
| Sternula antillarum (Least Tern) | 64 | 1.82 | 2.94 | 4.51 |
| Thalasseus maximus (Royal Tern) | 500 | 0.61 | 2.94 | 37.42 |
| Thalasseus sandvicensis (Sandwich Tern) | 5 | 0.00 | 0.00 | 0.38 |
| Tringa semipalmata (Willet) | 86 | 13.33 | 0.00 | 4.81 |
| All Birds | 1529 | 10.78 | 2.22 | 86.86 |
| Air Temp (°C) | Water Temp (°C) | Wind Speed (kph) | Salinity (ppt) | ||
|---|---|---|---|---|---|
| All Data | Mean | 23.5 | 24.1 | 8.1 | 33.3 |
| (n = 388) | 95% CI | 0.6 | 0.5 | 0.5 | 0.5 |
| Min–Max | 5.0–39.0 | 12.0–31.3 | 0–25.9 | 20.0–44.0 | |
| Summer | Mean | 28.0 | 28.8 | 6.5 | 29.2 |
| (n = 132) | 95% CI | 0.3 | 0.3 | 0.7 | 0.6 |
| Min–Max | 24.0–34.3 | 25.0–32.3 | 0–17.5 | 23.0–42.0 | |
| Fall | Mean | 23.0 | 23.7 | 8.9 | 34.4 |
| (n = 57) | 95% CI | 1.8 | 1.2 | 1.3 | 1.6 |
| Min–Max | 8.0–39.0 | 14.0–31.0 | 2.3–25.9 | 20.0–43.0 | |
| Winter | Mean | 18.6 | 18.7 | 9.4 | 35.3 |
| (n = 96) | 95% CI | 1.0 | 0.7 | 1.0 | 1.1 |
| Min–Max | 5.0–27.0 | 12.0–26.0 | 1.0–24.1 | 21.0–41.0 | |
| Spring | Mean | 22.6 | 23.5 | 8.4 | 36.0 |
| (n = 103) | 95% CI | 1.1 | 0.6 | 1.2 | 0.7 |
| Min–Max | 9.5–30.0 | 16.0–29.0 | 0–25.6 | 27.0–44.0 |
References
- Bildstein, K.L.; Bancroft, G.T.; Dugan, P.J.; Gordon, D.H.; Erwin, R.M.; Nol, E. Approaches to the conservation of coastal wetlands in the Western hemisphere. Wilson Bull. 1991, 103, 218–254. [Google Scholar]
- Piersma, T.; Chan, Y.C.; Mu, T.; Hassell, C.J.; Melville, D.S.; Peng, H.B.; Ma, Z.; Zhang, Z.; Wilcove, D.S. Loss of habitat leads to loss of birds: Reflecxtions on the Jiangsu, China, coastal development plans. Wader Study 2017, 124, 93–98. [Google Scholar] [CrossRef]
- Powell, G.V. Habitat use by wading birds in a subtropical estuary: Implications of hydrography. Auk 1987, 104, 740–749. [Google Scholar] [CrossRef]
- Schreiber, E.A.; Burger, J. Biology of Marine Birds; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Galbraith, H.; Jones, R.; Park, R.; Clough, J.; Herrod-Julius, S.; Harrington, B.; Page, G.W. Global climate change and sea level rise: Potential losses of intertidal habitat for shorebirds. Waterbirds 2002, 25, 173–183. [Google Scholar] [CrossRef]
- Gawlik, D.E. The effects of prey availavility on the numerical response of wading birds. Ecol. Monogr. 2002, 72, 329–346. [Google Scholar] [CrossRef]
- Goss-Custard, J.D. The Ecology of the Wash. III. Density-Related Behaviour and the Possible Effects of a Loss of Feeding Grounds on Wading Birds (Charadrii). J. Appl. Ecol. 1977, 14, 721–739. [Google Scholar] [CrossRef]
- Goss-Custard, J.D. Competition for Food and Interference among Waders. Ardea 1980, 55, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Goss-Custard, J.D.; Moser, M.E. Rates of Change in the Numbers of Dunlin, Calidris alpina, Wintering in British Estuaries in Relation to the Spread of Spartina anglica. J. Appl. Ecol. 1988, 25, 95–109. [Google Scholar] [CrossRef]
- Meire, P.M. Effects of a substantial reduction in intertidal area on numbers and densities of waders. Acta XX Congr. Int. Ornighologici 1991, 20, 2219–2227. [Google Scholar]
- Beck, M.W.; Brumbaugh, R.D.; Airoldi, L.; Carranza, A.; Coen, L.D.; Crawford, C.; Defeo, O.; Edgar, G.J.; Hancock, B.; Kay, M.C.; et al. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. BioScience 2011, 61, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Aristegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; pp. 447–588. [Google Scholar]
- Walters, L.J.; Sacks, P.E.; Campbell, D.E. Boating impacts and boat-wake resilient restoration of the easter oyster Crassostrea virginica in Mosquito Lagoon, Florida, USA. In Proceedings of the Indian River Lagoon Symposium, Fort Pierce, FL, USA, 13–14 February 2020. [Google Scholar]
- Baggett, L.P.; Powers, S.P.; Brumbaugh, R.D.; Coen, L.D.; Deangelis, B.M.; Greene, J.K.; Hancock, B.T.; Morlock, S.M.; Allen, B.L.; Breitburg, D.L.; et al. Guidelines for evaluating performance of osyter habitat restoration. Restor. Ecol. 2015, 23, 737–745. [Google Scholar] [CrossRef]
- Gregory, R.D.; Noble, D.; Field, R.; Marchant, J.; Raven, M.; Gibbons, D.W. Using birds as indicators of biodiversity. Ornis Hung. 2003, 12–13, 11–24. [Google Scholar]
- Gregory, R.D.; Strien, A.V. Wild bird indicators: Using composite population trends of birds as measures of environmental health. Ornithol. Sci. 2010, 9, 3–22. [Google Scholar] [CrossRef]
- Temple, S.A.; Wiens, J.A. Bird populations and environmental changes: Can birds be bio-indicators? Am. Birds 1989, 43, 260–270. [Google Scholar]
- Melvin, S.L.; Gawlik, D.E.; Scharff, T. Long-Term Movement Patterns for Seven Species of Wading Birds. Waterbirds 1999, 22, 411–416. [Google Scholar] [CrossRef]
- Shaffer, M.; Donnelly, M.; Walters, L. Does intertidal oyster reef restoration affect avian community structure and behavior in a shallow estuarine system? A post-restoration analysis. Fla. Field Nat. 2019, 47, 37–59. [Google Scholar]
- Luckenbach, M.W.; Coen, L.D.; Ross, P.G., Jr.; Stephen, J.A. Oyster reef habitat restoration: Relationships between oyster abundance and community development based on two studies in Virginia and South Carolina. J. Coast. Res. 2005, 21, 64–78. [Google Scholar]
- Rodney, W.S.; Paynter, K.T. Comparisons of macrofaunal assemblages on restored and non-restored oyster reefs in mesohaline regions of Chesapeake Bay in Maryland. J. Exp. Mar. Biol. Ecol. 2006, 335, 39–51. [Google Scholar] [CrossRef]
- Harris, K. Oyster Reef Restoration: Impacts on Infaunal Communities in a Shallow Water Estuary. Univ. Cent. Fla. Undergrad. Res. J. 2020, 11, 7–17. [Google Scholar]
- De Santiago, K.; Palmer, T.A.; Dumesnil, M.; Pollack, J.B. Rapid development of a restored oyster reef facilitates habitat provisioin for estuarine fauna. Restor. Ecol. 2019, 27, 870–880. [Google Scholar] [CrossRef]
- Meyer, D.L.; Townsend, E.C. Faunal Utilization of Created Intertidal Eastern Oyster (Crassostrea virginica) Reefs in the Southeastern United States. Estuaries 2000, 23, 34. [Google Scholar] [CrossRef]
- Grabowski, J.H.; Hughes, A.R.; Kimbro, D.L.; Dolan, M.A. How habitat setting influences restored oyster reef communities. Ecology 2005, 86, 1926–1935. [Google Scholar] [CrossRef]
- La Peyre, M.K.; Humphries, A.T.; Casas, S.M.; La Peyre, J.F. Temporal variation in development of ecosystem services from oyster reef restoration. Ecol. Eng. 2014, 63, 34–44. [Google Scholar] [CrossRef]
- Lewis, D.M.; Troast, B.V.; Glomb, J.C.; Cook, G.S. An ecological characterization of fish assemblages in Mosquito Lagoon, Florida. Southeast. Nat. 2020, 19, 491–510. [Google Scholar] [CrossRef]
- Skagen, S.K.; Oman, H.D. Dietary flexibility of shorebirds in the Western Hemisphere. Can. Field-Nat. 1996, 110, 419–444. [Google Scholar]
- Rezek, R.J.; Lebreton, B.; Roark, E.B.; Palmer, T.A. How does a restored oyster reef develop? An assessment based on stable isotopes and community metrics. Mar. Biol. 2017, 164, 54. [Google Scholar] [CrossRef]
- Eglington, S.M.; Noble, D.G.; Fuller, R.J. A meta-analysis of spatial relationships in species richness across taxa: Birds as indicators of wider biodiversity in temperate regions. J. Nat. Conserv. 2012, 20, 301–309. [Google Scholar] [CrossRef]
- Glass, K.A.; Watts, B.D. Osprey diet composition and quality in high- and low-salinity areas of lower Chesapeake Bay. J. Raptor Res. 2009, 43, 27–36. [Google Scholar] [CrossRef]
- Nellis, D.W. Common Coastal Birds of Florida and the Caribbean; Pineapple Press: Sarasota, FL, USA, 2001. [Google Scholar]
- Miranda, L.; Collazo, J.A. Food habits of 4 species of wading birds (Ardeidae) in a tropical mangrove swamp. Waterbirds 1997, 20, 413–418. [Google Scholar] [CrossRef]
- Kushlan, J.A. Feeding Ecology and Prey Selection in the White Ibis. Condor 1979, 81, 376–389. [Google Scholar] [CrossRef]
- Pranty, B.; Radamaker, K.A.; Kennedy, G. Birds of Florida; Lone Pine Publishing: Auburn, WA, USA, 2006. [Google Scholar]
- Barrett, R.T.; Camphuysen, K.C.J.; Anker-Nilssen, T.; Chardine, J.W.; Furness, R.W.; Garthe, S.; Huppop, O.; Leopold, M.F.; Montevecchi, W.A.; Viet, R.R. Diet studies of seabirds: A review and recommendations. ICES J. Mar. Sci. 2007, 64, 1675–1691. [Google Scholar] [CrossRef] [Green Version]
- Alsop, F.J., III. Birds of Florida; Dorling Kindersley, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Copertino, J.C.; Hromyak, J.; Sacks, P.; Walters, L.J. A survey of bird abundance and diversity in Mosquito Lagoon, Florida. Fla. Field Nat. 2022. in review. [Google Scholar]
- Walters, L.J.; Roman, A.; Stiner, J.; Weeks, D. Water Resource Management Plan, Canaveral National Seashore; National Park Service, Canaveral National Seashore: Titusville, FL, USA, 2001; p. 224. [Google Scholar]
- Philips, E.; Badylak, S.; Lasi, M.; Chamberlain, R.; Green, W.; Hall, L.; Hart, J.; Lockwood, J.; Miller, J.; Morris, L.; et al. From red tides to green and bron tides: Bloom dynamics in a restricted subtropical lagoon under shifting climatic conditions. Estuaries Coast 2015, 38, 889–904. [Google Scholar]
- Steward, J.; Virnstein, R.; Lasi, M.; Morris, L.; Miller, J.; Hall, L.; Tweedale, W. The impacts of the 2004 hurricanes on hydrology, water quality, and seagrass in the Central Indian River Lagoon, Florida. Estuaries Coast 2006, 29, 954–965. [Google Scholar] [CrossRef]
- Smith, N.P.; Pierce, F. Tidal and Nontidal Flushing of Florida’s Indian River Lagoon. Estuaries 1993, 16, 739–746. [Google Scholar] [CrossRef]
- Grabowski, J.; Brumbaugh, R.D.; Conrad, R.F.; Keeler, A.G. Economic valuation of ecosystem services provided by oyster reefs. Bioscience 2012, 632, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Garvis, S.K.; Sacks, P.E.; Walters, L.J. Formation, Movement, and Restoration of Dead Intertidal Oyster Reefs in Canaveral National Seashore and Mosquito Lagoon, Florida. J. Shellfish Res. 2015, 34, 251–258. [Google Scholar] [CrossRef]
- Wall, L.M.; Walters, L.J.; Grizzle, R.E.; Sacks, P.E. Recreational boating activity and its impact on the recruitment and survival of the oyster Crassostrea virginica on intertidal reefs in mosquito Lagoon, Florida. J. Shellfish Res. 2005, 24, 965–973. [Google Scholar] [CrossRef]
- Rumohr, H. Soft-bottom macrofauna: Collection, treatment, and quality assurance of samples. ICES J. Mar. Sci. 2009, 43, 20. [Google Scholar]
- Conway, A.; Courtney, J. Standardized North American Marsh Bird Monitoring Protocol. Waterbirds 2011, 34, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Schikorr, K.E.; Swain, H.M. Wading birds—Barometer of management strategies in the Indian River Lagoon. Bull. Mar. Sci. 1995, 57, 215–229. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.6.2; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 30 December 2021).
- RStudio Team. RStudio: Integrated Development for R; Version 1.3.5033; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com (accessed on 30 December 2021).
- Oksanen, J.; Blanchet, G.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package, Version 2.5.6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 30 December 2021).
- Clarke, K.R. Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 1993, 18, 143–177. [Google Scholar] [CrossRef]
- De Caceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. 2009. Available online: https://cran.r-project.org/package=indicspecies (accessed on 30 December 2021).
- Coen, L.D.; Brumbaugh, R.D.; Bushek, D.; Grizzle, R.; Luckenbach, M.W.; Posey, M.H.; Powers, S.P.; Tolley, S.G. Ecosystem services related to oyster restoration. Mar. Ecol.–Prog. Ser. 2007, 341, 303–307. [Google Scholar] [CrossRef]
- Brumbaugh, R.D.; Beck, M.W.; Coen, L.D.; Craig, L.; Hicks, P. A practitioners’ Guide to the Design and Monitoring of Shellfish Restoration Projects: An Ecosystem Services Approach; The Nature Conservancy: Arlington, VA, USA, 2006. [Google Scholar]
- Walters, L.; (University of Central Florida, Orlando, FL, USA). Personal communication, 2021.
- Nelson, G.E. Observations of the flocking habits of gulls and terns on the Mississippi. Q. J. Fla. Acad. Sci. 1962, 25, 303–306. [Google Scholar]
- Lenihan, H.S.; Peterson, C.H.; Byers, J.E.; Grabowski, J.H.; Thayer, G.W.; Colby, D.R. Cascading of habitat degradation: Oyster reefs invaded by refugee fishes escaping stress. Ecol. Appl. 2001, 11, 764–782. [Google Scholar] [CrossRef]
- Loch, J.M.H.; Walters, L.J.; Donnelly, M.L.; Cook, G.S. Restored coastal habitat can “reel in” juvenile sportfish: Population and community responses in the Indian River Lagoon, Florida, USA. Sustainability 2021, 13, 12832. [Google Scholar] [CrossRef]
- Mcintyre, N.E. Effects of forest patch size on avian diversity. Landsc. Ecol. 1995, 10, 85–99. [Google Scholar] [CrossRef]
- Erwin, R.M.; Allen, D.H.; Jenkins, D. Created versus natural coastal islands: Atlantic waterbird populations, habitat choices, and management implications. Estuaries 2003, 26, 949–955. [Google Scholar] [CrossRef]
- Zale, A.V.; Mulholland, R. Habitat suitability index models: Laughing gull. U.S. Fish Wildl. Serv. Biol. Rep. 1985, 82, 23. [Google Scholar]
- Troast, B. Exploring Multi-Scale Variation of Fish Community Diversity in a Dynamic Coastal Estuary. Master’s Thesis, University of Central Florida, Orlando, FL, USA, 2019. [Google Scholar]
- Kushlan, J.A.; Kushlan, M.S. Food of the white ibis in Southern Florida. Fla. Field Nat. 1975, 3, 31–38. [Google Scholar]
- Groves, S. Age-related differences in ruddy turnstone foraging and aggressive behavior. Auk 1978, 95, 95–103. [Google Scholar] [CrossRef]
- Warburg, M.R.; Llnsenmair, K.E.; Bercovitz, K. The effect of climate on the distribution and abundance of isopods. Zool. Symp. 1984, 53, 339–367. [Google Scholar]
- Kensley, B.; Nelson, W.G.; Schotte, M. Marine isopod biodiversity of the Indian River Lagoon, Florida. Bull. Mar. Sci. 1995, 57, 136–142. [Google Scholar]
- Brooks, A.R.; Bell, S.S. Colonization of a dynamic substrate: Factors influencing recruitment of the wood-boring isopod, Sphaeroma terebrans, onto red mangrove (Rhizophora mangle) prop roots. Oecologia 2001, 127, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Brusca, R. Effects of the root-boring isopod Sphaeroma peruvianum on red mangrove forests. Mar. Ecol. Prog. Ser. 1989, 57, 287–292. [Google Scholar] [CrossRef]
- Rehm, A.E. The Effects of the Wood-boring Isopod Sphaeroma terebrans on the Mangrove Communities of Florida. Environ. Conserv. 1976, 3, 47–57. [Google Scholar] [CrossRef]
- Xin, K.; Xie, Z.; Zhong, C.; Sheng, N.; Gao, C.; Xiao, X. Damage Caused by Sphaeroma to Mangrove Forests in Hainan, Dongzhaigang, China. J. Coast. Res. 2020, 36, 1197–1203. [Google Scholar] [CrossRef]
| Group | Example Species | Prey Items |
|---|---|---|
| Birds of Prey | Pandion haliaetus (Osprey) Haliaetus leucocephalus (Bald Eagle) | Fish [31,32] |
| Wading Birds | Egretta caerulea (Little Blue Heron) Ardea Herodias (Great Blue Heron) | Fish, Crustaceans, Worms [33,34,35] |
| Seabirds | Leucophaeus atricilla (Laughing Gull) Thalasseus maximus (Royal Tern) Pelacanus occidentalis (Brown Pelican) | Fish, Mollusks, Crustaceans [35,36] |
| Shorebirds | Arenaria interpes (Ruddy Turnstone) Tringa semipalmatta (Willet) Pluvialia squatarola (Blackbellied Plover) | Mollusks, Crustaceans, Worms, Insects [28,35,37] |
| (A) | |||
|---|---|---|---|
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p < 0.001 | p < 0.001 * | p < 0.001 * |
| 1 Month | p = 0.034 * | p = 0.004 * | p < 0.001 * |
| 6 Months | p = 0.809 | p < 0.001 * | p < 0.001 * |
| 1 Year | p = 0.003 * | p = 0.809 | p = 0.001 * |
| 2 Years | p = 0.664 | p = 0.018 * | p = 0.004 * |
| 3 Years | p = 0.218 | p = 0.648 | p = 0.091 |
| (B) | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p = 0.176 | p = 0.771 | p = 0.286 |
| 1 Month | p = 0.004 * | p = 0.131 | p < 0.001 * |
| 6 Months | p = 0.305 | p < 0.001 * | p < 0.001 * |
| 1 Year | p = 0.500 | p < 0.001 * | p < 0.001 * |
| 2 Years | p = 0.454 | p < 0.001 * | p = 0.002 * |
| 3 Years | p = 0.935 | p = 0.001 * | p = 0.001 * |
| (A) | |||
|---|---|---|---|
| Live | Restored | Dead | |
| 1 Month | p = 0.656 | p = 0.007 * | p = 0.275 |
| 6 Months | p = 0.103 | p = 0.014 * | p = 0.022 * |
| 1 Year | p = 0.019 * | p = 0.185 | p = 0.696 |
| 2 Years | p = 0.872 | p < 0.001 * | p = 0.064 |
| 3 Years | p < 0.001 * | p = 0.036 * | p = 0.002 * |
| (B) | |||
| Live | Restored | Dead | |
| 1 Month | p = 0.004 * | p = 0.075 | p = 0.946 |
| 6 Months | p < 0.001 * | p < 0.001 * | p = 0.822 |
| 1 Year | p = 0.022 * | p < 0.001 * | p = 0.625 |
| 2 Years | p < 0.001 * | p < 0.001 * | p = 0.244 |
| 3 Years | p < 0.001 * | p < 0.001 * | p = 0.304 |
| (A): Polychaetes | |||
|---|---|---|---|
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p < 0.001 * | p = 0.239 | p < 0.001 * |
| 1 Month | p = 0.005 * | p = 0.014 * | p < 0.001 * |
| 6 Months | p = 0.184 | p < 0.001 * | p < 0.001 * |
| 1 Year | p < 0.001 * | p = 0.724 | p < 0.001 * |
| 2 Years | p = 0.563 | p = 0.001 * | p < 0.001 * |
| 3 Years | p = 0.356 | p = 0.304 | p = 0.915 |
| (B): Amphipods | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p < 0.001 * | p = 0.450 | p < 0.001 * |
| 1 Month | p < 0.001 * | p = 0.007 * | p = 0.400 |
| 6 Months | p = 0.010 * | p < 0.001 * | p < 0.001 * |
| 1 Year | p < 0.001 * | p = 0.029 * | p = 0.074 |
| 2 Years | p = 0.005 * | p = 0.053 | p = 0.379 |
| 3 Years | p = 0.052 | p = 0.515 | p = 0.196 |
| (C): Isopods | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p = 0.209 | p = 0.409 | p = 0.666 |
| 1 Month | p = 0.041 * | p = 0.605 | p = 0.011 * |
| 6 Months | p = 0.531 | p < 0.001 * | p < 0.001 * |
| 1 Year | p = 0.156 | p = 0.010 * | p < 0.001 * |
| 2 Years | p = 0.888 | p = 0.805 | p = 0.698 |
| 3 Years | p = 0.352 | p < 0.001 * | p < 0.001 * |
| (D): Gastropods | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p = 0.001 * | p = 0.037 * | p = 0.207 |
| 1 Month | p = 0.260 | p = 0.004 * | p < 0.001 * |
| 6 Months | p = 0.997 | p = 0.996 | p < 0.001 * |
| 1 Year | p < 0.001 * | p = 0.119 | p < 0.001 * |
| 2 Years | p = 0.898 | p < 0.001 * | p < 0.001 * |
| 3 Years | p = 1.000 | p < 0.001 * | p < 0.001 * |
| (E): Bivalves | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p = 0.829 | p = 0.442 | p = 0.325 |
| 1 Month | p < 0.001 * | p = 0.006 * | p = 0.150 |
| 6 Months | p = 0.206 | p = 0.104 | p = 0.006 * |
| 1 Year | p = 1.000 | p = 0.007 * | p < 0.007 * |
| 2 Years | p = 0.260 | p = 0.013 * | p < 0.001 * |
| 3 Years | p = 0.001 * | p = 0.561 | p < 0.001 * |
| (F): Decapods | |||
| Restored vs. Live | Restored vs. Dead | Live vs. Dead | |
| Pre-Restoration | p = 0.039 * | p = 1.000 | p = 1.000 |
| 1 Month | p = 0.643 | p = 0.003 * | p < 0.001 * |
| 6 Months | p = 0.229 | p < 0.001 * | p < 0.001 * |
| 1 Year | p = 0.004 * | p = 0.254 | p < 0.002 * |
| 2 Years | p = 0.250 | p = 0.829 | p = 0.173 |
| 3 Years | p = 0.350 | p = 0.953 | p = 0.321 |
| Mean Reef Size (m2) ± 95% CI | Mean Bird Density ± 95% CI | Mean Foraging Bird Density ± 95% CI | Mean Loafing Bird Density ± 95% CI | |
|---|---|---|---|---|
| Live | 1543.800 ± 3114.135 | 0.024 ± 0.012 | 0.011 ± 0.006 | 0.004 ± 0.003 |
| Restored | 61.275 ± 62.370 | 0.049 ± 0.031 | 0.013 ± 0.008 | 0.019 ± 0.019 |
| Dead | 386.625 ± 576.407 | 0.198 ± 0.105 | 0.014 ± 0.006 | 0.118 ± 0.080 |
| (A). Representative Species for All Bird Observations by Reef Type. | |||
|---|---|---|---|
| Species | IndVal | A | B |
| Dead Oyster Reefs | |||
| Leucophaeus atricilla (Laughing Gull) | 0.359 | 0.813 | 0.159 |
| Thalasseus maximus (Royal Tern) | 0.329 | 0.979 | 0.110 |
| Arenaria interpres (Ruddy Turnstone) | 0.286 | 0.660 | 0.124 |
| Larus delawarensis (Ring-billed Gull) | 0.245 | 0.874 | 0,069 |
| Pelecanus occidentalis (Brown Pelican) | 0.220 | 1.000 | 0.048 |
| Sterna forsteri (Forster’s Tern) | 0.203 | 1.000 | 0.041 |
| Larus argentatus (Herring Gull) | 0.186 | 1.000 | 0.034 |
| Hydroprogne caspia (Caspian Tern) | 0.166 | 1.000 | 0.028 |
| Dead + Live Reefs | |||
| Tringa semipalmatta (Willet) | 0.318 | 1.000 | 0.101 |
| Eudocimus albus (White Ibis) | 0.278 | 0.928 | 0.083 |
| Pluvialia squatarola (Black-bellied Plover) | 0.166 | 1.000 | 0.028 |
| (B). Representative Species for foraging Birds by Reef Type. | |||
| Species | IndVal | A | B |
| Dead Oyster Reefs | |||
| Arenaria interpres (Ruddy Turnstone) | 0.293 | 0.735 | 0.117 |
| Live Oyster Reefs | |||
| Eudocimus albus (White Ibis) | 0.342 | 0.814 | 0.144 |
| Dead + Live Reefs | |||
| Tringa semipalmata (Willet) | 0.294 | 1.000 | 0.087 |
| Pluvialis squatarola (Black-bellied Plover) | 0.208 | 1.000 | 0.043 |
| (C). Representative Species for Loafing Birds by Reef Type. | |||
| Species | IndVal | A | B |
| Dead Oyster Reefs | |||
| Leucophaeus atricilla (Laughing Gull) | 0.357 | 0.838 | 0.151 |
| Thalasseus maximus (Royal Tern) | 0.332 | 1.000 | 0.110 |
| Larus delawarensis (Ring-billed Gull) | 0.233 | 0.874 | 0.062 |
| Pelecanus occidentalis (Brown Pelican) | 0.220 | 1.000 | 0.048 |
| Sterna forsteri (Forster’s Tern) | 0.203 | 1.000 | 0.041 |
| Ardea herodias (Great Blue Heron) | 0.186 | 1.000 | 0.034 |
| Tringa semipalmata (Willet) | 0.185 | 0.988 | 0.034 |
| Larus argentatus (Herring Gull) | 0.166 | 1.000 | 0.028 |
| Hydroprogne caspia (Caspian Tern) | 0.166 | 1.00 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copertino, J.L.; Harris, K.; Chute, L.; Walters, L.J. Impact of Oyster (Crassostrea virginica) Reef Restoration on Benthic Invertebrates and Coastal Birds in a Subtropical Estuary. Sustainability 2022, 14, 2371. https://doi.org/10.3390/su14042371
Copertino JL, Harris K, Chute L, Walters LJ. Impact of Oyster (Crassostrea virginica) Reef Restoration on Benthic Invertebrates and Coastal Birds in a Subtropical Estuary. Sustainability. 2022; 14(4):2371. https://doi.org/10.3390/su14042371
Chicago/Turabian StyleCopertino, Jessica L., Katherine Harris, Lyndsey Chute, and Linda J. Walters. 2022. "Impact of Oyster (Crassostrea virginica) Reef Restoration on Benthic Invertebrates and Coastal Birds in a Subtropical Estuary" Sustainability 14, no. 4: 2371. https://doi.org/10.3390/su14042371






