Abstract
The agri-food sector generates substantial quantities of waste material on farm and during the processing of these commodities, creating serious social and environmental problems. However, these wastes can be resources of raw material for the production of valuable chemicals with applications in various industrial sectors (e.g., food ingredients, nutraceuticals, bioderived fine chemicals, biofuels etc.). The recovery, purification and biotransformation of agri-food waste phytochemicals from this microbial spoilage-prone, complex agri-food waste material, requires appropriate fast pre-treatment and integration of various processes. This review provides a brief summary and discussion of the unique advantages and the importance of membrane technology in sustainable recycling of phytochemicals from some of the main agri-food sectors. Membrane-based pressure -driven processes present several advantages for the recovery of labile compounds from dilute streams. For example, they are clean technologies that can operate at low temperature (20–60 °C), have low energy requirements, there is no need for additional chemicals, can be quite automated and electrifiable, and have low space requirements. Based on their permselective properties based on size-, shape-, and charge-exclusion mechanisms, membrane-based separation processes have unpaired efficiency in fractionating biological components while presenting their properties. Pressure-driven membrane processes, such as microfiltration (MF), ultrafiltration (UF) and nanofiltration (NF), as well as other advanced membrane-based processes such as membrane bioreactors (MBR), membrane emulsification (ME) and membrane distillation (MD), are presented. The integration of various membrane technologies from the initial recovery of these phytochemicals (MF, UF, NF) to the final formulation (by ME) of commercial products is described. A good example of an extensively studied agri-food stream is the olive processing industry, where many different alternatives have been suggested for the recovery of biophenols and final product fabrication. Membrane process integration will deliver in the near future mature technologies for the efficient treatment of these streams in larger scales, with direct impact on the environmental protection and society (production of compounds with positive health effects, new job creation, etc.). It is expected that integration of these technologies will have substantial impact on future bio-based societies over forthcoming decades and change the way that these chemicals are currently produced, moving from petrochemical-based linear product fabrication to a sustainable circular product design based in agri-food waste biomass.
1. Agri-Food Waste Problem
The world’s population is expected to increase at a geometric rate, putting substantial pressure on natural resources capacity in respect to humanity’s energy and nutrition requirements. At the same time, fossil fuel-based resources are declining. Thus, there is an urgent need for alternatives able to replace current industrial production of fuels and chemicals. To achieve food and energy security for humankind, in a context of constrained resources and changing climate, without further compromising ecosystems quality and biodiversity, a multidimensional and integrated global strategy is necessary [1]. Waste management is considered as one of the most important challenges for the coming decades [2], as microbial, plant, and animal organic matter appropriate recycling can contribute towards a more sustainable future. In addition, food industry requires large amounts of clean, potable water and produces a considerable amount of wastewater during the agri-food raw material processing [3], thus due to clean water scarcity water recirculation is crucial.
In respect to agri-food waste management, nowadays it is estimated that more than 1.3–1.6 [4,5,6] billion tons of food are thrown away along the entire food supply chain worldwide [4,5,6], which is roughly equal to one-third of the global food production for human consumption and more than one-quarter of the global agricultural production [5]. Therefore, food waste losses have gained substantial attention from society and governments worldwide due to their economic, social, health and environmental dimensions [5]. These massive agri-food wastes quantities can occur both at the ‘on farm’ and ‘off farm’ levels. In general, food waste average composition is 82.5% w/w moisture and the remaining dry content 51.2% carbon, 7.2% hydrogen, 38.1% oxygen, 2.8% nitrogen and 0.7% w/w sulphur [7]. These wastes not only create safe disposal issues, but also contribute to negative environmental impacts, e.g., CO2 release, methane emissions, eutrophication, etc. [7,8,9,10]. It is estimated that about 4.4 billion metric tons of CO2 is released due to food waste disposal [7], which accounts for around 6% w/w of global CO2 emissions [11]. In the European Union (EU), food waste is approximately 130 million tons per year, of which more than 24% is at primary production, 23% is during processing and manufacturing, 5% is at the retail level, 39% is at the household level and 9% is from hospitality industry [1]. Up to 52% can therefore be easily recycled from the first three streams, as they are composed usually from a single biomass resource and they can be of a more defined chemical composition. Interestingly, in the EU, fruits and vegetables contribute almost 50% to total food losses [1]. It is estimated that the drink industries are generating around 26% of the total food waste, which makes them the first among the waste producing resources. The dairy industry, fruit and vegetable industry, and cereal industry follow at 21%, 14.8% and 12.9%, respectively [10]. These agri-food wastes include liquid streams, such as whey, olive mill or cork boiling wastewaters [12], or solids such as pomace (e.g., apple, citrus, peach, beetroot, etc.) and pericarp (e.g., citrus peel, pomegranate husk etc.) of fruits in the juice industry, etc. In addition, limited attention has been given to the waste biomass that comes from the plant material that consists of the leaves and stalks after pruning (in fields or glasshouses). Interestingly, if to these losses should be added the energy and other commodities employed during plant growing and the production of these wastes [4], this will lead to even worse estimations on the impact of this agri-food loses problem. However, all these waste resources constitute a valuable source of phytochemicals.
There are many different routes for utilizing agri-food waste material, with the more common being the energy production by incineration (fast but low yield and value option), their composting (slow and low value), their use as animal feed, the extraction of added value compound from them and their biotransformation by using appropriate microorganisms. Aside from the fact that they are of lower value, the first three options lead to downgrading of organic compounds that are not easily chemically synthesized and produce CO2 which contributes to global warming. The appropriate utilization of wastes from agri-food streams has been proven a valuable feedstock that can lead to the production of a wide range of intermediates with promising applications in different sectors, such as food ingredients, cosmetics, materials, biopolymers, biofuels etc. This is an opportunity for additional higher added-value revenue from these waste [13]. In addition, carbon remains in its organic form, not released as CO2, promoting thus a more sustainable bio-based circular economy. There are some recent comprehensive reviews in the agri-food waste field that cover re-use of waste resources [10,12,13]. These waste materials represent a cheap, renewable, and abundant feedstock useful for the recovery of several phytochemicals, which are often bioactive. Except for the recovery of phytochemicals, new products, biobased materials and biofuels, from these wastes can be achieved through their appropriate bioconversion [10]. Additionally, potable water is a resource under scarcity that is used in many instances in food processing operations. Thus, water recycling is gaining the scientific and societal attention [3]. This therefore represents an additional important opportunity as part of the valorization process of agri-food waste materials. Finally, during agri-food waste valorization it is important to evaluate the safety of the final products, especially when organic solvents are used instead of water for recovery and the final products are indented for human consumption [14]. This review focuses on the importance of membrane processes for the agri-food waste valorization and water recycling, providing evidence for membrane processes importance in the pre-treatment of agri-food waste, recovery of phytochemicals and final product formation.
2. Example of Waste Streams from Agri-Food Industries
Phytochemicals/bioactive compounds that exist in processing waste from popular fruits and vegetables have been presented in recent reviews [2,6,9,13,15,16,17,18,19,20] and research papers [12,21,22,23]. The most common groups of organic compounds in such waste are carbohydrates (pectins and their oligo-saccharides, starch, cellulose, dietary fibres, monosaccharides etc.), biophenols (lignin, phenolic acids, flavonoids, tannins, ellagitannins, etc.) [24], proteins, lipids, essential oils (e.g., terpenoids, hydrocarbons, aliphatic alcohols and ethers, lactones, polyacetylenes etc.), and pigments (anthocyanins, carotenoids, betalains, chlorophyll, etc.) [25,26]. The amounts of these compounds in these agri-food waste range from a few mg to some g per kg of waste and they have commercial values ranging from a few euros to some thousand euros per kg of final product, depending on the compound and its final purity after its recovery. Some characteristic examples of agri-food wastes and possible products able to be isolated from them are highlighted in the sections below.
2.1. Olive Processing Industry
Olive oil extraction dates back centuries Before Christ. Olives are considered one of the first and among the most important agricultural commodities in the Mediterranean [27]. Over the last three decades, olive oil consumption has increased worldwide, with its annual global production surpassing 3 million tons [28]. The increased olive oil consumption is attributed mainly to the scientific evidence about its health-promoting properties [16]. Currently, about 98% of the world’s olive oil is produced in the Mediterranean countries; thus, the olive oil industry has an important economic, environmental and social impact in this area. However, nowadays China is heavily investing in the olive oil industry, thus this figure is expected to change. The olive processing for the olive oil production generates large quantities of wastewaters and solids (olive pomace). The composition of the olive oil wastes depends on method of extraction, olives type and maturity, region, climatic conditions, and cultivation conditions [29,30]. There is an increasing trend for the olive oil industry to move from the use of the traditional three-phase to the two-phase production system, which releases less wastewater during processing and is thus considered more eco-friendly [28]. The waste obtained from the two-phase system is the olive pomace, a semi-solid slurry, composed of fragments of olive skin, pulp and stone (solid phase), water and oil [28]. This slurry usually has higher chemical oxygen demand (COD) values [31] in respect to three phase system, thus is more pollutant if disposed of. The traditional used three-phase olive mill production system is a water-intensive process [30] that generated large amounts of dark liquid effluents [27] called olive mill wastewaters (OMWWs) with high electric conductivity (~9.7 −13.8 [31,32,33] mS/cm), a pH value of 4.8–5.6 [31,32,33] and an average composition of 83–92% of water, 4–16% of organic compounds and 1–2% w/w of inorganic salts. OMWWs low pH is attributed to organic acids (such as malonic, citric, tartaric, lactic, fumaric and succinic) that are present. OMWWs come from the olive washing waters (about 5% w/w of the processed olives), the olive pulp water (40–50% w/w of the initial weight of olives), the water added to olive paste in the centrifugation step and the water coming from washing of equipment (5–10% w/w of the weight of processed olives) [27]. OMWWs COD and biological oxygen demand (BOD) are in the range of 40–220 g/L and 35–110 g/L, respectively [27], thus a cubic meter of OMWWs is considered to be equivalent to 100–200 m3 domestic sewage, in terms of pollution, if just disposed of [30]. Due to their physicochemical characteristics, only a small fraction of biophenols (2% w/w of the total phenolic content, TPC, expressed usually as gallic acid equivalent, GAE) passes into the oil phase [34]. The main part of these biophenols remains in the wastewaters (53% w/w TPC) and in the pomace (45% w/w TPC) in case of three-phase [34] or to the semi-solid slurry in the case of two-phase olive mills [28]. The typical value of total phenolic content in three–phase systems is in the range of 1.6–10.7 g GAE/L [35], depending on the water added during the process. Today more than 50 biophenols have been identified in OMWWs, with hydroxytyrosol and tyrosol being the most abundant [29]. It is worth underlining that the composition of biophenols strongly depends on their residence time in the untreated OMWW. The high molecular weight biophenols can be subject to enzyme/microbial degradation that converts them to low molecular weight biophenols, such as hydroxytyrosol and tyrosol. However, opposite reactions can take place due to biophenols oxidation and lead to higher molecular weight compounds. This implies that, when aiming at recovering specific biophenols, an appropriate treatment of the OMWWs must be designed to ensure the removal of enzymes and microorganisms that can have an effect on the biophenols chemical composition. Membrane processing appears as the most appropriate in this case, as membranes can relatively easily separate the particulates or microorganisms and the higher molecular weight compounds or enzymes from a liquid stream.
In addition to olive oil production, the table olive processing is of great economic importance [36]. The global production of table olives was about 2.5 million tons in 2012, with >90% processed around the Mediterranean region. Table olive processing is responsible for removing the naturally occurring bitter taste in raw olive fruit (attributed mainly to oleuropein glycoside), to make them edible and preserve them from deterioration. Substantial amounts of potable water are used in green and/or black olive processing (~3.9–7.5 and 0.9–1.9 m3/tones of olives processed, respectively). Table olive processing wastewater (TOPW) is a stream with high polluting load and it is difficult to treat it, as it is characterized by variable pH, salinity and concentrations of organic compounds (among them high biophenols concentrations) [36].
2.2. Wine Industry
Wine is one of the most commercially available alcoholic beverages worldwide and on average around 44 million tons of grapes are destined for the juice and winemaking industry every season [37]. Approximately 77.8 million tons of grape are produced annually, thus grape is one of the most widely evolved fruit crops [18]. Grape pomace represents the main waste of the wine industry. It mainly consists of grape skin, pulp and seeds. On average, 200 kg of grape pomace is produced per 1 ton of wine. Interestingly, the residues from wine and juice processing still contain about 70% of the biophenols found in grapes [37]. Grape pomaces contains substantial quantities of anthocyanins, procyanidins, flavonoids and stilbenes [18,22] that have important biological activities [18].
2.3. Coffee Industry
Coffee is one of the most popular consumed beverages worldwide [17,22] and considered as the second most valuable commodity, after petroleum and its derivatives [19]. Coffee is prepared from processed coffee beans. Coffee beans are actually the seeds present in coffee cherries (usually two beans per coffee cherry), which are covered with endosperm, endocarp, mesocarp, and epicarp layers (from the bean to the outside of the fruit). The first two layers are in direct contact with the beans. They are also known as silverskin and parchment. Parchment is a fibrous membrane comprised (w/w) of α-cellulose (40−49%), hemicellulose (25−32%), lignin (33−35%), and ash (0.5−1%) [38]. The coffee cherry processing and chemistry has been recently reviewed by Hejna [17]. This review provides some average values of the waste generated in the whole production cycle of roasted coffee, with the coffee parchment, silverskin and spent coffee grounds being equal to 35–61, 42 and 650 g/kg of the fresh coffee cherry. In addition, 1–20 dm3 of wastewaters are generated per kg of fresh coffee cherry treated [17]. The main waste from the final coffee consumption is coffee spent grounds and coffee silver skin. Around 10 million tons of coffee were produced in 2018, leading to ~7.9 million tons/annum of coffee residue. These residues contain active molecules [17,19,22] that can be recovered with the integration of appropriate technologies. Coffee spent grounds average composition includes oils (7.9–26.4%), crude fibers (19.7–22.1%), and different components such as alkaloids, biophenols and their esters, etc. [19]. Coffee spent grounds composition, as in case of other natural products, depends to a large extent on the origin of the coffee beans, the roasting conditions and the extraction process.
2.4. Dairy Industry
The dairy industry produces annually approximately 115 [39]–180 [40] million tons of whey, about 47% w/w [39,41] is disposed of in the environment, creating serious pollution problems, since it has a high BOD and COD [39,40]. Whey BOD and COD are in the range 30–60 and 50–100 g/L, respectively, depending on the cheese making process used [40]. Cheese whey is composed of ~93% water, 0.8% protein (~20% of total milk protein), 0.3% fat, 4.8% lactose and 0.5% w/w ash [41].
A fraction of the agri-food biomass from all the mentioned above categories can be recovered under appropriate processes integration. Remaining biomass can be used as carbon substrate in appropriate bioreactors for transforming these waste materials to added-value bio-based products (sweeteners, pigments, biopolymers, platform chemicals etc.) and/or biofuels (biodiesel, bioethanol, biomethane, biobutanol etc.).
3. Conventional Pressure Driven and Advanced Membrane Processes for Agri-Food Streams Valorization
3.1. Pressure Driven Membrane Processes
The use of membrane technologies for recovery, separation and an initial fractionation of organic compounds from the types of wastes described above is of intense research interest [24]. Membrane-based pressure-driven processes present several advantages for the recovery of labile compounds from dilute streams over other traditional separation methods. For example, they can operate at low temperature (20–60 °C), the solvent remains at the same phase (no latent heat losses, thus less energy requirements), have low energy requirements, there is no need for additional chemicals, can be quite automated and electrifiable, and have a low space requirement [30]. These advantages make them ideal for agri-food waste utilization and reduction in the environmental pollution. Furthermore, membrane technologies are able to minimize water consumption, as they represent a suitable solution for the treatment of wastewater and they allow recycling of process water under acceptable specifications for cleaning or irrigation [3,24]. The separation potential of membranes is linked directly to their productivity and it is dependent on a number of factors such as membrane material chemistry, pore size/molecular weight cut-off (MWCO) and operating conditions (e.g., transmembrane pressure (TMP), feed concentration and flow rate, pH, temperature, volume reduction or volume concentration factor, etc.) [24,42]. In pressure driven membrane processes, the main separation mechanism is dictated by the presence of pores and their size. Therefore, in microfiltration (MF, pore size > 0.1 μm) size exclusion is the main mechanism; as well as in ultrafiltration (UF, 10 nm < pore size < 100 nm). The main separation mechanism in MF and UF processes is the pore size-range of the membrane, which is mainly related to its MWCO, and in most cases to a lesser extent on molecular shape, charge and hydrophobicity. Besides size exclusion, macromolecular shape becomes significant, especially in the “tight UF” range and in nanofiltration (NF, 2 nm < pore size < 10 nm) size exclusion/steric hindrance and Donnan exclusion are dominant (it is worth recalling that, given the difficulty to measure the pore size in the tight NF range, pore size is commonly expressed as the MWCO of the molecule that is 90% rejected by the membrane); in reverse osmosis (RO) membranes are dense with no detectable pores and the separation occurs by solution diffusion mechanism [41,42]. These technical details of the pressure-driven membrane processes are summarized in Table 1. The appropriate processes integration of the mentioned pressure-driven processes (MF, UF, NF and/or RO) permits to separate, fractionate and/or concentrate molecules of interest under mild conditions. For all these pressure driven processes, one of the main challenges is the membrane fouling, due to cake formation and/or pore blockage, that causes permeate flux to decline over time, thus decreasing membrane process productivity. Table 2 summarizes some characteristic examples of pressure-driven membrane processes (operating conditions, membrane specifications, recovery and rejection ratio, membranes specifications, system components, etc.) available in the literature and their integration for added-value compound recovery from agri-food waste streams.

Table 1.
Summary of the technical details of the pressure-driven membrane processes.

Table 2.
Indicative membrane processes for added-value compound recovery from agri-food waste streams.
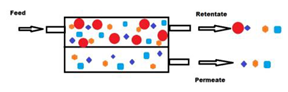
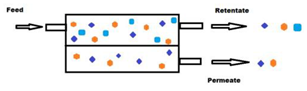
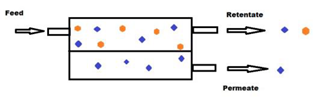
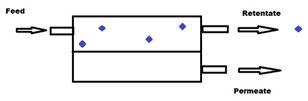
Membrane technologies used until today for agri-food waste valorization are mainly the relatively low pressure-driven processes (e.g., MF, UF and loose NF). The driving force (i.e., TMP) applied in these cases varies from a few hundred mbars to a couple of bars in the case of MF and UF, and up to 3–25 bars for the NF [42]. These processes are considered as physical separations which allow the separation of different compounds from a feed solution using a semipermeable, usually solid, barrier that allows the liquid solvent phase to pass through the barrier by applying appropriate pressure difference between the two sides of the barrier. This results in the feed solution being split into two streams, the permeate and the retentate fractions. The permeate fraction contains a significant portion of the solvent with the components that have permeated the membrane. The retentate fraction contains the remaining compounds retained or rejected by the membrane with the remaining amount of solvent, leading to an increase in the concentration of these compounds. Membrane filtration can be carried out in dead-end or cross-flow mode [41] (Figure 1a,b, respectively), but for industrial scale applications, the second case is preferred for process intensification.

Figure 1.
Schematic representation of dead-end (a) and cross-flow (b) membrane separation systems.
Membrane technologies are the most favorable choice for whey treatment, as whey is a dilute solution of valuable proteins and low molecular weight sugars. Thus, most of the whey components can be separated based on size [39,40]. The available membrane processes to recover whey protein were recently reviewed by Wen-qiong et al. [39], Argenta and Scheer [40]. Wen-qiong et al. [39] present evidence about the fouling mechanism, results of different membrane systems performance and economic analyses for whey treatment with membrane processes combination, whereas Argenta and Scheer [40], except the conventional pressure-driven separation processes, provide details about the importance of membrane surface modification, membrane distillation (MD) and integrated processes for whey treatment. Pázmándi et al. [47] studied the whey valorization by using a multistep process that involved membrane UF/NF to fractionate whey and the subsequent enzymatic conversion of lactose into galacto-oligosaccharides with a degree of polymerization (DP) of 3–5. Initially, in this study a partially demineralized whey was concentrated and diafiltered (DF) by UF to obtain whey protein isolates. The combined UF/DF procedure is well suited for generating whey protein isolate and recovering lactose. Over 97% of total lactose was recovered into the permeate, which was then concentrated by NF up to 330 g/L. Finally, with the use of β-galactosidase they were able to hydrolyse the whey-derived UF permeates consisting of lactose at low concentrations (<50 g/L), whereas transgalactosylation was dominant for streams concentrated by NF. NF was found to be particularly efficient in concentrating the UF permeate up to moderate lactose levels. A key feature of the enzymatic conversion is the competition between hydrolysis and transgalactosylation. It was shown that lactose hydrolysis is the predominant mechanism when using UF permeates, and transgalactosylation is particularly pronounced when using NF concentrates as substrates in the catalytic step.
The grape pomace valorization for the recovery of bioactive flavan-3-ols has been studied by Syed et al. [48]. In this study, the grape pomace was initially dried at 37 °C for 72 h before its usage. An aqueous ethanol mixture of 40% w/w was used as the optimum for the extraction under a stable solvent to solid ratio of 8:1. The extracts was centrifuged before the membrane processing. They test four NF membranes under nanofiltration and dia-nanofiltration mode. Dia-nanofiltration mode of operation was proved the most appropriate and 40% of the monomeric flavan-3-ols in the permeate were passed in this way (diafiltration volume of 2). The permeate then was concentrated by RO, allowing 90% of the solvent reusage. In the light of these results, higher dia-nanofiltration volumes, without adding new solvent, was probably possible.
There are recent research and/or review papers that provide comprehensive analysis of the literature concerning the use of membranes for the recovery of biological active compounds from other agri-food waste streams [39,41,42]. In addition, some of these articles provide evidence for the agri-food matrix pre-treatment for the recovery of these compounds and/or methodologies for their final formulation [40,41,49]. Various available pre-treatments of agri-food waste material valorization have been recently summarized [41,52] and some of the proposed methodologies are able to utilize the membrane technologies as an integrated part of the processing [29,49]. In general, tight UF and NF membranes have been recognized for their capability to recover phenolic compounds from several types of agri-food by-products [24,27,28,30,39,42,50,51,53,54]. Cassano et al. [42] provided a critical overview of the influence of membrane process operation parameters on the recovery of phenolic compounds from agri-food wastes with the use of such membranes.
Despite there not being much information on the energy consumption of membrane processes in the agri-food sector at the productive scale, it can be easily assumed that (i) the cost of pressure-driven membrane processes such as MF, UF, NF is not different from other sectors such as wastewater treatment and (ii) the cost of RO is much lower than in other sectors where more saline waters need to be treated; for example, operating costs for RO in agricultural and food treatment might not be limited by excessive osmotic pressure, as is the case for seawater desalination. As for MD, although it is driven by a thermal gradient as in distillation, MD has the advantage that it can be easily powered by low-grade heat such as industrial waste heat, solar energy, etc.
3.2. Membrane Bioreactors for the Agri-Food Biotransformation
One of the commonly used strategies for the management of agri-food industrial residues is the use of enzymes, especially hydrolytic. Plant cell walls are mainly composed of polysaccharides such as cellulose and pectins [55], thus cellulases and pectinases have been successfully used degrading them and enhancing the release of a variety of components, such as carotenoids [26], polyphenols [37,56], proteins [57], glycosides [58], oils [59,60] etc. Additionally, these enzymes can facilitate the release of smaller oligosaccharides that can easily be fermented to other added value products [61,62]. The application of enzymes for agri-food waste pre-treatment depends mainly on enzymes’ commercial availability and cost. The overall enzyme cost in an integrated process is directly linked to enzymatic activity and operating conditions, e.g., temperature, hydrolysis duration, pH, ability to use them for repeating cycles without loss of their activity etc.
Another important category of industrial enzymes relevant to such wastes is the lipases, which are lipolytic enzymes that hydrolyse triglycerides into free fatty acids and glycerol. Lipases can be used for the bioconversion of agro-industrial oily wastes to biofuels, and thus waste valorization [63].
Industrially important enzymes are typically produced from microbial cultures, and many agri-food industrial residues can be employed as the main carbon and nutrient substrates for these cultures, keeping the enzyme production cost low, preventing natural resource depletion, and protecting the environment [61,63].
Membrane bioreactors (MBRs) are systems in which a biotransformation is carried out by integrating a biocatalyst (e.g., an enzyme or microorganism) with a membrane separation processes in a combined step. The enzyme biocatalyst could be free (Figure 2a) or heterogenized (Figure 2b) in the membrane by different immobilization procedures, e.g., physical entrapment, covalent linkage, etc. In this case, the membrane has the double role of the separation and biotransformation, and the reactor is called a biocatalytic membrane reactor (BMR) [64]. A recent trend of both MBR and BMRs is the integrated use of nanoparticles (NPs) to compartmentalize the biocatalyst, which helps to recover/re-use the enzyme at the end of the process, to increase the immobilization surface and to reversibly remove the enzyme when aggressive membrane cleaning steps are needed [65].

Figure 2.
Scheme of membrane bioreactors with free (a) and immobilized enzyme (b) (BMR).
The importance of catalytic membrane reactors for the bioconversions of organic material has been recently reviewed [66] in respect to major types of enzymes used in biorefineries and the membrane processes to develop different MBRs configurations that facilitate the production of biofuels, phytotherapics and food ingredients. This work has highlighted both the advantages and the main drawbacks that can interfere with the development of this system at an industrial scale. There are examples of MBRs that have been used for agri-food waste valorization of oil palm empty fruit bunch, pre-treated wheat and rice straw, broomcorn seed flour, olive mill solid residue, and pre-treated corn stover. The use of MBRs for vegetable oils conversion to biodiesel is also discussed.
The use of MBRs to transform agri-food waste to added value compounds is increasing, since the easy integration of this technology with other membrane or non-membrane processes permits simultaneous waste purification and biotransformation, and in some cases also extraction of poor water soluble phytotherapics in organic solvents (e.g., integration with membrane emulsification [67,68]), promoting the development of intensified/integrated systems which fit well with green chemistry criteria. Examples of integrated membrane processes of agri-food waste valorization have been described where olive mill wastewaters (OMWWs) or olive leaves as source of a substrate to produce an important phythoterapic were used. In particular, both mentioned streams (vegetative waters and olive leaves) were used as source of oleuropein, a polyphenol which can be hydrolyzed by the action of a hydrolytic enzyme (β-glucosidase) to produce the potent phytotherapic oleuropein aglycone. OMWWs were used as a source of substrate and purified by an MF and UF step before the biotransformation in the BMR. This allowed a constant flux in the BMR and developed a continuous integrated membrane system [66]. In the case where olive leaves were used as source of oleuropein, the initial biomass was firstly treated by an innovative and sustainable microwave-assisted extraction [67], followed by biotransformation using an MBR with a free β-glucosidase. MBR was integrated with a membrane emulsification process to stabilize the poorly soluble oleuropein aglycon in ethyl acetate (90% of extraction) in mild operating conditions (25 °C, low shear stress and pressure less than 1 bar).
The enzymatic hydrolysis of xylan from coffee parchment in MBRs has been studied by Acosta-Fernandez et al. [38]. Xylooligosaccharides (XOs) composed of 2−20 units of xylose monomers have prebiotic characteristics, and thus exhibit great potential to be part of food as functional ingredients [38]. Coffee parchment represents a cheap raw waste material for producing XOs in a sustainable way. In that study, xylanase either as free enzyme in a solution or covalently immobilized on magnetic nanoparticles was used. Both MBRs are able to continuously produce reducing sugars as a function of time with an MW distribution in the range of prebiotic sugars (X1–X20); but only at a low substrate concentration when free enzyme in a stirred tank reactor (1 g/L; 97%) is used. The optimization of the residence time in MBR with the free enzyme allowed significant increase in the substrate conversion (about 85%) of prebiotic sugars at higher substrate concentration and this was attributed to the continuous removal of the inhibition products, present in the initial xylan solution, by the membrane process.
The major limitation in the development of BMR able to perform for long periods is the enzyme stability, which is jeopardized due to membrane fouling and subsequent membrane cleaning and maintenance. Reversible immobilization can be an alternative to easily remove the enzyme and preserve it during membrane cleaning [66]. It worth mentioning here that most BMR studies available in the open literature are still on a laboratory scale and with trial-and-error approach. The implementation of these systems in commercial scales requires more research effort on a prototype scale to prove the robustness of the technology. Furthermore, model-based predictive approaches for the selection of type of immobilization and membrane material for a given enzyme and reaction are still absent.
3.3. Membrane Emulsification for Final Products Formulation
The formulation of bioactive compounds in solid or liquid particles (emulsions, solid lipid and polymer particles) is a useful strategy adopted to improve bioavailability, mask undesirable tastes and control the release of the encapsulated compounds at a specific site. In this context, membrane emulsification (ME) is a technique that permits the production of loaded particles (such as oil-in-water and water-in-oil emulsions and solidified particles) with tuned size, in mild operative conditions and using low energy consumption relative to traditional devices (Figure 3a) [69]. This technique can also be used to extract poorly water-soluble compounds coming from hydrolytic biotransformation (Figure 3b) [67] or to heterogenize enzymes at the interface in heterogeneous reactions (Figure 3c) [70]. The ME is based on a drop-by-drop mechanism, where a dispersed phase is forced through the porous membrane into a continuous phase, which moves on the membrane surface to promote droplet dethatching [69].

Figure 3.
Applications of membrane emulsification for (a) formulation, (b) separation of recovered compounds and (c) enzyme heterogenization.
In recent years, this technology has received increasing research interest for valorization of bioactive compounds recovered from agri-food waste by the production of solid or liquid particles loaded with bioactive compounds recovered from the waste. However, to promote the production and purification of such biomolecules, a pre-treatment of the initial stream to purify/(bio)transform the compounds of interest is needed. Therefore, the use of agri-food waste bioactive compounds for formulation by ME is generally preceded by other purification steps, both membrane (MF, UF, NF, BMR) and non-membrane processes (centrifugation, chemical and/or physical pre-treatment); that are necessary to mitigate fouling and purify/fractionate/biotransform the target molecules.
Mazzei et al. [67] studied the production of purified non-commercially available phytotherapic compound (oleuropein aglycone) by MBR using as substrate oleuropein that has been recovered from olive leaves. Subsequently, the produced bioactive compound was extracted in a green organic solvent by ME. An extraction of the compound interest of 90% in ethyl acetate, which is considered as a green organic solvent, under mild extraction conditions was obtained. The membrane emulsification process has been used to produce two different formulations for oleuropein aglycone encapsulation. Oleuropein aglycone was encapsulated with an efficiency of 90% and 98% in solid lipids particles (SLPs) and PVA particles, respectively. The effectiveness of the membrane emulsification process for the preparation of oleuropein aglycone-loaded microparticles was indicated by the high uniformity of SLPs and PVA particles with respect to the polydisperse formulations produced by the homogenizer by using the same ingredients. The heterogeneous size distribution is undesirable for bioactive molecules delivery because it may cause unpredictable and unreproducible release profiles, also reducing the therapeutic effects.
4. Case Study: Water Purification and Recovery of Biophenols from Olive Industry Waste
As discussed in Section 2.1, above, important targets for the application of membrane technologies in the olive industries are the recovery of biophenols and the concurrent purification of water from OMWWs and TOPWs. Biophenols comprise a very large class of phytochemicals, members of which have widely differing properties in relation to their benefits (or otherwise) to humans and in the environment. Elevated polyphenol content in OMWWs is regularly associated with phytotoxicity, for example. Indeed, many polyphenolics are produced by plants as toxins to deter herbivores or competitor plant species. Removal of such biophenols from OMWWs is therefore desirable before the water can be recycled. On the other hand, many biophenols, including anthocyanins, tannins and flavonoids play roles as defensive antioxidants in plants [71]. These same antioxidant molecules can provide benefits to human health when consumed in appropriate quantities, and other biophenols have been attributed to have anti-inflammatory and anti-cancer effects [71]. Beneficial biophenols therefore provide targets for high-value products to be recovered from agri-food wastes.
Membrane technologies have been successfully used over the last two decades for separating, purifying, and concentrating bioactive phenolic compounds from OMWWs as an alternative to liquid–liquid extraction and adsorption/chromatographic separations. In particular, pressure-driven membrane cascades with membranes of decreasing MWCO have been suggested. This has proved a valid approach to overcome the wide range of molecular weights of phenolic compounds, but it is challenging to recover individual biophenols with high purities that are achievable only by chromatographic separations.
OMWWs from a three-phase olive mill were fractionated by di Lecce et al. [30] using a membrane cascade of MF and NF. MF system operated with the use of a tubular polypropylene membrane with pore size 0.2 μm, a TMP of 0.35 bar, an axial feed flow rate of 400 L/h and a temperature of 25 ± 2 °C. The resulting MF permeate was used as feed to a subsequent NF step. An NF spiral-wound membrane module was used at 22 ± 2 °C. Initially, an average TMP of six bars was used up to a final volume concentration factor (VCF) of 2.4, and a second set of experiments was performed at an average TMP of 15 bars up to a final VCF of 5.8. The results of this study suggested for the NF membrane a rejection higher than 98% towards COD, dry matter, phenolic compounds and antioxidant activity, independently of the VCF value.
In another study of Cassano et al. [27] a sequence of two UF (a hollow fiber and a flat sheet) followed by a NF (spiral-wound) membrane was investigated for achieving high levels of purification and a water fraction which can be discharged in aquatic systems or to be reused in the olive oil extraction process. The final NF membrane in this study was able to reject more than 95% and 100% of the TOC and low molecular weight biophenols, respectively.
OMWWs from a 3-phase and a 2-phase mill were subjected separately to a common two-step membrane filtration process using a novel vibratory system by Sedej et al. [29]. OMWWs samples were passed through a 100 μm screen to remove macro-solids, before membrane filtration. Two-step sequential membrane filtration of the OMWWs was conducted on a VSEP vibratory membrane separation system. Polyethersulfone UF membrane (PES-5/Tyvek, 7000 Da) was selected for the first filtration step to remove most of the suspended solids and large molecules, operated at 10.3 bar and on average 30 mL/min flow rate. The second filtration used a composite polyamide reverse osmosis membrane (ESPA, 40 Da), which purified the wastewater and concentrated the biophenols. The reverse osmosis retentate (RO-R) was enriched in biophenols stream, and the reverse osmosis permeate was a near to pure water stream, which was therefore suitable to be recycled into the milling process. Interestingly, the RO-R was spray-, freeze-, and infrared-dried only with an addition of 10% maltodextrin as a carrier to obtain solid material. The total biophenols in dried RO-R were in the range 0.15–0.58 mg gallic acid equivalents/g of dry weight for the two-phase system, and 1.38–2.17 mg gallic acid equivalents/g of dry weight for the three-phase system RO-R.
The recovery of biophenols from clarified olive pomace aqueous extracts were recently tested by Conidi et al. [54] with commercial polyamide UF and NF membranes. In this study, the aqueous extraction of biophenols from pomace was evaluated at laboratory scale under a range of liquid-to-solid ratio (L/S, 5 to 20 mL/g) and extraction temperatures (from 30 to 70 °C), keeping stable the extraction time at 60 min. The more appropriate extraction conditions were 70 °C and water-to-solid-ratio (L/S) of 5 mL/g that led to the higher total biophenols yield (2350 mg/L gallic acid). This aqueous extract was clarified by using a polypropylene MF tubular membrane module (nominal pore diameter of 0.2 µm), operated at TMP 0.32 bar and a temperature 27 ± 2 °C. The suspended solids were totally removed in this MF step, while most of the biophenols were recovered in the MF permeate. The NF membranes used showed higher permeation fluxes and lower fouling index when compared to UF membranes. In addition, NF membranes exhibited high rejection coefficients to biophenols. Membranes with MWCO in the range 150–500 Da were able to retain more than 70% of total biophenols under an optimal operating pressure of 25 bar.
Nunes et al. [28] studied the performance of two NF and an RO membrane using an aqueous olive pomace extract. For the extraction they used a 1:40 m/v water-to-solid-ratio at 40 ± 5 °C on a stir plate at 600 rpm for 2 h, and they filtered the solution through Whatman filter paper (No. 4). They found that the RO membrane was less affected by fouling (fouling index lower than 20%), had an almost constant permeate flux ~15 L/h·m2 during the whole operation until a volume concentration factor of 10 with the biophenols was totally rejected, whereas the rejection of total organic carbon and salts was 99.9 and 99.7%, respectively.
An innovative process design for water recovery and biophenols encapsulation of biophenols from OMWWs was developed by Bazzarelli et al. [49]. They used a combination of conventional pressure-driven processes (MF and NF) with alternative membrane operations (osmotic distillation, OD, and membrane emulsification, ME) to recover biophenols from the OMWWs biophenols. According to the process mass balance, the treatment of 1000 L of OMWWs was able to produce ~1.5 kg of phenolic compounds (85% of the initial phenolics) and 800 L (80% of the initial volume) of purified water, respectively. The final formulation was a water-in-oil emulsion (w/o) loaded with phenolic compounds with an encapsulation efficiency of 90% [49].
Recently, among membrane processes, there has been increasing interest in direct contact membrane distillation (DCMD) due to its advantages, such as its ability to operate at atmospheric pressure under a lower temperature than the normal boiling point of the feed solutions, the reduced fouling and concentration polarization in respect to other membrane processes, high retention of solutes, ability to treat very viscous solutions, and ability to use low grade energy. Furthermore, DCMD is not limited by high osmotic pressure and is able to reach concentration levels similar to those obtained in ordinary evaporation processes [35]. In a recent study, Tundis et al. [35] used DCMD to concentrate microfiltered OMWWs to produce fractions of phenolic compounds and they used a number of assays to evaluate the in vitro activity of the final fractions. They were able to produce by DCMD a concentrated fraction containing 2.8 g/L of biophenols (with hydroxytyrosol as the predominant compound at 2 mg/L) from microfiltered OMWWs. The feed and permeate temperatures in this case was 40 and 10 °C, respectively. Compared to the concentrations in the feed, MD retentate content was about five times greater for hydroxytyrosol and verbascoside, six times for tyrosol, and seven times greater for oleuropein. The average concentration factor of 5.4 for phenolic compounds in that case was in agreement with the weight reduction factor of the process, indicating no thermal degradation of bioactive compounds.
The long-term performance of a membrane bioreactor treating TOPW was studied by Patsios et al. [36]. The results of this study showed that after implementation of an appropriate protocol of active biomass acclimatization/proliferation, the semi-pilot scale MBR was able to operate continuously for 6 months with actual TOPW, under various conditions. Total organic carbon and polyphenol removal efficiencies were high (~ 91.5 and 82.8%, respectively), whereas the other basic nutrient (N and P) removal was also satisfactory. Interestingly, the permeability of fouled membranes could be fully restored by implementing chemical cleaning protocols. However, MBR effluent appeared with a yellowish tint and organic content that may require a final post-treatment to remove them, depending on local discharge standards. MBR definitely can serve as the basic treatment process in an integrated scheme for TOPW management to significantly reduce the initial organic load of these waste streams.
Based on literature data and the authors’ contribution to the field, a suitable integrated process to treat olive industry wastewaters and valorize their components is proposed in Figure 4. Here, in addition to the fractionation and concentration of biophenols (by MF, UF, NF, MD), and their bioconversion to debittered biophenols (by BMR) and formulation of emulsions (by ME), the possibility to achieve pure water (no detectable biophenols) with an RO step is highlighted. It is worth mentioning that stringent regulations in Europe about OMWWs management have imposed specific limitations to each EU country which developed an own national legislation (the maximum content of biophenols is 0.5 mg/L allowed in water to be discharged in the environment for most countries legislations [72]). To the best of the authors’ knowledge, there is no other technology so clean and low energy-demanding as membrane technology able to achieve such a level of water purity and biophenols recovery and valorization. It is worth mentioning that olive mill vegetative waters become “wastewaters” because they are collected and kept in open-air tanks. If they are intercepted and processed in line, being food processing waters, their content is suitable for edible use. On the other hand, concentrated fractions of biophenols seem suitable alternatives to synthetic fine chemicals, opening new venues for ecofriendly industrial treatments. One of the main limitations to their use could be their availability on a large scale; although the production of OMWWs is huge, it is a seasonal production, and the amount of biophenols might not be enough to satisfy industrial demand for massive production. Therefore, their use might be more interesting for niche industrial sectors. Figure 4 highlights the possibility to use organic residues for biogas production in anaerobic digester. This is possible thanks to the reduction in the mass of biophenols in the reduced retentate volume. In fact, since MF and UF do not retain biophenols, their concentration in the retentate is the same as the initial one, and since the retentate volume is much less than the initial one, the biophenols mass in the retentate is also reduced to an extend that it does not inhibit the biomass in the anaerobic fermenter. The biogas formed in the digester can be further upgraded by membrane gas separation (MGS) that is able to obtain biomethane (suitable for used in pipeline) from carbon dioxide (that can be purified to a degree suitable for food application) [73].

Figure 4.
Integrated process for olive mill wastewater treatment for water purification, biophenols recovery and biogas production. (MF: microfiltration, UF: ultrafiltration, NF: nanofiltration, BMR: biocatalytic membrane reactor, MD: membrane distillation, OD: osmotic distillation, ME: membrane emulsification, MGS: membrane gas separation).
5. Conclusions
From the previous sections analysis, it is evident that membranes are going to play a significant role in agri-food wastes valorization, environmental protection and water reclamation, as they demonstrate unique separation characteristics, e.g., they are able to perform in low temperatures, in general they do no require phase transition (thus have less energy requirements), they are easy to be controlled, they need less space for achieving a specific separation, etc. The appropriate membrane process integration can provide economically viable and environmentally sustainable solutions in larger scales to the agri-food industries. These solutions are able to provide high recovery of added-value chemicals from these streams, under low energy expenditure and even contribute to final formulation of the desired commercial product. The detailed and promising examples of olive processing wastes (olive oil, table olives and olive leaves) with use of various membrane processes support the wider adoption of these integrated processes in the real-world environment for the efficient and successful recycling of biophenols and water, leading to environmental protection with an apt impact to the society. Authors expect that membrane processes will play an important role in the sustainability of circular biobased industrial production, as they also significantly reduce carbon and water footprints.
Author Contributions
Conceptualization, E.H.P. and L.G.; writing—review and editing, all the authors have contributed to the writing; the final editing performed by E.H.P. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by International Exchanges 2019 Round 2 of The Royal Society, contract number IES\R2\192205.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the International Exchanges 2019 Round 2 of The Royal Society, contract number IES\R2\192205 for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Res. Cons. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, P. Valorization of food waste based on its composition through the concept of biorefinery. Cur. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Garnier, C.; Guiga, W.; Lameloise, M.L.; Degrand, L. Toward the reduction of water consumption in the vegetable-processing industry through membrane technology: Case study of a carrot-processing plant. Environ. Sci. Pol. Res. 2020, 27, 42685–42703. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Intern. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Amicarelli, V.; Bux, C. Food waste measurement toward a fair, healthy and environmental-friendly food system: A critical review. Brit. Food J. 2020, 123, 2907–2935. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.; Bezerra, F.; Pinto, R.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Res. Cons. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Makanjuola, O.; Arowosola, T.; Du, C. The utilization of food waste: Challenges and opportunities. J. Food Chem. Nanotechnol. 2020, 6, 182–188. [Google Scholar] [CrossRef]
- Mamma, D. Food Wastes: Feedstock for value-added products. Fermentation 2020, 6, 47. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food waste biorefinery: Pathway towards circular bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Amicarelli, V.; Lagioia, G.; Bux, C. Global warming potential of food waste through the life cycle assessment: An analytical review. Environ. Impact Assess. Rev. 2021, 91, 106677. [Google Scholar] [CrossRef]
- Squillaci, G.; la Cara, F.; Roseiro, L.B.; Marques, I.P.; Morana, A. Agro-industrial wastes as bioactive molecules source. Chem. Eng. Trans. 2021, 86, 37–42. [Google Scholar]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-products of agri-food industry as tannin-rich sources: A review of tannins’ biological activities and their potential for valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Intern. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Ibarz, R.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R.; Pérez-Fernández, M.; García-Juiz, S.; Osada, J.; Martín-Belloso, O.; et al. Valorization of agro-food by-products and their potential therapeutic applications. Food Bioprod. Process. 2021, 128, 247–258. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mitrouli, S.T.; Patsios, S.I.; Kazakli, M.; Karabelas, A.J. Valorization of pomegranate husk—Integration of extraction with nanofiltration for concentrated polyphenols recovery. J. Environ. Chem. Eng. 2020, 8, 103951. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Bosch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Karabelas, A.J. Lycopene recovery from tomato peel under mild conditions assisted by enzymatic pre-treatment and non-ionic surfactants. Acta Biochim. Pol. 2012, 59, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane–assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Sedej, I.; Milczarek, R.; Wang, S.C.; Sheng, R.; Avena–Bustillos, R.J.; Dao, L.; Takeoka, G. Membrane–filtered olive mill wastewater: Quality assessment of the dried phenolic–rich fraction. J. Food Sci. 2016, 81, E889–E896. [Google Scholar] [CrossRef]
- Di Lecce, G.; Cassano, A.; Bendini, A.; Conidi, C.; Giorno, L.; Toschi, G.T. Characterization of olivemill wastewater fractions treatment by integrated membrane process. J. Sci. Food Agric. 2014, 94, 2935–2942. [Google Scholar] [CrossRef]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Ghabbour, N.; Rharrabti, Y. Physicochemical and microbiological characterization of olive mill wastewater (OMW) from different regions of northern Morocco. Environ. Technol. 2020, 41, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Dahmen–Ben Moussa, I.; Maalej, A.; Masmoudi, M.A.; Feki, F.; Choura, S.; Baccar, N.; Jelail, L.; Karray, F.; Chamkha, M.; Sayadi, S. Effect of olive mill wastewaters on Scenedesmus sp. growth, metabolism and polyphenols removal. J. Sci. Food Agric. 2021, 101, 5508–5519. [Google Scholar] [CrossRef] [PubMed]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.-L.; Jellali, S. Olive mill waste water: From a pollutant to green fuels, agricultural water source, and bio–fertilizer. Part 2: Water recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of bioactive phenolic compounds in olive mill wastewater by direct contact membrane distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef]
- Patsios, S.I.; Papaioannou, E.H.; Karabelas, A.J. Long-term performance of a membrane bioreactor treating table olive processing wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 2253–2262. [Google Scholar] [CrossRef]
- Xavier Machado, T.D.O.; Portugal, I.B.M.; Padilha, C.V.D.S.; Ferreira Padilha, F.; dos Santos Lima, M. New trends in the use of enzymes for the recovery of polyphenols in grape byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef]
- Acosta–Fernandez, R.; Poerio, T.; Nabarlatz, D.; Giorno, L.; Mazzei, R. Enzymatic hydrolysis of xylan from coffee parchment in membrane bioreactors. Ind. Eng. Chem. Res. 2020, 59, 7346–7354. [Google Scholar] [CrossRef]
- Wen–qiong, W.; Yun–chao, W.; Xiao–feng, Z.; Rui–xia, G.; Mao–lin, L. Whey protein membrane processing methods and membrane fouling mechanism analysis. Food Chem. 2019, 289, 468–481. [Google Scholar] [CrossRef]
- Argenta, A.B.; Scheer, A.D.P. Membrane Separation Processes Applied to Whey: A Review. Food Rev. Int. 2020, 36, 499–528. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby–Figueroa, R.; Castro–Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro–food by–products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arboleda Meija, J.A.; Parpinello, G.P.; Versari, A.; Conidi, C.; Cassano, A. Microwave–assisted extraction and membrane–based separation of biophenols from red wine lees. Food Bioprod. Processing 2019, 117, 74–83. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of goat cheese whey through an integrated process of ultrafiltration and nanofiltration. Membranes 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, N.; Dadashi Firouzjaei, M.; Elliott, M.; Moradkhani, A.; Rahimpour, A. Preparation and modification of low–fouling ultrafiltration membranes for cheese whey treatment by membrane bioreactor. Case Stud. Chem. Environ. Eng. 2021, 4, 100137. [Google Scholar] [CrossRef]
- O’Halloran, J.; O’Sullivan, M.; Casey, E. Production of whey–derived DPP–IV inhibitory peptides using an enzymatic membrane reactor. Food Bioprocess Technol. 2019, 12, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Pázmándi, M.; Maráz, A.; Ladányi, M.; Kovács, Z. The impact of membrane pretreatment on the enzymatic production of whey–derived galacto–oligosaccharides. J. Food Process Eng. 2018, 41, e12649. [Google Scholar] [CrossRef]
- Syed, U.T.; Brazinha, C.; Crespo, J.G.; Ricardo–da–Silva, J.M. Valorisation of grape pomace: Fractionation of bioactive flavan–3–ols by membrane processing. Sep. Purif. Technol. 2017, 172, 404–414. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Piacentini, E.; Poerio, T.; Mazzei, R.; Cassano, A.; Giorno, L. Advances in membrane operations for water purification and biophenols recovery/valorization from OMWWs. J. Membr. Sci. 2016, 497, 402–409. [Google Scholar] [CrossRef]
- Uyttebroek, M.; Vandezande, P.; Van Dael, M.; Vloemans, S.; Noten, B.; Bongers, B.; Porto–Carrero, W.; Unamunzaga, M.M.; Bulut, M.; Lemmens, B. Concentration of phenolic compounds from apple pomace extracts by nanofiltration at lab and pilot scale with a techno–economic assessment. J. Food Process Eng. 2018, 41, e12629. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia–Castello, E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef] [Green Version]
- Uca, E.; Gulec, H.A. Recovery of phenolic compounds from pomegranate peels by sequential processes of water base extraction and ultrafiltration: Modelling of the process efficiency and fouling analysis. Waste Biomass Valorization 2021, 13, 511–523. [Google Scholar] [CrossRef]
- Conidi, C.; Egea–Corbacho, A.; Cassano, A. A combination of aqueous extraction and polymeric membranes as a sustainable process for the recovery of polyphenols from olive mill solid wastes. Polymers 2019, 11, 1868. [Google Scholar] [CrossRef] [Green Version]
- de Souza, T.S.P.; Kawaguti, H.Y. Cellulases, hemicellulases, and pectinases: Applications in the food and beverage industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Vardakas, A.T.; Shikov, V.T.; Dinkova, R.H.; Mihalev, K.M. Optimisation of the enzyme–assisted extraction of polyphenols from saffron (Crocus sativus L.) petals. Acta Sci. Pol. Technol. Aliment. 2021, 20, 359–367. [Google Scholar] [CrossRef]
- Dotsenko, G.; Lange, L. Enzyme enhanced protein recovery from green biomass pulp. Waste Biomass Valorization 2017, 8, 1257–1264. [Google Scholar] [CrossRef] [Green Version]
- Formigoni, M.; Milani, P.G.; Dacome, A.S.; Costa, S.C. Effect of enzymatic pretreatment on the extraction yield of Stevia rebaudiana leaves. Int. Food Res. J. 2018, 25, 1510–1514. [Google Scholar]
- Huda, M.S.; Nahar, N.; Monono, E.; Regmi, S. Oil recovery from fractionated dried distillers grains with solubles (Ddgs) using enzymes. Processes 2021, 9, 1507. [Google Scholar] [CrossRef]
- Rani, H.; Sharma, S.; Bala, M. Technologies for extraction of oil from oilseeds and other plant sources in retrospect and prospects: A review. J. Food Process Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Ramos, C.L.; Kuddus, M.; Rodriguez–Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic potential for the valorization of agro–industrial by–products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. Lignocellulolytic enzymes in biotechnological and industrial processes: A review. Sustainability 2020, 12, 7282. [Google Scholar] [CrossRef]
- Fibriana, F.; Upaichit, A.; Cheirsilp, B. Turning waste into valuable products: Utilization of agroindustrial oily wastes as the low–cost media for microbial lipase production. J. Phys. Conf. Ser. 2021, 1918, 052028. [Google Scholar] [CrossRef]
- Mazzei, R.; Drioli, E.; Giorno, L. Biocatalytic membranes and membrane bioreactors. Comp. Membr. Sci. Eng. 2010, 3, 195–212. [Google Scholar]
- Vitola, G.; Mazzei, R.; Poerio, T.; Barbieri, G.; Fontananova, E.; Büning, D.; Ulbricht, M.; Giorno, L. Influence of lipase immobilization mode on ethyl acetate hydrolysis in a continuous solid–gas biocatalytic membrane reactor. Bioconjug. Chem. 2019, 30, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; Yihdego Gebreyohannes, A.; Papaioannou, E.; Vankelecom, I.F.J.; Nunes, S.P.; Giorno, L. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery—A review. Bioresour. Technol. 2021, 335, 125248. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Nardi, M.; Poerio, T.; Bazzarelli, F.; Procopio, A.; Luisa Di Gioia, M.; Rizza, P.; Ceraldi, R.; Morelli, C.; et al. Production of plant–derived oleuropein aglycone by a combined membrane process and evaluation of its breast anticancer properties. Front. Bioeng. Biotechnol. 2020, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Mazzei, R.; Poerio, T.; Bazzarelli, F.; Wu, Z.; Li, K.; Giorno, L. Biorefinery of olive leaves to produce dry oleuropein aglycone: Use of homemade ceramic capillary biocatalytic membranes in a multiphase system. Chem. Eng. Sci. 2018, 185, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Piacentini, E.; Bazzarelli, F.; Poerio, T.; Albisa, A.; Mendoza, G.; Sebastian, V.; Giorno, L. Encapsulation of water–soluble drugs in Poly (vinyl alcohol) (PVA)–microparticles via membrane emulsification: Influence of process and formulation parameters on structural and functional properties. Mater. Today Commun. 2020, 24, 100967. [Google Scholar] [CrossRef]
- Piacentini, E.; Mazzei, R.; Giorno, L. Comparison between lipase performance distributed at the o/w interface by membrane emulsification and by mechanical stirring. Membranes 2021, 11, 137. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.-H. From fighting critters to saving lives: Polyphenols in plant defense and human health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef] [PubMed]
- Halalsheh, M.; Kassab, G.; Shatanawi, K. Impact of legislation on olive mill wastewater management: Jordan as a case study. Water Policy 2021, 23, 343–357. [Google Scholar] [CrossRef]
- Baena–Moreno, F.M.; le Sache, E.; Pastor–Perez, L.; Reina, T.R. Membrane–based technologies for biogas upgrading: A review. Environ. Chem. Lett. 2020, 18, 1649–1658. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).