Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
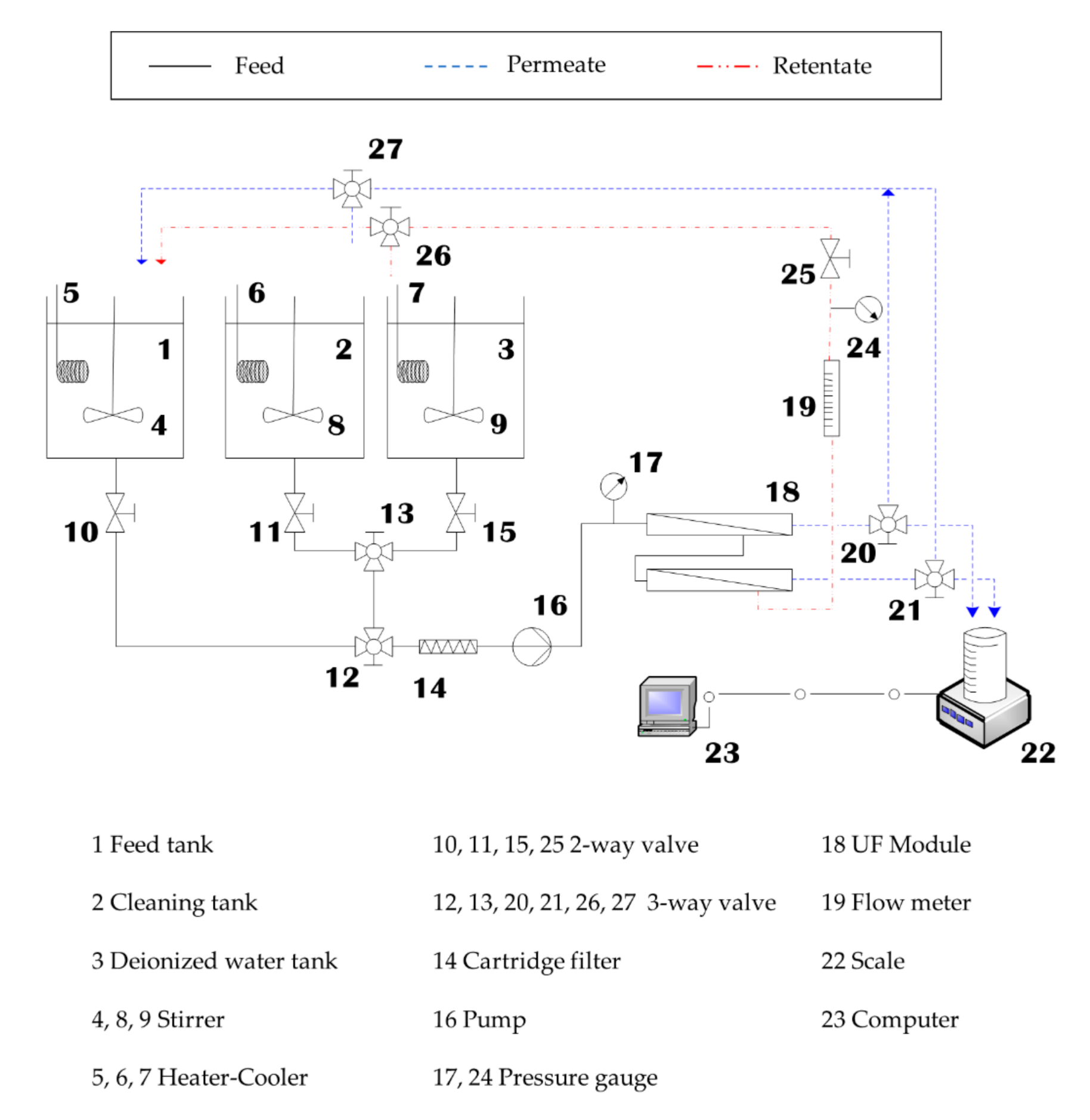
2.3. Ultrafiltration Procedure
2.4. Membrane Characterization
2.4.1. Membrane Permeability
2.4.2. Membrane Performance
2.5. Analytical Determination
2.6. Fouling Mechanisms
3. Results and Discussion
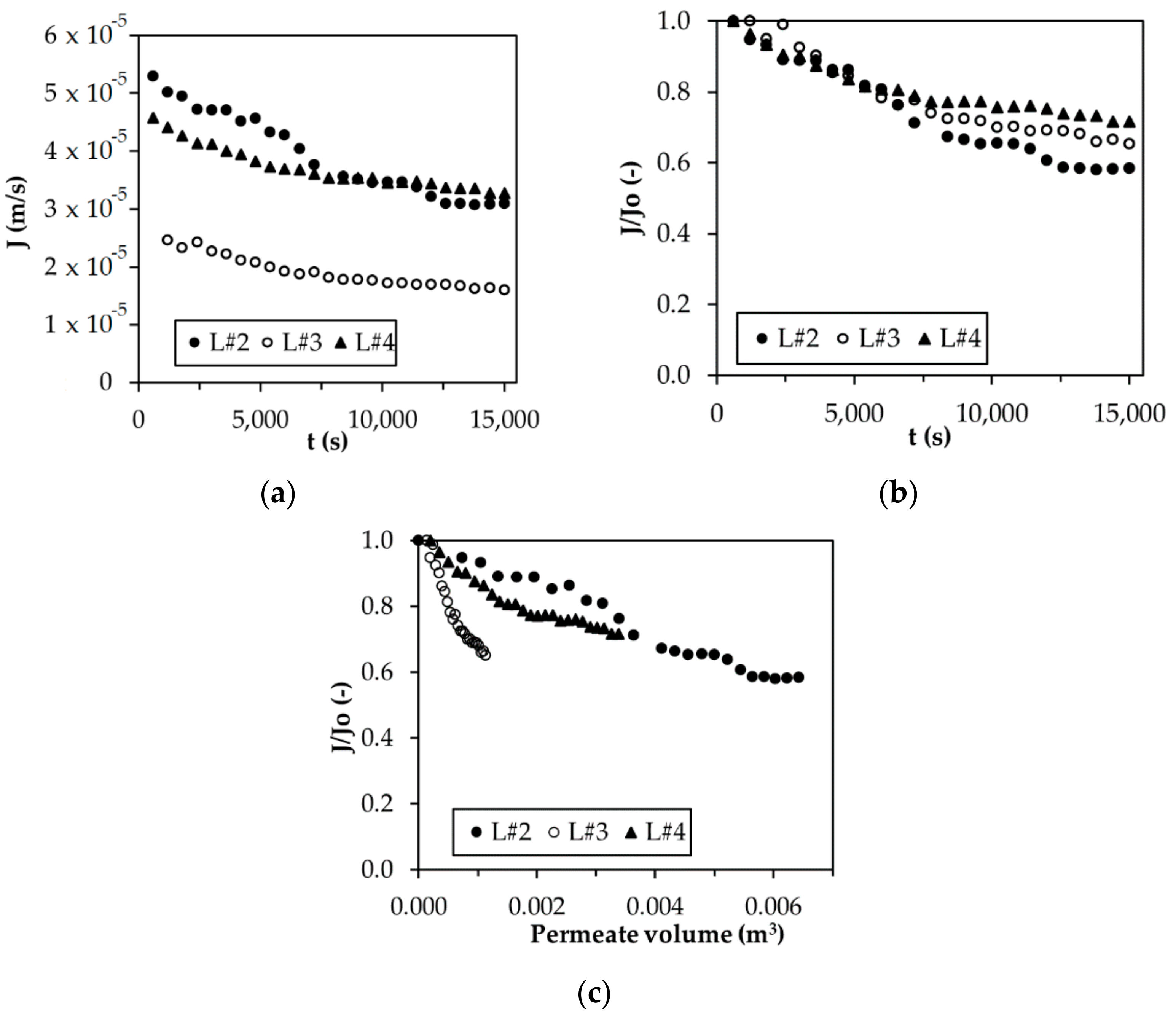
3.1. Flux Performance
3.2. Isolated Soy Protein Concentration
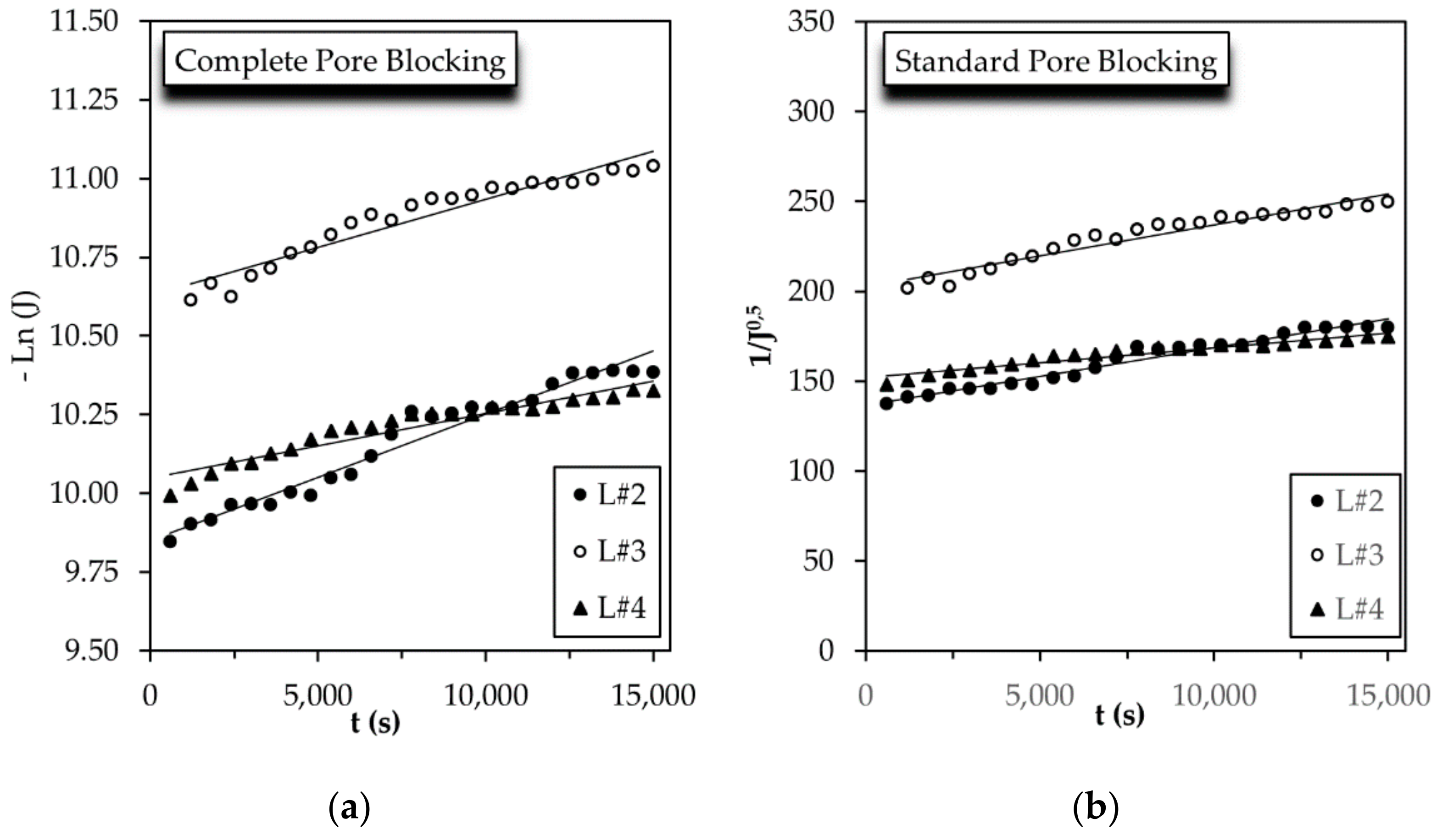
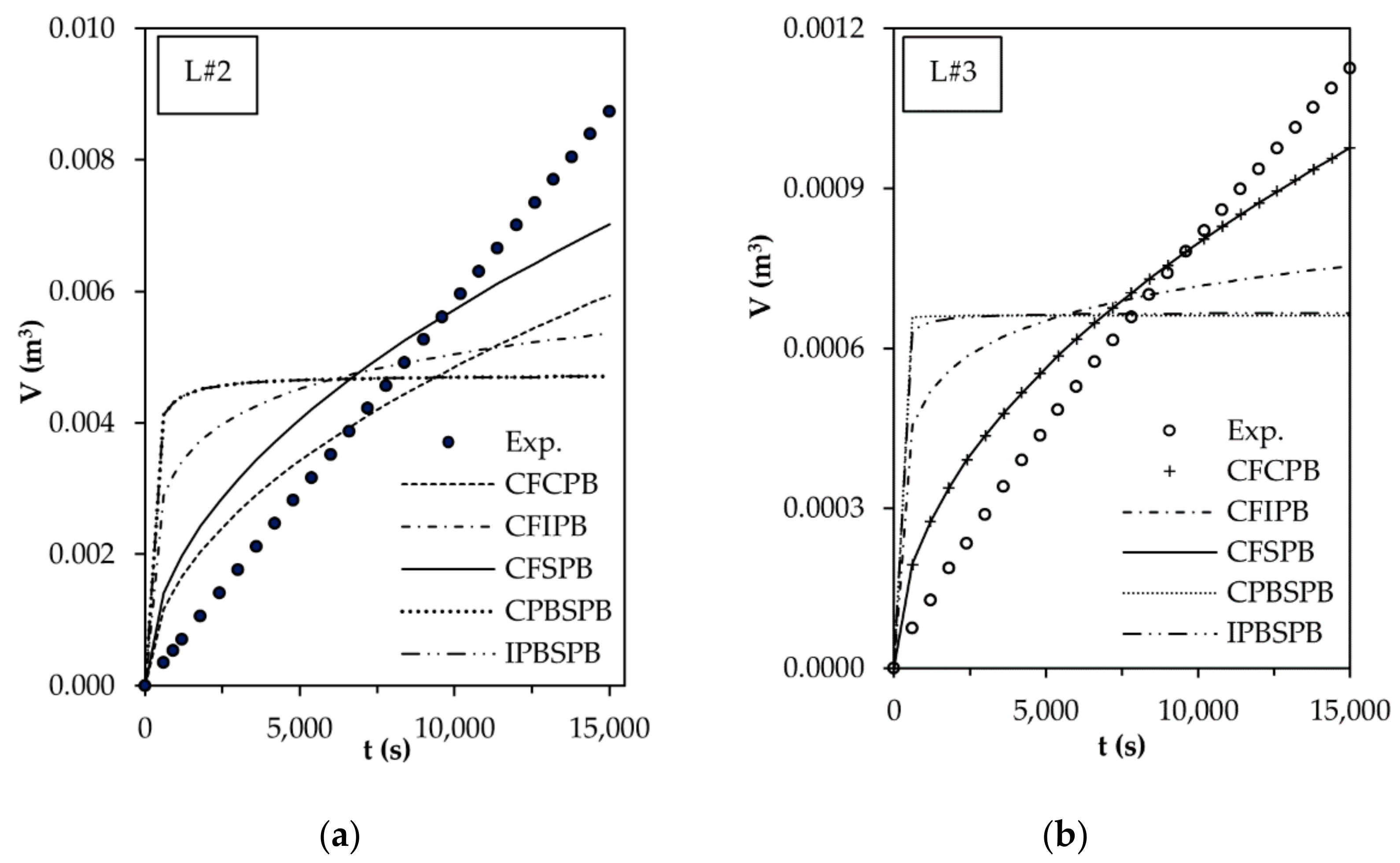
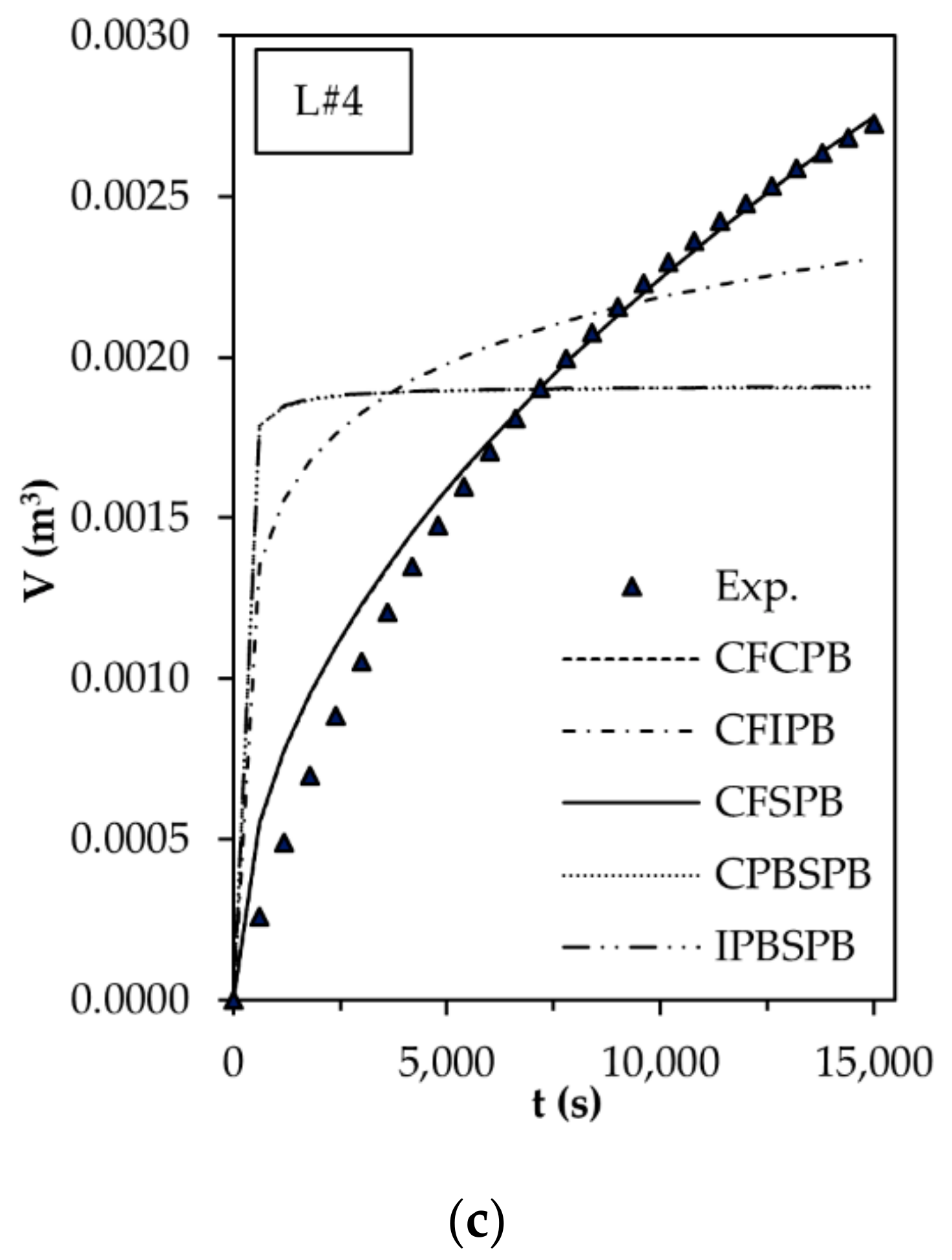
3.3. Flux Decline Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langevin, M.E.; Roblet, C.; Moresoli, C.; Ramassamy, C.; Bazinet, L. Comparative application of pressure- and electrically-driven membrane processes for isolation of bioactive peptides from soy protein hydrolysate. J. Membr. Sci. 2012, 403, 15–24. [Google Scholar] [CrossRef]
- Mondor, M.; Ali, F.; Ippersiel, D.; Lamarche, F. Impact of ultrafiltration/diafiltration sequence on the production of soy protein isolate by membrane technologies. Innov. Food Sci. Emerg. Technol. 2010, 11, 491–497. [Google Scholar] [CrossRef]
- Chove, E.; Grandison, A.S.; Lewis, M.J. Some functional properties of fractionated soy protein isolates obtained by microfiltration. Food Hydrocoll. 2007, 21, 1379–1388. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajó, J.C. Fractionation and Enzymatic Hydrolysis of Soluble Protein Present in Waste Liquors from Soy Processing. J. Agric. Food Chem. 2005, 53, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Domínguez, H.; Parajó, J.C. Ultrafiltration of industrial waste liquors from the manufacture of soy protein concentrates. J. Chem. Technol. Biotechnol. 2006, 81, 1252–1258. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Wilts, H.; Fecke, M.; Zeher, C. Economics of Waste Prevention: Second-Hand Products in Germany. Economies 2021, 9, 74. [Google Scholar] [CrossRef]
- Cassini, A.; Tessaro, I.C.; Marczak, L.; Pertile, C. Ultrafiltration of wastewater from isolated soy protein production: A comparison of three UF membranes. J. Clean. Prod. 2010, 18, 260–265. [Google Scholar] [CrossRef]
- Ali, F.; Ippersiel, D.; Lamarche, F.; Mondor, M. Characterization of low-phytate soy protein isolates produced by membrane technologies. Innov. Food Sci. Emerg. Technol. 2010, 11, 162–168. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Yea, M.; Cheryan, M.; Kumar, N.K. Ultrafiltration of soy protein concentrate: Performance and modelling of spiral and tubular polymeric modules. J. Membr. Sci. 2004, 244, 235–242. [Google Scholar] [CrossRef]
- Susanto, H.; Widiasa, I.N. Ultrafiltration fouling of amylose solution: Behavior, characterization and mechanism. J. Food Eng. 2009, 95, 423–431. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Zhao, W.; Shi, Q.; Wang, H.; Jiang, Z.; Zhu, S. Modification of polyethersulfone ultrafiltration membranes with phosphorylcholine copolymer can remarkably improve the antifouling and permeation properties. J. Membr. Sci. 2008, 322, 171–177. [Google Scholar] [CrossRef]
- Marcos, B.; Moresoli, C.; Skorepova, J.; Vaughan, B. CFD modeling of a transient hollow fiber ultrafiltration system for protein concentration. J. Membr. Sci. 2009, 337, 136–144. [Google Scholar] [CrossRef]
- Rajabzadeh, A.R.; Moresoli, C.; Marcos, B. Fouling behavior of electroacidified soy protein extracts during cross-flow ultrafiltration using dynamic reversible irreversible fouling resistances and CFD modeling. J. Membr. Sci. 2010, 361, 191–205. [Google Scholar] [CrossRef]
- Sosalagere, C.; Kehinde, B.A.; Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: A reviews. Food Chem. 2022, 366, 130494. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermia, J. Constant pressure blocking filtration laws-application to power law non Newtonian fluids. Inst. Chem. Eng. Trans. 1982, 60, 183–187. [Google Scholar]
- Bolton, G.; LaCasse, D.; Kuriyel, R. Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids. J. Membr. Sci. 2006, 277, 75–84. [Google Scholar] [CrossRef]
- Charfi, A.; Ben Amar, N.; Harmand, J. Analysis of fouling mechanisms in anaerobic membrane bioreactors. Water Res. 2012, 46, 2637–2650. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; De, S. A combined complete pore blocking and cake filtration model for steady-state electric field-assisted ultrafiltration. AIChE J. 2012, 58, 1435–1446. [Google Scholar] [CrossRef]
- Ruby Figueroa, R.A.; Cassano, A.; Drioli, A. Ultrafiltration of orange press liquor: Optimization for permeate flux and fouling index by response surface methodology. Sep. Purif. Technol. 2011, 80, 1–10. [Google Scholar] [CrossRef]
- de Barros, S.T.D.; Andrade, C.M.G.; Mendes, E.S.; Peres, L. Study of fouling mechanism in pineapple juice clarification by ultrafiltration. J. Membr. Sci. 2003, 215, 213–224. [Google Scholar] [CrossRef]
- Grossman, A.D.; Yang, Y.; Yogev, U.; Calero Camarena, D.; Oron, G.; Bernstein, R. Effect of ultrafiltration membrane material on fouling dynamics in a submerged anaerobic membrane bioreactor treating domestic wastewater. Environ. Sci. Water Res. Technol. 2019, 5, 1145–1156. [Google Scholar] [CrossRef]
- Reddy, A.V.R.; Mohan, D.J.; Bhattacharya, A.; Shah, V.J.; Ghosh, P.K. Surface modification of ultrafiltration membranes by preadsorption of a negatively charged polymer: I. Permeation of water soluble polymers and inorganic salt solutions and fouling resistance properties. J. Membr. Sci. 2003, 214, 211–221. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y.; Wang, D.M.; Bouyer, D.; Higuchi, A.; Lai, J.Y. PEGylation of anti-biofouling polysulfone membranes via liquid- and vapor-induced phase separation processing. J. Membr. Sci. 2012, 403, 47–57. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.K. Ultrafiltration membranes prepared from blends of polyethersulfone and poly(1-vinylpyrrolidone-co-styrene) copolymers. J. Membr. Sci. 2005, 262, 60–68. [Google Scholar] [CrossRef]
- Dong, H.B.; Xu, Y.Y.; Yi, Z.; Shi, J.L. Modification of polysulfone membranes via surface-initiated atom transfer radical polymerization. Appl. Surf. Sci. 2009, 255, 8860–8866. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Zhu, S.; Li, C.; Zhao, Y.; Jiang, Z. A facile method for synthesis of pegylated polyethersulfone and its application in fabrication of antifouling ultrafiltration membrane. J. Membr. Sci. 2007, 303, 204–212. [Google Scholar] [CrossRef]
- Saha, N.K.; Balakrishnan, M.; Ulbricht, M. Fouling control in sugarcane juice ultrafiltration with surface modified polysulfone and polyethersulfone membranes. Desalination 2009, 249, 1124–1131. [Google Scholar] [CrossRef]
- Susanto, H.; Arafat, H.; Janssen, E.M.L.; Ulbricht, M. Ultrafiltration of polysaccharide protein mixtures: Elucidation of fouling mechanisms and fouling control by membrane surface modification. Sep. Purif. Technol. 2008, 63, 558–565. [Google Scholar] [CrossRef]
- Korzhova, E.; Déon, S.; Koubaa, Z.; Fievet, P.; Lopatin, D.; Baranov, O. Modification of commercial UF membranes by electrospray deposition of polymers for tailoring physicochemical properties and enhancing filtration performances. J. Membr. Sci. 2020, 598, 117805. [Google Scholar] [CrossRef]
- Enfrin, M.; Wang, J.; Merenda, A.; Dumée, L.F.; Lee, J. Mitigation of membrane fouling by nano/microplastics via surface chemistry control. J. Membr. Sci. 2021, 633, 119379. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Jing, W.; Fan, Y.; Xu, N. Improving the filtration performance of ZrO2 membrane in non-polar organic solvents by surface hydrophobic modification. J. Membr. Sci. 2011, 375, 276–283. [Google Scholar] [CrossRef]
- Peyravi, M.; Rahimpour, A.; Jahanshahi, M.; Javadi, A.; Shockravi, A. Tailoring the surface properties of PES ultrafiltration membranes to reduce the fouling resistance using synthesized hydrophilic copolymer. Microporous Mesoporous Mater. 2012, 160, 114–125. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Jahanshahi, M.; Mansourpanah, Y.; Mortazavian, N. Development of high performance nano-porous polyethersulfone ultrafiltration membranes with hydrophilic surface and superior antifouling properties. Appl. Surf. Sci. 2009, 255, 9166–9173. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Membr. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S. Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: Preparation, morphology, performance and antifouling properties. J. Membr. Sci. 2007, 305, 299–312. [Google Scholar] [CrossRef]
- Peeva, P.D.; Knoche, T.; Pieper, T.; Ulbricht, M. Cross-flow ultrafiltration of protein solutions through unmodified and surface functionalized polyethersulfone membranes – Effect of process conditions on separation performance. Sep. Purif. Technol. 2012, 92, 83–92. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: 2. Theoretical analysis, polarization effects and thermal efficiency. J. Membr. Sci. 2013, 433, 180–191. [Google Scholar] [CrossRef]
- Eykens, L.; Hitsovac, I.; De Sitter, K.; Dotremont, C.; Pinoyd, L.; Nopens, I.; Van der Bruggen, B. Influence of membrane thickness and process conditions on direct contact membrane distillation at different salinities. J. Membr. Sci. 2016, 498, 353–364. [Google Scholar] [CrossRef]
- Mai, H.C. Application of cross-flow filtration technique in purification and concentration of juice from Vietnamese fruits. Beverages 2017, 3, 44. [Google Scholar] [CrossRef] [Green Version]
- Campos, P.R.F.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Trigueros, D.E.G.; Barros, S.T.D.; Pereira, N.C. Improvement on the concentrated grape juice physico-chemical characteristics by an enzymatic treatment and Membrane Separation Processes. An. Acad. Bras. Ciências 2016, 88, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.Y.; Mohammad, A.W.; Ng, L.Y.; Jahim, J.M. Membrane fouling mechanisms during ultrafiltration of skimmed coconut milk. J. Food Eng. 2014, 142, 190–200. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Cheng, Y.-H.; Paul, D.R.; Field, R.W.; Freeman, B.D. Fouling mechanisms in constant flux crossflow ultrafiltration. J. Membr. Sci. 2019, 574, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Lamdande, A.G.; Mittal, R.; Raghavarao, K.S.M.S. Flux evaluation based on fouling mechanism in acoustic field-assisted ultrafiltration for cold sterilization of tender coconut water. Innov. Food Sci. Emerg. Technol. 2020, 61, 102312. [Google Scholar] [CrossRef]
- Coelho, L.L.; Scaratti, G.; Hissanaga, A.M.; Oechsler, B.F.; José, H.J.; Moreira, R.F.P.M. Modeling and fouling control in a hybrid membrane process using CuO-catalytic membrane coupled to ozone. J. Environ. Chem. Eng. 2021, 9, 106138. [Google Scholar] [CrossRef]
- Cainglet, H.E.; Saavedra, T.; Bürgmayr, S.; Zhang, J.; Xie, Z.; Garnier, G.; Tanner, J. Recycled paper mill process water pre-treatment using ultrafiltration for water system closure. J. Water Process. Eng. 2021, 44, 102407. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Velswamy, K.; Gangasalam, A.; Ismail, A.F.; Goh, P.S.; Narayanan, A.; Abdullah, M.S. Performance evaluation of whey flux in dead-end and cross-flow modes via convolutional neural networks. J. Environ. Manag. 2022, 301, 113872. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rai, C.; Rai, P.; Majumdar, G.C.; De, S.; DasGupta, S. Mechanism of permeate flux decline during microfiltration of watermelon (Citrillus lanatus) juice. Food Bioprocess Technol. 2010, 3, 545–553. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Mayor, L.; Chorques, S.; Argüelles, A.; Vidal-Brotóns, D.; Gras, M.L. Reverse osmosis concentration of press liquid from orange juice solid wastes: Flux decline mechanisms. J. Food Eng. 2011, 106, 199–205. [Google Scholar] [CrossRef]
- Cassano, A.; Marchio, M.; Drioli, E. Clarification of blood orange juice by ultrafiltration: Analyses of operating parameters, membrane fouling and juice quality. Desalination 2007, 212, 15–27. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Yoshikawa, S.; Karasu, K.; Ookawara, O. A simple combination model for filtrate flux in cross-flow ultrafiltration of protein suspension. J. Membr. Sci. 2012, 403, 84–93. [Google Scholar] [CrossRef]
- Wang, F.; Tarabara, V.V. Pore blocking mechanisms during early stages of membrane fouling by colloids. J. Colloid Interface Sci. 2008, 328, 464–469. [Google Scholar] [CrossRef]
- Jafarzadeh, Y.; Yegani, R.; Sedaghat, M. Preparation, characterization and fouling analysis of ZnO/polyethylene hybrid membranes for collagen separation. Chem. Eng. Res. Des. 2015, 94, 417–427. [Google Scholar] [CrossRef]
- Heidari, S.; Etemadi, H.; Yegani, R. A comprehensive analysis of membrane fouling in microfiltration of complex linear macromolecules based on theoretical modeling and FESEM images. J. Chem. Technol. Biotechnol. 2021, 96, 360–373. [Google Scholar] [CrossRef]
- Akbari, A.; Yegani, R.; Pourabbas, B.; Behboudi, A. Fabrication and study of fouling characteristics of HDPE/PEG grafted silica nanoparticles composite membrane for filtration of Humic acid. Chem. Eng. Res. Des. 2016, 109, 282–296. [Google Scholar] [CrossRef]



| Ref. | PES (%) 1 | PEG (%) 1 | DMA (%) 1 | Temperature (°C) |
|---|---|---|---|---|
| L#2 | 18 | -- | 82 | 20 |
| L#3 | 15 | 3 | 82 | 20 |
| L#4 | 15 | 3 | 82 | 27 |
| Flux Decline Mechanism | n | Equation | |
|---|---|---|---|
| Complete pore blocking (CPB) | 2.0 | (8) | |
| Standard pore blocking (SPB) | 1.5 | (9) | |
| Intermediate pore blocking (IPB) | 1.0 | (10) | |
| Cake filtration (CF) | 0.0 | (11) |
| C#1 | C#2 | L#2 | L#3 | L#4 | |
|---|---|---|---|---|---|
| Permeability (m/s·bar) | 5.8 × 10−6 | 3.5 × 10−5 | 6.9 × 10−5 | 9.5 × 10−6 | 1.7 × 10−5 |
| Resistance (s·bar/m) | 1.72 × 105 | 2.86 × 104 | 1.45 × 104 | 1.05 × 105 | 5.88 × 104 |
| Cut-off (kDa) | 3 | 30 | n.d. | n.d. | n.d. |
| Material | PES | PES | PES | PES/PEG | PES/PEG |
| CPB | SPB | IPB | CF | |
|---|---|---|---|---|
| L#2 | R2 = 0.960 | R2 = 0.964 | R2 = 0.966 | R2 = 0.966 |
| Kcb = 0.00004 s−1 | Ksb = 0.0032 m−1 | Kib = 1.0344 m−1 | Kcf = 54,156 s m−2 | |
| L#3 | R2 = 0.925 | R2 = 0.937 | R2 = 0.948 | R2 = 0.965 |
| Kcb = 0.000030 s−1 | Ksb = 0.0035 m−1 | Kib = 1.5699 m−1 | Kcf = 163,602 s m−2 | |
| L#4 | R2 = 0.913 | R2 = 0.924 | R2 = 0.934 | R2 = 0.950 |
| Kcb = 0.0000205 s−1 | Ksb = 0.0017 m−1 | Kib = 0.5457 m−1 | Kcf = 29,153 s m−2 |
| Flux Decline Mechanism | Equation | Fitted Parameters | |
|---|---|---|---|
| CFCPB | Kcf (s·m−2); Kcb (s−1) | (12) | |
| CFIPB | Kcf (s·m−2); Kib (m−1) | (13) | |
| CFSPB | Kcf (s·m−2); Ksb (m−1) | (14) | |
| CPBSPB | Kcb (s−1); Ksb (m−1) | (15) | |
| IPBSPB | Kib (m−1); Ksb (m−1) | (16) |
| L#2 | L#3 | L#4 | |
|---|---|---|---|
| Cake Filtration—Complete Pore Blocking, CFCPB | SSE = 4.32 × 10−5 | SSE = 2.46 × 10−7 | SSE = 2.71 × 10−7 |
| Kcf = 8.44 × 108 s·m−2 | Kcf = 3.13 × 1010 s·m−2 | Kcf = 3.93 × 109 s·m−2 | |
| Kcb = 1 × 10−13 s−1 | Kcb = 0.00010 s−1 | Kcb = 0.00010 s−1 | |
| Cake Filtration—Intermediate Pore Blocking, CFIPB | SSE = 9.60 × 10−5 | SSE = 1.34 × 10−6 | SSE = 5.65 × 10−6 |
| Kcf = 0.0460 s·m−2 | Kcf = 0.2000 s·m−2 | Kcf = 0.2000 s·m−2 | |
| Kib = 1294.236 m−1 | Kib = 10,786.366 m−1 | Kib = 3357.282 m−1 | |
| Cake Filtration—Standard Pore Blocking, CFSPB | SSE = 2.68 × 10−5 | SSE = 2.49 × 10−7 | SSE = 2.75 × 10−7 |
| Kcf = 0.00254 s·m−2 | Kcf = 0.13158 s·m−2 | Kcf = 0.0166 s·m−2 | |
| Ksb=9.42 × 106 m−1 | Ksb=9.57 × 106 m−1 | Ksb=9.54 × 106 m−1 | |
| Complete Pore Blocking—Standard Pore Blocking, CPBSPB | SSE = 1.48 × 10−4 | SSE = 2.10 × 10−6 | SSE = 1.48 × 10−4 |
| Kcb = 10−7 s−1 | Kcb = 0.0318 s−1 | Kcb = 0.0017 | |
| Ksb = 422.61 m−1 | Ksb = 403.78 m−1 | Ksb = 1008.75 m−1 | |
| Intermediate Pore Blocking—Standard Pore Blocking, IPBSPB | SSE = 1.48·10−4 | SSE = 2.07·10−6 | SSE = 9.81 × 10−6 |
| Kib = 0.0210 m−1 | Kib = 0.0200 m−1 | Kib = 0.0210 m−1 | |
| Ksb = 422.59 m−1 | Ksb = 2997.98 m−1 | Ksb = 1045.74 m−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barredo-Damas, S.; Iborra-Clar, A.; Pascual-Garrido, J.; Iborra-Clar, M.I. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability 2021, 13, 13682. https://doi.org/10.3390/su132413682
Garcia-Castello EM, Rodriguez-Lopez AD, Barredo-Damas S, Iborra-Clar A, Pascual-Garrido J, Iborra-Clar MI. Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability. 2021; 13(24):13682. https://doi.org/10.3390/su132413682
Chicago/Turabian StyleGarcia-Castello, Esperanza M., Antonio D. Rodriguez-Lopez, Sergio Barredo-Damas, Alicia Iborra-Clar, Jairo Pascual-Garrido, and Maria Isabel Iborra-Clar. 2021. "Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions" Sustainability 13, no. 24: 13682. https://doi.org/10.3390/su132413682
APA StyleGarcia-Castello, E. M., Rodriguez-Lopez, A. D., Barredo-Damas, S., Iborra-Clar, A., Pascual-Garrido, J., & Iborra-Clar, M. I. (2021). Fabrication and Performance of Low-Fouling UF Membranes for the Treatment of Isolated Soy Protein Solutions. Sustainability, 13(24), 13682. https://doi.org/10.3390/su132413682








