Canola Seed Priming and Its Effect on Gas Exchange, Chlorophyll Photobleaching, and Enzymatic Activities in Response to Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
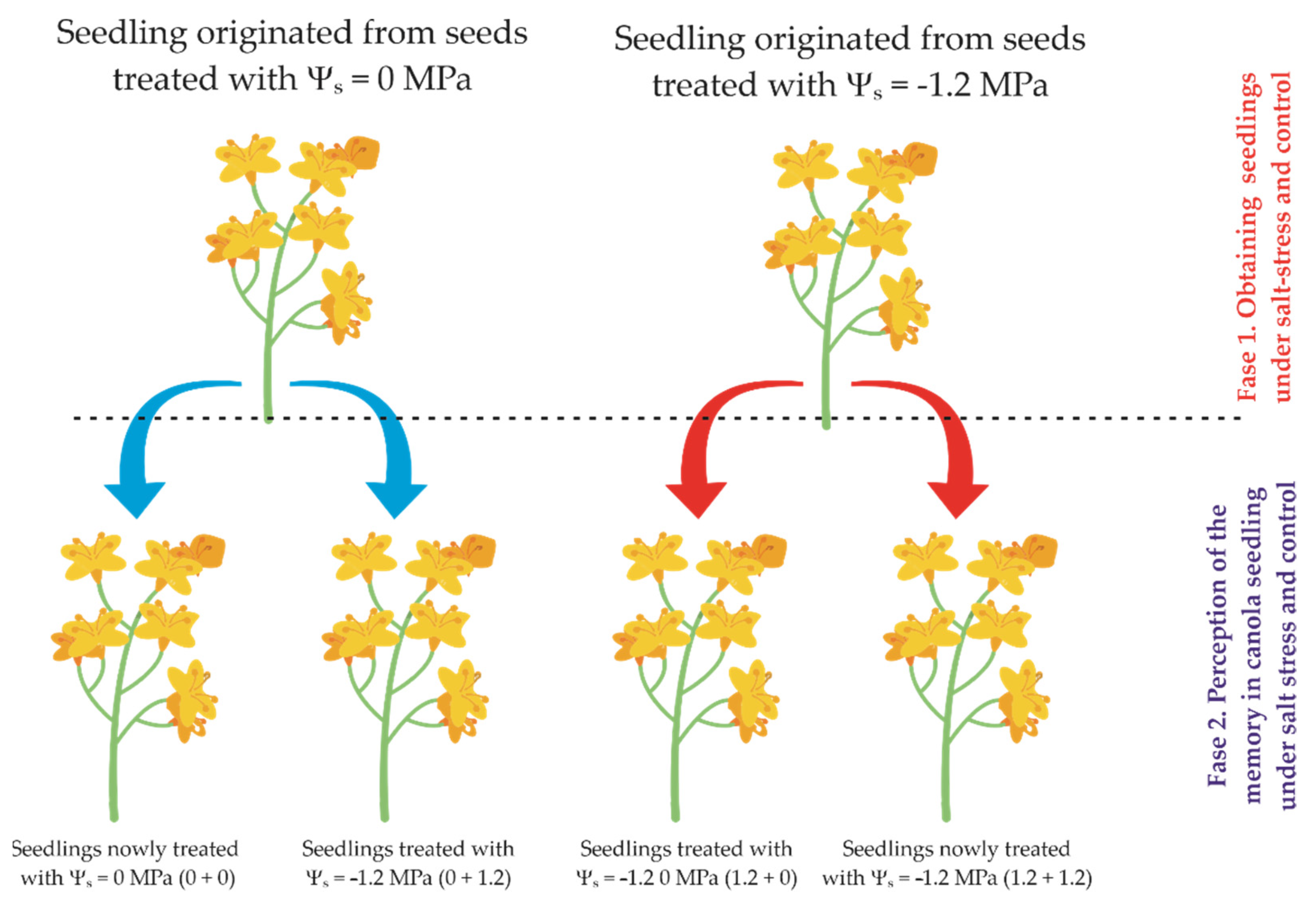
2.2. Experimental Design and Treatment
2.3. Plant Traits Measurements
2.3.1. Seed Germination and Plant Bank
2.3.2. Gas Exchange
2.3.3. Photosynthetic Pigments Analysis
2.3.4. Enzymatic Antioxidant Activity
2.4. Data Analysis
3. Results
3.1. Germination Features
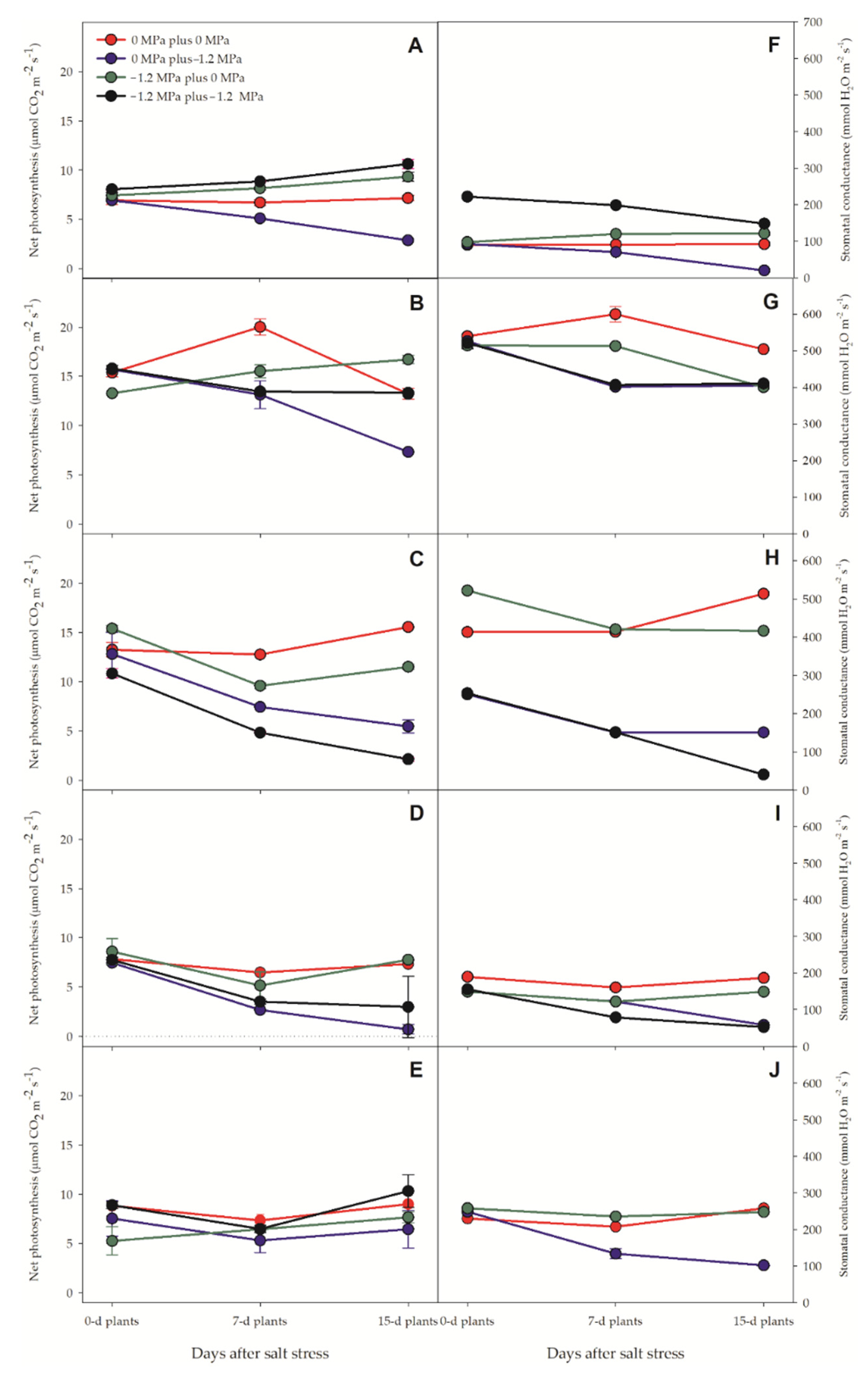
3.2. Gas Exchange
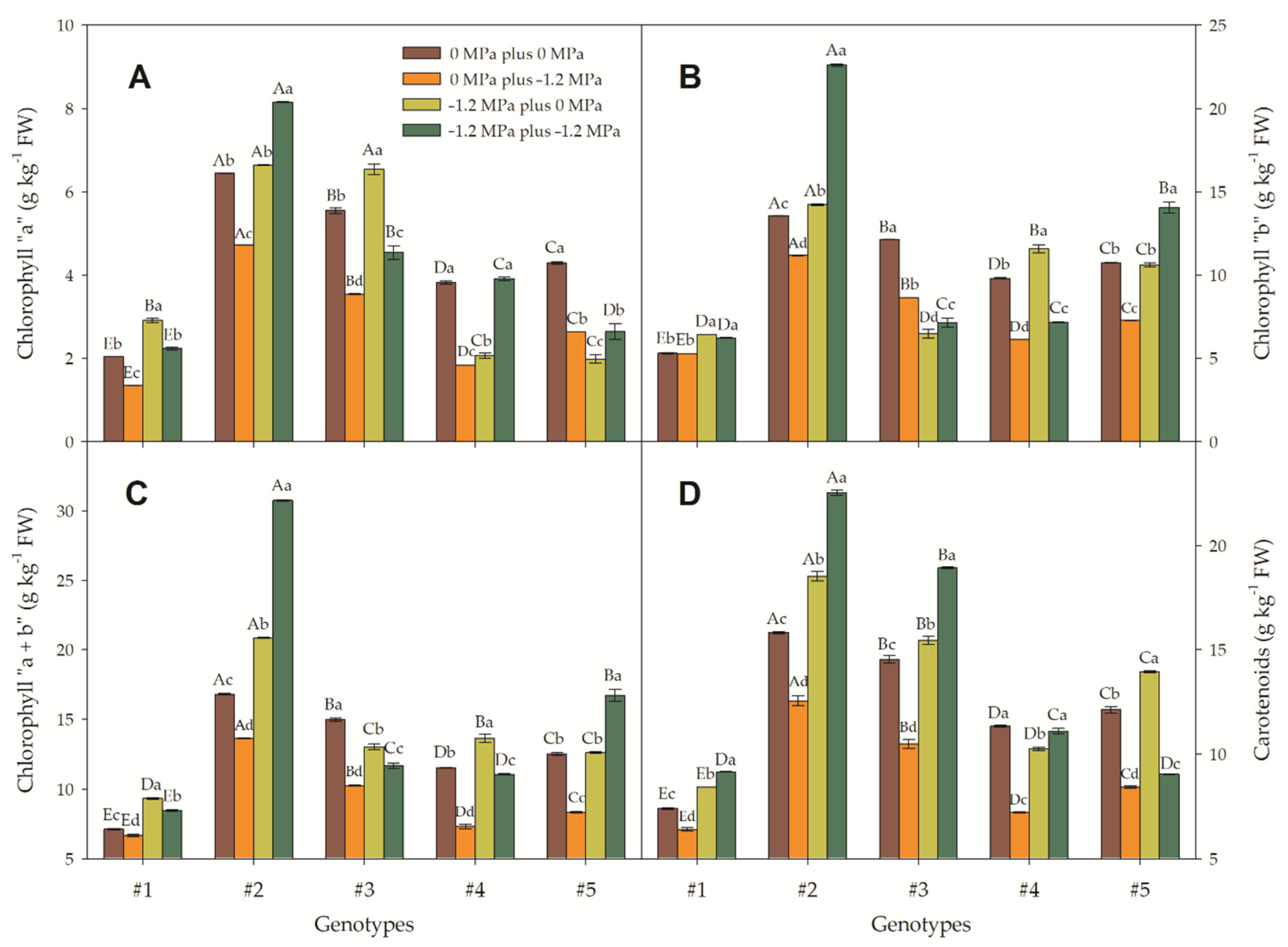
3.3. Interaction of Foliar Pigments and Salt-Stressed Canola Seedlings
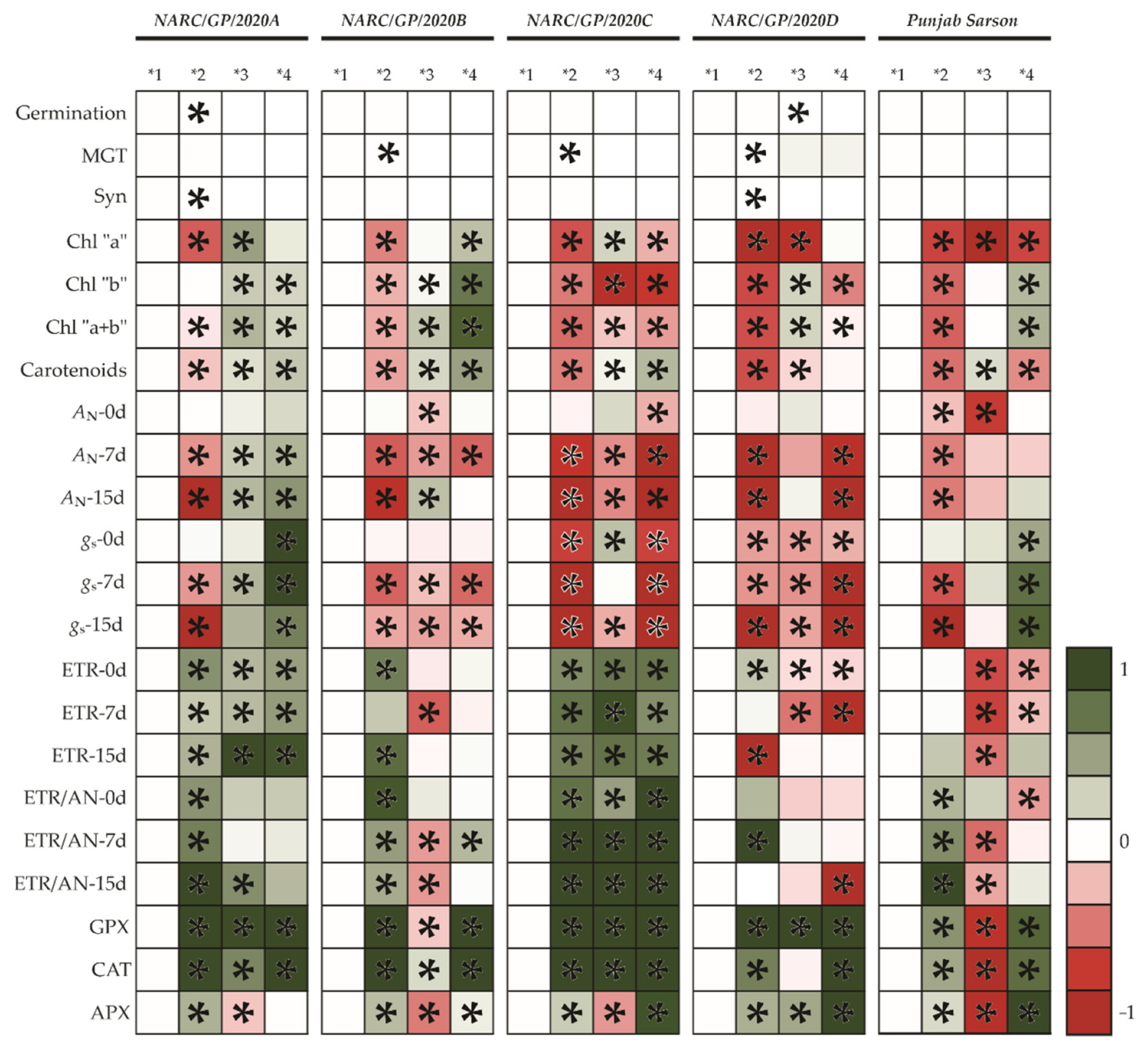
3.4. Enzymatic Responses in Different Canola Genotypes under Salt Stress
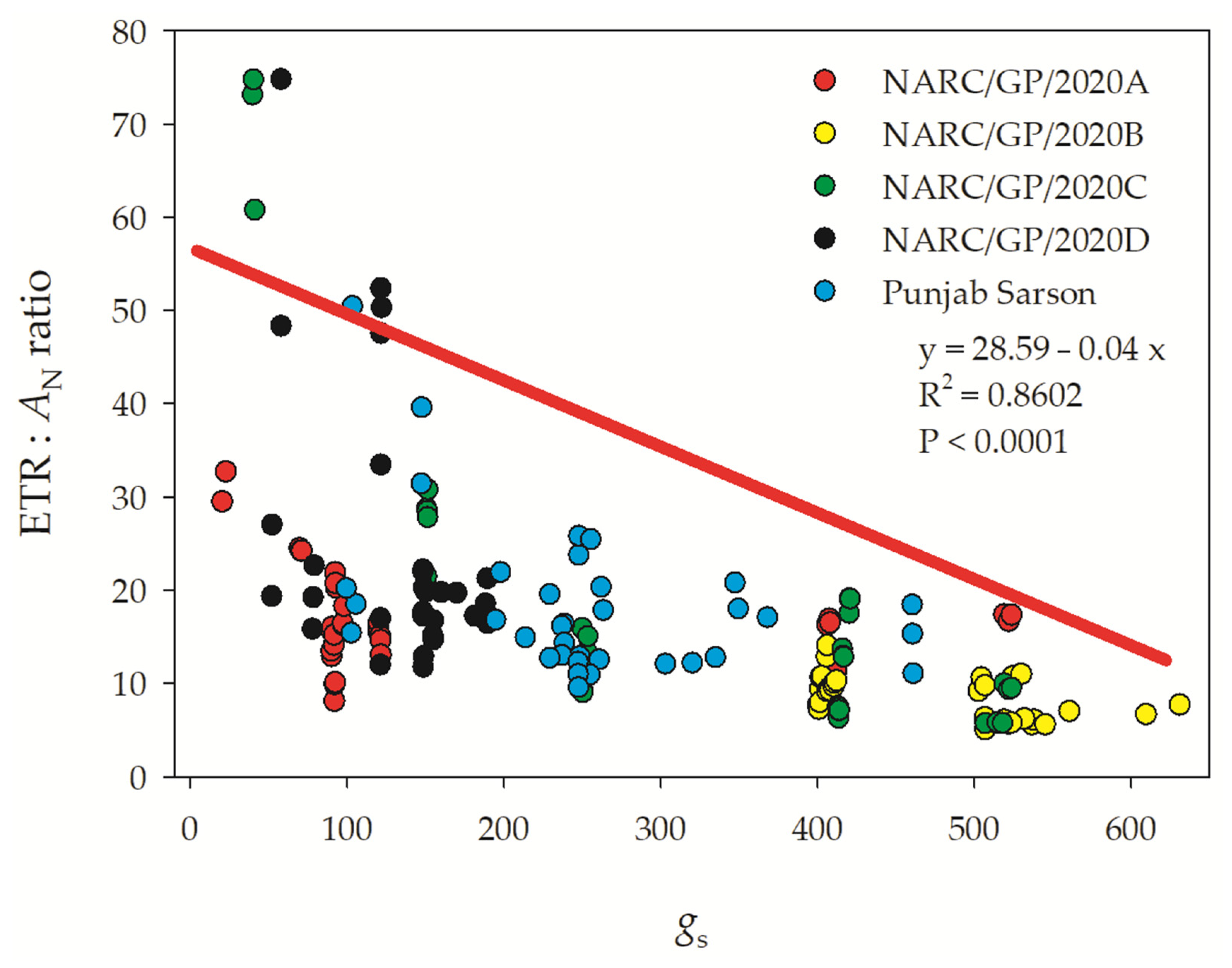
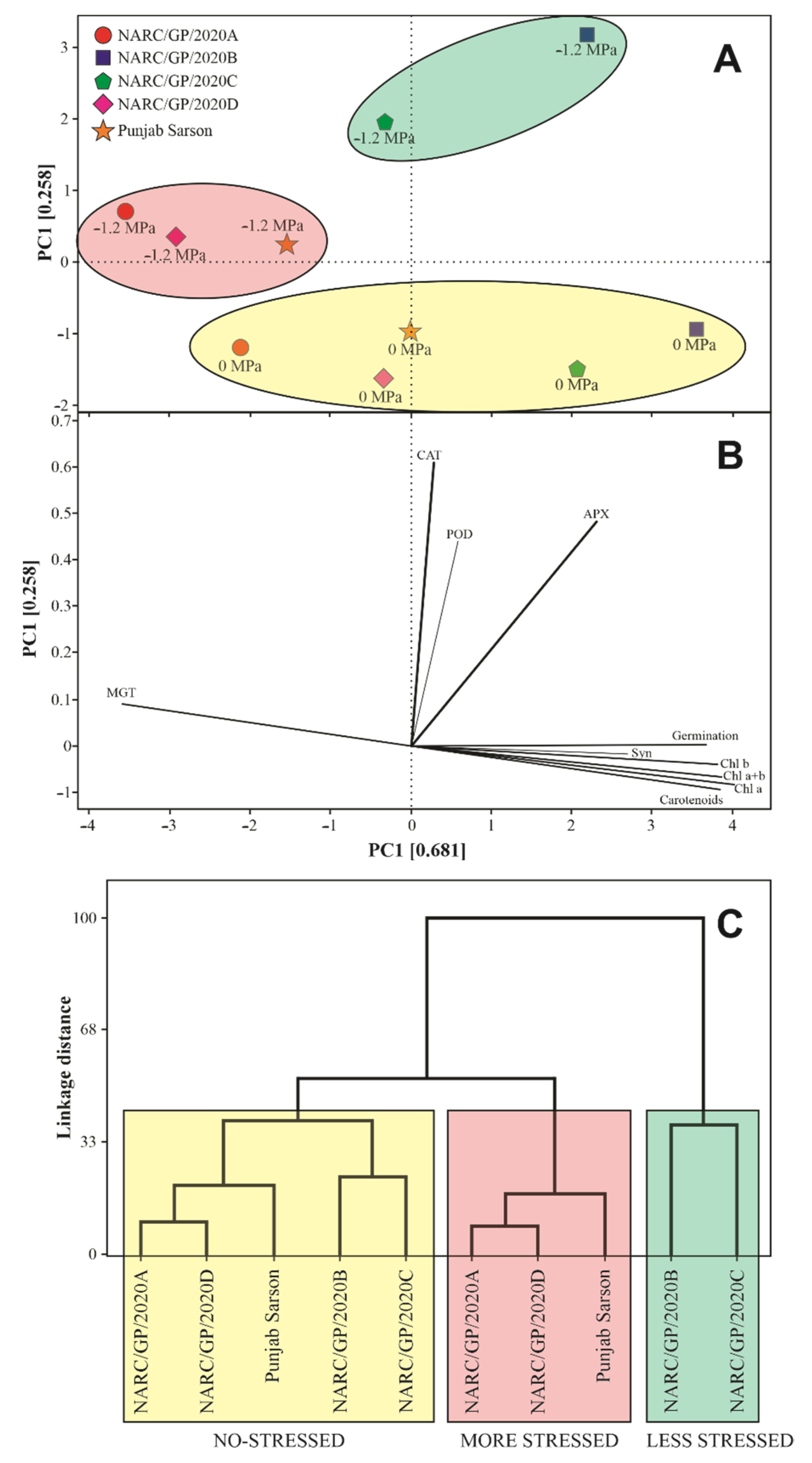
3.5. Salt Tolerance between Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.D. The ecological role of climate extremes: Current understanding and future prospects. J. Ecol. 2011, 99, 651–655. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Čanak, P.; Jeromela, A.M.; Vujošević, B.; Kiprovski, B.; Mitrović, B.; Alberghini, B.; Zanetti, F. Is drought stress tolerance affected by biotypes and seed size in the emerging oilseed crop Camelina? Agronomy 2020, 10, 1856. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.E.N.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Mattana, E.; Pritchard, H.W. Seeds of future past: Climate change and the thermal memory of plant reproductive traits. Biol. Rev. 2019, 94, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Tol, R.S.J. The economic impacts of climate change. Rev. Environ. Econ. Policy 2018, 12, 1. [Google Scholar] [CrossRef]
- Wheeler, T.; Braum, J.V. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Mcnutt, M. Climate change impacts. Science 2013, 341, 435. [Google Scholar] [CrossRef]
- Aymen, E.M.; Zhani, K.; Meriem, B.F.; Hannachi, C. Seed priming for better growth and yield of safflower (Carthamus tinctorius) under saline condition. J. Stress Physiol. Biochem. 2012, 8, 135–143. [Google Scholar]
- Hafeez, M.B.; Raza, A.; Zahra, N.; Shaukat, K.; Akram, M.Z.; Iqbal, S.; Basra, S.M.A. Gene regulation in halophytes in conferring salt tolerance. In Handbook of Bioremediation; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Elsevier: London, UK, 2021; pp. 341–370. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodrígues-Páez, L.A. Salinity in Jatropha curcas: A review of physiological, biochemical, and molecular factors involved. Agriculture 2022, 12, 594. [Google Scholar] [CrossRef]
- Raymer, P.L. Canola: An emerging oilseed crop. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; Volume 1, pp. 122–126. [Google Scholar]
- Tang, X.; Lee, J.; Chen, W.N. Engineering the fatty acid metabolic pathway in Saccharomyces cerevisiae for advanced biofuel production. Metab. Eng. Commun. 2015, 2, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Janßen, H.J.; Steinbüchel, A. Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnol. Biofuels. 2014, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Hanai, T.; Liao, J. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 July 2022).
- MINFAL. Agricultural Statistic of Pakistan. Available online: https://www.pbs.gov.pk/content/agriculture-statistics (accessed on 22 July 2022).
- Steppuhn, H.; Volkmar, K.; Miller, P. Comparing canola, field pea, dry bean, and durum wheat crops grown in saline media. Crop Sci. 2001, 41, 1827–1833. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Sen, A.; Puthur, J.T. Seed priming-induced physiochemical and molecular events in plants coupled to abiotic stress tolerance: An overview. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D., Fujita, M., Huang, B., Eds.; Elsevier: London, UK, 2020; pp. 303–316. [Google Scholar]
- Saddhe, A.A.; Saddhe, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, T.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Sarath, N.G.; Manzil, S.A.; Ali, S.; Alsahli, A.B.; Puthur, J.T. Physio-anatomical modifications and elemental allocation pattern in Acanthus ilicifolius L. subjected to zinc stress. PLoS ONE 2022, 17, e0263753. [Google Scholar] [CrossRef]
- Kinoshita, T.; Sekil, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Villagómez-Aranda, A.L.; Feregrino-Pérez, A.A.; García-Ortega, L.F.; González-Chavira, M.M.; Torres-Pacheco, I.; Guevara-González, R.G. Activating stress memory: Eustressors as potential tools for plant breeding. Plant Cell Rep. 2022, 41, 1481–1498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.V.; Somidi, A.K.R.; Dalai, A.K. Preparation and properties evaluation of biolubricants derived from canola oil and canola biodiesel. J. Agric. Food Chem. 2015, 63, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Isla, F.; Alfaro, O.B.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application ‘GerminaQuant for R’. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Corte-Real, N.; Miranda, P.V.V.C.; Endres, L.; Souza, E.R.; Pompelli, M.F. Tolerance to salinity in Jatropha curcas are genotype-dependent. Braz. J. Dev. 2019, 5, 22169–22199. [Google Scholar] [CrossRef][Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Pompelli, M.F.; Barata-Luís, R.M.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass. Bioenerg. 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Santo, A.; Mattana, E.; Hugot, L.; Spinosi, P.; Bacchetta, G. Seed germination and survival of the endangered psammophilous Rouya polygama (Apiaceae) in different light, temperature and NaCl conditions. Seed Sci. Res. 2014, 24, 331–339. [Google Scholar] [CrossRef]
- Prado, F.E.; Boero, C.; Gallardo, M.; Gonzalez, J.A. Effect of NaCl on germination, growth and soluble sugars content in Chenopodium quinoa Wild. seed. Bot. Bull. Acad. Sin. 2000, 41, 27–34. [Google Scholar]
- Khan, M.A.; Gul, B.; Weber, D.J. Germination responses of Salicornia rubra to temperature and salinity. J. Arid. Environ. 2000, 45, 207–214. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Effects of salinity, temperature and light on germination of invasive Prosopis juliflora (Sw.) D.C. J. Arid. Environ. 2005, 61, 555–565. [Google Scholar] [CrossRef]
- Johnston, S.K.; Crowley, R.H.; Murray, D.S. Separating seed by species with CaCl2 solutions. Weed Sci. 2017, 26, 213–215. [Google Scholar] [CrossRef]
- Darrudi, R.; Hassandokht, M.R.; Nazer, V. Effects of KNO3 and CaCl2 on seed germination of Rheum khorasanicum B. Baradaran & A. Jafari. J. Appl. Sci. Res. 2014, 10, 171–175. [Google Scholar]
- Islam, R.; Mukherjee, A.; Hossin, M. Effect of osmopriming on rice seed germination and seedling growth. J. Bangladesh Agril. Univ. 2012, 10, 15–20. [Google Scholar] [CrossRef]
- Jam, B.J.; Shekari, F.; Azimi, M.R.; Zangani, E. Effect of priming by salicylic acid on germination and seedling growth of safflower seeds under CaCl2 stress. Int. J. Agric. 2012, 2, 1097–1105. [Google Scholar]
- Harmeet, S.; Kaur, J.R.; Kaur, J.S.; Sandhu, S.S.; Harrajdeep, K.; Kamaljit, G. Seed priming techniques in field crops—A review. Agric. Rev. 2015, 36, 251–264. [Google Scholar]
- Afzal, I.; Butt, A.; Rehman, H.U.; Basra, A.; Afzal, A. Alleviation of salt stress in fine aromatic rice by seed priming. Aust. J. Crop. Sci. 2012, 6, 1401–1407. [Google Scholar]
- Reimer-Michalski, E.V.; Conrath, U. Innate immune memory in plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; Cerdá, A.; Martínez, V. Does calcium ameliorate the negative effect of NaCl on melon root water transport by regulating aquaporin activity? New Phytol. 2000, 145, 439–447. [Google Scholar] [CrossRef]
- Rasmann, S.; De Vos, M.; Casteel, C.L.; Tian, D.; Halitschke, R.; Sun, J.Y.; Agraval, A.A.; Felton, F.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, S.; Szechyńska-Hebda, M. Secret life of plants: From memory to intelligence. Plant Signal. Behav. 2010, 5, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.T.; Siddiqui, S.A.; Rathore, M.S. Seed germination, seedling growth and seedling development associated physiochemical changes in Salicornia brachiata (Roxb.) under salinity and osmotic stress. Aquat. Bot. 2020, 166, 103272. [Google Scholar] [CrossRef]
- Bhatt, A.; Phondani, P.C.; Pompelli, M.F. Seed maturation time influences the germination requirements of perennial grasses in desert climate of Arabian Gulf. Saudi J. Biol. Sci. 2018, 25, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Bhat, N.R.; Lozano-Isla, F.; Gallacher, D.; Santo, A.; Batista-Silva, W.; Fernandes, D.; Pompelli, M.F. Germination asynchrony is increased by dual seed bank presence in two desert perennial halophytes. Botany 2019, 97, 639–649. [Google Scholar] [CrossRef]
- Bhatt, A.; Gallacher, D.; Jarma-Orozco, A.; Pompelli, M.F. Seed mass, dormancy and germinability variation among maternal plants of four Arabian halophytes. Seed Sci. Res. 2022, 32, 53–61. [Google Scholar] [CrossRef]
- Bhatt, A.; Daibes, L.F.; Gallacher, D.; Jarma-Orozco, A.; Pompelli, M.F. Water stress inhibits germination while maintaining embryo viability of subtropical wetland seeds: A functional approach with phylogenetic contrasts. Front Plant Sci. 2022, 13, 906771. [Google Scholar] [CrossRef]
- Allen, E.; Alvarez, S. International Rules for Seed Testing 2020; The International Seed Testing Association: Bassersdorf, Switzerland, 2020. [Google Scholar]
- Futrell, M.C. New concepts in chemical seed treatment of agronomic crops. J. Environ. Qual. 1972, 1, 240–243. [Google Scholar] [CrossRef]
- Bybordi, A. The influence of salt stress on seed germination, growth and yield of canola cultivars. Not. Bot. Hort. Agrobot. Cluj 2010, 38, 128–133. [Google Scholar]
- Moosavi, S.A.; Gharineh, M.H.; Afshari, R.T.; Ebrahimi, A. Effects of some heavy metals on seed germination characteristics of canola (Brassica napus), wheat (Triticum aestivum) and safflower (Carthamus tinctorious) to evaluate phytoremediation potential of these crops. J. Agric. Sci. 2012, 4, 11–19. [Google Scholar]
- Zheng, G.H.; Gao, Y.P.; Willen, R.W.; Gusta, L.V. Canola seed germination and seedling emergence from pre-hydrated and re-dried seeds subjected to salt and water stresses at low temperatures. Ann. Appl. Biol. 1998, 132, 339–348. [Google Scholar] [CrossRef]
- Somagh, J.A.; Mousav, S.M.; Omidi, H.; Mohammadian, E.; Hemmati, M. Canola seed germination and seedling growth in response to saline condition and bio-priming. Iran J. Plant Physiol. 2017, 7, 2149–2156. [Google Scholar]
- Silva, J.V.; Lacerda, C.F.; Costa, P.H.A.; Enéas Filho, J.; Gomes Filho, E.; Prisco, J.T. Physiological responses of NaCl stressed cowpea plants grown in nutrient solution supplemented with CaCl2. Braz. J. Plant Physiol. 2003, 15, 99–105. [Google Scholar] [CrossRef]
- Nedjimi, B.; Daoud, Y. Ameliorative effect of CaCl2 on growth, membrane permeability and nutrient uptake in Atriplex halimus subsp. schweinfurthii grown at high (NaCl) salinity. Desalination 2009, 249, 163–666. [Google Scholar] [CrossRef]
- Latef, A.A.H.A. Ameliorative effect of calcium chloride on growth, antioxidant enzymes, protein patterns and some metabolic activities of canola (Brassica napus L.) under seawater stress. J. Plant Nutr. 2011, 34, 1303–1320. [Google Scholar] [CrossRef]
- Bybordi, A. Effect of ascorbic acid and silicium on photosynthesis, antioxidant enzyme activity, and fatty acid contents in canola exposure to salt stress. J. Integr. Agric. 2012, 11, 1610–1620. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Zafar, Z.U.; Ashraf, M. Glycinebetaine improved photosynthesis in canola under salt stress: Evaluation of chlorophyll fluorescence parameters as potential indicators. J. Agron. Crop. Sci. 2015, 201, 428–442. [Google Scholar] [CrossRef]
- Nazarbeygi, E.; Yazdi, H.L.; Naseri, R.; Soleimani, R. The effects of different levels of salinity on proline and A-, B- Chlorophylls in canola. Am. Eurasian J. Agric. Environ. Sci. 2011, 10, 70–74. [Google Scholar]
- Mukhtar, E.; Siddiqi, E.H.; Bhatti, K.H. Gas exchange attributes can be valuable selection criteria for salinity tolerance in canola cultivars (Brassica napus L.). Pak. J. Bot. 2013, 45, 35–40. [Google Scholar]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. What determines rates of photosynthesis per unit nitrogen in Eucalyptus seedlings? Funct. Plant Biol. 2004, 31, 1169–1178. [Google Scholar] [CrossRef]
- Tuba, Z.; Lichtenthaler, H.; Csintalan, Z.; Nagy, Z.; Szente, K. Loss of chlorophylls, cessation of photosynthesis CO2 assimilation and respiration in the poikilochlorophyllous plant Xerophyta scabrida during desiccation. Physiol. Plant 1996, 96, 383–388. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of qhy leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J. Agron. Crop. Sci. 2021, 208, 314–334. [Google Scholar] [CrossRef]
- Alam, M.; Juraimi, A.S.; Rafii, M.; Abdul Hamid, A. Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane (Portulaca oleracea L.) accessions. Biomed. Res. Int. 2015, 2015, 105695. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, O.O.; Mendes, K.R.; Martins, S.V.C.; Batista-Silva, W.; dos Santos, M.A.; Figueirôa, J.M.; Souza, E.R.; Fernandes, D.; Araújo, W.L.; Pompelli, M.F. Physiological parameters and plasticity as key factors to understand pioneer and late successional species in the Atlantic Rainforest. Acta Physiol. Plant 2019, 41, 145. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, C. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak. J. Bot. 2009, 41, 87–98. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, H.; Chu, Y.; Sun, C.; Wei, N.; Yang, M.; Zheng, C. Effects of salt stress on chlorophyll fluorescence and the antioxidant system in Ginkgo biloba L. seedlings. Hort. Sci. 2019, 54, 2125–2133. [Google Scholar] [CrossRef]
- Sali, A.; Rusinovci, I.; Fetahu, S.; Gashi, B.; Simeonovska, E.; Rozman, L. The effect of salt stress on the germination of maize (Zea mays L.) seeds and photosynthetic pigments. Acta Agric. Slov. 2015, 105, 85–94. [Google Scholar]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Fonseca-Pereira, P.; Daloso, D.M.; Gago, J.; Nunes-Nesi, A.; Araújo, W.L. On the role of the plant mitochondrial thioredoxin system during abiotic stress. Plant Signal. Behav. 2019, 14, 1592536. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, R.; Hussain, M.; Dong-Jin, L. Modulation of enzymatic antioxidants improves the salinity resistance in canola (Brassica napus). Int. J. Agric. Biol. 2012, 14, 465–468. [Google Scholar]
- Xu, D.; Wang, W.; Gao, T.; Fang, X.; Gao, X.; Li, J.; Bu, H.; Mu, J. Calcium alleviates decreases in photosynthesis under salt stress by enhancing antioxidant metabolism and adjusting solute accumulation in Calligonum mongolicum. Conserv. Physiol. 2017, 5, cox060. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. C. R. Biol. 2007, 330, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Ahmad, S.D.; Sabir, S.M.; Awan, S.I.; Shah, A.H.; Abbas, S.R.; Shafique, S.; Khan, F.; Chaudhary, A. Potential antioxidant activities improve salt tolerance in ten varieties of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2013, 4, 69–76. [Google Scholar] [CrossRef]
- Sairam, R.; Srivastava, G.; Agarwal, S.; Meena, R. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Campos, M.L.O.; Hsie, B.S.; Granja, J.A.A.; Correia, R.M.; Silva, S.R.S.; Almeida-Cortez, J.S.; Pompelli, M.F. Photosynthesis and antioxidant activity mechanisms in Jatropha curcas L. under salt stress. Braz. J. Plant Physiol. 2012, 24, 55–67. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.G.; Ribeiro, R.V.; Vieira, S.A. Photoprotective function of energy dissipation by thermal processes and photorespiratory mechanisms in Jatropha curcas plants during different intensities of drought and after recovery. Environ. Exp. Bot. 2015, 110, 36–45. [Google Scholar] [CrossRef]
- Corte-Real, N.; Oliveira, M.S.; Jarma-Orozco, A.; Fernandes, D.; dos Santos, M.A.; Endres, L.; Calsa, T., Jr.; Pompelli, M.F. Comparative analysis of salt-induced changes in the leaves proteome of two contrasting Jatropha curcas genotypes. Braz. J. Dev. 2020, 6, 39845–39872. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Ulfat, M.; Athar, H.U.R.; Ashraf, M.; Akram, N.A.; Jamil, A. Appraisal of physiological and biochemical selection criteria for evaluation of salt tolerance in canola (Brassica napus L.). Pak. J. Bot. 2007, 39, 1593–1608. [Google Scholar]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful ‘‘memories’’ of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Baroi, C.; Mahto, S.; Niu, C.; Dalay, A.K. Biofuel production from green seed canola oil using zeolites. Appl. Catal. A Gen. 2014, 469, 18–32. [Google Scholar] [CrossRef]
- Silva, L.F.L.; Gonçalves, W.M.; Maluf, W.R.; Resende, L.V.; Sarmiento, C.M.; Licursi, V.; Moretto, P. Energy balance of biodiesel production from canola. Cienc. Rural 2017, 47, e20151084. [Google Scholar] [CrossRef][Green Version]
- Smyth, S.J.; Phillips, P.W.B.; Keer, W.A. Food security and the evaluation of risk. Glob. Food Sec. 2015, 4, 16–23. [Google Scholar] [CrossRef]
- Jayas, D.S. Storing grains for food security and sustainability. Agric. Res. 2002, 1, 21–24. [Google Scholar] [CrossRef]
- Saeed, M.F.; Jamal, A.; Ahmad, I.; Ali, S.; Shah, G.M.; Husnain, S.K.; Farooq, A.; Wang, J. Storage cnditions deteriorate cotton and wheat seeds quality: An assessment of farmers’ awareness in Pakistan. Agronomy 2020, 10, 1246. [Google Scholar] [CrossRef]

| Symbol | Genotype’s Name |
|---|---|
| #1 | NARC/GP/2020A |
| #2 | NARC/GP/2020B |
| #3 | NARC/GP/2020C |
| #4 | NARC/GP/2020D |
| #5 | Punjab Sarson |
| Genotype | Osmotic Potential (MPa) | GRP (%) | MGT (Days) | SYN |
|---|---|---|---|---|
| NARC/GP/2020A | 0 | 40.90 ± 2.40 Ca | 9.27 ± 0.10 Aa | 0.12 ± 0.01 Aa |
| −1.2 | 18.10 ± 2.69 Cb | 9.42 ± 0.51 Aa | 0.06 ± 0.01 Db | |
| NARC/GP/2020B | 0 | 66.20 ± 0.28 Aa | 8.13 ± 0.03 Bb | 0.12 ± 0.01 Aa |
| −1.2 | 59.10 ± 0.14 Ab | 8.57 ± 0.08 Ba | 0.12 ± 0.01 Aa | |
| NARC/GP/2020C | 0 | 51.30 ± 4.95 Ba | 8.52 ± 0.12 Bb | 0.11 ± 0.01 Aa |
| −1.2 | 35.50 ± 4.95 Bb | 9.42 ± 0.13 Aa | 0.12 ± 0.01 Aa | |
| NARC/GP/2020D | 0 | 36.00 ± 3.39 Ca | 8.92 ± 0.17 ABb | 0.10 ± 0.01 Aa |
| −1.2 | 21.00 ± 2.26 Cb | 9.44 ± 0.12 Aa | 0.08 ± 0.01 Cb | |
| Punjab Sarson | 0 | 32.40 ± 4.24 Ca | 9.15 ± 0.04 Aa | 0.10 ± 0.01 Aa |
| −1.2 | 29.00 ± 1.98 Ca | 9.02 ± 0.14 Aa | 0.10 ± 0.01 Ba |
| Chlorophyll “a” | |||||
| SV | DF | SS | MS | Fcal | p |
| Genotype | 4 | 157.706 | 39.426 | 2513.142 | ≤0.001 |
| Osmotic potential (ψs) | 3 | 24.343 | 8.114 | 517.223 | ≤0.001 |
| Genotype × ψs | 12 | 31.775 | 2.648 | 168.787 | ≤0.001 |
| Residual | 40 | 0.628 | 0.016 | ||
| Total | 59 | 214.452 | 3.635 | ||
| Chlorophyll “b” | |||||
| SV | DF | SS | MS | Fcal | p |
| Genotype | 4 | 606.833 | 151.708 | 2984.923 | ≤0.001 |
| Osmotic potential (ψs) | 3 | 110.144 | 36.715 | 722.378 | ≤0.001 |
| Genotype × ψs | 12 | 298.910 | 24.909 | 490.099 | ≤0.001 |
| Residual | 40 | 2.033 | 0.051 | ||
| Total | 59 | 1017.920 | 17.253 | ||
| Chlorophyll “a + b” | |||||
| SV | DF | SS | MS | Fcal | p |
| Genotype | 4 | 1047.547 | 261.887 | 3966.766 | ≤0.001 |
| Osmotic potential (ψs) | 3 | 337.793 | 112.598 | 1705.503 | ≤0.001 |
| Genotype × ψs | 12 | 378.505 | 31.542 | 477.764 | ≤0.001 |
| Residual | 40 | 2.641 | 0.066 | ||
| Total | 59 | 1766.486 | 29.940 | ||
| Carotenoids | |||||
| SV | DF | SS | MS | Fcal | p |
| Genotype | 4 | 710.744 | 177.686 | 3899.852 | ≤0.001 |
| Osmotic potential (ψs) | 3 | 228.031 | 76.010 | 1668.271 | ≤0.001 |
| Genotype × ψs | 12 | 147.788 | 12.316 | 270.304 | ≤0.001 |
| Residual | 40 | 1.822 | 0.046 | ||
| Total | 59 | 1088.386 | 18.447 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, W.; Afridi, M.Z.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Székely, Á.; Zia, A.; Khan, M.A.; Jarma-Orozco, A.; Pompelli, M.F. Canola Seed Priming and Its Effect on Gas Exchange, Chlorophyll Photobleaching, and Enzymatic Activities in Response to Salt Stress. Sustainability 2022, 14, 9377. https://doi.org/10.3390/su14159377
Iqbal W, Afridi MZ, Jamal A, Mihoub A, Saeed MF, Székely Á, Zia A, Khan MA, Jarma-Orozco A, Pompelli MF. Canola Seed Priming and Its Effect on Gas Exchange, Chlorophyll Photobleaching, and Enzymatic Activities in Response to Salt Stress. Sustainability. 2022; 14(15):9377. https://doi.org/10.3390/su14159377
Chicago/Turabian StyleIqbal, Waleed, Muhammad Zahir Afridi, Aftab Jamal, Adil Mihoub, Muhammad Farhan Saeed, Árpád Székely, Adil Zia, Muhammad Awais Khan, Alfredo Jarma-Orozco, and Marcelo F. Pompelli. 2022. "Canola Seed Priming and Its Effect on Gas Exchange, Chlorophyll Photobleaching, and Enzymatic Activities in Response to Salt Stress" Sustainability 14, no. 15: 9377. https://doi.org/10.3390/su14159377
APA StyleIqbal, W., Afridi, M. Z., Jamal, A., Mihoub, A., Saeed, M. F., Székely, Á., Zia, A., Khan, M. A., Jarma-Orozco, A., & Pompelli, M. F. (2022). Canola Seed Priming and Its Effect on Gas Exchange, Chlorophyll Photobleaching, and Enzymatic Activities in Response to Salt Stress. Sustainability, 14(15), 9377. https://doi.org/10.3390/su14159377










