Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest
Abstract
1. Introduction
2. Materials and Methods
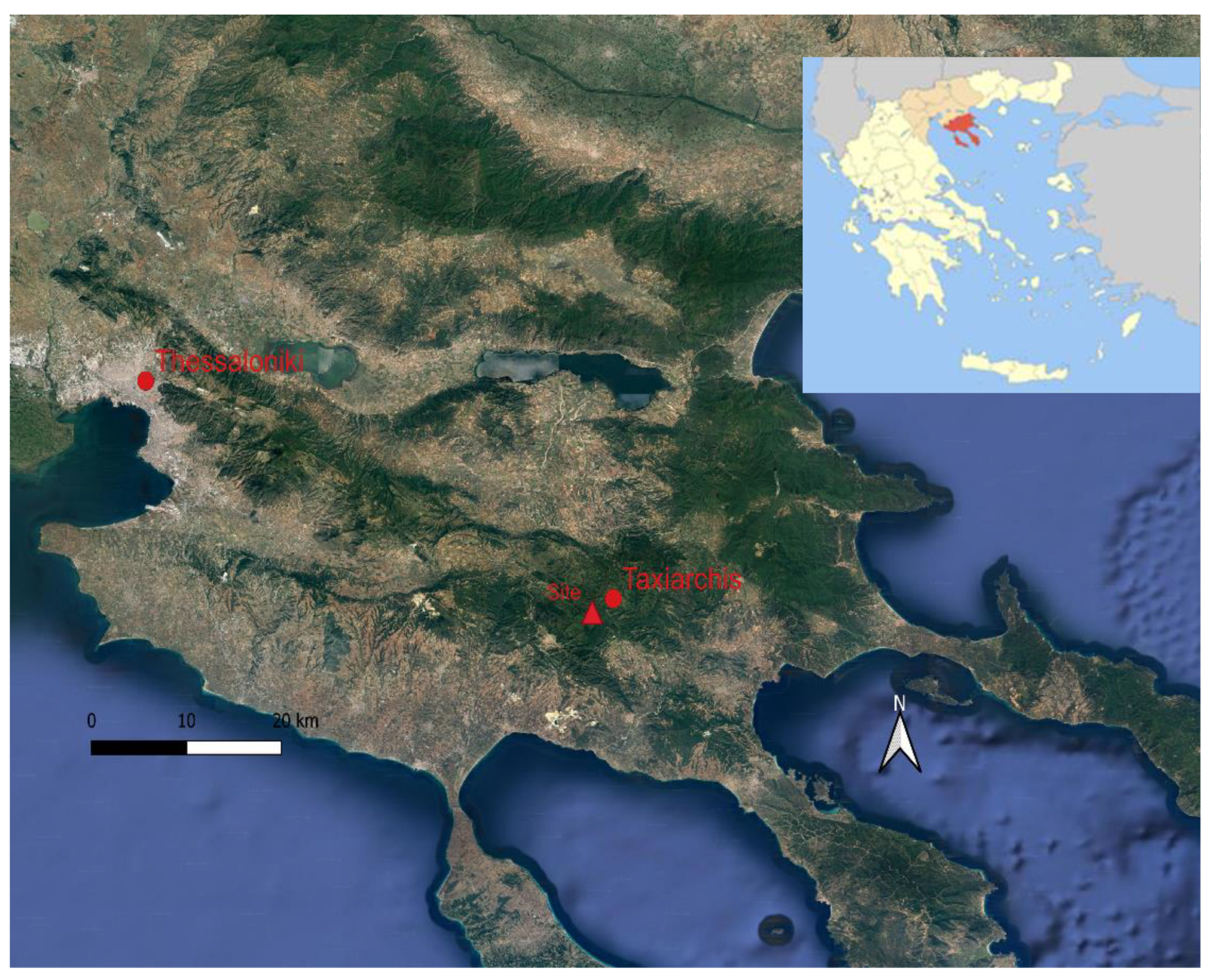
2.1. Study Area
2.2. Sampling Design and Experimental Section
2.2.1. Field Data Collection for the Estimation of Aboveground Live Tree Biomass
2.2.2. Dead Wood Sampling
2.2.3. Litter and Soil Sampling
2.3. Laboratory Analyses
2.4. Statistical Analysis
3. Results
3.1. Stand Structural Characteristics of the Studied Forest
3.2. Biomass and Carbon Pools in the Aboveground Living Part of the Forest Ecosystem
3.2.1. Biomass Prediction Allometric Equations
3.2.2. Estimation of Aboveground Living Biomass at Forest Stand Level per Hectare
3.3. Biomass and Carbon Accumulation in Standing and Fallen Dead Wood per Hectare
3.4. Biomass and Carbon Accumulation in Forest Litter and Soil Per Hectare
3.5. Estimation of Total Stand Biomass and Ecosystem Carbon Pools per Hectare
4. Discussion
5. Conclusions—Application in Forest Management
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Makineci, E.; Tolunay, D.; Son, Y. Estimating the effect of abandoning coppice management on carbon sequestration by oak forests in Turkey with a modeling approach. Sci. Total Environ. 2018, 640, 400–405. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, C.; Zhao, X.; Fousseni, F.; Wang, J.; Dai, H.; Yang, S.; Zuo, Q. Allometric biomass equations for 12 tree species in coniferous and broadleaved mixed forests, Northeastern China. PLoS ONE 2018, 13, e0186226. [Google Scholar] [CrossRef]
- Fazan, L.; Song, Y.G.; Kozlowski, G. The Woody Planet: From Past Triumph to Manmade Decline. Plants 2020, 9, 1593. [Google Scholar] [CrossRef]
- Węgiel, A.; Polowy, K. Aboveground Carbon Content and Storage in Mature Scots Pine Stands of Different Densities. Forests 2020, 11, 240. [Google Scholar] [CrossRef]
- Köhl, M.; Lasco, R.; Cifuentes, M.; Jonsson, Ö.; Korhonen, K.T.; Mundhenk, P.; de Jesus Navar, J.; Stinson, G. Changes in forest production, biomass and carbon: Results from the 2015 UN FAO Global Forest Resource Assessment. For. Ecol. Manag. 2015, 352, 21–34. [Google Scholar] [CrossRef]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest Carbon Management: A Review of Silvicultural Practices and Management Strategies Across Boreal, Temperate and Tropical Forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Sedjo, R.A. The carbon cycle and global forest ecosystem. Water Air Soil Pollut. 1993, 70, 295–307. [Google Scholar] [CrossRef]
- Sombroek, W.G.; Nachtergaele, F.O.; Hebel, A. Amounts, dynamics and sequestering of carbon in tropical and subtropical soils. Ambio 1993, 22, 417–426. [Google Scholar]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change, 2nd ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Whittaker, R.H.; Likens, G.E. Primary production: The biosphere and man. Hum. Ecol. 1973, 1, 357–369. [Google Scholar] [CrossRef]
- Song, B.L.; Yan, M.J.; Hou, H.; Guan, J.H.; Shi, W.Y.; Li, G.Q.; Du, S. Distribution of soil carbon and nitrogen in two typical forests in the semiarid region of the Loess Plateau, China. Catena 2016, 143, 159–166. [Google Scholar] [CrossRef]
- Ahmed, I.U. Forest Soil C: Stock and Stability under Global Change. New Perspect. For. Sci. 2018, 37, 37–67. [Google Scholar]
- Papaioannou, E.A.; Stefanou, S.; Gakis, S.F.; Netsikas, E.N.; Mavridis, A. Growth, forest floor, and soil chemical analysis comparison between two management practices in chestnut forests of Greece. J. Sustain. For. 2019, 38, 526–541. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, S.Q.; Liu, S.; Oeding, J. A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 2013, 10, 3691. [Google Scholar] [CrossRef]
- Dixon, R. Silvicultural options to conserve and sequester carbon in forest systems: Preliminary economic assessment. Crit. Rev. Environ. Sci. Technol. 2009, 27 (Suppl. 1), 139–149. [Google Scholar] [CrossRef]
- Manolis, E.N.; Zagas, T.D.; Poravou, C.A.; Zagas, D.T. Biomass assessment for sustainable bioenergy utilization in a Mediterranean forest ecosystem in northwest Greece. Ecol. Eng. 2016, 91, 537–544. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Hatakeyama, K.; Ohkubo, T.; Takeuchi, D. Dynamics of secondary forest and its perspective under the Satoyama system in Saitama prefecture, Japan. J. For. Res. 2020, 25, 51–57. [Google Scholar] [CrossRef]
- Adame, P.; Hynynen, J.; Cañellas, I.; Del Río, M. Individual-tree diameter growth model for rebollo oak (Quercus pyrenaica Willd.) coppices. For. Ecol. Manag. 2008, 255, 1011–1022. [Google Scholar] [CrossRef]
- Bruckman, V.J.; Yan, S.; Hochbichler, E.; Glatzel, G. Carbon pools and temporal dynamics along a rotation period in Quercus dominated high forest and coppice with standards stands. For. Ecol. Manag. 2011, 262, 1853–1862. [Google Scholar] [CrossRef]
- Salomón, R.; Rodriguez-Calcerrada, J.; Zafra, E.; Morales-Molino, C.; Rodriguez-Carcía, A.; González-Doncel, I.; Oleksyn, J.; Zytkowiak, R.; López, R.; Miranda, J.C.; et al. Unearthing the roots of degradation of Quercus pyrenaica coppices: A root-to-shoot imbalance caused by historical management? For. Ecol. Manag. 2016, 363, 200–211. [Google Scholar] [CrossRef]
- Noormets, A.; Epron, D.; Domec, J.C.; McNulty, S.G.; Fox, T.; Sun, G.; King, J.S. Effects of forest management on productivity and carbon sequestration: A review and hypothesis. For. Ecol. Manag. 2015, 355, 124–140. [Google Scholar] [CrossRef]
- Vacca, A.; Aru, F.; Ollesch, G. Short-term impact of coppice management on soil in a Quercus ilex L. stand of Sardinia. Land Degrad. Dev. 2017, 28, 553–565. [Google Scholar] [CrossRef]
- Nabuurs, G.-J.; Delacote, P.; Ellison, D.; Hanewinkel, M.; Hetemäki, L.; Lindner, M. By 2050 the Mitigation Effects of EU Forests Could Nearly Double through Climate Smart Forestry. Forests 2017, 8, 484. [Google Scholar] [CrossRef]
- Applied Silviculture; Giahoudi-Giapouli: Thessaloniki, Greece, 1989.
- Ciancio, O.; Corona, P.; Lamonaca, A.; Portoghesi, L.; Travaglini, D. Conversion of clearcut beech coppices into high forests with continuous cover: A case of study in central Italy. For. Ecol. Manag. 2006, 224, 235–240. [Google Scholar] [CrossRef]
- Mattioli, W.; Ferrari, B.; Giuliarelli, D.; Mancini, L.D.; Portoghesi, L.; Corona, P. Conversion of mountain beech coppices into high forest: An example for ecological intensification. Environ. Manag. 2015, 56, 1159–1169. [Google Scholar] [CrossRef]
- Gonçalves, A.C. Stand Structure Alterations in Forest Stands. JOJ Hortic. Arboric. 2018, 1, 555564. [Google Scholar] [CrossRef]
- O’Hara, K.L. Multiaged Silviculture: Managing for Complex Forest Stand Structures; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Hoff, C.; Rambal, S.; Joffre, R. Simulating carbon and water flows and growth in a Mediterranean evergreen Quercus ilex coppice using the FOREST-BGC model. For. Ecol. Manag. 2002, 164, 121–136. [Google Scholar] [CrossRef]
- Kneifl, M.; Kadavý, J.; Knott, R.; Adamec, Z.; Drápela, K. An inventory of tree and stand growth empirical modelling approaches with potential application in coppice forestry (a review). Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 53, 195. [Google Scholar] [CrossRef]
- Prada, M.; Bravo, F.; Berdasco, L.; Canga, E.; Martínez-Alonso, C. Carbon sequestration for different management alternatives in sweet chestnut coppice in northern Spain. J. Clean. Prod. 2016, 135, 1161–1169. [Google Scholar] [CrossRef]
- IPCC. 2019. Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland.
- Tzamtzis, I.; Ganatsas, P. Land use, land-use change and their effect on greenhouse gas emissions and removals from Greek forests. Int. J. Glob. Warm. 2020, 22, 111–131. [Google Scholar] [CrossRef]
- Coomes, D.A.; Allen, R.B.; Sčoty, N.A.; Goulding, C.; Beets, P. Designing systems to monitor carbon stocks in forests and shrublands. For. Ecol. Manag. 2002, 164, 89–108. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, F.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Cienciala, E.; Apltauer, J.; Exnerová, Z.; Tatarinov, F. Biomass functions applicable to oak trees grown in Central-European forestry. J. For. Sci. 2008, 3, 109–120. [Google Scholar] [CrossRef]
- Suchomel, C.; Pyttel, P.; Becker, G.; Bauhus, J. Biomass equations for sessile oak (Quercus petraea (Matt.) Liebl.) and hornbeam (Carpinus betulus L.) in aged coppiced forests in southwest Germany. Biomass Bioenergy 2012, 46, 722–730. [Google Scholar] [CrossRef]
- Aguilar, R.; Ghilardi, A.; Vega, E.; Skutsch, M.; Oyama, K. Sprouting productivity and allometric relationships of two oak species managed for traditional charcoal making in central Mexico. Biomass Bioenergy 2012, 36, 192–207. [Google Scholar] [CrossRef]
- Theodoropoulos, K. Definition and Classification of Plant sociological Units in University Forest Taxiarhis Chalkidiki; AUTH: Thessaloniki, Greek, 1991. [Google Scholar]
- Cai, S.; Kang, X.; Zhang, L. Allometric models for aboveground biomass of ten tree species in northeast China. Ann. For. Res. 2013, 56, 105–122. [Google Scholar]
- Litton, C.M.; Ryan, M.G.; Tinker, D.B.; Knight, D.H. Belowground and aboveground biomass in young postfire lodgepole pine forests of contrasting tree density. Can. J. For. Res. 2003, 33, 351–363. [Google Scholar] [CrossRef]
- Xiao, C.W.; Yuste, J.C.; Janssens, I.A.; Roskams, P.; Nachtergale, L.; Carrara, A.; Sanchez, B.Y.; Ceulemans, R. Above- and belowground biomass and net primary production in a 73-year-old Scots pine forest. Tree Physiol. 2003, 23, 505–516. [Google Scholar] [CrossRef]
- Wang, C. Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For. Ecol. Manag. 2006, 222, 9–16. [Google Scholar] [CrossRef]
- Kralicek, K.; Huy, B.; Poudel, K.P.; Temesgen, H.; Salas, C. Simultaneous estimation of above- and below-ground biomass in tropical forests of Viet Nam. For. Ecol. Manag. 2017, 390, 147–156. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ji, C.J.; Han, W.X. Above- and belowground biomass allocation in Tibetan grasslands. J. Veg. Sci. 2009, 20, 177–184. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Chen, X.; Tian, X.; Wang, X.; Luo, G. Biomass allocation patterns across China’s terrestrial biomes. PloS ONE 2014, 9, e93566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, J.; Zhu, B. Forest biomass and root–shoot allocation in northeast China. For. Ecol. Manag. 2008, 255, 4007–4020. [Google Scholar] [CrossRef]
- Makineci, E.; Ozdemir, E.; Caliskan, S.; Yilmaz, E.; Kumbasli, M.; Keten, A.; Beskardes, V.; Zengin, H.; Yilmaz, H. Ecosystem carbon pools of coppice-originated oak forests at different development stages. Eur. J. For. Res. 2015, 134, 319–333. [Google Scholar] [CrossRef]
- Tsitsoni, T.; Ganatsas, P.; Zagas, T.; Tsakaldimi, M. Dynamics of postfire regeneration of Pinus brutia Ten. In an artificial forest ecosystem of northern Greece. Plant Ecol. 2004, 171, 165–174. [Google Scholar] [CrossRef]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Balboa-Murias, M.A.; Rojo, A.; Álvarez, J.G.; Merino, A. Carbon and nutrient stocks in mature Quercus robur L. stands in NW Spain. Ann. For. Sci. 2006, 63, 557–565. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root:shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Easdale, T.A.; Richardson, S.J.; Marden, M.; England, J.G.; Gayoso-Aguilar, J.; Guerra-Cárcamo, J.E.; McCarthy, J.K.; Paul, K.I.; Schwendenmann, L.; Brandon, A.M. Root biomass allocation in southern temperate forests. For. Ecol. Manag. 2019, 453, 117542. [Google Scholar] [CrossRef]
- Paletto, A.; De Meo, I.; Cantiani, P.; Ferretti, F. Effects of forest management on the amount of deadwood in Mediterranean oak ecosystems. Ann. For. Sci. 2014, 71, 791–800. [Google Scholar] [CrossRef]
- Mladenova, D.; Tsavkov, E.; Bardarov, N. Study on the density of oak wood. Innov. Wood Work. Ind. Engeenering Des. 2017, 1, 65–68. [Google Scholar]
- Renninger, H.J.; Carlo, N.; Clark, K.L.; Schäfer, K.V.R. Modeling respiration from snags and coarse woody debris before and after an invasive gypsy moth disturbance. J. Geophys. Res. Biogeosci. 2014, 119, 630–644. [Google Scholar] [CrossRef]
- Di Cosmo, L.; Gasparini, P.; Paletto, A.; Nocetti, M. Deadwood basic density values for national-level carbon stock estimates in Italy. For. Ecol. Manag. 2013, 295, 51–58. [Google Scholar] [CrossRef]
- Meincken, M.; Tyhoda, L. Biomass Quality. In Bioenergy from Wood; Springer: Dordrecht, The Netherlands, 2014; Volume 26, pp. 169–187. [Google Scholar]
- Schelhaas, M.J.; Van Esch, P.W.; Groen, T.A.; De Jong, B.H.J.; Kanninen, M.; Liski, J.; Masera, O.; Mohren, G.M.J.; Nabuurs, G.J.; Palosuo, T.; et al. CO2FIX V 3.1. In A Modelling Framework for Quantifying Carbon Sequestration in Forest Ecosystems; Alterra-Centrum Ecosystemen: Wageningen, The Netherlands, 2004; Alterra-rapport No 1068. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical Analysis. In Methods of Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell: Oxford, UK, 1986; pp. 285–344. [Google Scholar]
- Somogyi, Z.; Cienciala, E.; Mäkipää, R.; Muukkonen, P.; Lehtonen, A.; Weiss, P. Indirect methods of large-scale forest biomass estimation. Eur. J. For. Res. 2007, 126, 197–207. [Google Scholar] [CrossRef]
- Cotillas, M.; Espelta, J.M.; Sánchez-Costa, E.; Sabaté, S. Aboveground and belowground biomass allocation patterns in two Mediterranean oaks with contrasting leaf habit: An insight into carbon stock in young oak coppices. Eur. J. For. Res. 2016, 135, 243–252. [Google Scholar] [CrossRef]
- Zianis, D.; Muukkonen, P.; Mäkipää, R.; Mencuccini, M. Biomass and stem volume equations for tree species in Europe. In Silva Fennica Monographs; Finnish Society of Forest Science: Helsinki, Finland, 2005; Volume 4, 63p. [Google Scholar]
- Norušis, M. IBM SPSS Statistics 19 Guide to Data Analysis; Addison Wesley: Hoboken, NJ, USA, 2011. [Google Scholar]
- The European Green Deal, Brussels 11.12.2019 COM (2019) 640 final. Available online: https://ec.europa.eu/info/sites/default/files/european-green-deal-communication_en.pdf (accessed on 2 October 2022).
- Ohtsuka, T.; Shizu, Y.; Nishiwaki, A.; Yashiro, Y.; Koizumi, H. Carbon cycling and net ecosystem production at an early stage of secondary succession in an abandoned coppice forest. J. Plant Res. 2010, 123, 393–401. [Google Scholar] [CrossRef]
- Terzaghi, M.; Montagnoli, A.; DiIorio, A.; Scippa, G.S.; Chiatante, D. Fine-root carbon and nitrogen concentration of European beech (Fagus sylvatica L.) in Italy Prealps: Possible implications of coppice conversion to high forest. Front. Plant Sci. 2013, 4, 192. [Google Scholar] [CrossRef]
- Lee, J.; Tolunay, D.; Makineci, E.; Çömez, A.; Son, Y.M.; Son, Y. 2016. Estimating the age dependent changes in carbon stocks of Scots pine (Pinus sylvestris L.) stands in Turkey. Ann. For. Sci. 2016, 73, 523–531. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Han, S.H.; Kim, S.; Roh, Y.; Salim, K.A.; Pietsch, S.A.; Son, Y. Estimating carbon dynamics in an intact lowland mixed dipterocarp forest using a forest carbon model. Forests 2017, 8, 114. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Z.; Piao, S.; Chen, A. Terrestrial vegetation carbon sinks in China, 1981–2000. Sci. China Ser. D Earth Sci. 2007, 50, 1341–1350. [Google Scholar] [CrossRef]
- Hui, D.; Deng, Q.; Tian, H.; Luo, Y. Climate change and carbon sequestration in forest ecosystems. In Handbook of Climate Change Mitigation and Adaptation, 2nd ed.; Chen, W.Y., Suzuki, T., Lackner, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 555–594. [Google Scholar]
- Wang, W.; Lei, X.; Ma, Z.; Kneeshaw, D.D.; Peng, C. Positive relationship between aboveground carbon stocks and structural diversity in spruce-dominated forest stands in New Brunswick, Canada. For. Sci. 2011, 57, 506–515. [Google Scholar]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Konopka, B.; Pajtik, J.; Moravcik, M.; Lukac, M. Biomass partitioning and growth efficiency in four naturally regenerated forest tree species. Basic Appl. Ecol. 2010, 11, 234–243. [Google Scholar] [CrossRef]
- Green, P.; Peterken, G.F. Variation in the amount of dead wood in the woodlands of the Lower Wye Valley, UK in relation to the intensity of management. For. Ecol. Manage. 1997, 98, 229–238. [Google Scholar] [CrossRef]
- Verkerk, P.J.; Lindner, M.; Zanchi, G.; Zudin, S. Assessing impacts of intensified biomass removal on deadwood in European forests. Ecol. Indic. 2011, 11, 27–35. [Google Scholar] [CrossRef]
- Kirby, K.J.; Reid, C.M.; Thomas, R.C.; Goldsmith, F.B. Preliminary estimates of fallen dead wood and standing dead trees in managed and unmanaged forests in Britain. J. Appl. Ecol. 1998, 35, 148–155. [Google Scholar] [CrossRef]


| Tree Level | Forest Stand Level | ||||||
|---|---|---|---|---|---|---|---|
| DBH | H | CBH | CL | Density | Basal Area | Stand Volume | |
| (cm) | (m) | (m) | (m) | (Trees/ha) | (m2) | (m3 ha−1) | |
| Max. | 44.50 | 23.50 | 16.50 | 12.70 | 1936.7 | 35.85 | 270.93 |
| Mean. | 15.49 | 12.97 | 6.21 | 6.83 | 1463.3 | 34.85 | 252.89 |
| Min. | 4.00 | 1.50 | 0.50 | 0.50 | 1080.0 | 34.02 | 237.99 |
| St error | 0.19 | 0.11 | 0.09 | 0.05 | 185.6 | 0.48 | 13.10 |
| DBH (cm) | H (m) | CBH (m) | CL (m) | Age (Years) | |
|---|---|---|---|---|---|
| Max. | 31.00 | 23.50 | 9.30 | 14.00 | 83 |
| Mean. | 20.52 | 18.79 | 8.05 | 10.83 | 77 |
| Min. | 12.00 | 14.08 | 5.80 | 6.28 | 66 |
| St error | 1.85 | 0.86 | 0.45 | 0.62 | 2.4 |
| Tree Parts | Fresh Weight kg (%) | Moisture Content (%) | Dry Weight (Biomass) kg (%) | Carbon Content kg (DwX0.47) | CO2 Amount kg (CX3.67) | ||
|---|---|---|---|---|---|---|---|
| Stem | 324.438 ± 52.7 | 74.25 | 58.61 ± 1.4 | 203.24 ± 31.9 | 76.47 | 95.53 | 350.57 |
| Branches | 72.936 ± 21.1 | 16.69 | 70.98 ± 3.2 | 43.255 ± 12.0 | 16.28 | 20.33 | 74.61 |
| Foliage | 39.572 ± 6.9 | 9.06 | 107.39 ± 3.3 | 19.273 ± 3.4 | 7.25 | 9.06 | 33.24 |
| Total | 436.946 ± 70.5 | 100 | 64.41 ± 1.6 | 265.768 ± 48.5 | 100 | 124.91 | 458.42 |
| Model | a | b | R2 | Sig. | SEE a | SEE b | RD (%) |
|---|---|---|---|---|---|---|---|
| AGB = a (DBH) b | 0.1279 | 2.4852 | 0.97 | *** | 0.052 | 0.185 | 6.59 |
| STB = a (DBH) b | 0.229 | 2.214 | 0.96 | *** | 0.140 | 0.189 | 8.00 |
| BRB = a (DBH) b | 0.000046 | 4.400 | 0.98 | *** | 0.0000 | 0.272 | 16.31 |
| FB = a (DBH) b | 0.013 | 2.374 | 0.87 | *** | 0.016 | 0.380 | 21.53 |
| Tree Parts | Biomass t ha−1 | Carbon Content Mg ha−1 | CO2 Amount Mg ha−1 |
|---|---|---|---|
| Stem | 195.56 | 91.91 | 337.32 |
| Branches | 41.63 | 19.57 | 71.81 |
| Foliage | 18.54 | 8.71 | 31.40 |
| Total | 255.73 | 120.19 | 441.11 |
| Category of Dead Wood | Volume m3 ha−1 | Biomass t ha−1 | Carbon Content Mg ha−1 | CO2 Amount Mg ha−1 |
|---|---|---|---|---|
| Standing | 2.81 (0.44) | 1.97 (0.37) | 0.98 (0.19) | 3.60 (0.72) |
| Fallen | 0.94 (0.30) | 0.40 (0.16) | 0.20 (0.08) | 0.73 (0.32) |
| Total | 3.75 (0.56) | 2.37 (0.48) | 1.18 (0.25) | 4.33 (0.93) |
| Layer | Biomass Accumulation | Carbon Content | CO2 Amount | |
|---|---|---|---|---|
| t ha−1 | (%) | Mg ha−1 | Mg ha−1 | |
| Litter layer | ||||
| P | 6.78 | 21.78 | 2.51 | 9.207 |
| H | 9.79 | 31.45 | 3.62 | 13.299 |
| F | 14.56 | 46.77 | 5.38 | 19.766 |
| Total in litter | 31.13 | 100.00 | 11.51 | 42.272 |
| Soil layer | Organic matter | |||
| 0–20 cm | 41.72 | 55.00 | 24.20 | 88.814 |
| 20–50 cm | 34.14 | 45.00 | 19.80 | 72.666 |
| Total in soil | 75.86 | 100.00 | 44.00 | 161.480 |
| Part of the Forest Ecosystem | Biomass Accumulation | Carbon Content | CO2 Amount Mg ha−1 | |
|---|---|---|---|---|
| t ha−1 | Mg ha−1 | % | ||
| Above ground living biomass (AGB) | 255.73 | 120.19 | 57.75 | 441.11 |
| Below ground living biomass (BGB = AGB × 0.26) | 66.49 | 31.25 | 15.01 | 114.69 |
| Dead wood (standing and fallen) | 2.37 | 1.18 | 0.57 | 4.33 |
| Litter | 31.13 | 11.51 | 5.53 | 42.27 |
| Soil | 75.86 | 44.00 | 21.14 | 161.48 |
| TOTAL | 431.58 | 208.13 | 100.00 | 763.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganatsas, P.; Tsakaldimi, M.; Karydopoulos, T.; Petaloudi, L.-M.; Papaemmanouil, A.; Papadopoulos, S.; Gerochristou, S. Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest. Sustainability 2022, 14, 13764. https://doi.org/10.3390/su142113764
Ganatsas P, Tsakaldimi M, Karydopoulos T, Petaloudi L-M, Papaemmanouil A, Papadopoulos S, Gerochristou S. Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest. Sustainability. 2022; 14(21):13764. https://doi.org/10.3390/su142113764
Chicago/Turabian StyleGanatsas, Petros, Marianthi Tsakaldimi, Theodoros Karydopoulos, Lydia-Maria Petaloudi, Alexandros Papaemmanouil, Sotirios Papadopoulos, and Sofia Gerochristou. 2022. "Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest" Sustainability 14, no. 21: 13764. https://doi.org/10.3390/su142113764
APA StyleGanatsas, P., Tsakaldimi, M., Karydopoulos, T., Petaloudi, L.-M., Papaemmanouil, A., Papadopoulos, S., & Gerochristou, S. (2022). Carbon Pools in a 77 Year-Old Oak Forest under Conversion from Coppice to High Forest. Sustainability, 14(21), 13764. https://doi.org/10.3390/su142113764







