Improving Access to Export Market for Fresh Vegetables through Reduction of Phytosanitary and Pesticide Residue Constraints
Abstract
1. Introduction
2. Review of Determinants of Quality in Exported Fresh Vegetables
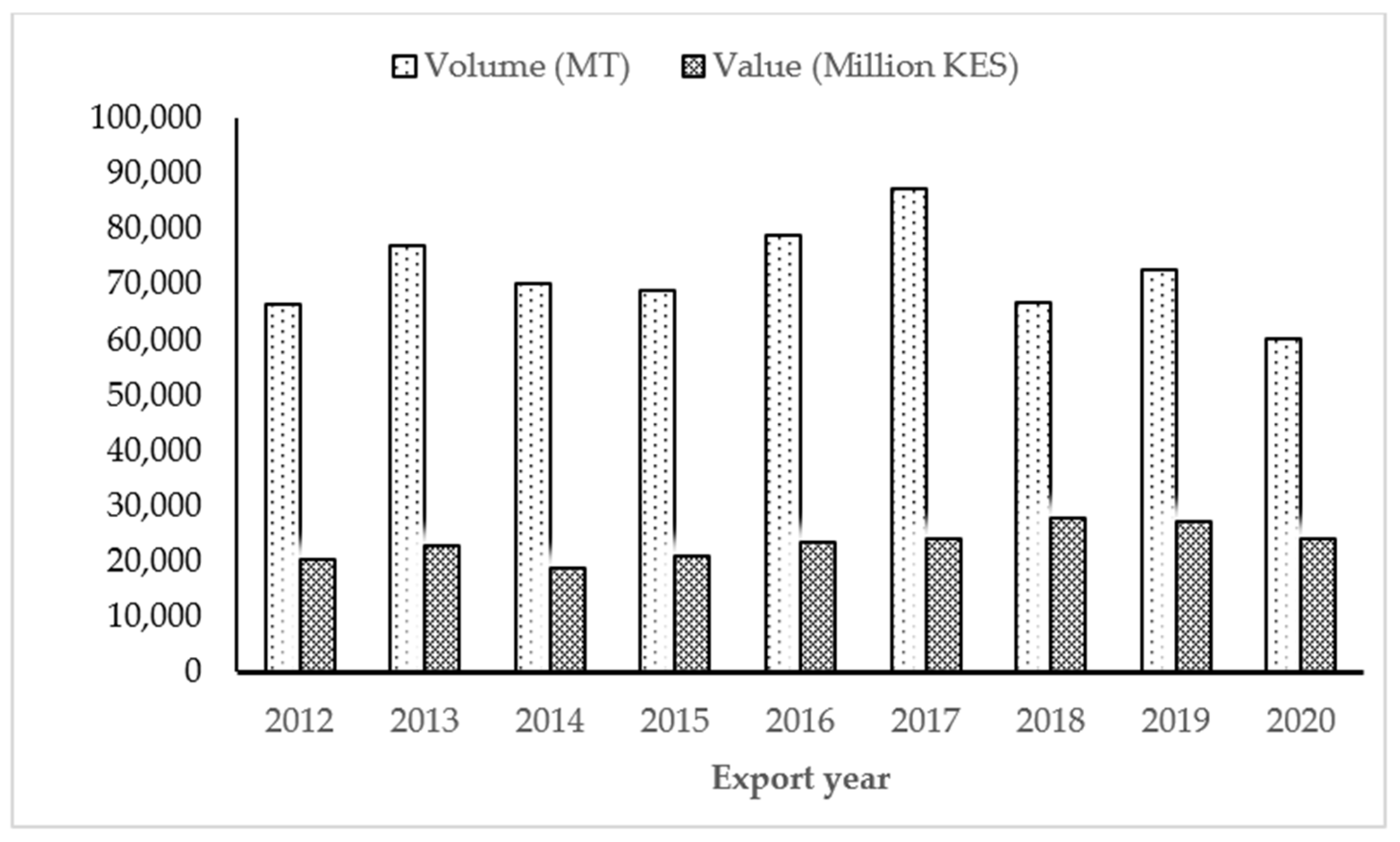
2.1. The Status of Fresh Vegetable for Niche Markets in Kenya
2.2. Pests of Phytosanitary Concern in Fresh Vegetables
2.3. Microbial Contaminants of Fresh Vegetables
2.4. Strategies Used by Farmers in Managing Pests in Fresh Vegetables
2.5. Problems Associated with Use of Synthetic Pesticides
2.6. Fresh Produce Export Requirements and Their Implications
3. Four-Tiered Approach to Compliance with Phytosanitary and Pesticide Residue Requirements
3.1. Establishment of Structured Grower Systems for Exporters
3.2. Strict Enforcement of Standards
3.3. Investment in Research and Adoption of Innovative Technologies
3.4. Awareness Creation and Training
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Research Solutions Africa (RSA) Ltd. Report of a Study on Fresh Vegetables Market in Kenya; Desk Review; Research Solutions Africa: Nairobi, Kenya, 2015. [Google Scholar]
- Horticultural Crops Directorate (HCD). Horticulture Validated Report 2015–2016; Agriculture and Food Authority: Nairobi, Kenya, 2016. [Google Scholar]
- The Horticultural Crops Directorate (HCD). Validated Report 2016–2017; Agriculture and Food Authority: Nairobi, Kenya, 2019. [Google Scholar]
- Tyce, M. A ‘Private-sector Success Story’? Uncovering the role of politics and the state in Kenya’s horticultural export sector. J. Dev. Stud. 2020, 56, 1877–1893. [Google Scholar] [CrossRef]
- Kenya National Bureauof Statistics (KNBS). Statistical Abstract; Kenya National Bureau of Statistics: Nairobi, Kenya, 2021. [Google Scholar]
- Irandu, E.M. Factors influencing growth of horticultural exports in Kenya: A gravity model analysis. GeoJournal 2018, 84, 877–887. [Google Scholar] [CrossRef]
- Fresh Produce Exporters Association of Kenya (FPEAK). Latest Industry News and Statistics, Interceptions. Available online: https://fpeak.org/downloads/newsletters/ (accessed on 12 December 2021).
- Mordor Intelligence. Morocco Fruits and Vegetables Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/fruits-and-vegetables-industry-in-morocco (accessed on 14 February 2022).
- Capobianco-Uriarte, M.D.L.M.; Aparicio, J.; De Pablo-Valenciano, J.; Casado-Belmonte, M.D.P. The European tomato market. An approach by export competitiveness maps. PLoS ONE 2021, 16, e0250867. [Google Scholar] [CrossRef]
- Siringoringo, H.; Tintri, D.; Kowanda, A. Problems Faced by Small and Medium Business In Exporting Products. Delhi Bus. Rev. 2009, 10, 49–61. [Google Scholar] [CrossRef]
- Macharia, I. Pesticides and Health in Vegetable Production in Kenya. BioMed Res. Int. 2015, 2015, 241516. [Google Scholar] [CrossRef] [PubMed]
- Nguetti, J.H.; Imungi, J.K.; Okoth, M.W.; Wangâ, J.; Mbacham, W.F.; Mitema, S.E. Assessment of the knowledge and use of pesticides by the tomato farmers in Mwea Region, Kenya. Afr. J. Agric. Res. 2018, 13, 379–388. [Google Scholar] [CrossRef][Green Version]
- Intracen. Export Potential Map. Spot Export Opportunities for Trade Development. Export Potential, Kenya. Available online: https://exportpotential.intracen.org/en/products/treemap?fromMarker=i&exporter=404&toMarker=w&market=w&whatMarker=k (accessed on 20 February 2022).
- Centre for the Promotion of Imports from Developing Countries (CBI). Buyer Requirements: Fresh Fruit and Vegetables. CBI Market Intelligence. Available online: www.cbi.eu/market-information (accessed on 18 January 2021).
- Fulano, A.M.; Lengai, G.M.W.; Muthomi, J.W. Phytosanitary and Technical Quality Challenges in Export Fresh Vegetables and Strategies to Compliance with Market Requirements: Case of Smallholder Snap Beans in Kenya. Sustainability 2021, 13, 1546. [Google Scholar] [CrossRef]
- Business Daily. Chemical Ban Hits Vegetable Exports to the EU Market. 23 February 2013. Available online: https://www.businessdailyafrica.com/bd/economy/chemical-ban-hits-vegetable-exports-to-the-eu-market-2025298 (accessed on 12 June 2019).
- Business Daily. Illegal Horticulture Exports Risk Kenya’s EU Market. 2014. Available online: https://www.businessdailyafrica.com/news/Illegal-horticulture-exports-risk-Kenya-s-EU-market/539546-2329850-ryrmj2z/index.html (accessed on 20 December 2019).
- Fresh Produce Exporters Association of Kenya (FPEAK), 2021. Update on the State of the Horticulture Industry in Kenya 2020. Available online: https://fpeak.org/update-on-the-state-of-the-horticulture-industry-in-kenya-2021/ (accessed on 12 February 2022).
- Kenya Plant Health Inspectorate Service (KEPHIS). Annual Report and Financial Statements 2019. Available online: www@kephis.org (accessed on 28 May 2021).
- Koigi, B. EU Policy on Kenyan Exports Creating a Local Health Crisis. EURACTIV.de. 2016. Available online: https://www.euractiv.com/section/development-policy/news/eu-policy-on-kenyan-exports-creating-a-local-health-crisis/ (accessed on 16 March 2019).
- O’Reilly, R.K. Kenyan Vegetable Farmers’ IPM adoption: Barriers and Impacts. MSc Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2020. [Google Scholar]
- Chemeltorit, P.; Saavedra, Y.; Gema, J. Food Traceability in the Domestic Horticulture Sector in Kenya: An Overview; 3R Research Report 003; 3R Kenya Project, African Centre for Technology Studies, ICIPE: Nairobi, Kenya, 2018. [Google Scholar]
- Martinez, M.G.; Poole, N. The development of private fresh produce safety standards: Implications for developing Mediterranean exporting countries. Food Policy 2004, 29, 229–255. [Google Scholar] [CrossRef]
- The East African. South Africa Lifts 10-Year Ban on Kenya’s Avocado. Available online: https://www.theeastafrican.co.ke/tea/business/south-africa-lifts-10-year-ban-on-kenya-s-avocado-1399534 (accessed on 11 December 2018).
- FAO. Fruit and Vegetables—Your Dietary Essentials. The International Year of Fruits and Vegetables, 2021; Background Paper; FAO: Rome, Italy, 2020. [Google Scholar]
- Fruit Logistica. A Collection of Key Production, Import and Export Information, Market Trends and Patterns of Trade for Europe’s Fresh Fruit and Vegetable Business. In European Statistics Handbook; Messe Berlin GmbH: Berlin, Germany, 2021. [Google Scholar]
- Faria, J. Export Volume of Vegetables from Kenya between January 2019 and July 2021 (in 1000 Metric Tons). Monthly Export Volume of Vegetables from Kenya 2019–2021. Available online: https://www.statista.com/statistics/1130896/monthly-export-volume-of-vegetables-from-kenya/ (accessed on 1 March 2022).
- Ashebre, K.M. On opportunities and potential in Ethiopia for production of fruits and vegetables. Int. J. Afr. Asian Stud. 2015, 15, 41–48. [Google Scholar]
- Kenya Plant Health Inspectorate Service (KEPHIS). KEPHIS Laboratory Re-Accredited to Meet International Plant Export Requirements. KEPHIS News. December 2015. Available online: https://kephis.org/images/docs/december2015enewsletter.pdf. (accessed on 18 June 2017).
- Sumitra, A.; Kanojia, A.K.; Kumar, A.; Mogha, N.; Sahu, V. Biopesticide formulation to Control Tomato Lepidopteran Pest Menace. Curr. Sci. 2012, 102, 1051–1057. [Google Scholar]
- Leach, A.; Reiners, S.; Fuchs, M.; Nault, B. Evaluating integrated pest management tactics for onion thrips and pathogens they transmit to onion. Agric. Ecosyst. Environ. 2017, 250, 89–101. [Google Scholar] [CrossRef]
- Din, N.; Ashraf, M.; Hussain, S. Effect of different non-chemical and chemical measures against onion thrips. J. Entomol. Zool. Stud. 2016, 4, 10–12. [Google Scholar]
- Roy, S.K.; Ali, M.S.; Mony, F.T.Z.; Islam, M.S.; Matin, M.A. Chemical Control of Whitefly and Aphid Insect Pest of French Bean (Phaseolus vulgaris L.). J. Biosci. Agric. Res. 2014, 2, 69–75. [Google Scholar] [CrossRef][Green Version]
- Ángel, D.; Jorge, E.; Martínez, H.; Santamaria, G.; Parada, P.; Ebratt, R. Identification and distribution of whiteflies (Hemip-tera: Aleyrodidae) in tomato crops (Solanum lycopersicum) in Cundinamarca (Colombia). Agron. Colomb. 2016, 34, 42–50. [Google Scholar] [CrossRef]
- European Commission. Final Report of an Audit Carried out in Kenya from 12–22nd November 2013 in order to Evaluate the System of Official Controls for the Export of Plants and Plant Products to the European Union, Ref DG(SANCO) 2010-8707; European Commission; Directorate-General for Health and Food Safety: Brussel, Belgium, 2014. [Google Scholar]
- Daily Nation. “Pepper Exporters Suffer Setback as Stubborn Pest Infests Crop” Saturday Edition 21st April 2018. Available online: https://nation.africa/kenya/business/seedsofgold/Pepper-exporters-suffer-setback-as-stubborn-pest-infests-crop/2301238-4491060-vgtj3mz/index.html (accessed on 25 April 2019).
- Daily Nation. EU Rejects French Beans over Use of Banned Spray. Thursday Edition 14th February 2013. Available online: https://nation.africa/kenya/business/eu-rejects-french-beans-over-use-of-banned-spray--849036?view=htmlamp (accessed on 25 April 2019).
- Massebo, F.; Tefera, Z. Status of Bactrocerainvadens (Diptera: Tephritidae) in mango-producing areas of Arba Minch, southwestern Ethiopia. J. Insect Sci. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis Complex of Fruit Flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the inte-gration of biopesticides with other biological approaches for their management with a focus on the Pacific Region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Foba, C.N.; Salifu, D.; Lagat, Z.O.; Gitonga, L.M.; Akutse, K.S.; Fiaboe, K.K.M. Species composition, distribution, and seasonal abundance of Liriomyza leafminers (Diptera: Agromyzidae) under different vegetable production systems and agroecological zones in Kenya. Environ. Entomol. 2015, 44, 223–232. [Google Scholar] [CrossRef] [PubMed]
- European Union Notification System for Plant Health Interceptions (EUROPHYT). Interceptions of Harmful Organisms in Commodities Imported into the Eumember States and Switzerland. 2014, p. 184. Available online: http://ec.europa.eu/food/plant/plant_health_biosafety/europhyt/docs/2013_interceptions_en.pdf (accessed on 6 October 2014).
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef]
- Bhilwadikar, T.; Pounraj, S.; Manivannan, S.; Rastogi, N.K.; Negi, P.S. Decontamination of microorganisms and pesticides from fresh fruits and vegetables: Acomprehensive review from common household processes to modern techniques. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1003–1038. [Google Scholar] [CrossRef]
- Food and Agriculture Organizations of the United Nations (FAO) CODEX Alimentarius International Food Standards: CO-DEX, S. 293: 2008. Standard for Tomatoes. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B293-2008%252FCXS_293e.pdf (accessed on 16 September 2018).
- Seto, K.C.; Dhakal, A.S.; Bigio, H.; Blanco, G.C.; Delgado, D.; Dewar, L.; Huang, A.; Inaba, A.; Kansal, S.; Lwasa, J.E.; et al. Human Settlements, Infrastructure and Spatial Planning; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Iyoha, O.; Agoreyo, F. Bacterial Contamination of Ready to Eat Fruits Sold in and around Ugbowo Campus of University of Benin (Uniben), Edo State, Nigeria. Br. J. Med. Med. Res. 2015, 7, 155–160. [Google Scholar] [CrossRef]
- Dada, E.O.; Olusola-Makinde, O.O. Microbial and parasitic contamination on vegetables collected from retailers in main market, Akure, Nigeria. Am. J. Microbiol. Res. 2015, 3, 112–117. [Google Scholar]
- Aderinoye-Abdulwahab, S.A.; Salami, S.T. Assessment of organic fertilizer usage by vegetable farmers in Asa Local Government area of Kwara State. Niger. Agrosearch 2017, 17, 101–114. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.A.; Buys, M.E. Irrigation water and microbiological safety of fresh produce; South Africa as a case study: A review. Afr. J. Agric. Res. 2012, 7, 4848–4857. [Google Scholar] [CrossRef]
- Lamuka, P.O. Public Health Measures: Challenges of Developing Countries in Management of Food Safety. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; Volume 4, pp. 20–26. [Google Scholar]
- Ogumo, E.O.; Kunyanga, C.N.; Okoth, M.W.; Kimenju, J.W. Correlation between Time of Harvesting and Duration before Cooling on the Microbial Quality of French Bean (Phaseolus vulgaris L.). Int. J. Sci. 2018, 4, 8–15. [Google Scholar] [CrossRef]
- Kunyanga, C.; Amimo, J.; Njue, L.K.; Chemining’wa, G. Consumer risk exposure to chemical and microbial hazards through consumption of fruits and vegetables in Kenya. Food Sci. Qual. Manag. 2018, 78, 59–69. [Google Scholar]
- Maina, J.; Ndung’U, P.; Muigai, A.; Kiiru, J. Antimicrobial resistance profiles and genetic basis of resistance among non-fastidious Gram-negative bacteria recovered from ready-to-eat foods in Kibera informal housing in Nairobi, Kenya. Access Microbiol. 2021, 3, 000236. [Google Scholar] [CrossRef]
- Kibitok, S.K.; Nduko, J.M. Evaluation of microbial contamination of consumed fruits and vegetables salad (Kachumbari) around Egerton University, Kenya. J. Food Safe Hyg. 2016, 2, 26–29. [Google Scholar]
- Kutto, E.K.; Ngigi, M.W.; Karanja, N.; Kange’the, E.; Bebora, L.C.; Lagerkvist, C.J.; Mbuthia, P.G.; Njagi, L.W.; Okello, J.J. Bacterial contamination of kale (Brassica oleracea Acephala) along the supply chain in Nairobi and its environment. East Afr. Med. J. 2011, 88, 46–53. [Google Scholar]
- Birech, R.; Bernhard, F.; Joseph, M. Towards Reducing synthetic pesticides imports in favour of locally available botani-cals in Kenya. In Proceedings of the International Agricultural Research for Development, Bonn, Germany, 11–13 October 2006; pp. 8–12. [Google Scholar]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of Pesticide Residues in Commonly Used Fruits and Vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Halimatunsadiah, A.B.; Norida, M.; Omar, D.; Kamarulzaman, N.H. Application of pesticide in pest management: The case of lowland vegetable growers. Int. Food Res. J. 2016, 23, 85–94. [Google Scholar]
- Goufo, P.; Mofor, C.T.; Fontem, D.A.; Ngnokam, D. High efficacy of extracts of Cameroon plants against tomato late blight disease. Agron. Sustain. Dev. 2008, 28, 567–573. [Google Scholar] [CrossRef]
- Owusu-Boateng, G.; Amuzu, K.K. A survey of some critical issues in vegetable crops farming along River Oyansia in Opeibea and Dzorwulu, Accra-Ghana. Glob. Adv. Res. J. Physl. Appl. Sci. 2013, 2, 24–31. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Nampeera, E.L.; Nonnecke, G.R.; Blodgett, S.L.; Tusiime, S.M.; Masinde, D.M.; Wesonga, J.M.; Murungi, L.K.; Baidu-Forson, J.J.; Abukutsa-Onyango, O.M. Farmers’ Knowledge and Practices in the Management of Insect Pests of Leafy Amaranth in Kenya. J. Integr. Pest Manag. 2019, 10, 31. [Google Scholar] [CrossRef]
- Pires, R.C.D.M.; Folegatti, M.V.; Tanaka, M.A.D.S.; Passos, F.A.; Ambrosano, G.M.B.; Sakai, E. Water levels and soil mulches in relation to strawberry diseases an yield in a greenhouse. Sci. Agricola 2007, 64, 575–581. [Google Scholar] [CrossRef][Green Version]
- Smith, A.H.; Liburd, O.E. Intercropping, Crop Diversity and Pest Management; University of Florida, IFAS Extension: Gainesville, FL, USA, 2012; pp. 1–7. [Google Scholar]
- Henze, J.; Abukutsa-Onyango, M.; Opiyo, A. Production and Marketing of African Indigenous Leafy Vegetables. Training Manual for Extension Offcers and Practitioners; Humboldt-Universität zu Berlin: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Marek, L.F.; Lee, D.K.; Thapa, S.B.; Hahn, V.; Bradshaw, J.D. Effects from early planting of late-maturing sun-flowers on damage from primary insect pests in the United States. Helia 2016, 39, 45–56. [Google Scholar] [CrossRef]
- Mutisya, D.L.; Karanja, D.R.; Kisilu, R.K. Economic advantage of sorghum harvest at soft dough grain stage to prevent bird damage. Cogent Food Agric. 2016, 2, 1259141. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Herms, D.A.; Cardina, J.; Moore, R.H. Climate Change and Pest Management: Unanticipated Consequences of Trophic Dislocation. Agronomy 2018, 8, 7. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Rwomushana, I.; Mugambi, I.; Makale, F.; Lamontagne-Godwin, J.; Chacha, D.; Kibwage, P.; Oluyali, J.; Day, R. Crop losses and economic impact associated with papaya mealybug (Paracoccus marginatus) infestation in Kenya. Int. J. Pest Manag. 2020, 1–14. [Google Scholar] [CrossRef]
- Infonet-Biovision. Crops. Available online: http://www.infonet-biovision.org/default/ct/118/crops (accessed on 28 February 2022).
- Azandémè-Hounmalon, G.Y.; Fellous, S.; Kreiter, S.; Fiaboe, K.K.M.; Subramanian, S.; Kungu, M.; Martin, T. Dispersal Behavior of Tetranychus evansi and T. urticae on Tomato at Several Spatial Scales and Densities: Implications for Integrated Pest Management. PLoS ONE 2014, 9, e95071. [Google Scholar] [CrossRef]
- Ochieng, S.O.; Nderitu, P.W. Biocontrol approach to management of greenpeach aphid Myzus persicae in garden peas for a sustainable ecosystem. J. Horticult. For. 2011, 3, 231–237. [Google Scholar]
- Macharia, I.; Mithöfer, D.; Waibel, H. Pesticide handling practices by vegetable farmer in Kenya. Environ. Dev. Sustain. 2012, 15, 887–902. [Google Scholar] [CrossRef]
- Pest Control Products Board (PCPB). Pest Control Products Registered for Use in Kenya, 9th ed.; Pest Control Products Board (PCPB): Embu, Kenya, 2015; pp. 1–365. [Google Scholar]
- The East African. Kenya at Risk of Losing EU Market for Peas, Beans Due to Falling Standards. Saturday Edition 25th April 2015. Available online: https://www.theeastafrican.co.ke/tea/news/east-africa/kenya-at-risk-of-losing-eu-market-for-peas-beans-due-to-falling-standards-1335328 (accessed on 13 May 2018).
- United States Agency for International Development-Kenya Horticulture Competitiveness Project (USAID-KHCP). Global Competitiveness Study: Benchmarking Kenya’s Horticulture Sector for Enhanced Export Competitiveness; Fintrac. Inc.: Nairobi, Kenya, 2015. [Google Scholar]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Tsimbiri, P.F.; Moturi, W.N.; Sawe, J.; Henley, P.; Bend, J.R. Health Impact of Pesticides on Residents and Horticultural Workers in the Lake Naivasha Region, Kenya. Occup. Dis. Environ. Med. 2015, 3, 24–34. [Google Scholar] [CrossRef]
- Sarwar, M. The dangers of pesticides associated with public health and preventing of the risks. Int. J. Bioinform. Biomed. Eng. 2015, 1, 130–136. [Google Scholar]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruits and vegetables. TrAC Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Porto, A.L.M.; Melgar, G.Z.; Kasemodel, M.C.; Nitschke, M. Biodegradation of pesticides. In Pesticides in the Modern World-Pesticides Use and Management; InTech: London, UK, 2011. [Google Scholar]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Heard, M.S.; Baas, J.; Dorne, J.L.; Lahive, E.; Robinson, A.G.; Rortais, A.; Hesketh, H. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species? Sci. Total Environ. 2017, 578, 357–365. [Google Scholar] [CrossRef]
- Karaagac, S.U. Insecticide Resistance. In Insecticides-Advances in Integrated Pest Management; InTech: London, UK, 2012; Available online: https://www.intechopen.com/chapters/25687 (accessed on 8 October 2016).
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K. REffects of pesticides on environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- United Nations. Standard Layout for UNECE Explanatory Brochures on Fresh Fruit and Vegetables (FFV) 2015 Edition United Nations New York. Available online: https://unece.org/fileadmin/DAM/trade/agr/standard/fresh/StandardLayout/FFVBrochureLayout_2015_e.pdf (accessed on 26 January 2017).
- European Commission. Pesticide Residues. 2018. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=download.MRL (accessed on 1 April 2021).
- Michel, P. Buyer Requirements: Fresh Fruits and Vegetables, CBI Market Intelligence, 2015. Available online: www.cbi.edu/disclaimer (accessed on 20 May 2016).
- Rao, E.J.O.; Brümmer, B.; Qaim, M. Farmer Participation in Supermarket Channels, Production Technology, and Efficiency: The Case of Vegetables in Kenya. Am. J. Agric. Econ. 2012, 94, 891–912. [Google Scholar] [CrossRef]
- Sciabarrasi, M. The Big Five Risks Faced by Farmers. Available online: https://nevegetable.org/big-five-risks-faced-farmers (accessed on 8 January 2022).
- Yeung, M.T.; Kerr, W.A.; Coomber, B.; Lantz, M.; McConnel, A. The Economics of International Harmonization of MRLs [CHAPTER]. In Declining International Cooperation on Pesticide Regulation; Palgrave Macmillan: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Pest Control Products Board. Pest Control Products Registered for Use in Kenya. 2018. Available online: https://www.pcpb.go.ke/crops/ (accessed on 5 June 2020).
- Dijkxhoorn, Y.; Bremmer, J.; Kerklaan, E. Towards Integrated Pest Management in East Africa; A Feasibility Study; LEI Wageningen UR (University & Research Centre): Wageningen, Poland, 2013; pp. 1–48. [Google Scholar]
- Ngutu, M.; Bukachi, S.; Olungah, C.O.; Kiteme, B.; Kaeser, F.; Haller, T. The Actors, Rules and Regulations Linked to Export Horticulture Production and Access to Land and Water as Common Pool Resources in Laikipia County, Northwest Mount Kenya. Land 2018, 7, 110. [Google Scholar] [CrossRef]
- Grasswitz, T.R. Integrated pest management (IPM) for small-scale farms in developed economies: Challenges and opportu-nities. Insects 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Integrated Pest Management of Major Pests and Diseases in Eastern Europe and the Caucasus; FAO: Rome, Italy, 2017. [Google Scholar]
- Kunbhar, S.; Rajput, L.B.; Gilal, A.A.; Channa, A.; Sahito, J.G.M. Impact of botanical pesticides against sucking insect pests and their insect predators in brinjal crop. J. Entomol. Zool. Stud. 2018, 6, 83–87. [Google Scholar]
- Dehariya, S.; Shukla, A.; Barde, S.; Ahirwar, K. Efficacy of Botanical Pesticides against Sucking Insects Pests in Brinjal. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1930–1935. [Google Scholar] [CrossRef][Green Version]
- Amoabeng, B.W.; Johnson, A.; Gurr, G.M. Natural enemy enhancement and botanical insecticide source: A review of dual use companion plants. Appl. Entomol. Zool. 2019, 54, 1–19. [Google Scholar] [CrossRef]
- Nath, U.; Puzari, A. Ethnobotanical study on pesticidal plants used in Southwest Nagaland, India for the development of eco-friendly pest control system. Acta Ecol. Sin. 2021. [Google Scholar] [CrossRef]
- Vidyasagar, G.M.; Tabassum, N. Antifungal investigations on plant essential oils; A Review. Int. J. Pharm. Pharm Sci. 2013, 5, 19–28. [Google Scholar]
- Muthomi, J.W.; Fulano, A.M.; Wagacha, J.M.; Mwang’ombe, A.W. Management of snap bean insect pests and diseases by use of antagonistic fungi and plant extracts. Sustain. Agric. Res. 2017, 6. [Google Scholar] [CrossRef]
- Charles, S.; Walter, O.; Kambale, V.; Muller, K.; Lusenge, V.; Jules, N.; Mariamu, B.; Guy, B.; Sivirihauma, C.; Ocimati, W.; et al. Diversity of cultural practices used in banana plantations and possibilities for fine-tuning: Case of North Kivu and Ituri provinces, eastern Democratic Republic of Congo. Afr. J. Agric. Res. 2017, 12, 2163–2177. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Abdul-Baki, A.A.; Mill, D.J.; Thorpe, K.W. Enhanced pest management with cover crop mulches. Acta Hortic. 2004, 638, 135–140. [Google Scholar] [CrossRef]
- Max, J.F.J.; Schmidt, L.; Mutwiwa, U.N.; Kahlen, K. Effects of shoot pruning and inflorescence thinning on plant growth, yield and fruit quality of greenhouse tomatoes in a tropical climate. J. Agric. Rural Dev. Trop. Subtrop. 2016, 117, 45–56. [Google Scholar]
- Lassiter, B.R.; Jordan, D.L.; Wilkerson, G.G.; Shew, B.B.; Brandenburg, R.L. Influence of Cultural and Pest Management Prac-tices on Performance of Runner, Spanish, and Virginia Market Types in North Carolina. Adv. Agric. 2016, 2016, 5795373. [Google Scholar]
- Sarker, P.; Rahman, M.; Das, B. Effect of Intercropping with Mustard with Onion and Garlic on Aphid Population and Yield. J. Bio-Sci. 1970, 15, 35–40. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, Z.; Ahmad, W.; Paul, B.; Paul, S.; Aggarwal, C.; Akhtar, M.S. Insect pest resistance: An alternative approach for crop protection. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 257–282. [Google Scholar]
- Abrol, D.P.; Shankar, U. Integrated Pest Management. In Breeding Oilseed Crops for Sustainable Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 523–549. [Google Scholar]
- Devi, M.S.; Roy, K. Comparable study on different coloured sticky traps for catching of onion thrips, Thrips tabaci Lindeman. J. Entomol. Zool. Stud. 2017, 5, 669–671. [Google Scholar]
- Saha, T.; Chandran, N. Chemical Ecology and Pest Management: A Review. Int. J. Cardiovasc. Sci. 2017, 5, 618–621. [Google Scholar]
- Rao, K.S.; Vishnupriya, R.; Ramaraju, K. Efficacy and Safety Studies onPredatory Mite, Neoseiuluslongispinosus (Evans) against Two-Spotted Spider Mite, Tetranychusurticae Koch under Laboratory and Greenhouse Conditions. J. Entomol. Zool. Stud. 2017, 4, 835–839. [Google Scholar]
- Wall, G.L. Farm-to-Table Food Safety for Colorado Produce Crops: A Web-Based Approach for Promoting Good Agricultural and Handling Practices. Doctoral Thesis, Colorado State University, Fort Collins, CO, USA, 2011. [Google Scholar]
- Nirmala, G. Impact of Good Agricultural Practices (GAP) on Small Farm Development: Knowledge and Adoption levels of Farm Women of Rainfed Areas. Indian Res. J. Ext. Educ. 2016, 15, 153–156. [Google Scholar]
- Akkaya, F.; Yalcin, R.; Ozkan, B. Good agricultural practices (GAP) and its implementation in Turkey. International Sym-posium on Improving the Performance of Supply Chains in the Transitional Economies. Acta Hortic. 2005, 699, 47–52. [Google Scholar]
- Nono-Womdim, R.; Ojiewo, C.; Abang, M.; Olouch, M.O. Good agricultural practices for African indigenous vegetables. Scr. Hort. 2012, 15, 83–89. [Google Scholar]
- Bihn, E.; Wall, G.; Fisk, C.; Humiston, M.; Pahl, D.; Stoeckel, D.; Way, R.; Woods, K. Produce Safety Alliance National Curriculum; Version 1.1; Available in English and Spanish; Produce Safety Alliance, Cornell University: Ithaca, NY, USA, 2017. [Google Scholar]
- IPPC. International Phytosanitary Conference—Kenya Agenda Item: 11.2 International Plant Protection Convention. International Phytosanitary Conference—Proposal to Establish an IPPC Format for a Regular Phytosanitary Conference: The “International Phytosanitary Conference. International Plant Protection Convention 04_SPG_2017_Oct. 2017. Available online: https://www.ippc.int/static/media/files/publication/en/2017/09/04_SPG_2017_Oct_KenyaProposal-2017-09-25.pdf (accessed on 6 April 2019).
- KEPHIS. Report of the Second Phytosanitary Confrerence, Theme: “Phytosanitary Systems for Safe Trade and Food Security”. 2018. Available online: frica-cope.org/phytosanitary-conference-2021/images/conference-report/3a5-proceedings-of-the-2nd-phytosanitary-conference-2018-final-03092018.pdf (accessed on 12 February 2022).
- Kenya Bureau of Standards (KBS). Standards Development, about Our Standards. Available online: https://www.kebs.org/index.php?option=com_content&view=article&id=124&Itemid=129 (accessed on 20 February 2022).
- Ministry of Agriculture, Livestock, Fisheries and Cooperatives (MoALFC). The National Food Policy. 2021. Available online: https://kilimo.go.ke/wp-content/uploads/2022/02/Draft-Food-Safety-Policy-2021.pdf (accessed on 18 February 2022).
- Ministry of Industrialization, Forging New Trade and Investment Partnerships between Africa and the European Union. Available online: https://www.industrialization.go.ke/index.php/media-center/speeches-by-cabinet-secretary/616-forging-new-trade-and-investment-partnerships-between-africa-and-the-european-union (accessed on 28 February 2022).
- International Plant protection Convention (IPPC). Identification of challenges and best practice Sub-saharan Africa FAO Region/Anglophone countries. In Proceedings of the Regional Workshop for the Review of Phytosanitary Surveillance in the Context of the IPPC Standard (ISPM6), Alisa Hotel, Accra, Ghana, 6–8 February 2012. [Google Scholar]
- Razin, A.; Taktarova, S.; Semenov, V. Innovative and investment development of vegetable growing. MATEC Web Conf. 2018, 212, 07010. [Google Scholar] [CrossRef][Green Version]
- Christiaan, A.; Rolf, A. Greenhouse Technology Adoption among Small and Medium Scale Tomato Farmers in Kenya. Master’s Thesis, Wageningen University, Wageningen, Poland, 2020. [Google Scholar]
- FAO. Good Agricultural Practices for Greenhouse Vegetable Production in the South East European Countries. 2017. Available online: https://bib.irb.hr/datoteka/889849.FAO_GAP_for_greenhouse_vegetable_production.pdf (accessed on 10 October 2020).
- Global Agriculture Productivity. Investing in Innovation and Infrastructure in the International Year of Fruits and Vegetables. Available online: https://globalagriculturalproductivity.org/investing-in-innovation-and-infrastructure-in-the-international-year-of-fruits-and-vegetables/ (accessed on 30 May 2021).
- Kenya Plant Health Inspectorate Service (KEPHIS). Phytosanitary Services; Kenya Plant Health Inspectorate Service (KEPHIS): Nairobi, Kenya, 2022. [Google Scholar]
- Centre of Phytosanitary Excellence (COPE). Phytosanitary Curricula for in-Service Short Term Training Courses; Centre of Phytosanitary Excellence (COPE): Nairobi, Kenya, 2008. [Google Scholar]
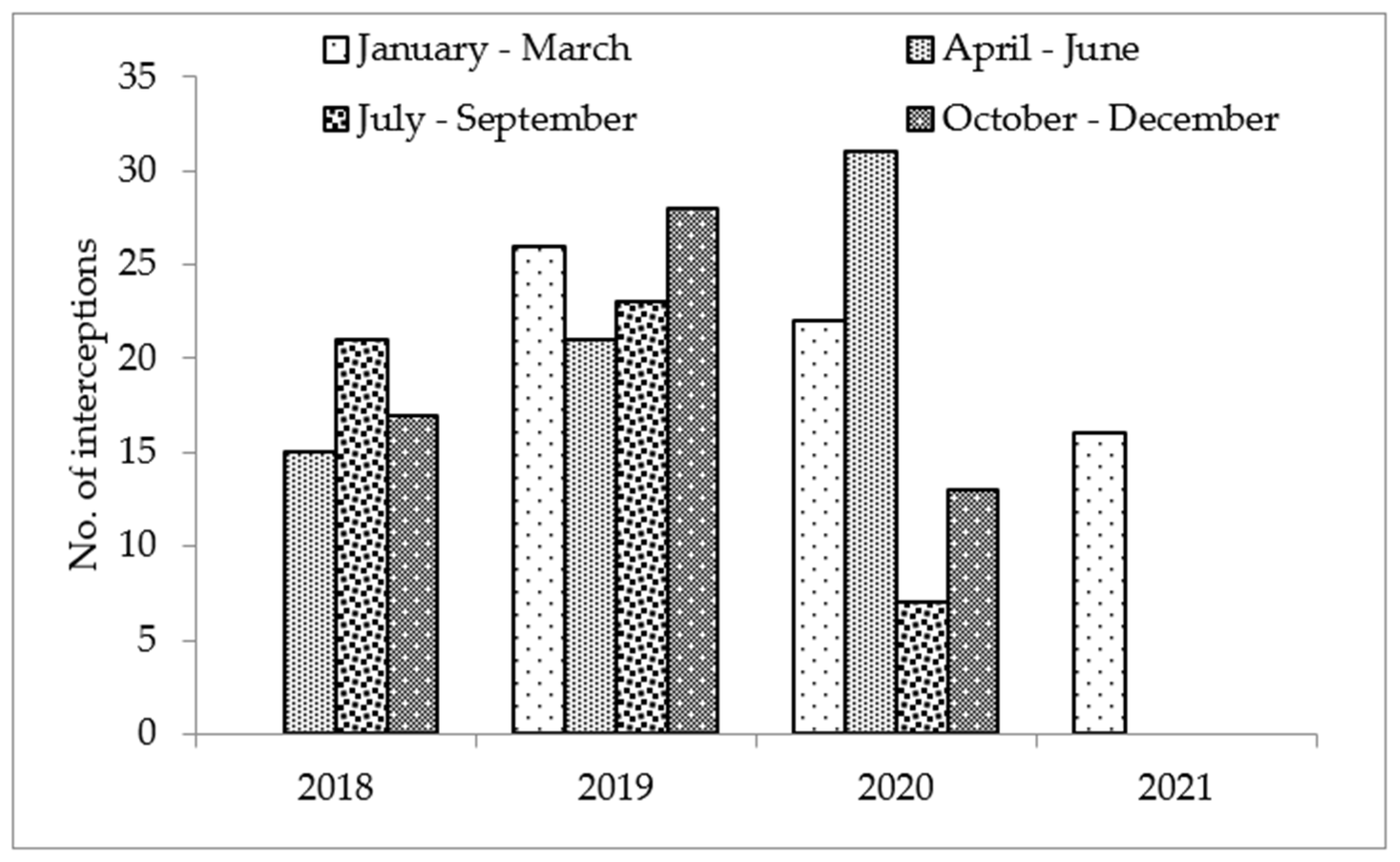
| Harmful Organisms | Percentage |
|---|---|
| Spodoptera frugiperda | 9.3 |
| Spodoptera littoralis | 9.3 |
| Thaumatotibia leucotreta | 14.7 |
| Bemisia tabaci | 13.3 |
| Liriomyza huidobrensis | 10.7 |
| Tephritidae | 10.7 |
| Liriomyza | 12.0 |
| Liriomyza sativae | 10.7 |
| Thrips | 8.0 |
| Scirtothrips aurantii | 1.3 |
| Microorganism | Detected on | Place Sourced | Reference |
|---|---|---|---|
| Enterobacteriacea | French beans | Harvested from commercial planting | [52] |
| Tomatoes, kales, amaranth leaves | Markets and retail outlet in urban and peri-urban towns | [53] | |
| Escherichia coli | Ready-to-eat kales, cabbage, and nightshades | Vending points | [54] |
| Corriander, onions, tomatoes, and chili | Vending points | [55] | |
| Salmonella spp. | Ready-to-eat kales, cabbage, and nightshades | Vending points | [54] |
| Kales | Vegetable growing peri-urban areas around Nairobi | [56] | |
| Corriander, onions, tomatoes, and chili | Vending points | [55] | |
| Listeria monocytogenes | French beans | Harvested from commercial planting | [52] |
| Staphylococcus aureus | Tomatoes, kales, amaranth leaves | Markets and retail outlet in urban and peri-urban towns | [53] |
| French beans | Harvested from commercial planting | [52] |
| Type of Control | Farmer’s Strategy | Target Pest | Reference |
|---|---|---|---|
| Physical | Washing plants with strong jet of water | Arthropod pests | [70] |
| handpicking | Pod borers | [71] | |
| Remove and destroy infested plant material. | Red spider mite | [66] | |
| Cultural | Field sanitation | [66] | |
| Planting resistant varieties | Snap bean insect pests | [66] | |
| Weeding | Spider mites | [72] | |
| Crop rotation | [70] | ||
| Planting early in the season | Bean fly | [70] | |
| Intercropping | Thrips | [66] | |
| Biological | Organic production | [70] | |
| Use of predatory mite | Western flower thrips | [70] | |
| Use of botanicals | Pyrethrins-Aphids, whiteflies; Azadirachtin-thrips, leafminers, etc. | [73] | |
| Chemical | Use of synthetic pesticides (cypermethrine, deltamethrin, fenvalerate, imidacloprid, triazopho, dimethoate) | Bean fly, aphids, whiteflies | [73,74] |
| Integrated | Use of multiple interventions | Targets all pests | [70] |
| Active Ingredient | EU MRL (mg/Kg) | MRL mg/Kg | Year Adopted |
|---|---|---|---|
| Aldrin and Dieldrin | Leafy vegetables | 0.05 | 1997 |
| Abamectin | Tomatoes | 0.09 | 2015 |
| Snow peas | 0.01 | ||
| French beans | 0.03 | ||
| Azoxystrobin | Fruiting vegetables | 3 | 2009 |
| Acetamiprid | Cabbage | 0.01 | 2017 |
| Coriander | 0.01 | ||
| Bifenthrin | Peas with pods | 0.9 | 2016 |
| Flowering brassicas | 0.4 | ||
| Chlorpyrifos | Bulb vegetables | 0.01 | 2003 |
| Root and tuber vegetables | 0.05 | ||
| Cypermethrin | Leafy vegetables | 0.7 | 2009 |
| Deltamethrin | Leafy vegetables | 2 | 2006 |
| Dimethoate | Brussel sprouts | 0.1 | 2009 |
| Legume vegetables | 0.01 | ||
| Carrots | 0.03 | ||
| Imidacloprid | Peas with pods | 5 | 2009 |
| Paraquat | Leafy vegetables | 0.07 | 2006 |
| Metalaxyl | Chinese cabbage | 0.02 | 2017 |
| Eggplants | 0.01 | ||
| Beans with pods | 0.02 | ||
| Peas with pods | 0.02 | ||
| Cyantraniliprole | Peas with pods | 2 | 2018 |
| Peas without pods | 0.3 | ||
| Beans with pods | 1.5 | ||
| Beans without pods | 0.3 | ||
| Chlorothalonil | Bulb vegetables | 0.01 | 2016 |
| Sweet pepper | 0.01 | ||
| Broccoli | 0.01 | ||
| Asparagus | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengai, G.M.W.; Fulano, A.M.; Muthomi, J.W. Improving Access to Export Market for Fresh Vegetables through Reduction of Phytosanitary and Pesticide Residue Constraints. Sustainability 2022, 14, 8183. https://doi.org/10.3390/su14138183
Lengai GMW, Fulano AM, Muthomi JW. Improving Access to Export Market for Fresh Vegetables through Reduction of Phytosanitary and Pesticide Residue Constraints. Sustainability. 2022; 14(13):8183. https://doi.org/10.3390/su14138183
Chicago/Turabian StyleLengai, Geraldin M. W., Alex M. Fulano, and James W. Muthomi. 2022. "Improving Access to Export Market for Fresh Vegetables through Reduction of Phytosanitary and Pesticide Residue Constraints" Sustainability 14, no. 13: 8183. https://doi.org/10.3390/su14138183
APA StyleLengai, G. M. W., Fulano, A. M., & Muthomi, J. W. (2022). Improving Access to Export Market for Fresh Vegetables through Reduction of Phytosanitary and Pesticide Residue Constraints. Sustainability, 14(13), 8183. https://doi.org/10.3390/su14138183








