Improved Food Waste Stabilization and Valorization by Anaerobic Digestion Through Supplementation of Conductive Materials and Trace Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaerobic Seed
2.2. Food Waste
2.3. Conductive Material
2.4. Analytical Methods
2.5. Biochemical Methane Potential Assays
3. Results and Discussion
3.1. Biogas Production
3.2. Methane Yields
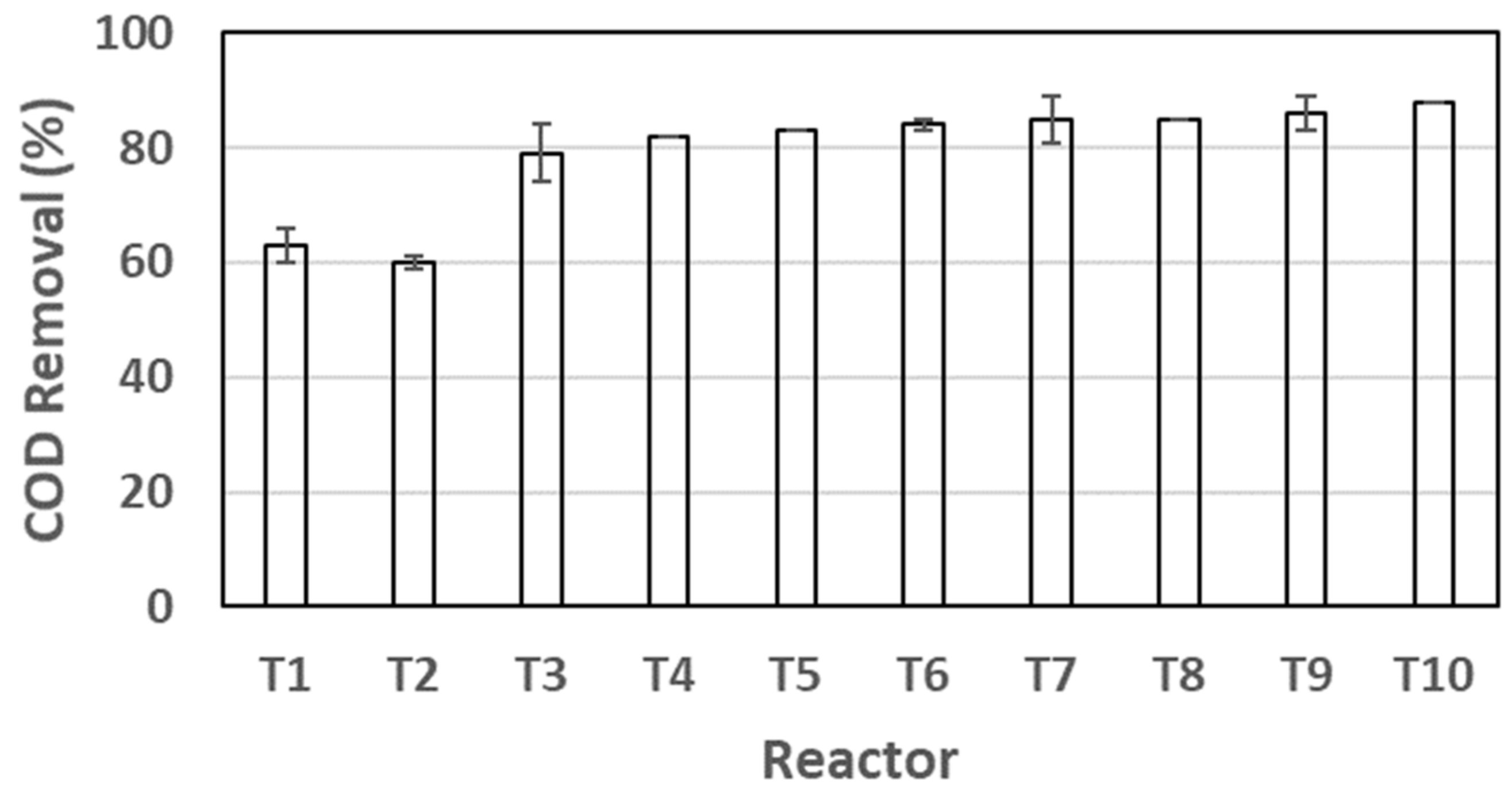
3.3. Anaerobic Stabilization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustavvson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Food Wastage Footprint Impacts on Natural Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107752-8. [Google Scholar]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Lin, C.; Pfaltzgraff, L.; Herrero-Davila, L.; Mubofu, E.; Abderrahim, S.; Clark, J.; Koutinas, A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Posmanik, R.; Labatut, R.; Kim, A.; Usack, J.; Tester, J.; Angenent, L. Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour. Technol. 2017, 233, 134–143. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Ngoc, B.D.T.; Chiu-Yue, L.; Gopalakrishnan, K. Waste-to-wealth for valorization of food waste to hydrogen and methane towards creating a sustainable ideal source of bioenergy. J. Clean. Prod. 2016, 122, 29–41. [Google Scholar]
- Alkaya, E.; Demirer, G.N. Minimizing and adding value to seafood processing wastes. Food Bioprod. Process. 2016, 100, 195–202. [Google Scholar] [CrossRef]
- Tampio, E.; Ervasti, S.; Paavola, T.; Heaven, S.; Banks, C.; Rintala, J. Anaerobic digestion of autoclaved and untreated food waste. Waste Manag. 2014, 34, 370–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erguder, T.H.; Demirer, G.N. Organic acid production from the organic fraction of municipal solid waste and cow manure in leaching bed reactors. Environ. Eng. Manag. J. 2016, 15, 24–87, 2495. [Google Scholar]
- Calicioglu, O.; Demirer, G.N. Biogas production from waste microalgal biomass obtained from nutrient removal of domestic wastewater. Waste Biomass Valorization 2016, 7, 1397–1408. [Google Scholar] [CrossRef]
- Ramírez-Arpide, F.R.; Demirer, G.N.; Gallegos-Vázquez, C.; Hernández-Eugenio, G.; Santoyo-Cortés, V.H.; Espinosa-Solares, T. Life cycle assessment of biogas production through anaerobic co-digestion of nopal cladodes and dairy cow manure. J. Clean. Prod. 2018, 172, 2313–2322. [Google Scholar] [CrossRef]
- Gur, E.; Demirer, G.N. Anaerobic digestibility and biogas production capacity of pistachio processing wastewater in UASB reactors. J. Environ. Eng. 2019, 145, 8. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pre-treatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Anaerobic digestion of food waste-Effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Capson-Tojo, G.; Ruiz, D.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Accumulation of propionic acid during consecutive batch anaerobic digestion of commercial food waste. Bioresour. Technol. 2017, 245, 724–733. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energ. Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef] [Green Version]
- Cruz Viggi, C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic cocultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz Viggi, C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S. Enhancing methane production from food waste fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Voelklein, M.A.; O’Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, S.; Guo, J.; Zhou, J.; Dong, R. Performance and kinetic evaluation of semi-continuously fed anaerobic digesters treating food waste: Role of trace elements. Bioresour. Technol. 2015, 178, 297–305. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Achinas, S.; Euverink, G.J.W. Elevated biogas production from the anaerobic co-digestion of farmhouse waste: Insight into the process performance and kinetics. Waste Manag. Res. 2019, 37, 1240–1249. [Google Scholar] [CrossRef]
- Zhao, M.X.; Ruan, W.Q. Biogas performance from co-digestion of Taihu algae and kitchen wastes. Energy Convers. Manag. 2013, 75, 21–24. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international inter-laboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Pham, C.H.; Triolo, J.M.; Cu, T.T.T.; Pedersen, L.; Sommer, S.G. Validation and recommendation of methods to measure biogas production potential of animal manure. Asian-Australas J. Anim. Sci. 2013, 26, 864–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Brown, R.C.; Wen, Z. Biochar as an additive in anaerobic digestion of municipal sludge: Biochar properties and their effects on the digestion performance. ACS Sustain. Chem. Eng. 2020, 8, 6391–6401. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Leon, P.A.I.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Alberto, D.R.; Repa, K.S.; Hegde, S.; Miller, C.W.; Trabold, T.A. Novel production of magnetite particles via thermochemical processing of digestate from manure and food waste. IEEE Magn. Lett. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Thines, K.; Abdullah, E.; Mujawar, M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for wastewater and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Meng, Y.; Li, S.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Evaluating biomethane production from anaerobic mono- and co-digestion of food waste and floatable oil (FO) skimmed from food waste. Bioresour. Technol. 2015, 185, 7–13. [Google Scholar] [CrossRef]
- Labatut, R.; Angenent, L.T.; Scott, N. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Lin, J.; Zuo, J.; Gan, L.; Li, P.; Liu, F.; Wang, K.; Chen, L.; Gan, H. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 2011, 23, 1403–1408. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [PubMed]
- Agyeman, F.; Tao, W. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumara, G.; Xu, K. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Meyer-Kohlstock, D.; Haupt, T.; Heldt, E.; Heldt, N.; Kraft, E. Biochar as additive in biogas-production from bio-waste. Energies 2016, 9, 247. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Latif, M.A.; Ahmad, A.; Ghufran, R.; Wahid, Z.A. Effect of temperature and organic loading rate on upflow anaerobic sludge blanket reactor and CH4 production by treating liquidized food waste. Environ. Prog. Sustain. Energy 2012, 31, 114–121. [Google Scholar] [CrossRef]
- Chan, P.C.; de Toledo, R.A.; Shim, H. Anaerobic co-digestion of food waste and domestic wastewater—Effect of intermittent feeding on short and long chain fatty acids accumulation. Renew. Energy 2018, 124, 129–135. [Google Scholar] [CrossRef]

| Parameter | Seed | Food Waste | |
|---|---|---|---|
| First Exp. Setup | Second Exp. Setup | ||
| COD (mg/L) | 8883 ± 46 | 91,455 ± 12,065 | 62810 ± 7710 |
| TS (mg/L) | 33,500 ± 300 | 118,700 ± 7700 | 77,200 ± 2500 |
| VS (mg/L) | 21,400 ± 200 | 111,900 ± 7200 | 71,500 ± 2700 |
| Total N (mg/L) | 265 ± 66 | 561 ± 66 | 595 ± 85 |
| Total P (mg/L) | 686 ± 140 | 464 ± 127 | 1792 ± 78 |
| pH | 8.9 | 4.2 | 6.3 |
| Reactor | Trace Metals | Biochar (g/L) | Magnetite (g/L) | |
|---|---|---|---|---|
| First Experimental Setup | C1 | - | 0 | 0 |
| C2 | + | 0 | 0 | |
| T1 | - | 20 | 0 | |
| T2 | + | 20 | 0 | |
| C3 | - | 0 | 0 | |
| C4 | + | 0 | 0 | |
| T3 | - | 20 | 0 | |
| T4 | + | 20 | 0 | |
| Second Experimental Setup | C5 | + | 0 | 0 |
| T5 | + | 2.0 | 0 | |
| T6 | + | 5.0 | 0 | |
| T7 | + | 10.0 | 0 | |
| T8 | + | 0 | 2.0 | |
| T9 | + | 0 | 5.0 | |
| T10 | + | 0 | 10.0 |
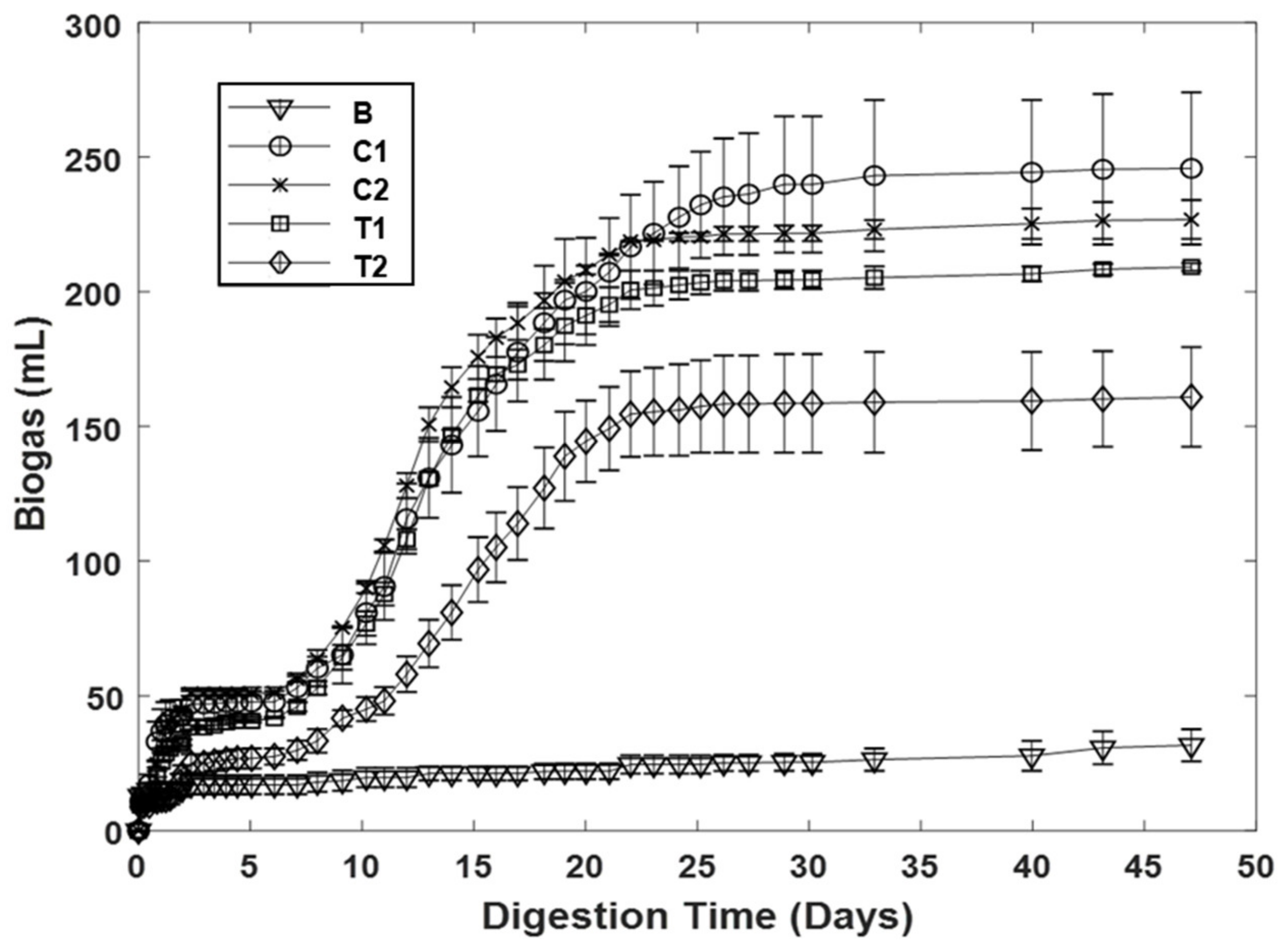
| Reactor | Biogas Production | Methane Yield | |||
|---|---|---|---|---|---|
| Methane Content (%) | Volume (mL) | Change Relative to Control (%) | mL CH4/g VS | Change Relative to Control (%) | |
| C1 | 45.6 ± 6.8 | 245.8 ± 40.0 | - | 293.0 ± 12.0 | - |
| C2 | 41.0 ± 1.8 | 226.8 ± 10.2 | - | 238.4 ± 5.2 | - |
| T1 | - | 209.2 ± 2.0 | -12.7 ± 18.9 | - | - |
| T2 | - | 160.9 ± 26.2 | −29.4 ± 11.8 | - | - |
| C3 | 60.8 ± 2.0 | 441.4 ± 12.6 | - | 372.0 ± 6.8 | - |
| C4 | 64.0 ± 0.3 | 436.2 ± 32.6 | - | 386.4 ± 12.3 | - |
| T3 | 55.6 ± 6.9 | 460.6 ± 16.3 | 4.3 ± 1.0 | 358.5 ± 21.2 | -3.7 ± 5.6 |
| T4 | 58.5 ± 0 | 400.9 ± 98.7 | −9.3 ± 22.4 | 386.6 ± 16.8 | 0.0 ± 1.6 |
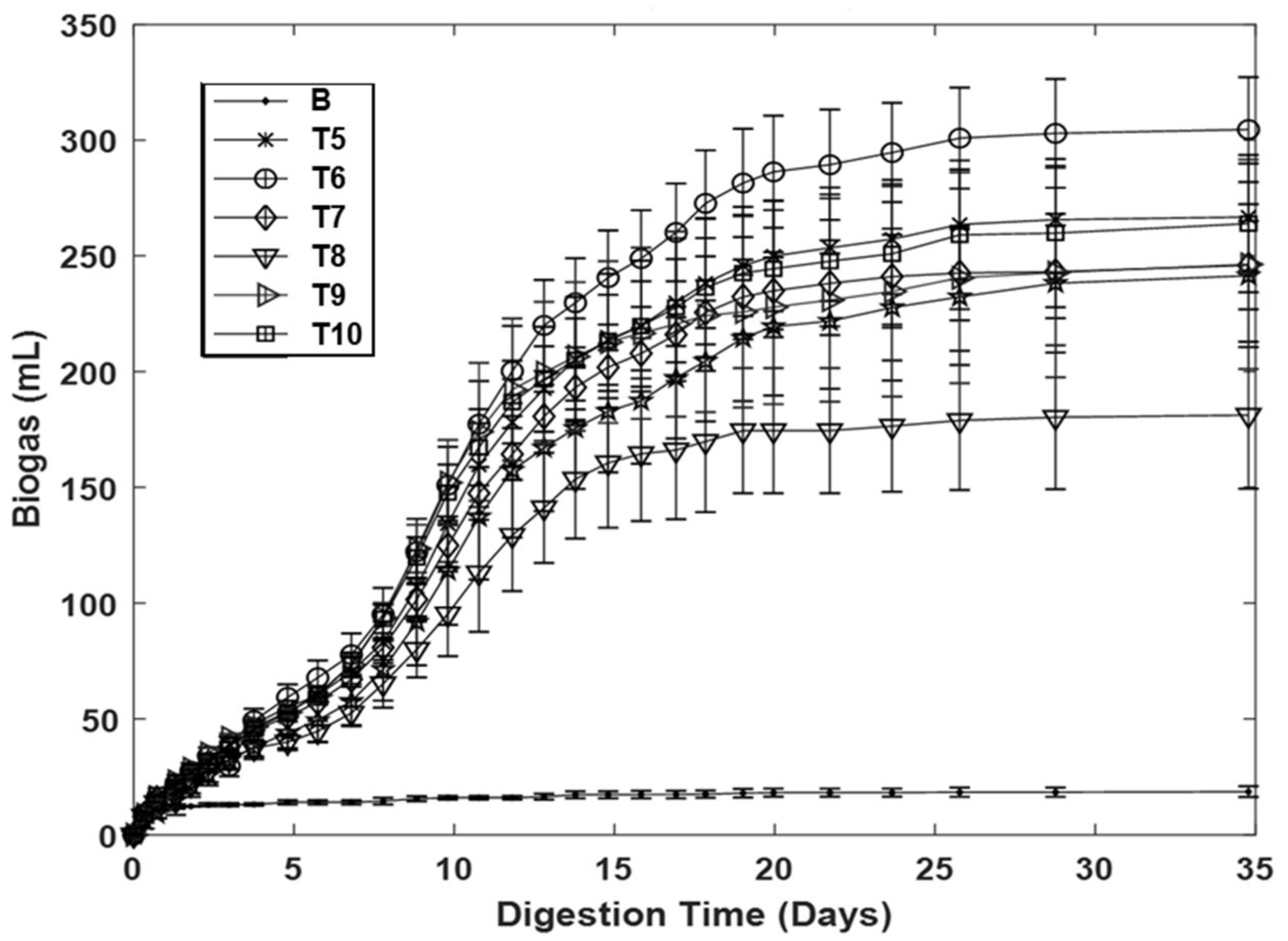
| C5 | 65.5 ± 0.6 | 241.4 ± 31.0 | - | 408.9 ± 21.2 | - |
| T5 | 63.7 ± 0.5 | 267 ± 23.3 | 11.2 ± 6.5 | 442.0 ± 3.4 | 8.3 ± 6.8 |
| T6 | 68.1 ± 0.9 | 305.1 ± 23.2 | 27.3 ± 9.5 | 545.2 ± 36.2 | 33.2 ± 2.8 |
| T7 | 61.2 ± 0.7 | 246.0 ± 19.8 | 2.5 ± 7.0 | 389.7 ± 19.4 | −4.7 ± 0.3 |
| T8 | 59.8 ± 1.1 | 181.4 ± 32.4 | −25.3 ± 5.4 | 272.9 ± 43.2 | −33.6 ± 10.0 |
| T9 | 58.1 ± 0.9 | 246.2 ± 45.4 | 1.2 ± 8.2 | 370.0 ± 35.4 | −9.7 ± 5.6 |
| T10 | 60.2 ± 1.0 | 264.0 ± 30.8 | 9.5 ± 1.8 | 413.3 ± 24.2 | 1.0 ± 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akturk, A.S.; Demirer, G.N. Improved Food Waste Stabilization and Valorization by Anaerobic Digestion Through Supplementation of Conductive Materials and Trace Elements. Sustainability 2020, 12, 5222. https://doi.org/10.3390/su12125222
Akturk AS, Demirer GN. Improved Food Waste Stabilization and Valorization by Anaerobic Digestion Through Supplementation of Conductive Materials and Trace Elements. Sustainability. 2020; 12(12):5222. https://doi.org/10.3390/su12125222
Chicago/Turabian StyleAkturk, A. Sinan, and Goksel N. Demirer. 2020. "Improved Food Waste Stabilization and Valorization by Anaerobic Digestion Through Supplementation of Conductive Materials and Trace Elements" Sustainability 12, no. 12: 5222. https://doi.org/10.3390/su12125222




