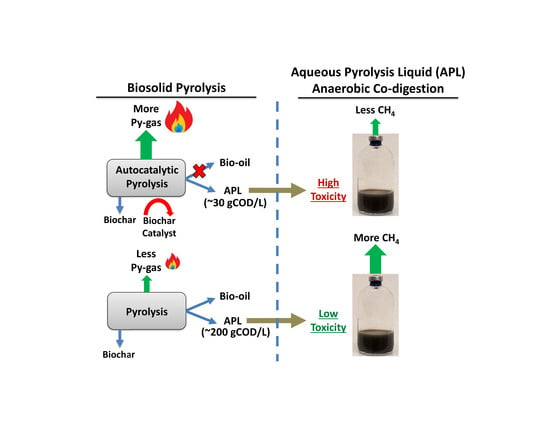
Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. APL Production and APL NH3-N Air Stripping
2.2. Short-Term Semi-Continuous Anaerobic Digestion
2.3. Long-Term Semi-Continuous Anaerobic Digestion
2.4. Synthetic Primary Sludge
2.5. Analytical Methods
2.6. DNA Extraction and Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. APL Composition
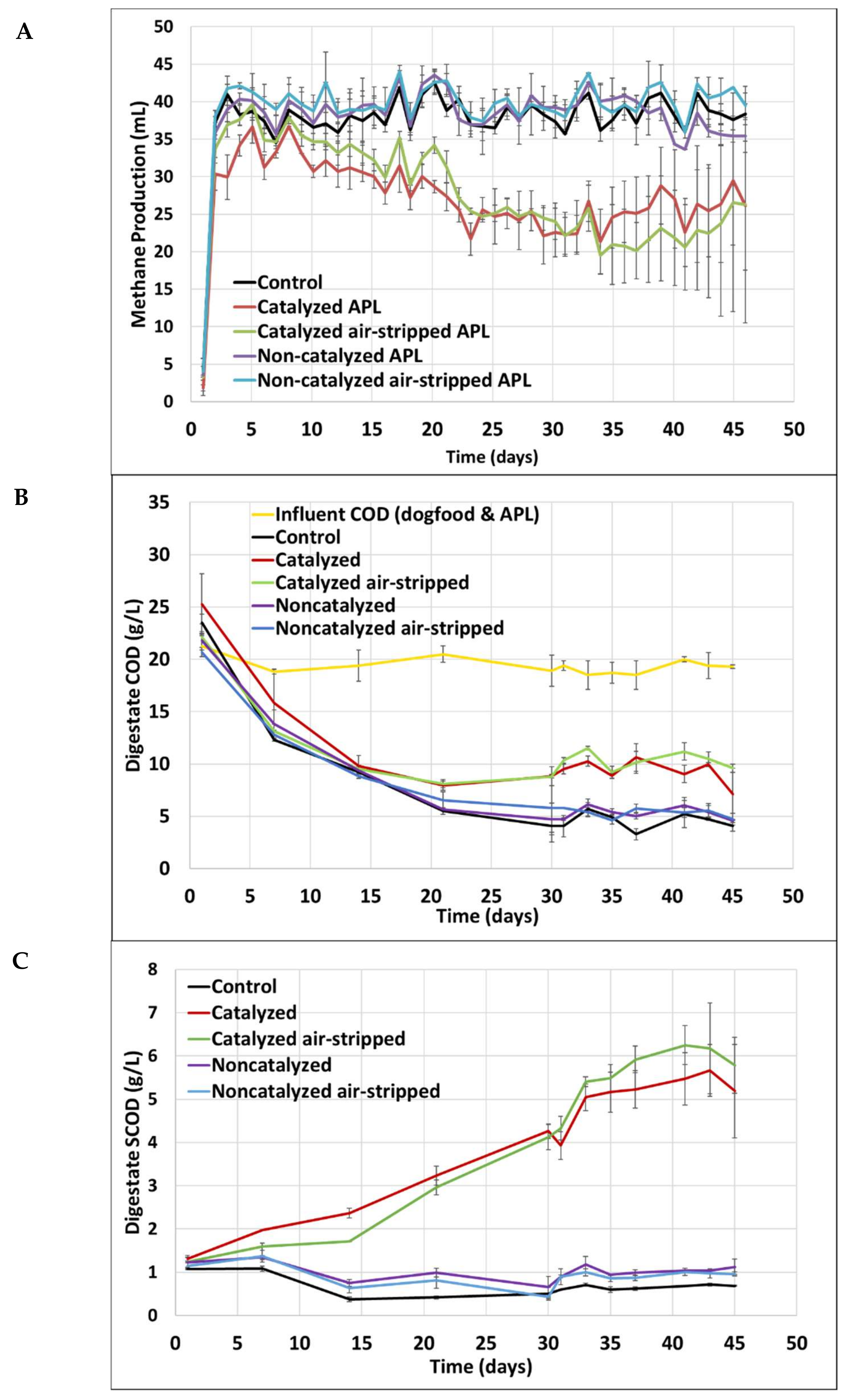
3.2. Short-Term Semi-Continuous Anaerobic Digestion
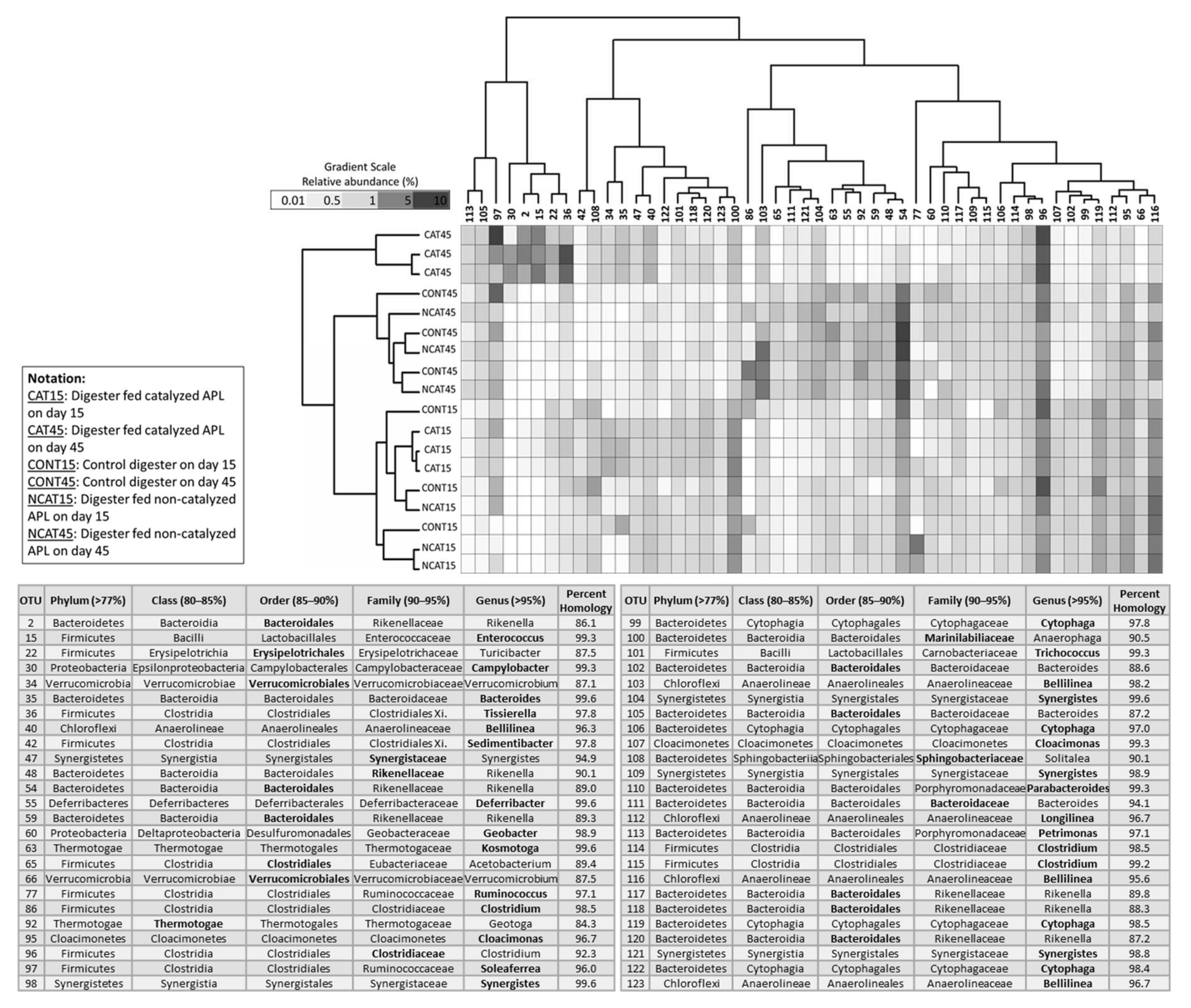
3.3. Short-Term Digestion Microbial Community Analysis
3.3.1. Archaea Community
3.3.2. Bacteria Community
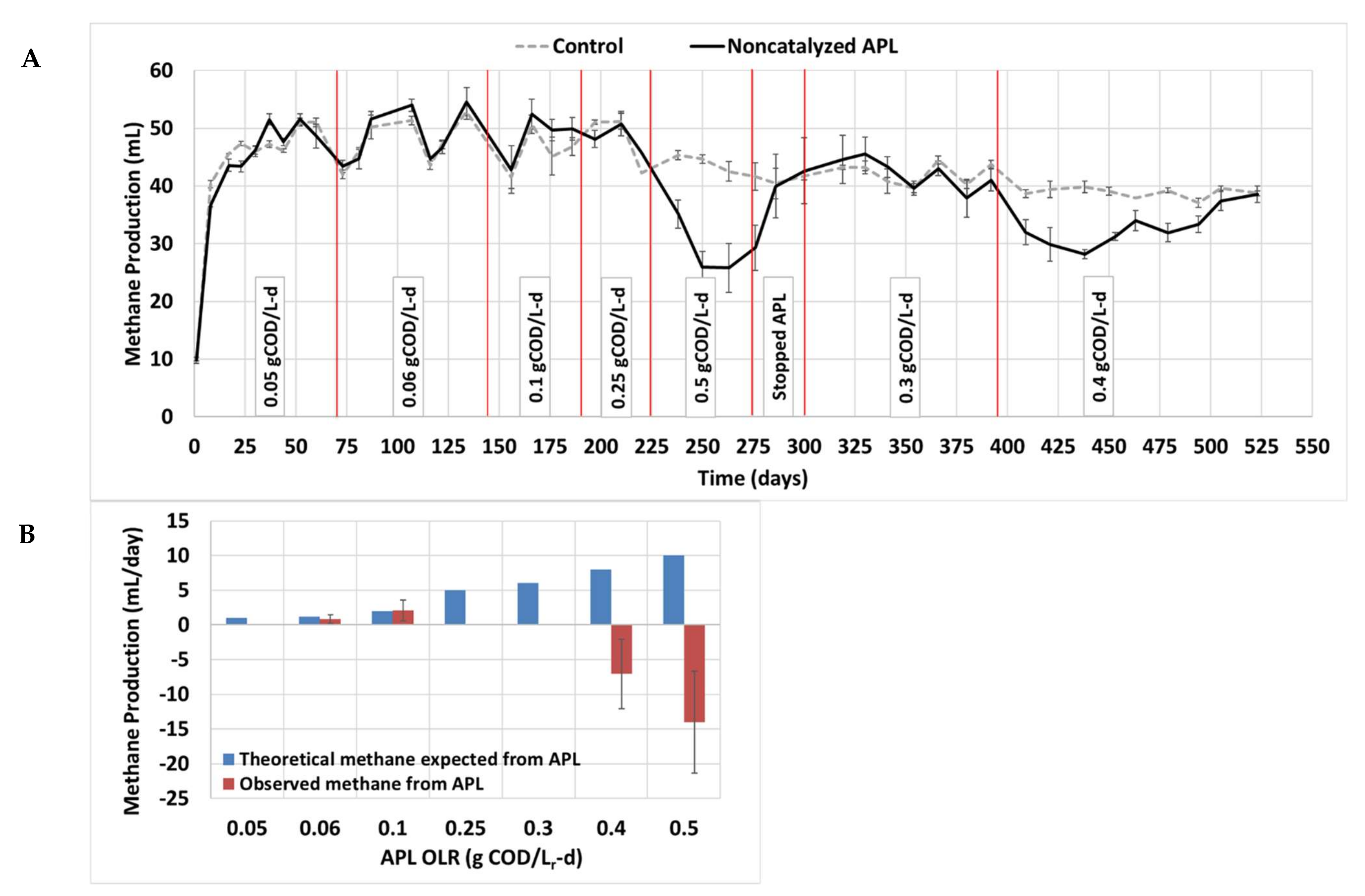
3.4. Long-Term Semi-Continuous Anaerobic Digestion
3.5. Inhibition by APL and Future Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Singer, S.; Tong, Y.; Kimbell, L.; Anderson, E.; Hughes, M.; Zitomer, D.; McNamara, P. Characteristics and applications of biochars derived from wastewater solids. Renew. Sustain. Energy Rev. 2018, 90, 650–664. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, J.H.; Su, T.Y.; Chang, Y.M. Characterization of bio-oil from induction-heating pyrolysis of food-processing sewage sludges using chromatographic analysis. Bioresour. Technol. 2009, 100, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; Zitomer, D.H.; Miller, T.R.; Weirich, C.A.; McNamara, P.J. Emerging investigators series: Pyrolysis removes common microconstituents triclocarban, triclosan, and nonylphenol from biosolids. Environ. Sci. Water Res. Technol. 2016, 2, 282–289. [Google Scholar] [CrossRef]
- Kimbell, L.K.; Kappell, A.D.; McNamara, P.J. Effect of pyrolysis on the removal of antibiotic resistance genes and class I integrons from municipal wastewater biosolids. Environ. Sci. Water Res. Technol. 2018, 4, 1807–1818. [Google Scholar] [CrossRef]
- McNamara, P.J.; Koch, J.D.; Liu, Z.; Zitomer, D.H. Pyrolysis of Dried Wastewater Biosolids Can Be Energy Positive. Water Environ. Res. 2016, 88, 804–810. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Ábrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Seyedi, S. Anaerobic Co-digestion of Aqueous Liquid from Biosolids Pyrolysis. Master’s Thesis, Marquette University, Milwaukee, WI, USA, 2018. [Google Scholar]
- Liu, Z.; Mayer, B.K.; Venkiteshwaran, K.; Seyedi, S.; Raju, A.S.K.; Zitomer, D.; McNamara, P.J. The state of technologies and research for energy recovery from municipal wastewater sludge and biosolids. Curr. Opin. Environ. Sci. Health 2020, 14, 31–36. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, B.S.; Kim, J.S. Recovery of oils with high caloric value and low contaminant content by pyrolysis of digested and dried sewage sludge containing polymer flocculants. Energy Fuels 2008, 22, 1335–1340. [Google Scholar] [CrossRef]
- Hübner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion–Use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef]
- Torri, C.; Fabbri, D. Biochar enables anaerobic digestion of aqueous phase from intermediate pyrolysis of biomass. Bioresour. Technol. 2014, 172, 335–341. [Google Scholar] [CrossRef]
- Li, H.; Xu, Q.; Xue, H.; Yan, Y. Catalytic reforming of the aqueous phase derived from fast-pyrolysis of biomass. Renew. Energy 2009, 34, 2872–2877. [Google Scholar] [CrossRef]
- Seyedi, S.; Venkiteshwaran, K.; Zitomer, D. Toxicity of Various Pyrolysis Liquids from Biosolids on Methane Production Yield. Front. Energy Res. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; McNamara, P.; Zitomer, D. Autocatalytic Pyrolysis of Wastewater Biosolids for Product Upgrading. Environ. Sci. Technol. 2017, 51, 9808–9816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Brown, R.C.; Wen, Z. Anaerobic digestion of aqueous phase from pyrolysis of biomass: Reducing toxicity and improving microbial tolerance. Bioresour. Technol. 2019, 292, 121976. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Parry, D.L.; Lewis, F.M.; Vandenburgh, S.; Haug, R.T.; Amador, J.; Way, S.E.E. Pyrolysis of Dried Biosolids for Increased Biogas Production. Proc. Water Environ. Fed. 2012, 2012, 1128–1139. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Qiu, L.; Yao, Y.; Sun, G.; Guo, X. Anaerobic digestion of swine manure using aqueous pyrolysis liquid as an additive. Renew. Energy 2020, 147, 2484–2493. [Google Scholar] [CrossRef]
- Wen, C.; Moreira, C.M.; Rehmann, L.; Berruti, F. Feasibility of anaerobic digestion as a treatment for the aqueous pyrolysis condensate (APC) of birch bark. Bioresour. Technol. 2020, 307, 123199. [Google Scholar] [CrossRef]
- Specifications: Milorganite. Available online: https://www.milorganite.com/professionals/product-information/specifications (accessed on 13 April 2020).
- Fertilizer Basics |Milorganite. Available online: https://www.milorganite.com/lawn-care/fertilizer-basics (accessed on 14 April 2020).
- Benn, N.; Zitomer, D. Pretreatment and Anaerobic Co-digestion of Selected PHB and PLA Bioplastics. Front. Environ. Sci. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.Y.; Nevin, K.P.; Lovley, D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Benn, N.; Seyedi, S.; Zitomer, D. Methane yield and lag correlate with bacterial community shift following bioplastic anaerobic co-digestion. Bioresour. Technol. Rep. 2019, 7, 100198. [Google Scholar] [CrossRef]
- Speece, R.E. Anaerobic Biotechnology and Odor/Corrosion Control for Municipalities and Industries; Archae Press: Nashville, TN, USA, 2008. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; APHA: Washington DC, USA, 1998. [Google Scholar]
- Carey, D.E.; Zitomer, D.H.; Kappell, A.D.; Choi, M.J.; Hristova, K.R.; McNamara, P.J. Chronic exposure to triclosan sustains microbial community shifts and alters antibiotic resistance gene levels in anaerobic digesters. Environ. Sci. Process. Impacts 2016, 18, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Venkiteshwaran, K.; Milferstedt, K.; Hamelin, J.; Zitomer, D.H. Anaerobic digester bioaugmentation influences quasi steady state performance and microbial community. Water Res. 2016, 104, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Kappell, A.D.; Kimbell, L.K.; Seib, M.D.; Carey, D.E.; Choi, M.J.; Kalayil, T.; Fujimoto, M.; Zitomer, D.H.; McNamara, P.J. Removal of antibiotic resistance genes in an anaerobic membrane bioreactor treating primary clarifier effluent at 20 °C. Environ. Sci. Water Res. Technol. 2018, 4, 1783–1793. [Google Scholar] [CrossRef]
- Falk, M.W.; Song, K.G.; Matiasek, M.G.; Wuertz, S. Microbial community dynamics in replicate membrane bioreactors–Natural reproducible fluctuations. Water Res. 2009, 43, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Regueiro, L.; Lema, J.M. Microbial management of anaerobic digestion: Exploiting the microbiome-functionality nexus. Curr. Opin. Biotechnol. 2015, 33, 103–111. [Google Scholar] [CrossRef]
- Xu, K.; Liu, H.; Chen, J. Effect of classic methanogenic inhibitors on the quantity and diversity of archaeal community and the reductive homoacetogenic activity during the process of anaerobic sludge digestion. Bioresour. Technol. 2010, 101, 2600–2607. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Chaplin, A.V.; Shcherbakova, V.A.; Suzina, N.E.; Kafarskaia, L.I.; Bozhenko, V.K.; Efimov, B.A. Ruthenibacterium lactatiformans gen. nov., sp. nov., an anaerobic, lactate-producing member of the family Ruminococcaceae isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2016, 66, 3041–3049. [Google Scholar] [CrossRef]
- Vos, P.D.; Garrity, G.M.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K. Bergey’s Manual of Systematic Bacteriology—Vol 3: The Firmicutes; Springer-Verlag: New York, NY, USA, 2009; ISBN 9780387950419. [Google Scholar]
- L’Haridon, S.; Jiang, L.; Alain, K.; Chalopin, M.; Rouxel, O.; Beauverger, M.; Xu, H.; Shao, Z.; Jebbar, M. Kosmotoga pacifica sp. nov., a thermophilic chemoorganoheterotrophic bacterium isolated from an East Pacific hydrothermal sediment. Extremophiles 2014, 18, 81–88. [Google Scholar] [CrossRef]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., Sp. nov., An anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, P.P.; Zbinden, R.; Altwegg, M. Novel anaerobic, Gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 2002, 12, 1263–1266. [Google Scholar]
- O’Cuív, P.; Klaassens, E.S.; Durkin, A.S.; Harkins, D.M.; Foster, L.; McCorrison, J.; Torralba, M.; Nelson, K.E.; Morrison, M. Draft genome sequence of Turicibacter sanguinis PC909, isolated from human feces. J. Bacteriol. 2011, 193, 1288–1289. [Google Scholar]
- Verbarg, S.; Rheims, H.; Emus, S.; Frühling, A.; Kroppenstedt, R.M.; Stackebrandt, E.; Schumann, P. Erysipelothrix inopinata sp. nov., isolated in the course of sterile filtration of vegetable peptone broth, and description of Erysipelotrichaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, P.; De Ley, J. Proposal for a New Family, Campylobacteraceae. Int. J. Syst. Evol. Microbiol. 1991, 41, 451–455. [Google Scholar] [CrossRef]
- Collins, M.D.; Shah, H.N. Reclassification of Bacteroides praeacutus Tissier (Holdeman and Moore) in a New Genus, Tissierella, as Tissierella praeacuta. Int. J. Syst. Bacteriol. 1986, 36, 461–463. [Google Scholar] [CrossRef]
- Sieber, J.R.; Sims, D.R.; Han, C.; Kim, E.; Lykidis, A.; Lapidus, A.L.; McDonnald, E.; Rohlin, L.; Culley, D.E.; Gunsalus, R.; et al. The genome of Syntrophomonas wolfei: New insights into syntrophic metabolism and biohydrogen production. Environ. Microbiol. 2010, 12, 2289–2301. [Google Scholar] [CrossRef]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. The division “Synergistes. ” Anaerobe 2007, 13, 99–106. [Google Scholar] [CrossRef]
- Madigou, C.; Poirier, S.; Bureau, C.; Chapleur, O. Acclimation strategy to increase phenol tolerance of an anaerobic microbiota. Bioresour. Technol. 2016, 216, 77–86. [Google Scholar] [CrossRef]
- Fang, H.H.; Zhou, G.M. Degradation of phenol and p-cresol in reactors. Water Sci. Technol. 2000, 42, 237–244. [Google Scholar] [CrossRef]
- Alvares, A.B.C.; Diaper, C.; Parsons, S.A. Partial oxidation by ozone to remove recalcitrance from wastewaters—A review. Environ. Technol. 2001, 22, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Beltran-Heredia, J.; Torregrosa, J.; Acero, J.L. Improvement of the anaerobic biodegradation of olive mill wastewaters by prior ozonation pretreatment. Bioprocess Eng. 1997, 17, 169–175. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. 2019, 37, 107414. [Google Scholar] [CrossRef] [PubMed]

| APL | COD (g/L) | NH3-N (g/L) | VFA Concentration as Acetic Acid (g/L) |
|---|---|---|---|
| Non-catalyzed | 202 ± 4.3 | 63 ± 0.1 | 29 ± 1.6 |
| Non-catalyzed, air-stripped | 198 ± 7.8 | 13 ± 0.6 | 33 ± 1.1 |
| Catalyzed | 33 ± 1.1 | 72 ± 3.4 | 0.06 ± 0.009 |
| Catalyzed, air-stripped | 17 ± 0.7 | 25 ± 0.3 | BD |
| Co-Digestate Feed | Total VFA Concentration as Acetic Acid (mg/L) | Acetic Acid Concentration (mg/L) | COD Concentration (g/L) | SCOD Concentration (g/L) | Methane Production (mL/d) |
|---|---|---|---|---|---|
| None (Control) | BD | BD | 4.1 ± 0.52 | 0.7 ± 0.02 | 38.8 ± 1.8 |
| Catalyzed | 2010 ± 43 | 1500 ± 164 | 7.1 ± 2.5 | 5.2 ± 1.1 | 28.0 ± 4.0 |
| Catalyzed air-stripped | 2070 ± 34 | 1530 ± 153 | 9.6 ± 0.4 | 5.8 ± 0.65 | 28.0 ± 5.9 |
| Non-catalyzed | 50.0 ± 16 | BD | 4.5 ± 0.16 | 0.9 ± 0.05 | 38.0 ± 2.7 |
| Non-catalyzed air-stripped | BD | BD | 4.7 ± 0.57 | 1.1 ± 0.2 | 40.4 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyedi, S.; Venkiteshwaran, K.; Benn, N.; Zitomer, D. Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge. Sustainability 2020, 12, 3441. https://doi.org/10.3390/su12083441
Seyedi S, Venkiteshwaran K, Benn N, Zitomer D. Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge. Sustainability. 2020; 12(8):3441. https://doi.org/10.3390/su12083441
Chicago/Turabian StyleSeyedi, Saba, Kaushik Venkiteshwaran, Nicholas Benn, and Daniel Zitomer. 2020. "Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge" Sustainability 12, no. 8: 3441. https://doi.org/10.3390/su12083441
APA StyleSeyedi, S., Venkiteshwaran, K., Benn, N., & Zitomer, D. (2020). Inhibition during Anaerobic Co-Digestion of Aqueous Pyrolysis Liquid from Wastewater Solids and Synthetic Primary Sludge. Sustainability, 12(8), 3441. https://doi.org/10.3390/su12083441