Four-Year Monitoring Survey of Pesticide Residues in Tomato Samples: Human Health and Environmental Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sample Collection and Processing
2.3. Extraction Procedure
2.4. UHPLC-MS/MS Analysis and Method Validation
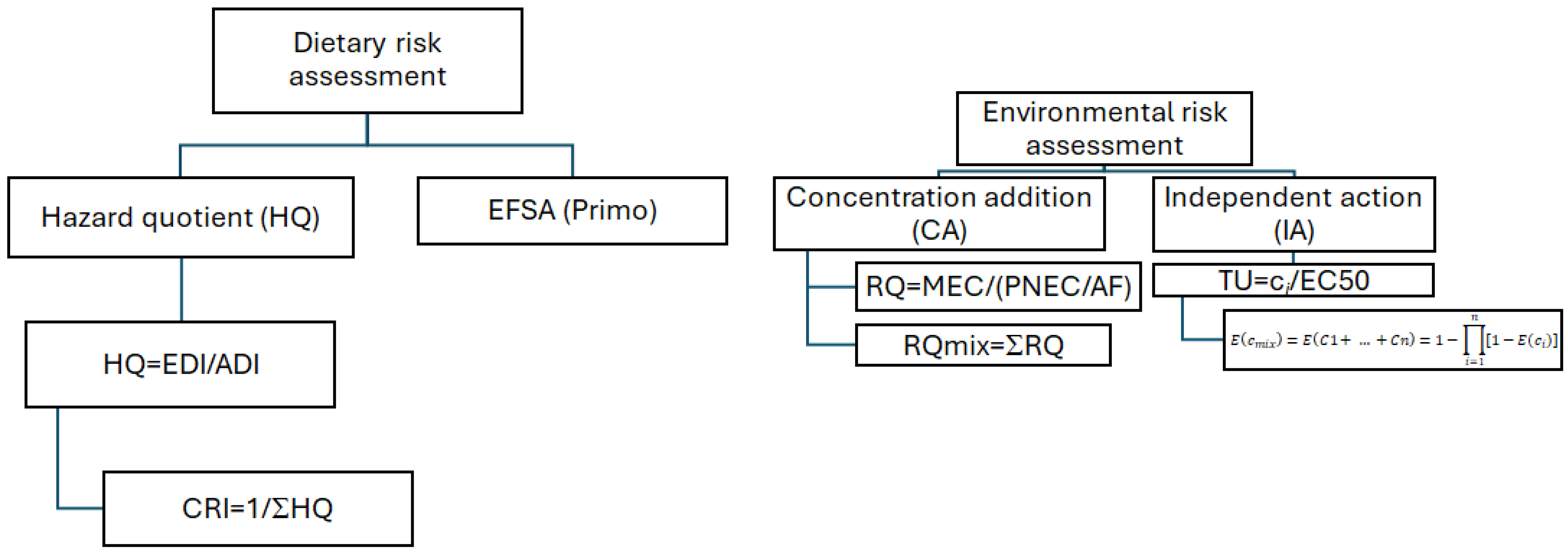
2.5. Risk Assessment
3. Results and Discussion
3.1. Method Validation
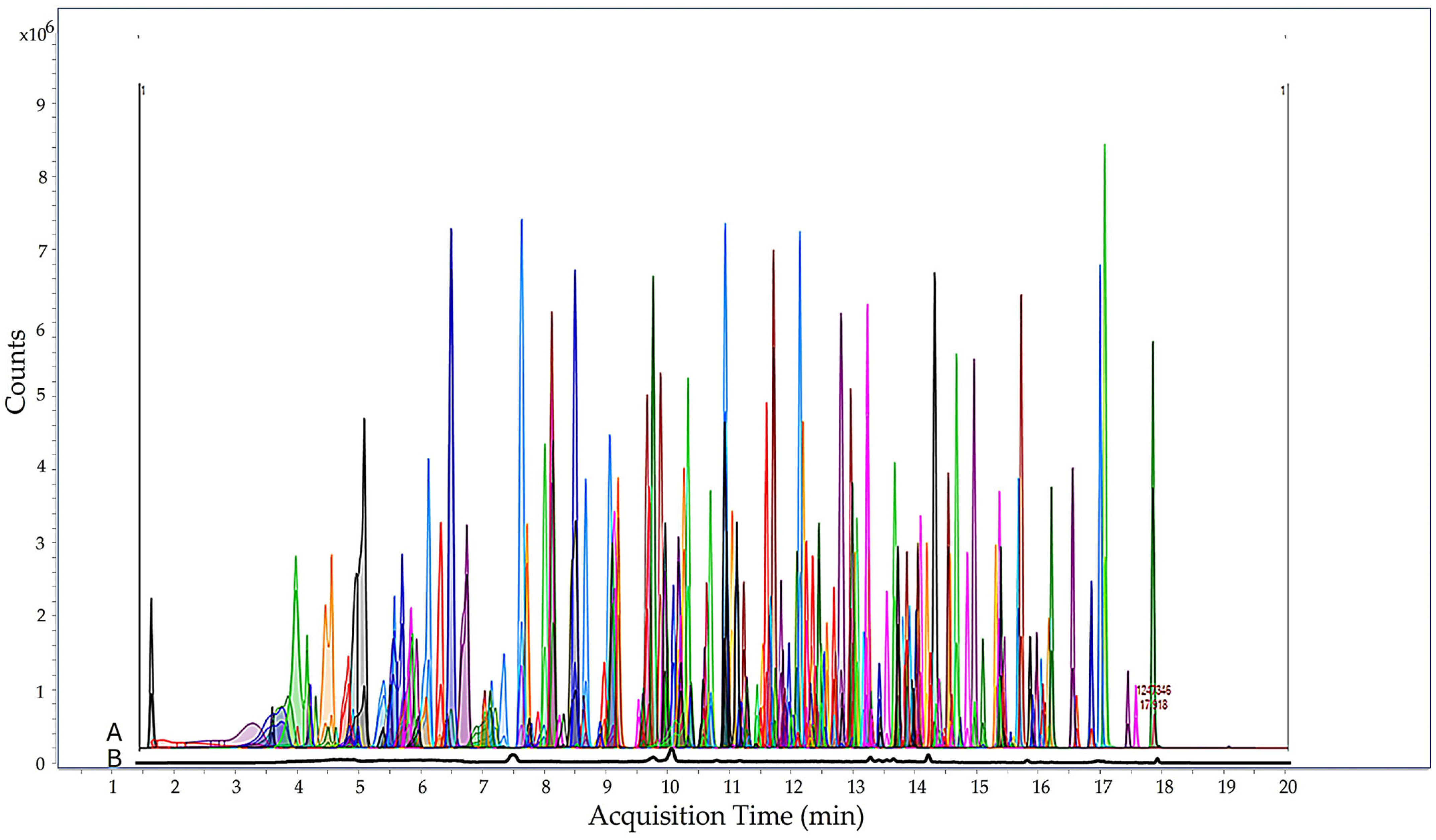
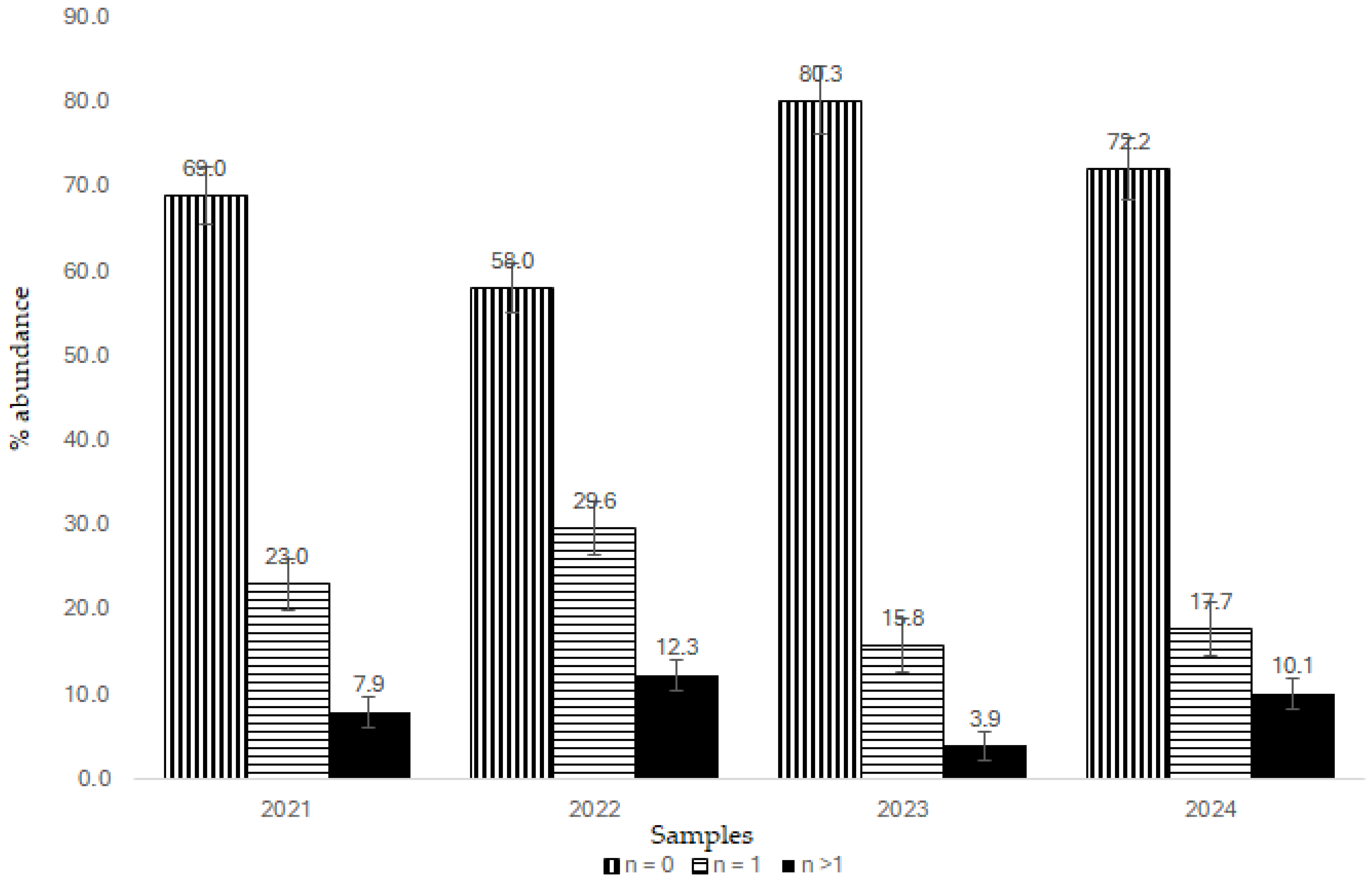
3.2. Monitoring Assessment of Pesticide Residues in Tomato Samples
3.3. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudi, M.; Ruan, D.H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Yang, G.; Wang, Y.; Liu, F.; Chen, C. A review of cumulative risk assessment of multiple pesticide residues in food: Current status, approaches and future perspectives. Trends Food Sci. Technol. 2024, 144, 104340. [Google Scholar] [CrossRef]
- Wesseler, J.H.H. The EU’s farm-to-fork strategy: An assessment from the perspective of agricultural economics. Appl. Econ. Perspect Policy 2022, 44, 13239. [Google Scholar] [CrossRef]
- Integrated Pest Management (IPM). European Commission, Food Safety. Available online: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/integrated-pest-management-ipm_en (accessed on 2 June 2025).
- European Commission. Regulation (EC) no 396/2005 of the European Parliament and of the council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, L70, 1–16. [Google Scholar]
- Djekic, I.; Smigic, N.; Udovicki, B.; Tomic, N. “Zero Residue” Concept—Implementation and Certification Challenges. Standards 2023, 3, 177–186. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; López-Ruiz, R.; Romero-González, R.; Garrido Frenich, A. Multiresidue Methods for Determination of Pesticides and Related Contaminants in Food by Liquid Chromatography. In Liquid Chromatography, 3rd Edition, Applications, Vol 2 Handbooks in Separation Science; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C.F., Riekkola, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 21; pp. 705–732. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2024/989 of 2 April 2024 Concerning A Coordinated Multiannual Control Programme of The Union for 2025, 2026 and 2027 to Ensure Compliance With Maximum Residue Levels of Pesticides and to Assess The Consumer Exposure to Pesticide Residues in and on Food of Plant and Animal Origin and Repealing Implementing Regulation (EU) 2023/731; European Union: Luxembourg, 2024; Available online: http://data.europa.eu/eli/reg_impl/2024/989/oj (accessed on 2 June 2025).
- The Fruit and Vegetable Sector in the EU—A Statistical Overview. Eurostat Data Extracted in January and February 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?oldid=656462 (accessed on 5 March 2025).
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef]
- European Commission. European Commission Regulation (EC) No 10235/2016 of the European Parliament and of the Council of 23 February 2021 on Evaluation of Data Submitted to Confirm MRLs Following the Review of Existing MRLs; European Union: Luxembourg, 2021. [Google Scholar]
- PAN (Piano di Azione Nazionale). Piano di Azione Nazionale per l’Uso Sostenibile dei Prodotti Fitosanitari. Decreto 22 Gennaio 2014—Ai SensI dell’Articolo 6 del Decreto Legislativo 14 Agosto 2012, n. 150 Recante: Attuazione della Direttiva 2009/128/CE che Istituisce un Quadro per l’Azione Comunitaria ai Fini dell’Utilizzo Sostenibile dei Pesticidi; Ministero delle Politiche Agricole Alimentari e Forestali: Rome, Italy, 2014.
- Ministero della Salute. Controllo Ufficiale sui Residui dei Prodotti Fitosanitari negli Alimenti—Rapporto 2020—Ministero della Salute 0018801-24/11/2022-GAB-MDS-A; Ministero della Salute: Rome, Italy, 2022. Available online: https://www.salute.gov.it/new/sites/default/files/imported/C_17_pubblicazioni_3284_allegato.pdf (accessed on 11 June 2025).
- Ouakhssase, A.; Addi, E.A. Monitoring 432 potential pesticides in tomatoes produced and commercialized in Souss Massa region Morocco, using LC-MS/MS and GC-MS/MS. Environ. Pol. 2023, 337, 122611. [Google Scholar] [CrossRef]
- El Sherif, D.F.; Elabasy, N.N.; Sayed, M.A. Monitoring and risk assessment of pesticide residues in tomatoes and cucumbers traded in Fayoum governorate markets, Egypt. FJARD 2024, 38, 616–627. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2002/63/EC of 11 July 2002 Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in or on Products of Plant and Animal Origin Repealing Directive 79/700/EEC; European Commission: Luxembourg, 2002. [Google Scholar]
- Corrias, F.; Atzei, A.; Lai, C.; Dedola, F.; Ibba, E.; Zedda, G.; Canu, F.; Angioni, A. Effects of Industrial Processing on Pesticide Multiresidues Transfer from Raw Tomatoes to Processed Products. Foods 2020, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- European Commission Directorate-General for Health and Food Safety. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/11312/2021 Rev. 1); European Commission: Luxembourg, 2021. [Google Scholar]
- EURACHEM/CITAC. EURACHEM/CITAC Guide Quantifying Uncertainty in Analytical Measurement, 3rd ed.; EURACHEM/CITAC: London, UK, 2012. [Google Scholar]
- Regulation (EC) No 1107/2009 of the European Parliament of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: http://data.europa.eu/eli/reg/2009/1107/oj (accessed on 11 June 2025).
- European Food Safety Authority (EFSA). Pesticide Residue Intake Model—EFSA PRIMo Revision 3.1 (Update of EFSA PRIMo Revision 3); Technical Report, approved 22 March 2019; EFSA: Parma, Italy, 2019. [Google Scholar] [CrossRef]
- Simon, T. Environmental Risk Assessment. A Toxicological Approach, 2nd ed.; available at VitalSource Bookshelf; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 2020; ISBN E-Book 9780429286001. [Google Scholar]
- Di Piazza, G.; Dujardin, B.; Levorato, S.; Medina, P.; Mohimont, L.; Solazzo, E.; Costanzo, V. Prioritisation of pesticides and target organ systems for dietary cumulative risk assessment based on the 2019–2021 monitoring cycle. EFSA J. 2024, 22, e8554. [Google Scholar] [CrossRef]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hameyd, P.Y.; Sebestyene, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Ecotoxicological assessment of pharmaceuticals and personal care products using predictive toxicology approaches. Green Chem. 2020, 22, 1458. [Google Scholar] [CrossRef]
- Price, P.S.; Han, X. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int. J. Environ. Res. Public Health 2011, 8, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Document SANTE/11813/2017. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commission: Luxembourg, 2017. [Google Scholar]
- European Commission. EU Pesticides Database. 2023. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 11 June 2025).
- Grosssteiner, I.; Mienne, A.; Lucas, L.; Yvonnet, P.L.; Trenteseaux, C.; Fontaine, K.; Sarda, X. Cumulative risk assessment with pesticides in the framework of MRL setting. EFSA J. 2023, 21 (Suppl. S1), e211009. [Google Scholar] [CrossRef]
- Choubbane, H.; Ouakhssase, A.; Chahid, A.; Taourirte, M.; Aamouche, A. Pesticides in fruits and vegetables from the Souss Massa region, Morocco. Food Addit. Contam. B Surveill. 2022, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, V.; Calitri, A.; Casamassima, F.P.; Della Rovere, I.; Ingegno, M.T.; Iammarino, M. Monitoring of Pesticides in Fruit and Vegetables Marketed in Italy during 2020/2021 and Food Safety Evaluations. In Proceedings of the 14th European Pesticide Residue Workshop (EPRW 2022), Bologna, Italy, 19–23 September 2022. Abstract PT–07. [Google Scholar]
- Li, Z.; Nie, J.; Yan, Z.; Cheng, Y.; Lan, F.; Huang, Y.; Chen, Q.; Zhao, X.; Li, A. A monitoring survey and dietary risk assessment for pesticide residues on peaches in China. Regul. Toxicol. Pharmacol. 2018, 97, 152–162. [Google Scholar] [CrossRef]
- EFSA Scientific Report. The 2021 European Union report on pesticide residues in food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef]
- Weisner, O.; Frische, T.; Liebmann, L.; Reemtsma, T.; Roß-Nickoll, M.; Schäfer, R.B.; Schäffer, A.; Scholz-Starke, B.; Vormeier, P.; Knillmann, S.; et al. Risk from pesticide mixtures—The gap between risk assessment and reality. Sci. Total Environ. 2021, 796, 149017. [Google Scholar] [CrossRef]
- EFSA (EFSA Panel on Plant Protection Products and their Residues). Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters. EFSA J. 2013, 11, 3290. [Google Scholar] [CrossRef]
- Backhaus, T.; Faust, M. Predictive Environmental Risk Assessment of Chemical Mixtures: A Conceptual Framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef]
- Backhaus, T.; Altenburger, R.; Faust, M.; Frein, D.; Frische, T.; Johansson, P.; Kehrer, A.; Porsbring, T. Proposal for environmental mixture risk assessment in the context of the biocidal product authorization in the EU. Environ. Sci. Eur. 2013, 25, 1–9. [Google Scholar] [CrossRef]
- Dietrich, C.; Wang, M.; Ebeling, M.; Gladbach, A. An efficient and pragmatic approach for regulatory aquatic mixture risk assessment of pesticides. Environ. Sci. Eur. 2022, 34, 16. [Google Scholar] [CrossRef]
- Cedergreen, N.; Christensen, A.M.; Kamper, A.; Kudsk, P.; Mathiassen, S.K.; Streibig, J.C.; Sorensen, H. A Review of independent action compared to concentration addition as reference models for mixture of compounds with different molecular target sites. Environ. Toxicol. Chem. 2008, 27, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A.; Backhaus, T.; Faust, M. State of the Art Report on Mixture Toxicity; Report to the EU Commission, Directorate General for the Environment, No. 070307/2007/485103/ETU/D.1; EU Commission: Brussels, Belgium, 2009. [Google Scholar]
- Laetz, C.A.; Baldwin, D.H.; Hebert, V.R.; Stark, J.D.; Scholz, N.L. The synergistic toxicity of pesticide mixtures: Implications for risk assessment and the conservation of endangered Pacific salmon. Environ. Health Perspect. 2009, 117, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.T.; Vo, P.L.T.; Truong, L. Developmental neurotoxicity of pesticide mixtures in zebrafish: Synergistic effects on behavior and acetylcholinesterase activity. Chemosphere 2022, 301, 134416. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Peer review of the pesticide risk assessment of theactive substance acetamiprid. Conclusion on Pesticide Peer review. EFSA J. 2016, 14, 4610. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance cymoxanil. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2008, 167, 1–116. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Available online: https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/113501/113501-068.pdf (accessed on 11 June 2025).
- European Food Safety (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance azoxystrobin. Conclusion on Pesticide Peer review. EFSA J. 2010, 8, 1542. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Evaluation of the new active BOSCALID in the product FILAN FUNGICIDE. Australian Pesticides and Veterinary Medicines Authority 2004. Available online: https://www.apvma.gov.au/sites/default/files/publication/13621-prs-boscalid.pdf (accessed on 11 June 2025).
- European Food Safety (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance mandipropamid. Conclusion on Pesticide Peer review. EFSA J. 2012, 10, 2935. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance dimethomorph. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2006, 82, 1–69. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion on the peer review of the pesticide risk assessment of the activesubstance myclobutanil. Conclusion on Pesticide Peer review. EFSA J. 2010, 8, 1682. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance tetraconazole. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2008, 152, 1–86. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance penconazole. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2008, 175, 1–104. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance tebuconazole. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2008, 176, 1–109. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance zoxamide. Conclusion on Pesticide Peer review. EFSA J. 2017, 15, 4980. [Google Scholar] [CrossRef][Green Version]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance spinosad. Conclusion on Pesticide Peer review. EFSA J. 2018, 16, 5252. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance pyraclostrobin. Conclusion on Pesticide Peer review. EFSA J. 2025, 23, e9257. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance clofentezin. Conclusion on Pesticide Peer review. EFSA J. 2021, 19, 6817. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance difenconazole. Conclusion on Pesticide Peer review. EFSA J. 2011, 9, 1967. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance ametotractin. Conclusion on Pesticide Peer review. EFSA J. 2012, 10, 2921. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance metaflumizone. Conclusion on Pesticide Peer review. EFSA J. 2013, 11, 3373. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance emamectin. Conclusion on Pesticide Peer review. EFSA J. 2012, 10, 2955. [Google Scholar] [CrossRef]
- European Food Safety (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance etofenprox. Conclusion on Pesticide Peer review. EFSA Sci. Rep. 2008, 213, 1–131. [Google Scholar] [CrossRef]
| Adults | Toddlers | ||||
|---|---|---|---|---|---|
| Pesticide | ADI (mg/kg/d) | EDI (mg/kg bw/d) | HQ | EDI (mg/kg bw/d) | HQ |
| Acetamiprid | 0.005 * | 1.26 × 10−4 | 2.53 × 10−2 | 1.25 × 10−4 | 2.49 × 10−2 |
| Acetamiprid | 0.025 | 1.26 × 10−4 | 5.05 × 10−3 | 1.25 × 10−4 | 4.98 × 10−3 |
| Cymoxanil | 0.013 | 5.39 × 10−5 | 4.15 × 10−3 | 5.32 × 10−5 | 4.09 × 10−3 |
| Metalaxyl | 0.08 | 6.48 × 10−5 | 8.11 × 10−4 | 6.39 × 10−5 | 7.99 × 10−4 |
| Azoxystrobin | 0.2 | 1.18 × 10−4 | 5.92 × 10−4 | 1.17 × 10−4 | 5.84 × 10−4 |
| Boscalid | 0.04 | 3.49 × 10−4 | 8.73 × 10−3 | 3.44 × 10−4 | 8.61 × 10−3 |
| Mandipropamid | 0.15 | 3.77 × 10−5 | 2.51 × 10−4 | 3.71 × 10−5 | 2.48 × 10−4 |
| Dimethomorph | 0.05 | 1.02 × 10−4 | 2.04 × 10−3 | 1.01 × 10−4 | 2.02 × 10−3 |
| Myclobutanil | 0.025 | 4.06 × 10−5 | 1.62 × 10−3 | 4.00 × 10−5 | 1.60 × 10−3 |
| Tetraconazole | 0.004 | 5.58 × 10−5 | 1.39 × 10−2 | 5.50 × 10−5 | 1.38 × 10−2 |
| Penconazole | 0.03 | 2.49 × 10−2 | 9.37 × 10−4 | 2.77 × 10−5 | 9.24 × 10−4 |
| Tebuconazole | 0.03 | 4.09 × 10−3 | 3.94 × 10−3 | 1.17 × 10−4 | 3.89 × 10−3 |
| Zoxamide | 0.5 | 7.99 × 10−4 | 2.15 × 10−4 | 1.06 × 10−4 | 2.12 × 10−4 |
| Spinosad (sum A + D) | 0.024 | 5.84 × 10−4 | 3.10 × 10−3 | 7.34 × 10−5 | 3.06 × 10−3 |
| Pyraclostrobin | 0.03 | 8.61 × 10−3 | 2.11 × 10−3 | 6.25 × 10−5 | 2.08 × 10−3 |
| Clofentezin | 0.017 | 2.48 × 10−4 | 2.00 × 10−3 | 3.35 × 10−5 | 1.97 × 10−3 |
| Difenoconazole | 0.01 | 2.02 × 10−3 | 2.33 × 10−2 | 2.30 × 10−4 | 2.30 × 10−2 |
| Ametoctradin | 10 | 1.60 × 10−3 | 1.07 × 10−5 | 1.06 × 10−4 | 1.06 × 10−5 |
| Metaflumizone | 0.01 | 1.38 × 10−2 | 1.01 × 10−3 | 1.00 × 10−5 | 1.00 × 10−3 |
| Emamectin | 0.0005 | 2.49 × 10−2 | 4.51 × 10−2 | 2.23 × 10−5 | 4.45 × 10−2 |
| Etofenprox | 0.03 | 4.04 × 10−5 | 1.35 × 10−3 | 3.98 × 10−5 | 1.33 × 10−3 |
| Plant Protection Product | Toxicity Class (EPA) | NOAEL mg·Kg bw·d | Toxicity Activity | Chemical Class | Main Uses |
|---|---|---|---|---|---|
| Acetamiprid | II/III | 10 | Acetylcholine, nicotinic receptors | neonicotinoid | insecticide |
| Cymoxanil | II | 0.01 | Inhibits cytochrome c oxidase in the fourth complex of the electron transport chain | organic nitriles | fungicide |
| Metalaxyl | III | 9.4 | Disrupts fungal nucleic acid synthesis by inhibiting RNA polymerase I | phenylamide | fungicide |
| Azoxystrobin | III/IV | 20 | Quinone outside inhibitor (QoI)-type | strobilurin | fungicide |
| Boscalid | III/IV | 22 | Mitochondrial damage | aromatic anilides | fungicide |
| Mandipropamid | IV | 247.6 | Inhibitor of phospholipid biosynthesis | mandelamide | fungicide |
| Dimethomorph | III | 16 | Endocrine disruptor | morpholine group | fungicide |
| Myclobutanil | II | 2.5 | Induce liver microsomal enzymes | triazole | fungicide |
| Tetraconazole | III/IV | 2.95 | Interact with nuclear receptors (constitutive androstane receptor, CAR; pregnane X receptor, PXR), liver toxicity. | triazole | fungicide |
| Penconazole | III | 69 | Increase oxidative damage and modify enzyme activity | triazole | fungicide |
| Tebuconazole | III/IV | 3 | Activate nuclear receptors (aryl hydrocarbon receptor, AHR), trigger oxidative stress and apoptosis. | triazole | fungicide |
| Zoxamide | III/IV | 50 | Mitotic arrest by disrupting cell division, binding to β-tubulin and inhibiting tubulin polymerisation | benzamide | fungicide |
| Spinosad (Sum A + D) | III | 4.89 | Nicotinic acetylcholine receptor | spynosin | insecticide |
| Pyraclostrobin | IV | 3.4 | Quinone outside inhibitor (QoI)-type | strobilurin | fungicide |
| Clofentezin | III | 1.95 | Effect on the liver (enzyme induction) Affects thyroid function and impairs steroid hormone regulation | tetrazine | acaricide |
| Difenoconazole | III/IV | 3.7 | Disruptions to the endocrine system and nervous system | dioxolanes | fungicide |
| Ametoctradin | IV | 1000 | Disrupting mitochondrial energy production | triazolopyrimidine | fungicide |
| Metaflumizone | IV | 40 | Sodium channel blocker | semicarbazone | insecticide |
| Emamectin | II | 0.6 | Chloride channel activator by binding gamma aminobutyric acid (GABA) receptor and glutamate-gated chloride channels disrupting nerve signals within arthropods | second-generation avermectin | insecticide |
| Etofenprox | IV | 23 | Disturbs insect nervous systems following direct contact or ingestion | pyrethroid derivative | insecticide |
| 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|
| Residues (mg/kg ± RSD%) * | ||||
| min | 0.058 ± 3.61 | 0.068 ± 5.59 | 0.067 ± 5.27 | 0.104 ± 9.17 |
| max | 0.228 ± 3.80 | 1.061 ± 1.27 | 0.584 ± 9.77 | 0.403 ± 5.73 |
| median | 0.133 ± 11.46 | 0.204 ± 2.46 | 0.188 ± 1.96 | 0.260 ± 1.53 |
| CRI adult | ||||
| min | 22.64 ± 16.03 | 21.90 ± 1.26 | 81.12 ± 9.03 | 54.14 ± 13.84 |
| max | 3096.03 ± 20.10 | 879.30 ± 9.15 | 1250.86 ± 5.07 | 679.97 ± 10.10 |
| median | 446.49 ± 4.14 | 237.70 ± 4.98 | 177.53 ± 2.63 | 115.61 ± 4.49 |
| CRI toddler | ||||
| min | 22.96 ± 16.03 | 22.21 ± 1.26 | 82.26 ± 9.03 | 54.91 ± 13.84 |
| max | 3139.64 ± 20.10 | 891.68 ± 9.15 | 1268.48 ± 5.07 | 689.54 ± 10.10 |
| median | 452.78 ± 4.14 | 241.03 ± 4.97 | 180.03 ± 2.63 | 117.24 ± 4.49 |
| 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|
| CA | ||||
| min | 0.10 | 0.01 | 0.52 | 0.00 |
| max | 14.10 | 15.36 | 235.0 | 0.95 |
| median | 0.46 | 10.93 | 1.86 | 0.93 |
| IA | ||||
| min | 0.01 | 0.01 | 0.22 | 0.00 |
| max | 1.37 | 15.25 | 5.64 | 00.095 |
| median | 0.51 | 2.62 | 1.56 | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzei, A.; Bouakline, H.; Corrias, F.; Angioni, A. Four-Year Monitoring Survey of Pesticide Residues in Tomato Samples: Human Health and Environmental Risk Assessment. J. Xenobiot. 2025, 15, 171. https://doi.org/10.3390/jox15050171
Atzei A, Bouakline H, Corrias F, Angioni A. Four-Year Monitoring Survey of Pesticide Residues in Tomato Samples: Human Health and Environmental Risk Assessment. Journal of Xenobiotics. 2025; 15(5):171. https://doi.org/10.3390/jox15050171
Chicago/Turabian StyleAtzei, Alessandro, Hamza Bouakline, Francesco Corrias, and Alberto Angioni. 2025. "Four-Year Monitoring Survey of Pesticide Residues in Tomato Samples: Human Health and Environmental Risk Assessment" Journal of Xenobiotics 15, no. 5: 171. https://doi.org/10.3390/jox15050171
APA StyleAtzei, A., Bouakline, H., Corrias, F., & Angioni, A. (2025). Four-Year Monitoring Survey of Pesticide Residues in Tomato Samples: Human Health and Environmental Risk Assessment. Journal of Xenobiotics, 15(5), 171. https://doi.org/10.3390/jox15050171









