Innovative Analytical Approaches for Food Pesticide Residue Detection: Towards One Health-Oriented Risk Monitoring
Abstract
1. Introduction
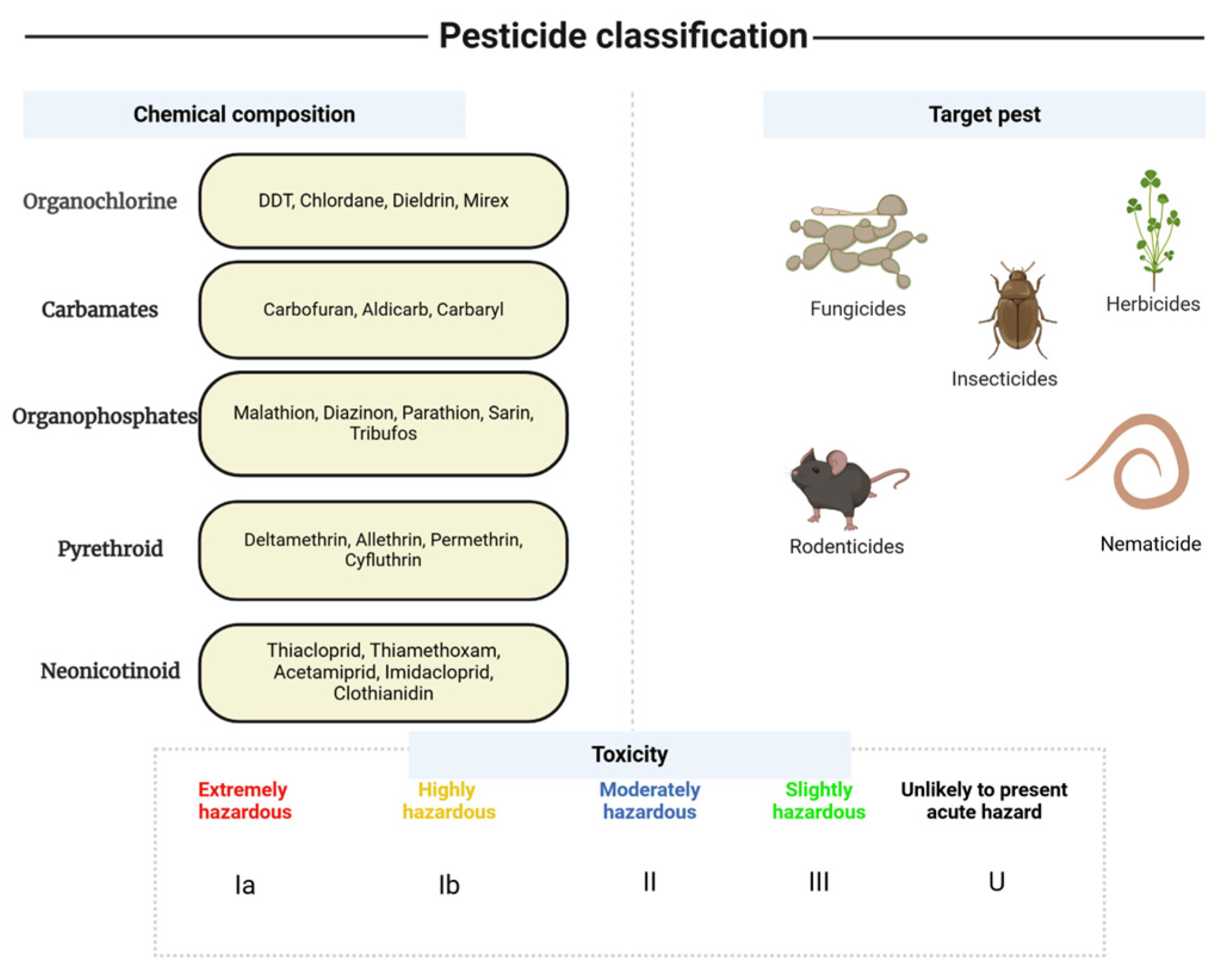
2. Pesticide Types, Characteristics, and Regulatory Framework
2.1. OCPs

2.2. OPPs
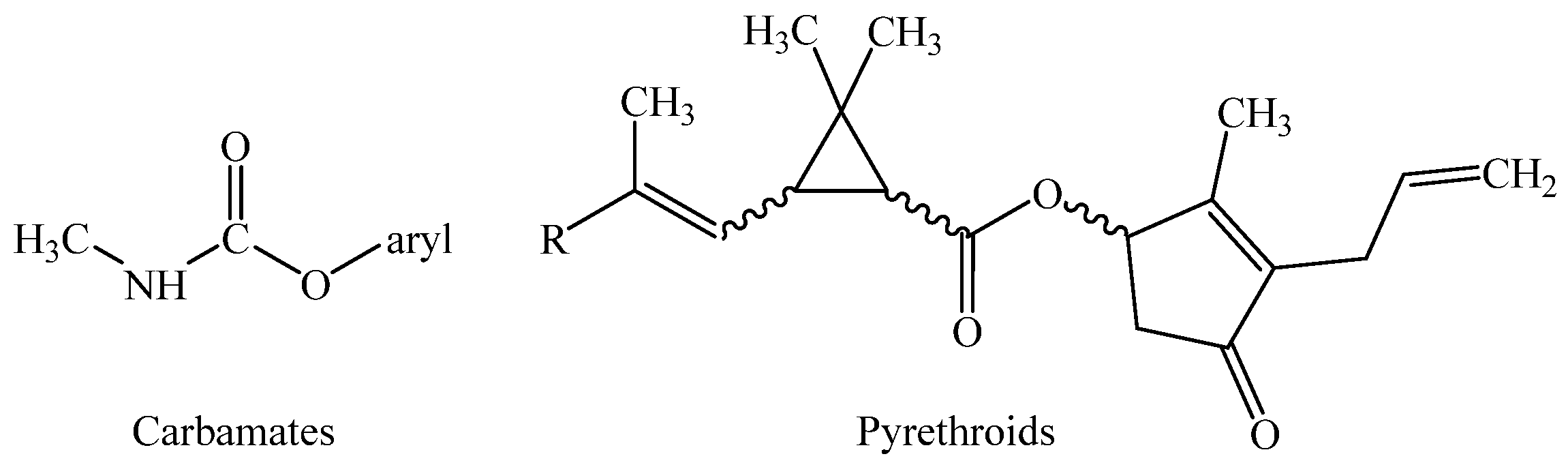
2.3. Carbamates
2.4. Pyrethroids
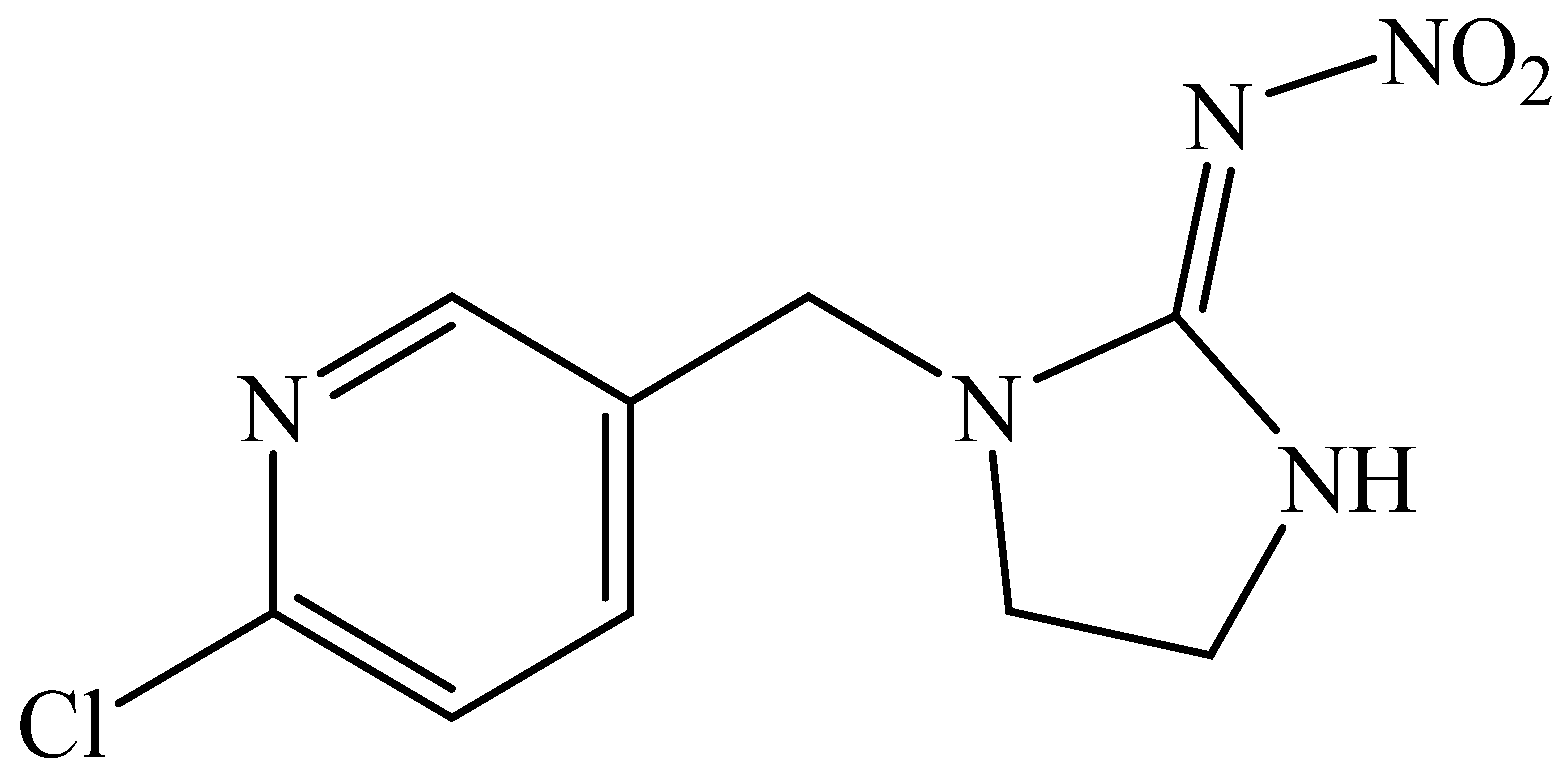
2.5. Neonicotinoids
2.6. Regulatory Framework
3. Sample Pretreatment
3.1. MAE (Microwave-Assisted Extraction)
3.2. ASE (Accelerated Solvent Extraction)
3.3. SPE (Solid Phase Extraction)
3.4. dSPE (Dispersive Solid Phase Extraction)
3.5. MSPD (Matrix Solid-Phase Dispersion)
3.6. SPME (Solid-Phase Microextraction)
3.7. SFE (Supercritical Fluid Extraction)
3.8. QuEChERS
3.9. HF-LPME (Hollow Fiber Liquid-Phase Microextraction)
3.10. DLLME (Dispersive Liquid–Liquid Microextraction)
3.11. SDME (Single Drop Microextraction)
3.12. CSDF-ME (Continuous Sample Drop Flow Microextraction)
4. Detection Methods
4.1. Chromatography Techniques
4.2. Rapid Technologies
4.2.1. Biosensors
| Transductor | Food Matrix | Pesticide | Characteristics | LOD | Ref. |
|---|---|---|---|---|---|
| Electrochemical | - | Paraoxon | 20 consecutive measurements → the operational stability = 94.13% Storage stability (60 days) = 70% | 0.17 nM | [188] |
| Electrochemical | Cabbage | Dichlorvos | Good stability | 0.23 nM | [189] |
| Electrochemiluminescence | Rape Pineapple | Methyl parathion | Highly sensitive | - | [190] |
| Fluorescence | Tea | Quinalphos Thiamethoxam Propargite Hexaconazole | High stability Simultaneous detection of 4 pesticides in a single sample | 0.2 ng/mL | [140] |
| Fluorescence | Orange juice Apple juice | Ethyl parathion | High sensitivity Simpler synthetic protocol | 2.40 pM | [45] |
| Colorimetric | Fruits Vegetables | Chlorpyrifos Profenofos Cypermethrin | High sensitivity Good linear response | 0.235 mg/L 4.891 mg/L 4.053 mg/L | [191] |
| Colorimetric | Pear Rice Cabbage | Parathion | Wide linear range 0.01–50 µg·L−1 | 2.04 ng·L−1 | [192] |
| SERS | - | Profenofos Acetamiprid Carbendazim | Stable and significantly short measurement time Low detection limit | 0.0021 ng mL−1 0.0046 ng mL−1 0.0061 ng mL−1 | [193] |
| SERS | Apples | Methyl parathion | Paper-based substrate Superior reproducibility Good stability and sensitivity | 0.011 µg/cm2 | [44] |
4.2.2. ELISA (Enzyme-Linked Immunosorbent Assays)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRLs | Maximum Residue Limits |
| GC | Gas Chromatography |
| MS | Mass Spectrometry |
| LC | Liquid Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| OCPs | Organochlorine Pesticides |
| OPPs | Organophosphates Pesticides |
| DDT | p,p′-dichlorodiphenyltrichloroethane |
| DDE | Dichlorodiphenyldichloroethylene |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| EPA | Environmental Protection Agency |
| IARC | International Agency for Research on Cancer |
| AChE | Acetylcholinesterase |
| EFSA | European Food Safety Authority |
| GAP | Good Agricultural Practices |
| HBGVs | Health-Based Guidance Values |
| ADI | Acceptable Daily Intake |
| UL | Tolerable Upper Intake Level |
| SFC | Supercritical Fluid Chromatography |
| MAE | Microwave-Assisted Extraction |
| PFAS | Polyfluoroalkyl Substances |
| LOD | Limit Of Detection |
| LOQ | Limit Of Quantification |
| ASE | Accelerated Solvent Extraction |
| QuEChERS | Quick, Easy, Cheap, Rugged, Effective and Safe |
| PSA | Primary Secondary Amine |
| SPE | Solid Phase Extraction |
| dSPE | Dispersive Solid Phase Extraction |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| SPME | Solid-Phase Microextraction |
| MSPD | Matrix Slid-Phase Dispersion |
| BiT-MSPD | Balls-in-Tube Matrix Solid-Phase Dispersion |
| SFE | Supercritical Fluid Extraction |
| HF-LPME | Hollow Fiber Liquid-Phase Microextraction |
| PF | Pre-concentration Factor |
| EF | Enrichment Factor |
| ER | Extraction Recovery |
| RR | Recovery Rate |
| DLLME | Dispersive Liquid–Liquid Microextraction |
| SDME | Single Drop Microextraction |
| CSDF-ME | Continuous Sample Drop Flow Microextraction |
| CFME | Continuous Flow Microextraction |
| LPME | Liquid-Phase Microextraction |
| UAE | Ultrasound-Assisted Extraction |
| HF-PLM | Hollow Fiber-Protected Liquid-Phase Microextraction |
| G-HF-LPME | Graphene-Reinforced Hollow Fiber Liquid-Phase Microextraction |
| G-HC-LPME | Gas-assisted Hollow Fiber Liquid-Phase Microextraction |
| HS-SPME | Headspace Solid-Phase Microextraction |
| NPD | Nitrogen Phosphorus Detector |
| ECD | Electron Capture Detector |
| FID | Flame Ionization Detector |
| FPD | Flame Photometric Detector |
| HRMS | High-Resolution Mass Spectrometer |
| Q-TOF/MS | Quadrupole-Time-of-Flight Mass Spectrometry |
| FL | Fluorescence |
| SERS | Surface-Enhanced Raman Spectroscopy or Scattering |
| UV | Ultraviolet |
| ELISA | Enzyme-Linked Immune Sorbent Assays |
References
- Ouyang, M.; Liu, T.; Yuan, X.; Xie, C.; Luo, K.; Zhou, L. Nanomaterials-Based Aptasensors for Rapid Detection and Early Warning of Key Food Contaminants: A Review. Food Chem. 2025, 462, 140990. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Trincă, L.C. Heavy Metals in Foods and Beverages: Global Situation, Health Risks and Reduction Methods. Foods 2023, 12, 3340. [Google Scholar] [CrossRef]
- Popa, I.D.; Şchiriac, E.C.; Ungureanu, D.; Cuciureanu, R. Immune Response in Rats Following Administration of Honey with Sulfonamides Residues. Rev. Rom. Med. Lab. 2012, 20, 63–72. [Google Scholar]
- Hashimi, M.H.; Hashimi, R.; Ryan, Q. Toxic Effects of Pesticides on Humans, Plants, Animals, Pollinators and Beneficial Organisms. Asian Plant Res. J. 2020, 5, 37–47. [Google Scholar] [CrossRef]
- Bashir, K.; Shikha, S.; Rattu, G.; Jan, K.; Krishna, P.M.; Pattanayek, S.K. Pesticide Residues and Their Detection Techniques in Foods Using Sensors—A Review. J. Food Sci. Technol. 2024, 62, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Jadhav, S.; Sonone, S.; Singh Sankhla, M.; Kumar, R. Microalgae Based Sustainable Bioremediation of Water Contaminated by Pesticides. Biointerface Res. Appl. Chem. 2021, 12, 149–169. [Google Scholar] [CrossRef]
- Hoffmann, V.; Paul, B.; Falade, T.; Moodley, A.; Ramankutty, N.; Olawoye, J.; Djouaka, R.; Lekei, E.; de Haan, N.; Ballantyne, P.; et al. A One Health Approach to Plant Health. CABI Agric. Biosci. 2022, 3, 62. [Google Scholar] [CrossRef]
- Botnaru, A.A.; Lupu, A.; Morariu, P.C.; Pop, O.L.; Nedelcu, A.H.; Morariu, B.A.; Cioancă, O.; Di Gioia, M.L.; Lupu, V.V.; Avasilcai, L.; et al. Balancing Health and Sustainability: Assessing the Benefits of Plant-Based Diets and the Risk of Pesticide Residues. Nutrients 2025, 17, 727. [Google Scholar] [CrossRef]
- Dhuldhaj, U.P.; Singh, R.; Singh, V.K. Pesticide Contamination in Agro-Ecosystems: Toxicity, Impacts, and Bio-Based Management Strategies. Environ. Sci. Pollut. Res. 2023, 30, 9243–9270. [Google Scholar] [CrossRef]
- Falkenberg, T.; Ekesi, S.; Borgemeister, C. Integrated Pest Management (IPM) and One Health—A Call for Action to Integrate. Curr. Opin. Insect. Sci. 2022, 53, 100960. [Google Scholar] [CrossRef]
- Gavahian, M.; Pallares, N.; Al Khawli, F.; Ferrer, E.; Barba, F.J. Recent Advances in the Application of Innovative Food Processing Technologies for Mycotoxins and Pesticide Reduction in Foods. Trends Food Sci. Technol. 2020, 106, 209–218. [Google Scholar] [CrossRef]
- Garud, A.; Pawar, S.; Patil, M.S.; Kale, S.R.; Patil, S. A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact. Cureus 2024, 16, e67945. [Google Scholar] [CrossRef]
- Louppis, A.P.; Kontominas, M.G. Recent Developments (2020–23) on the Use of LC in the Determination of Food Contaminants. Separations 2024, 11, 342. [Google Scholar] [CrossRef]
- Botnaru, A.A.; Lupu, A.; Morariu, P.C.; Jităreanu, A.; Nedelcu, A.H.; Morariu, B.A.; Anton, E.; Di Gioia, M.L.; Lupu, V.V.; Dragostin, O.M.; et al. Neurotoxic Effects of Pesticides: Implications for Neurodegenerative and Neurobehavioral Disorders. J. Xenobiot. 2025, 15, 83. [Google Scholar] [CrossRef]
- Caba, I.-C.; Ștreangă, V.; Dobrin, M.-E.; Jităreanu, C.; Jităreanu, A.; Profire, B.; Apotrosoaei, M.; Focșa, A.-V.; Caba, B.; Agoroaei, L. Clinical Assessment of Acute Organophosphorus Pesticide Poisoning in Pediatric Patients Admitted to the Toxicology Emergency Department. Toxics 2022, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Boneva, I.; Yaneva, S.; Danalev, D. Development and Validation of Method for Determination of Organophosphorus Pesticides Traces in Liver Sample by GC-MS/MS-Ion Trap. Acta Chromatogr. 2021, 33, 188–194. [Google Scholar] [CrossRef]
- Tucker, S.; Dumitriu, G.-D.; Teodosiu, C. Pesticides Identification and Sustainable Viticulture Practices to Reduce Their Use: An Overview. Molecules 2022, 27, 8205. [Google Scholar] [CrossRef] [PubMed]
- Sel, S.; Er, E.Ö.; Koyuncu, İ. Development of an Analytical Method for the Determination of Pesticides in Tropical Fruits by LC-QTOF-MS/MS after QuEChERS Extraction Sample Cleanup and DLLME Preconcentration. Methods Appl. Fluoresc. 2024, 12, 015008. [Google Scholar] [CrossRef]
- Chormey, D.S.; Zaman, B.T.; Kasa, N.A.; Bakırdere, S. Liquid Phase Microextraction Strategies and Their Application in the Determination of Endocrine Disruptive Compounds in Food Samples. TrAC Trends Anal. Chem. 2020, 128, 115917. [Google Scholar] [CrossRef]
- Rani, P.; Nanda, B.P.; Narang, R.K.; Bhatia, R. Advancements in Solvent Microextraction: Recent Developments and Diverse Applications in the Modern Era. Sep. Sci. Plus 2024, 7, 2300243. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Lehotay, S.J.; Michlig, N.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Critical Review and Re-Assessment of Analyte Protectants in Gas Chromatography. J. Chromatogr. A 2020, 1632, 461596. [Google Scholar] [CrossRef]
- Brinco, J.; Guedes, P.; Gomes da Silva, M.; Mateus, E.P.; Ribeiro, A.B. Analysis of Pesticide Residues in Soil: A Review and Comparison of Methodologies. Microchem. J. 2023, 195, 109465. [Google Scholar] [CrossRef]
- Shalaby, A.A.; El-Sheikh, E.S.A.; Refaat, A.M.; Ragheb, D.A. Residue Analysis and Associated Risk Assessment of Hexythiazox and Spinosad Applied on Strawberry Plants. Egypt. J. Chem. 2022, 65, 489–498. [Google Scholar] [CrossRef]
- Gai, T.; Nie, J.; Ding, Z.; Wu, W.; Liu, X. Progress of Rapid Detection of Pesticides in Fruits and Vegetables. Front. Food Sci. Technol. 2023, 3, 1253227. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Tsekouras, V.; Mercader, J.V.; Abad-Fuentes, A.; Kintzios, S. Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce. Biosensors 2024, 14, 311. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Pulkrabova, J.; Hajslova, J. Optical Screening Methods for Pesticide Residue Detection in Food Matrices: Advances and Emerging Analytical Trends. Foods 2021, 10, 88. [Google Scholar] [CrossRef]
- Ansari, I.; Magdy El-Kady, M.; Muniyan, S. A Review on the Fatal Impact of Pesticide Toxicity on Environment and Human Health 16. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Dawson, A.H.; Eddleston, M.; Senarathna, L.; Mohamed, F.; Gawarammana, I.; Bowe, S.J.; Manuweera, G.; Buckley, N.A. Acute Human Lethal Toxicity of Agricultural Pesticides: A Prospective Cohort Study. PLoS Med. 2010, 7, e1000357. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-Baranowska, I. A Holistic Study of Neonicotinoids Neuroactive Insecticides—Properties, Applications, Occurrence, and Analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Kardani, F.; Jelyani, A.Z.; Hashemi, M.; Rashedinia, M.; Shariati, S.; Mirzaei, R.; Mahdavinia, M.; Noori, S.M.A. Determination of 323 Pesticide Residues in Iran’s Cereal by GC-MS and HPLC-UV Combined with QuEChERS Extraction and Mixed-Mode SPE Clean-up Method. J. Food Compos. Anal. 2023, 124, 105670. [Google Scholar] [CrossRef]
- Pany, B.K.; Sahu, G.; Pattnaik, M.; Pany, K.; Jena, D.; Kumar Pal, A. Effect of Organochlorine Pesticides on Living Organisms and Environment Chemical Science Review and Letters Effect of Organochlorine Pesticides on Living Organisms and Environment. Chem. Sci. Rev. Lett. 2020, 9, 682–686. Available online: https://chesci.com/wp-content/uploads/2020/09/10_CS2051063_p682-686.pdf (accessed on 13 August 2025).
- Madrigal, J.M.; Sargis, R.M.; Persky, V.; Turyk, M.E. Multiple Organochlorine Pesticide Exposures and Measures of Sex Steroid Hormones in Adult Males: Cross-Sectional Findings from the 1999–2004 National Health and Nutrition Examination Survey. Int. J. Hyg. Environ. Health 2021, 231, 113609. [Google Scholar] [CrossRef]
- Parada, H.; Sun, X.; Tse, C.K.; Engel, L.S.; Olshan, A.F.; Troester, M.A. Plasma Levels of Dichlorodiphenyldichloroethene (DDE) and Dichlorodiphenyltrichloroethane (DDT) and Survival Following Breast Cancer in the Carolina Breast Cancer Study. Environ. Int. 2019, 125, 161–171. [Google Scholar] [CrossRef]
- Ugalde-Resano, R.; Gamboa-Loira, B.; Mérida-Ortega, Á.; Rincón-Rubio, A.; Flores-Collado, G.; Piña-Pozas, M.; López-Carrillo, L. Exposure to Organochlorine Pesticides and Female Breast Cancer Risk According to Molecular Receptors Expression: A Systematic Review and Meta-Analysis of Epidemiological Evidence. Curr. Environ. Health Rep. 2023, 10, 442–458. [Google Scholar] [CrossRef]
- Freire, C.; Koifman, R.J.; Sarcinelli, P.; Rosa, A.C.; Clapauch, R.; Koifman, S. Long Term Exposure to Organochlorine Pesticides and Thyroid Function in Children from Cidade Dos Meninos, Rio de Janeiro, Brazil. Environ. Res. 2012, 117, 68–74. [Google Scholar] [CrossRef]
- Bandow, N.; Conrad, A.; Kolossa-Gehring, M.; Murawski, A.; Sawal, G. Polychlorinated Biphenyls (PCB) and Organochlorine Pesticides (OCP) in Blood Plasma—Results of the German Environmental Survey for Children and Adolescents 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2020, 224, 113426. [Google Scholar] [CrossRef] [PubMed]
- Rapini, R.; Marrazza, G. Biosensor Potential in Pesticide Monitoring. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar]
- Zhao, W.; Lu, J.; Lai, Y.; Hou, Y.; Zhao, X.; Wei, Q.; Zou, X.; Gou, Z. Occurrences, Possible Sources, and Risk Impacts of Organochlorine Pesticides in Soil of Changchun Central Urban Area, Northeast China. Sustainability 2023, 15, 16801. [Google Scholar] [CrossRef]
- Adeyinka, G.C.; Moodley, B.; Birungi, G.; Ndungu, P. Evaluation of Organochlorinated Pesticide (OCP) Residues in Soil, Sediment and Water from the Msunduzi River in South Africa. Environ. Earth Sci. 2019, 78, 223. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, Y.; Chao, M.; Zhang, Y.; Cheng, W.; Xu, H.; Zhang, L.; Tao, Q.; Da, Q. Association between Organophosphorus Insecticides Exposure and Osteoarthritis in Patients with Arteriosclerotic Cardiovascular Disease. BMC Public Health 2024, 24, 1873. [Google Scholar] [CrossRef]
- Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules 2022, 27, 618. [Google Scholar] [CrossRef]
- Wu, G.; Shi, W.; Zheng, L.; Wang, X.; Tan, Z.; Xie, E.; Zhang, D. Impacts of Organophosphate Pesticide Types and Concentrations on Aquatic Bacterial Communities and Carbon Cycling. J. Hazard Mater. 2024, 475, 134824. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Khan, I.M.; Wang, Z.; Ma, X. Flexible Paper-Based SERS Substrate Strategy for Rapid Detection of Methyl Parathion on the Surface of Fruit. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 231, 118104. [Google Scholar] [CrossRef]
- Sharma, D.; Wangoo, N.; Sharma, R.K. Sensing Platform for Pico-Molar Level Detection of Ethyl Parathion Using Au–Ag Nanoclusters Based Enzymatic Strategy. Talanta 2021, 221, 121267. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Wu, C.; Qi, X.; Jiang, S.; Zhou, T.; Xiao, H.; Li, W.; Lu, D.; Feng, C.; et al. Early-Life Carbamate Exposure and Intelligence Quotient of Seven-Year-Old Children. Environ. Int. 2020, 145, 106105. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Huang, J.; Wu, J.; Zhang, Q. Evaluation of Supercritical Fluid Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Pesticide Residues in Grain. J. Sep. Sci. 2024, 47, e2300623. [Google Scholar] [CrossRef] [PubMed]
- Tomasevic, A.; Mijin, D.; Marinkovic, A.; Cvijetic, I.; Gasic, S. Photocatalytic Degradation of Carbamate Insecticides: Effect of Different Parameters. Pestic. I Fitomedicina 2019, 34, 193–200. [Google Scholar] [CrossRef]
- Gu, M.; Chen, S.; Jiang, J.; Liao, J.; Long, T.; Xie, Y. Do Pyrethroid Pesticide Residues in Chinese Mitten Crab Aquaculture Areas Have an Impact on the Ecological Environment?—A Case Study of Yangcheng Lake. J. Hazard Mater. 2024, 135920. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liao, G.; Chen, L.; Qian, Y.; Yan, X.; Qiu, J. Pesticide Residues in Animal-Derived Food: Current State and Perspectives. Food Chem. 2024, 438, 137974. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids—From Zero to Hero in Insecticide Chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Ensley, S.M. Neonicotinoids. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 521–524. [Google Scholar]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of Neonicotinoid Insecticides to Honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef]
- Yari, K.; Rahmani, A.; Asgari, G.; Azarian, Q.; Bhatnagar, A.; Leili, M. Degradation of Imidacloprid Pesticide in Aqueous Solution Using an Eco-Friendly Electrochemical Process. Desalination Water Treat. 2017, 86, 150–157. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, K.H.-C.; Huang, J.-C.; Lai, H.-T.; Uapipatanakul, B.; Roldan, M.J.M.; Macabeo, A.P.G.; Ger, T.-R.; Hsiao, C.-D. Physiological Effects of Neonicotinoid Insecticides on Non-Target Aquatic Animals—An Updated Review. Int. J. Mol. Sci. 2021, 22, 9591. [Google Scholar] [CrossRef]
- Basley, K.; Goulson, D. Neonicotinoids Thiamethoxam and Clothianidin Adversely Affect the Colonisation of Invertebrate Populations in Aquatic Microcosms. Environ. Sci. Pollut. Res. 2018, 25, 9593–9599. [Google Scholar] [CrossRef]
- Gautam, P.; Dubey, S.K. Biodegradation of Neonicotinoids: Current Trends and Future Prospects. Curr. Pollut. Rep. 2023, 9, 410–432. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y. Photodegradation of Neonicotinoid Insecticides Nitenpyram, Thiacloprid, and Acetamiprid in Water and Soil Environments. Bull. Environ. Contam. Toxicol. 2024, 113, 66. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Xiao, Q.; Li, Z.; Jia, X.; Hu, W.; Liu, K.; Lu, S. Urinary Neonicotinoid Insecticides in Children from South China: Concentrations, Profiles and Influencing Factors. Chemosphere 2022, 291, 132937. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-W.; Huang, Y.-F.; Fang, L.-J.; Chen, M.-L. Prenatal and Childhood Neonicotinoid Exposure and Neurodevelopment: A Study in a Young Taiwanese Cohort. Sci. Total Environ. 2024, 946, 174232. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Smaoui, S.; Varzakas, T. Chemical Food Safety in Europe Under the Spotlight: Principles, Regulatory Framework and Roadmap for Future Directions. Foods 2025, 14, 1628. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2023 European Union Report on Pesticide Residues in Food. EFSA J. 2025, 23, e9398. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hougaard Bennekou, S.; Koutsoumanis, K.; Machera, K.; Naegeli, H.; Nielsen, S.; et al. Statement on the Derivation of Health-Based Guidance Values (HBGVs) for Regulated Products That Are Also Nutrients. EFSA J. 2021, 19, e06479. [Google Scholar] [CrossRef]
- Brancato, A.; Brocca, D.; Ferreira, L.; Greco, L.; Jarrah, S.; Leuschner, R.; Medina, P.; Miron, I.; Nougadere, A.; Pedersen, R.; et al. Use of EFSA Pesticide Residue Intake Model (EFSA PRIMo Revision 3). EFSA J. 2018, 16, e05147. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- El-Sheikh, E.S.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef]
- Drabińska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jeleń, H.H. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules 2023, 28, 7985. [Google Scholar] [CrossRef]
- Ezati, M.; Moinfar, S.; Mohammadi, S.; Khayatian, G. A Continuous Sample Drop Flow-Based Microextraction Method for Spectrophotometric Determination of Cobalt with 1-(2-Pyridylazo)-2-Naphthol in Water Samples. J. Anal. Chem. 2021, 76, 172–179. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Papadakis, E.-N.; Maggalou, M.G.; Karaoglanidis, G.S.; Samanidou, V.F.; Menkissoglu-Spiroudi, U. Development of a Microwave-Assisted Extraction Protocol for the Simultaneous Determination of Mycotoxins and Pesticide Residues in Apples by LC-MS/MS. Appl. Sci. 2021, 11, 10931. [Google Scholar] [CrossRef]
- Zondo, S.; Mahlambi, P. Comparison of Soxhlet and Microwave-assisted Extractions Efficiency for the Determination of Herbicides in Soil and Maize Crop: Cumulative and Health Risks Assessment. eFood 2024, 5, e177. [Google Scholar] [CrossRef]
- Barchańska, H.; Czalicka, M.; Giemza, A. Simultaneous Determination of Selected Insecticides and Atrazine in Soil by Mae–GC–ECD. Arch. Environ. Prot. 2013, 39, 27–40. [Google Scholar] [CrossRef]
- Tian, Q.; Li, H.; Chen, L.; Han, B. Microwave-Assisted “One-Pot” Acidolysis and Extraction for the Rapid Determination of Mancozeb in Fruit and Vegetable Samples. J. Food Qual. 2024, 2024, 2577585. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Wei, J.; Ji, Y.; Bai, X.; Shao, Y.; Li, H.; Gao, R.; Wu, Z.; Peng, Z.; et al. Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application. Atmosphere 2022, 13, 818. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Labella, G.F.; Panseri, S.; Britti, D.; Galbiati, F.; Villa, R.; Arioli, F. Accelerated Solvent Extraction by Using an ‘in-Line’ Clean-up Approach for Multiresidue Analysis of Pesticides in Organic Honey. Food Addit. Contam. Part A 2017, 34, 809–818. [Google Scholar] [CrossRef]
- Horvat, T.; Jakovljević, I.; Sever Štrukil, Z.; Pehnec, G. Optimisation of ASE for Determination of Organic Compounds Bound to Particulate Matter. Kem. U Ind. 2024, 73, 507–513. [Google Scholar] [CrossRef]
- Morariu, I.D.; Avasilcai, L.; Cioanca, O.; Morariu, B.-A.; Vieriu, M.; Tanase, C. The Effects of Honey Sulfonamides on Immunological and Hematological Parameters in Wistar Rats. Medicina 2022, 58, 1558. [Google Scholar] [CrossRef]
- Morariu, I.D. Immunochemical Assay of Chloramphenicol in Honey. Farmacia 2019, 67, 235–239. [Google Scholar] [CrossRef]
- Belmonte, I.d.S.; Pizzolato, T.M.; Gama, M.R. Quaternary Ammonium Pesticides: A Review of Chromatography and Non-Chromatography Methods for Determination of Pesticide Residues in Water Samples. Trends Environ. Anal. Chem. 2022, 35, e00171. [Google Scholar] [CrossRef]
- Avellar, Á.; Prates, G.; Alves, L.; Prestes, O.; Adaime, M.; Zanella, R. Multiresidue and Multiclass Determination of Current-Use Pesticides (Cups) in Water Samples by SPE and UHPLC-MS/MS. Quim Nova 2024, 47, e20240051. [Google Scholar] [CrossRef]
- Lee, H.; Cho, Y.; Jung, G.; Kim, H.; Jeong, W. Comparison of Recovery Efficiency and Matrix Effect Reduction in Pesticide Residue Analysis: QuEChERS with d-SPE, SPE, and FaPEx in Apples and Korean Cabbage. Anal. Methods 2023, 15, 3709–3716. [Google Scholar] [CrossRef]
- Yun, D.Y.; Bae, J.Y.; Kang, Y.J.; Lim, C.U.; Jang, G.H.; Eom, M.O.; Choe, W.J. Simultaneous Analysis of 272 Pesticides in Agricultural Products by the QuEChERS Method and Gas Chromatography with Tandem Mass Spectrometry. Molecules 2024, 29, 2114. [Google Scholar] [CrossRef]
- Zgoła-Grześkowiak, A.; Grześkowiak, T.; Ligor, M.; Frankowski, R. Achievements and Challenges of Matrix Solid-Phase Dispersion Usage in the Extraction of Plants and Food Samples. Processes 2024, 12, 1146. [Google Scholar] [CrossRef]
- Zuin, V.G.; Yariwake, J.H.; Lanças, F.M. Analysis of Pesticide Residues in Brazilian Medicinal Plants: Matrix Solid Phase Dispersion versus Conventional (European Pharmacopoeia) Methods. J. Braz. Chem. Soc. 2003, 14, 304–309. [Google Scholar] [CrossRef]
- Souza, M.R.R.; Jesus, R.A.; Costa, J.A.S.; Barreto, A.S.; Navickiene, S.; Mesquita, M.E. Applicability of Metal–Organic Framework Materials in the Evaluation of Pesticide Residues in Egg Samples of Chicken (Gallus Gallus Domesticus). J. Fur Verbraucherschutz Und Leb. 2021, 16, 83–91. [Google Scholar] [CrossRef]
- Kemmerich, M.; Demarco, M.; Bernardi, G.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Balls-in-Tube Matrix Solid Phase Dispersion (BiT-MSPD): An Innovative and Simplified Technique for Multiresidue Determination of Pesticides in Fruit Samples. J. Chromatogr. A 2020, 1612, 460640. [Google Scholar] [CrossRef]
- Nolvachai, Y.; Amaral, M.S.S.; Herron, R.; Marriott, P.J. Solid Phase Microextraction for Quantitative Analysis—Expectations beyond Design? Green Anal. Chem. 2023, 4, 100048. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Kobakhidze, T.; Morton, D. Solid-Phase Microextraction Techniques and Application in Food and Horticultural Crops. Molecules 2023, 28, 6880. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Rosendo, L.M.; Brinca, A.T.; Pires, B.; Catarro, G.; Rosado, T.; Guiné, R.P.F.; Araújo, A.R.T.S.; Anjos, O.; Gallardo, E. Miniaturized Solid Phase Extraction Techniques Applied to Natural Products. Processes 2023, 11, 243. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, Y.; Huang, L.; Xiang, Z.; Liu, J.; Liu, S.; Chen, Z. In Vivo Tracing of Triazole Pesticides in Chinese Cabbage via a Novel Solid-Phase Microextraction Fiber. Food Control 2024, 156, 110143. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Wang, S.; Ma, Z.; Feng, J.; Pei, H.; Liu, Y. Simultaneous Determination of Three Herbicide Residues in Wheat Flour Based on the Hollow Fiber Supported Carbon Dots. J. Food Compos. Anal. 2022, 108, 104426. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, A.B.M.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A Review on the Composition, Extraction and Applications of Phenolic Compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Green and Selective Supercritical Fluid Extraction of Essential Oil and Cannabidiol from Cannabis sativa L. Can. J. Chem. Eng. 2025, 103, 3637–3646. [Google Scholar] [CrossRef]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Mirsadeghi, S.; Pourmortazavi, S.M. Supercritical Fluid Extraction of Pesticides and Insecticides from Food Samples and Plant Materials. Crit. Rev. Anal. Chem. 2020, 51, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Morariu, I.D.; Avasilcai, L.; Vieriu, M.; Panainte, A.D.; Bibire, N. Novel Multiresidue Method for the Determination of Eight Trichothecene Mycotoxins in Pollen Samples Using QuEChERS-Based GC-MS/MS. Rev. De Chim. 2017, 68, 304–306. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Junaid, A.M.; Lawal, A.R.; Ibrahim, H.B.; Tan, G.H. Determination of Pesticide Residues in Beans Using QuEChERS Technique Coupled to Gas Chromatography-Mass Spectrometry: Multivariate Optimization of CEN and AOAC Methods. Food Chem. 2025, 463, 141464. [Google Scholar] [CrossRef] [PubMed]
- Tasic, A.M.; Ninković, M.; Pavlović, I. Validation and Application of a Method for Determination of Multi-Class Pesticides in Muscle Chicken Breast Fillets Using QuEChERS Extraction and GC/MS. J. Vet. Res. 2024, 68, 223–232. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of Pesticide Residues in Food Matrices Using the QuEChERS Methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Ma, X.; Wang, J.; Wu, Q.; Wang, C.; Wang, Z. Extraction of Carbamate Pesticides in Fruit Samples by Graphene Reinforced Hollow Fibre Liquid Microextraction Followed by High Performance Liquid Chromatographic Detection. Food Chem. 2014, 157, 119–124. [Google Scholar] [CrossRef]
- Kaenjun, T.; Tangtreamjitmun, N. Spectrophotometric Determination of O-Phenylphenol in Canned Drinks Using Three-Phase Hollow-Fiber Liquid Phase Microextraction. Food Chem. 2025, 463, 141204. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Pakade, V.E.; Ncube, S.; Tutu, H.; Chimuka, L. Application of Hollow Fibre-Liquid Phase Microextraction Technique for Isolation and Pre-Concentration of Pharmaceuticals in Water. Membranes 2020, 10, 311. [Google Scholar] [CrossRef]
- Moema, D.; Makwakwa, T.A.; Gebreyohannes, B.E.; Dube, S.; Nindi, M.M. Hollow Fiber Liquid Phase Microextraction of Fluoroquinolones in Chicken Livers Followed by High Pressure Liquid Chromatography: Greenness Assessment Using National Environmental Methods Index Label (NEMI), Green Analytical Procedure Index (GAPI), Analytical GREEnness Metric (AGREE), and Eco Scale. J. Food Compos. Anal. 2023, 117, 105131. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.K.; Rasmussen, K.E.; Pedersen-Bjergaard, S. Environmental and Bioanalytical Applications of Hollow Fiber Membrane Liquid-Phase Microextraction: A Review. Anal. Chim. Acta 2008, 624, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Arain, M.B.; Yamini, Y.; Shah, N.; Kazi, T.G.; Pedersen-Bjergaard, S.; Tajik, M. Hollow Fiber-Based Liquid Phase Microextraction Followed by Analytical Instrumental Techniques for Quantitative Analysis of Heavy Metal Ions and Pharmaceuticals. J. Pharm. Anal. 2020, 10, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, M.H.; Cunha, S.C.; Fernandes, J.O. Determination of Pesticide Residues in Soybeans Using QuEChERS Followed by Deep Eutectic Solvent-Based DLLME Preconcentration Prior to Gas Chromatography-Mass Spectrometry Analysis. J. Chromatogr. A 2024, 1727. [Google Scholar] [CrossRef]
- Pourhossein, M.; Khadem, M.; Omidi, F.; Heravizadeh, O.R.; Shahtaheri, S.J. Development of a Green Single Drop Microextraction Based on Deep Eutectic Solvent and HPLC-UV for Trace Residue Analysis of Three Frequent-Used Pesticides. Iran. J. Public Health 2023, 52, 2440–2449. [Google Scholar] [CrossRef]
- Wahab, S.; Muzammil, K.; Nasir, N.; Khan, M.S.; Ahmad, M.F.; Khalid, M.; Ahmad, W.; Dawria, A.; Reddy, L.K.V.; Busayli, A.M. Advancement and New Trends in Analysis of Pesticide Residues in Food: A Comprehensive Review. Plants 2022, 11, 1106. [Google Scholar] [CrossRef]
- Moinfar, S.; Jamil, L.A.; Sami, H.Z. Determination of Organophosphorus Pesticides in Juice and Water by Modified Continuous Sample Drop Flow Microextraction Combined with Gas Chromatography–Mass Spectrometry. Food Anal. Methods 2020, 13, 1050–1059. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Moinfar, S. Determination of Trimethoprim in Milk, Water and Plasma Using Protein Precipitation Combined with Liquid Phase Microextraction Method. J. Food Compos. Anal. 2023, 118, 105224. [Google Scholar] [CrossRef]
- Moinfar, S.; Jamil, L.A.; Sami, H.Z.; Ataei, S. An Innovative Continuous Sample Drop Flow Microextraction for GC–MS Determination of Pesticides in Grape Juice and Water Samples. J. Food Compos. Anal. 2021, 95, 103695. [Google Scholar] [CrossRef]
- Jamil, L.A. Optimization of New Sample Preparation Technique for the Determination of Methadone and Codeine in Plasma Sample by GC-FID. J. Braz. Chem. Soc. 2020, 31, 580–588. [Google Scholar] [CrossRef]
- Méndez-Barredo, L.H.; Monribot-Villanueva, J.L.; Bojórquez-Velázquez, E.; Elizalde-Contreras, J.M.; Guerrero-Analco, J.A.; Ruiz-May, E. Comparative Evaluation of Different Extraction Methods for Identification and Quantification of Glyphosate in Fortified Corn Flour. J. Mex. Chem. Soc. 2023, 67, 213–226. [Google Scholar] [CrossRef]
- Han, W.; Tang, H.; Zhao, L.; Li, Y.; Men, D.; Dong, M.; Han, L.; Wang, W. A Modified Method for the Simultaneous Determination of Fluindapyr and Its Metabolites Residues in Vegetables and Fruits by SPE and UHPLC MS/MS. J. Food Compos. Anal. 2025, 137, 106972. [Google Scholar] [CrossRef]
- Bakhshizadeh Aghdam, M.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. Partially Carbonized Cellulose Filter Paper as a Green Adsorbent for the Extraction of Pesticides from Fruit Juices. J. Chromatogr. A 2021, 1648, 462220. [Google Scholar] [CrossRef]
- Yin, Y.; Fan, C.; Cheng, L.; Shan, Y. Efficient and Sensitive Detection of Organophosphate Pesticides in Orange Juice Using Dispersed Solid-Phase Extraction Based on Amorphous UiO-66. J. Sep. Sci. 2025, 48, e70066. [Google Scholar] [CrossRef]
- Nazari Koloujeh, M.; Iranifam, M.; Fathi, A.A.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. Development of COF@MOF Nanocomposite-Based Dispersive Solid-Phase Microextraction for the Extraction of Pesticides from Strawberries. Food Anal. Methods 2025, 18, 682–693. [Google Scholar] [CrossRef]
- Mujahid, M.; Latif, S.; Ahmed, M.; Shehzadi, W.; Imran, M.; Ahmad, M.; Asari, A.; Jehangir, M.; Mahmud, Z. Modified Matrix Solid Phase Dispersion-HPLC Method for Determination of Pesticide Residue in Vegetables and Their Impact on Human Health: A Risk Assessment. Front. Chem. 2022, 10, 1084350. [Google Scholar] [CrossRef]
- Yin, R.; Gao, L.; Qin, D.; Chen, L.; Niu, N. Preparation of Porous Carbon-Based Molecularly Imprinted Polymers for Separation of Triazine Herbicides in Corn. Microchim. Acta 2022, 189, 23. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, W.; Xu, Y.; Chen, Q.; Jiao, T.; Wei, J.; Chen, Q.; Chen, X. Development of a Hydrophilic-Lipophilic-Balanced Copolymer@zirconium-Based Metal-Organic Framework-Based Solid-Phase Microextraction Probe for the Trace Determination of Organophosphorus Pesticides in Tea Infusions. Talanta 2025, 281, 126823. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zang, X.; Li, H.; Liu, J.; Chang, Q.; Zhang, S.; Wang, C.; Wang, Z. Solid-Phase Microextraction of Organophosphorous Pesticides from Food Samples with a Nitrogen-Doped Porous Carbon Derived from g-C3N4 Templated MOF as the Fiber Coating. J. Hazard Mater. 2020, 384, 121430. [Google Scholar] [CrossRef] [PubMed]
- Delińska, K.; Yavir, K.; Kloskowski, A. Head-Space SPME for the Analysis of Organophosphorus Insecticides by Novel Silica IL-Based Fibers in Real Samples. Molecules 2022, 27, 4688. [Google Scholar] [CrossRef]
- Nakamura, K.; Otake, T.; Hanari, N. Quantitative Determination of Organophosphorus, Pyrethroid, and Dithiolane Pesticide Residues in Brown Rice Using Supercritical Fluid Extraction and Liquid Chromatography–Tandem Mass Spectrometry. J. AOAC Int. 2023, 106, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Otake, T.; Hanari, N. Evaluation of Supercritical Fluid Extraction for the Determination of Neonicotinoid Pesticides in Green Onion. J. Environ. Sci. Health Part B 2020, 55, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Lee, H.S.; Park, J.S.; Lee, S.J.; Shin, H.S.; Chung, Y.M.; Choi, H.N.; Jung, Y.H.; Oh, J.H.; Yun, S.S. Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method. Foods 2021, 10, 2090. [Google Scholar] [CrossRef]
- Muiambo, S.L.; Chaúque, E.F.C.; Gulamussen, N.J.; Chimuka, L.; Morifi, E.; Nyambe, I. Modified QuEChERS Method for the Extraction and Quantification of Persistent Organic Compounds in Vegetables from Mozambican Local Markets. J. Hazard. Mater. Adv. 2023, 10, 100262. [Google Scholar] [CrossRef]
- Abasalizadeh, A.; Sorouraddin, S.M.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. Development of a Green Approach Based on DµSPE Combined with Deep Eutectic Solvent-Based DLLME for the Extraction of Some Pesticides from Vegetable Samples Prior to GC–FID and GC–MS. J. Iran. Chem. Soc. 2022, 19, 4699–4707. [Google Scholar] [CrossRef]
- Szarka, A.; Búčiková, K.; Kostić, I.; Hrouzková, S. Development of a Multiresidue QuEChERS–DLLME—Fast GC–MS Method for Determination of Selected Pesticides in Yogurt Samples. Food Anal. Methods 2020, 13, 1829–1841. [Google Scholar] [CrossRef]
- Raoufi, A.; Raoufi, A.M.; Ismailzadeh, A.; Soleimani Rad, E.; Kiaeefar, A. Application of Hollow Fiber-Protected Liquid-Phase Microextraction Combined with GC-MS in Determining Endrin, Chlordane, and Dieldrin in Rice Samples. Environ. Geochem. Health 2023, 45, 5261–5277. [Google Scholar] [CrossRef]
- Jamil, L.A.; Sami, H.Z.; Aghaei, A.; Moinfar, S.; Ataei, S. Combination of Modified Ultrasound-Assisted Extraction with Continuous Sample Drop Flow Microextraction for Determination of Pesticides in Vegetables and Fruits. Microchem. J. 2021, 160, 105692. [Google Scholar] [CrossRef]
- Sassolas, A.; Prieto-Simón, B.; Marty, J.-L. Biosensors for Pesticide Detection: New Trends. Am. J. Anal. Chem. 2012, 03, 210–232. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Moreno-González, D. Current Role of Mass Spectrometry in the Determination of Pesticide Residues in Food. Separations 2022, 9, 148. [Google Scholar] [CrossRef]
- Ciscato, C.H.P.; Bertoni Gebara, A.; Henrique Monteiro, S. Pesticide Residue Monitoring of Brazilian Fruit for Export 2006–2007. Food Addit. Contam. Part B 2009, 2, 140–145. [Google Scholar] [CrossRef]
- Mutengwe, M.T.; Chidamba, L.; Korsten, L. Pesticide Residue Monitoring on South African Fresh Produce Exported over a 6-Year Period. J. Food Prot. 2016, 79, 1759–1766. [Google Scholar] [CrossRef]
- John, P.J.; Bakore, N.; Bhatnagar, P. Assessment of Organochlorine Pesticide Residue Levels in Dairy Milk and Buffalo Milk from Jaipur City, Rajasthan, India. Environ. Int. 2001, 26, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Wepener, V. Review of Scientific Literature on Available Methods of Assessing Organochlorine Pesticides in the Environment. Heliyon 2023, 9, e22142. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K.; Kalpana, D. Automation in Analytical Chemistry: The Role of AI in Chromatography. Int. J. Appl. Pharm. 2024, 16, 14–21. [Google Scholar] [CrossRef]
- Singh, Y.R.; Shah, D.B.; Kulkarni, M.; Patel, S.R.; Maheshwari, D.G.; Shah, J.S.; Shah, S. Current Trends in Chromatographic Prediction Using Artificial Intelligence and Machine Learning. Anal. Methods 2023, 15, 2785–2797. [Google Scholar] [CrossRef]
- Sinha, N.; Ray, S. Application of Carbon Quantum Dots Derived from Waste Tea for the Detection of Pesticides in Tea: A Novel Biosensor Approach. ACS Omega 2024, 9, 50201–50213. [Google Scholar] [CrossRef]
- Sindhu, S.; Manickavasagan, A. Nondestructive Testing Methods for Pesticide Residue in Food Commodities: A Review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1226–1256. [Google Scholar] [CrossRef]
- Keklik, M.; Golge, O.; González-Curbelo, M.Á.; Kabak, B. Determination of Pesticide Residues in Vine Leaves Using the QuEChERS Method and Liquid Chromatography-Tandem Mass Spectrometry. Foods 2024, 13, 909. [Google Scholar] [CrossRef]
- Gormez, E.; Golge, O.; González-Curbelo, M.Á.; Kabak, B. Pesticide Residues in Mandarins: Three-Year Monitoring Results. Molecules 2023, 28, 5611. [Google Scholar] [CrossRef]
- Penelope Mabunda, K.; Rejoice Maseko, B.; Ncube, S. Development and Application of a New QuEChERS-Molecularly Imprinted Solid Phase Extraction (QuEChERS-MISPE) Technique for Analysis of DDT and Its Derivatives in Vegetables. Food Chem. 2024, 436, 137747. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.M.; De Queiroz, M.E.L.R.; Rodrigues, A.A.Z.; de Oliveira, A.F.; Libardi, V.M.; de Freitas, J.F. Determination of Pesticide Residues in Oat Flour Using Low-Temperature Partition Extraction and GC–MS Analysis. J. Food Sci. Technol. 2024, 61, 2402–2410. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, B.; Pang, C.; Liu, L.; Zhou, Q. Determination of Multiple Pesticide Residues and Dietary Intake Risk Assessment of 35 Pesticides in Beet from Five Provinces of Northern China. Sugar Tech. 2024, 27, 393–406. [Google Scholar] [CrossRef]
- Morariu, I.-D.; Schiriac, E.-C.; Matiut, D.; Cuciureanu, R. Method Validation for Simultaneous Determination of 12 Sulfonamides in Honey Using Biochip Array Technology. Farmacia 2012, 60, 143–154. [Google Scholar]
- Bessaire, T.; Savoy, M.-C.; Ernest, M.; Christinat, N.; Badoud, F.; Desmarchelier, A.; Carrères, B.; Chan, W.-C.; Wang, X.; Delatour, T. Enhanced Surveillance of >1100 Pesticides and Natural Toxins in Food: Harnessing the Capabilities of LC-HRMS for Reliable Identification and Quantification. Foods 2024, 13, 3040. [Google Scholar] [CrossRef]
- Malm, L.; Liigand, J.; Aalizadeh, R.; Alygizakis, N.; Ng, K.; Fro̷kjær, E.E.; Nanusha, M.Y.; Hansen, M.; Plassmann, M.; Bieber, S.; et al. Quantification Approaches in Non-Target LC/ESI/HRMS Analysis: An Interlaboratory Comparison. Anal. Chem. 2024, 96, 16215–16226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Daka, Z.; Yao, L.; Dong, J.; Zhang, Y.; Li, P.; Zhang, K.; Ji, S. Recent Progress in the Application of Chromatography-Coupled Mass-Spectrometry in the Analysis of Contaminants in Food Products. Food Chem. X 2025, 27, 102397. [Google Scholar] [CrossRef]
- Goh, M.S.; Lam, S.D.; Yang, Y.; Naqiuddin, M.; Addis, S.N.K.; Yong, W.T.L.; Luang-In, V.; Sonne, C.; Ma, N.L. Omics Technologies Used in Pesticide Residue Detection and Mitigation in Crop. J. Hazard Mater. 2021, 420, 126624. [Google Scholar] [CrossRef]
- Shi, Y.; Jin, H.-F.; Ma, X.-R.; Cao, J. Highly Sensitive Determination of Multiple Pesticide Residues in Foods by Supercritical Fluid Chromatography Coupled with Ion Mobility Quadrupole Time-of-Flight Mass Spectrometry. Food Res. Int. 2024, 175, 113769. [Google Scholar] [CrossRef]
- Bauer, A.; Luetjohann, J.; Rohn, S.; Jantzen, E.; Kuballa, J. Development of a Suspect Screening Strategy for Pesticide Metabolites in Fruit and Vegetables by UPLC-Q-Tof-MS. Food Anal. Methods 2018, 11, 1591–1607. [Google Scholar] [CrossRef]
- Donley, N.; Cox, C.; Bennett, K.; Temkin, A.M.; Andrews, D.Q.; Naidenko, O.V. Forever Pesticides: A Growing Source of PFAS Contamination in the Environment. Environ. Health Perspect. 2024, 132, 75003. [Google Scholar] [CrossRef]
- Zahra, Z.; Song, M.; Habib, Z.; Ikram, S. Advances in Per- and Polyfluoroalkyl Substances (PFAS) Detection and Removal Techniques from Drinking Water, Their Limitations, and Future Outlooks. Emerg. Contam. 2025, 11, 100434. [Google Scholar] [CrossRef]
- Almeida, M.O.; Oloris, S.C.S.; Faria, V.H.F.; Ribeiro, M.C.M.; Cantini, D.M.; Soto-Blanco, B. Optimization of Method for Pesticide Detection in Honey by Using Liquid and Gas Chromatography Coupled with Mass Spectrometric Detection. Foods 2020, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Elmi, M.; Ghane, T.; Daraei, B.; Eskandari, S.; Mohammadpour, A.; Amirahmadi, M.; Mousavi Khaneghah, A. Monitoring of Pesticide Residue in Pistachio Nut Samples by LC/MS-MS. Food Chem. 2024, 437, 137848. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.; Wei, J.; Tong, K.; Xie, Y.; Chang, Q.; Yu, X.; Li, B.; Lu, M.; Fan, C.; et al. Multi-Residue Analytical Method Development and Dietary Exposure Risk Assessment of 345 Pesticides in Mango by LC-Q-TOF/MS. Food Control 2025, 170, 111016. [Google Scholar] [CrossRef]
- Phichitsaenyakorn, H.; Bunkoed, O. A Porous Composite Sorbent Incorporating a Metal Organic Framework and Zinc Oxide in Cryogel to Extract Carbamate Pesticides. Microchem. J. 2024, 204, 111156. [Google Scholar] [CrossRef]
- Mohamadi, S.; Akbari-adergani, B.; Sadighara, P.; Jannat, B.; Abdoli, N.; Mirzaei, G.; Zeinali, T. Quantitative Analysis of Multiclass Pesticide Residues in Spinach, Iran. Appl. Food Res. 2023, 3, 100368. [Google Scholar] [CrossRef]
- Chen, G.; Shi, L.; Wang, J.; Zhu, S.; Sheng, J.; Yang, X.; Xu, H. Pesticide Residues in Rice Planted in South and Southwest China. Food Addit. Contam. Part B 2023, 16, 176–184. [Google Scholar] [CrossRef]
- Hammood, M.K.; Arif, M.A. Optimization and Validation of a GC-FID/QuEChERS Method for Quantitative Determination of Spiromesifen Residues in Tomato Fruits, Leaves and Soil Matrices. Anal. Bioanal. Chem. Res. 2024, 11, 447–461. [Google Scholar]
- Lobato, A.; Fernandes, V.C.; Pacheco, J.G.; Delerue-Matos, C.; Gonçalves, L.M. Organochlorine Pesticide Analysis in Milk by Gas-Diffusion Microextraction with Gas Chromatography-Electron Capture Detection and Confirmation by Mass Spectrometry. J. Chromatogr. A 2021, 1636, 461797. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Gao, S.; Wei, J.; Chen, T. The Determination Methods of Pesticide Multi-Residues and the Degradation Dynamics in Bitter Melon and Cucumber. J. Food Sci. Technol. 2025. [Google Scholar] [CrossRef]
- Ramadan, M.F.A.; Abdel-Hamid, M.M.A.; Altorgoman, M.M.F.; AlGaramah, H.A.; Alawi, M.A.; Shati, A.A.; Shweeta, H.A.; Awwad, N.S. Evaluation of Pesticide Residues in Vegetables from the Asir Region, Saudi Arabia. Molecules 2020, 25, 205. [Google Scholar] [CrossRef]
- Ramadevi, R.; Ramachandraiah, C.; Reddy, G.V.S. Development of a Multi Residue Method for the Quantification of 45 Pesticides Using Gc-Ms/Ms and Study of Peeling Effect on Pesticide Residues in Citrus Fruits. Orient. J. Chem. 2023, 39, 1145–1155. [Google Scholar] [CrossRef]
- Kim, M.; Cho, M.; Kim, S.-H.; Lee, Y.; Jo, M.-R.; Moon, Y.-S.; Im, M.-H. Monitoring and Risk Assessment of Pesticide Residues in Fishery Products Using GC–MS/MS in South Korea. Toxics 2024, 12, 299. [Google Scholar] [CrossRef]
- Zheng, K.; Zheng, H.; Yu, Y.; Su, J.; Chen, L.; Zheng, M.; Liu, L.; Wu, X.; Chen, D.; Meng, X. Simultaneous Determination of Four Pesticides Residues in Rice by Modified QuEChERS Coupled with GC-MS/MS. J. Food Compos. Anal. 2024, 133, 106396. [Google Scholar] [CrossRef]
- Bustamante, C.M.; Bravo, N.; Ruiz, P.; Grimalt, J.O.; Garí, M. Method Optimization for a Simultaneous Determination of Neonicotinoid, Carbamate/Thiocarbamate, Triazole, Organophosphate and Pyrethroid Pesticides and Their Metabolites in Urine Using UPLC-MS/MS. J. Chromatogr. A 2024, 1730, 465054. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Ulloa, P.; Ramos, C.; Correa, A.; Molinett, S. Analysis of Multi-Pesticide Residues and Dietary Risk Assessment in Fresh Tomatoes (Lycopersicum esculentum) from Local Supermarkets of the Metropolitan Region, Chile. Toxics 2021, 9, 249. [Google Scholar] [CrossRef]
- Crocoli, L.C.; Ramires, N.; Moura, S. Determination of Pesticide Residues in Grapes Consumed in Natura and for Juice and Wine Production by High-Performance Liquid Chromatography with High Resolution Mass Spectrometry (HPLC-HRMS). Anal. Lett. 2023, 56, 1454–1464. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Jiao, B.; Zhao, Q.; Wang, C.; Cui, Y.; He, Y.; Li, J. Determination, Quality, and Health Assessment of Pesticide Residues in Kumquat in China. Foods 2023, 12, 3423. [Google Scholar] [CrossRef]
- Wang, B.; Shi, L.; Ren, P.; Qin, S.; Li, J.; Cao, J. Dissipation and Dietary Risk Assessment of the Fungicide Pyraclostrobin in Apples Using Ultra-High Performance Liquid Chromatography–Mass Spectrometry. Molecules 2024, 29, 4434. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.; Ghoudi, K.; Meetani, M.A. Determination and Health Risk Assessment of Carbamate Pesticide Residues in Date Palm Fruits (Phoenix dactylifera) Using QuEChERS Method and UHPLC-MS/MS. Sci. Rep. 2024, 14, 13064. [Google Scholar] [CrossRef]
- Diao, Z.; Di, S.; Qi, P.; Liu, Z.; Wang, Z.; Zhao, H.; Wang, M.; Zhang, C.; Wang, X. Stereoselective Study on Chiral Fungicide Metconazole in Four Kinds of Fruits: Absolute Configuration, SFC-MS/MS Enantioseparation, Degradation and Risk Assessment. Food Chem. 2024, 438, 137944. [Google Scholar] [CrossRef]
- Zušťáková, V.; Dušek, M.; Jandovská, V.; Olšovská, J. Screening and Quantification of Pesticide Residues in Ciders by Liquid Chromatography-High Resolution Mass Spectrometry. Czech J. Food Sci. 2023, 41, 29–35. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Han, X. Selection of Internal Standards for Accurate Quantification of Complex Lipid Species in Biological Extracts by Electrospray Ionization Mass Spectrometry—What, How and Why? Mass Spectrom. Rev. 2017, 36, 693–714. [Google Scholar] [CrossRef]
- Rimayi, C.; Odusanya, D.; Mtunzi, F.; Tsoka, S. Alternative Calibration Techniques for Counteracting the Matrix Effects in GC–MS-SPE Pesticide Residue Analysis—A Statistical Approach. Chemosphere 2015, 118, 35–43. [Google Scholar] [CrossRef]
- Mirres, A.C.d.M.; Silva, B.E.P.d.M.d.; Tessaro, L.; Galvan, D.; de Andrade, J.C.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Dong, J.; Yang, H.; Li, Y.; Liu, A.; Wei, W.; Liu, S. Fluorescence Sensor for Organophosphorus Pesticide Detection Based on the Alkaline Phosphatase-Triggered Reaction. Anal. Chim. Acta 2020, 1131, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, B.; Wang, X.; Shen, J.; Zhao, B. Detection of Organophosphorus Pesticides by Nanogold/Mercaptomethamidophos Multi-Residue Electrochemical Biosensor. Food Chem. 2021, 354, 129511. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, Z.; Li, X. Advances in Pesticide Biosensors: Current Status, Challenges, and Future Perspectives. Anal. Bioanal. Chem. 2013, 405, 63–90. [Google Scholar] [CrossRef]
- Sekhwama, M.; Mpofu, K.; Sivarasu, S.; Mthunzi-Kufa, P. Applications of Microfluidics in Biosensing. Discov. Appl. Sci. 2024, 6, 303. [Google Scholar] [CrossRef]
- Kant, T.; Shrivas, K.; Tejwani, A.; Tandey, K.; Sharma, A.; Gupta, S. Progress in the Design of Portable Colorimetric Chemical Sensing Devices. Nanoscale 2023, 15, 19016–19038. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, J.; Yang, Y.; Yu, W.; Chen, Y.; Lin, P.; Liang, K. Smartphone-Coupled Three-Layered Paper-Based Microfluidic Chips Demonstrating Stereoscopic Capillary-Driven Fluid Transport towards Colorimetric Detection of Pesticides. Anal. Bioanal. Chem. 2022, 414, 1759–1772. [Google Scholar] [CrossRef]
- Peng, S.; Wang, A.; Lian, Y.; Zhang, X.; Zeng, B.; Chen, Q.; Yang, H.; Li, J.; Li, L.; Dan, J.; et al. Smartphone-Based Molecularly Imprinted Sensors for Rapid Detection of Thiamethoxam Residues and Applications. PLoS ONE 2021, 16, e0258508. [Google Scholar] [CrossRef] [PubMed]
- Akdag, A.; Işık, M.; Göktaş, H. Conducting Polymer-Based Electrochemical Biosensor for the Detection of Acetylthiocholine and Pesticide via Acetylcholinesterase. Biotechnol. Appl. Biochem. 2021, 68, 1113–1119. [Google Scholar] [CrossRef]
- Hu, H.; Wang, B.; Li, Y.; Wang, P.; Yang, L. Acetylcholinesterase Sensor with Patterned Structure for Detecting Organophosphorus Pesticides Based on Titanium Dioxide Sol-gel Carrier. Electroanalysis 2020, 32, 1834–1842. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Ling, J.; Li, J. An Electrochemiluminescence Biosensor for Detection of Methyl Parathion with a Novel Probe Ru-MIL-100 via the Acetylcholinesterase Inhibition Coupled with the Reaction Between HAc and Triethylamine. Luminescence 2025, 40, e70073. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, X.; Zhang, W.; Chen, M.; Feng, D.; Zhao, Y.; Zhu, Y. Simultaneous and Sensitive Detection of Three Pesticides Using a Functional Poly (Sulfobetaine Methacrylate)-Coated Paper-Based Colorimetric Sensor. Biosensors 2023, 13, 309. [Google Scholar] [CrossRef]
- Chen, G.; Jin, M.; Yan, M.; Cui, X.; Wang, Y.; Zheng, W.; Qin, G.; Zhang, Y.; Li, M.; Liao, Y.; et al. Colorimetric Bio-Barcode Immunoassay for Parathion Based on Amplification by Using Platinum Nanoparticles Acting as a Nanozyme. Microchim. Acta 2019, 186, 339. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, Y.; Xiao, Y.; Li, Z.; Sheng, E.; Dai, Z. A Silver@gold Nanoparticle Tetrahedron Biosensor for Multiple Pesticides Detection Based on Surface-Enhanced Raman Scattering. Talanta 2021, 234, 122585. [Google Scholar] [CrossRef]
- Arsawiset, S.; Sansenya, S.; Teepoo, S. Nanozymes Paper−based Analytical Device for the Detection of Organophosphate Pesticides in Fruits and Vegetables. Anal. Chim. Acta 2023, 1267, 341377. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Y.; Jia, L.; Chang, T.; Chen, J.; Li, Y.; Ma, Z.; Zhan, Y.; Yang, H. An Electrochemical Biosensor with Perovskite/AuNPs Composite for Sensitive Determination of Fenitrothion. Microchem. J. 2025, 216, 114593. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; de Almeida Rodrigues, P.; Joshi, N.; Ferrari, R.G.; Conte-Junior, C.A. Nucleic Acid-Based Nanobiosensor (NAB) Used for Salmonella Detection in Foods: A Systematic Review. Nanomaterials 2022, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- El Alami, A.; Lagarde, F.; Tamer, U.; Baitoul, M.; Daniel, P. Enhanced Raman Spectroscopy Coupled to Chemometrics for Identification and Quantification of Acetylcholinesterase Inhibitors. Vib. Spectrosc. 2016, 87, 27–33. [Google Scholar] [CrossRef]
- Nie, Y.; Teng, Y.; Li, P.; Liu, W.; Shi, Q.; Zhang, Y. Label-Free Aptamer-Based Sensor for Specific Detection of Malathion Residues by Surface-Enhanced Raman Scattering. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Chen, H.; An, L.; Li, M.; Liu, H.; Jin, Z.; Ma, H.; Ma, J.; Zhou, J.; Duan, R.; Zhang, D.; et al. A Self-Assembled 3D Nanoflowers Based Nano-ELISA Platform for the Sensitive Detection of Pyridaben. Food Chem. 2024, 445, 138756. [Google Scholar] [CrossRef]
- Dávila, E.L.; Romero, O.R.; Du Laing, G.; Spanoghe, P. Du Use of Enzyme-Linked ImmunoSorbent Assay Technique to Monitor Pesticide Residues in Horticultural Crops. J. Chem. Eng. Theor. Appl. Chem. 2021, 78, 199–209. [Google Scholar]
- López Dávila, E.; Houbraken, M.; Gil Unday, Z.; Romero Romero, O.; Du Laing, G.; Spanoghe, P. ELISA, a Feasible Technique to Monitor Organophosphate, Carbamate, and Pyrethroid Residues in Local Vegetables. Cuban Case Study. SN Appl. Sci. 2020, 2, 1487. [Google Scholar] [CrossRef]
- Li, H.; He, S.; Liu, G.; Li, C.; Ma, Z.; Zhang, X. Residue and Dissipation Kinetics of Toosendanin in Cabbage, Tobacco and Soil Using IC-ELISA Detection. Food Chem. 2021, 335, 127600. [Google Scholar] [CrossRef]
- Jia, M.; E, Z.; Zhai, F.; Bing, X. Rapid Multi-Residue Detection Methods for Pesticides and Veterinary Drugs. Molecules 2020, 25, 3590. [Google Scholar] [CrossRef] [PubMed]


| Extraction | Advantages | Limitations |
|---|---|---|
| MAE | High extraction efficiency Green and eco-friendly Automation Low solvent consumption | Poor extraction performance for non-polar/volatile compounds Not suitable for thermally unstable analytes |
| ASE | Reduced solvent and time consumption Simple operation and eco-friendly | High cost of equipment and maintenance Handling of extraction cells can be challenging |
| SPE | Simple, cost-effective, widely available Variety of sorbents for diverse pesticide properties | Risk of co-eluting interferences |
| dSPE | Requires minimal equipment Efficient cleanup of fatty acids, pigments, and sugars | Risk of analyte loss during cleanup |
| MSPD | Low sample and solvent consumption Suitable for multi-residue analysis | Requires optimization Sorbent handling |
| SPME | Solvent-free/minimal solvent Simple and flexible Reusable fibers | Limited commercial availability of specialized fibers |
| SFE | Reduced solvent use Prevents oxidation/degradation Environmentally friendly | Mainly extracts non-polar compounds Requires high-purity CO2 Expensive equipment |
| QuEChERS | Fast and simple Minimal equipment Broad analyte range | May need modifications for fatty/complex matrices |
| HF-LPME | Very low solvent usage Eco-friendly | Limited mainly to polar analytes |
| DLLME | Suitable for hydrophobic analytes Fast Low cost | Restricted solvent choices Involves multiple stages |
| SDME | Very eco-friendly (few µL solvents) Simple and cost-effective | Reduced reproducibility |
| CSDF-ME | Minimal solvent use | Limited validation |
| Pretreatment | Food Matrix | Pesticide | EF | ER | RR (%) | Ref. |
|---|---|---|---|---|---|---|
| MAE | Maize | Atrazine Glyphosate Mesotrione | - | - | 80–98 | [71] |
| MAE | Fruits and vegetables | Mancozeb | - | - | 81–112 | [73] |
| MAE | Apples | Thiamethoxam | - | - | 61–112 | [70] |
| ASE | Soy products | 230 pesticides | - | - | 70–120 | [74] |
| ASE | Corn flour | Glyphosate | - | - | 109.19 ± 8.26 | [114] |
| SPE | Fruits and vegetables | Fluindapyr + metabolites | - | - | 71–118 | [115] |
| SPE | Packed fruit juice | Ametryn Chlorpyrifos Clodinafob-propargyl Fenpropathrin Oxadiazon Diniconazole Penconazole | 452–751 | 45–75 | 85–101 | [116] |
| dSPE | Orange juice | OPPs | - | - | 95.35–110.75 | [117] |
| dSPE-DLME | Strawberries | Hexaconazole Oxadiazon Tebuconazole Clodinafop-propargyl Difenoconazole | 365–405 | 73–81 | - | [118] |
| MSPD | Eggplant Capsicum Apple gourd Cauliflower Sponge gourd | Diafenthiuron Lufenuron Azoxystrobin Difenoconazole Chlorothalonil | - | - | 88.5–116.9 | [119] |
| MSPD | Corn | Triazines | - | - | 92.6–104.7 | [120] |
| SPME | Tea | OPPs | - | - | 73.12–101.20 | [121] |
| SPME | Fruits and vegetables | OPPs | - | - | 82.6–118 | [122] |
| HS-SPME | Grapefruit Cucumber | OPPs | - | - | 85–118 | [123] |
| SFE | Brown rice | OPPs Pyrethroid Dithiolane | - | - | 96.4–105.0 | [124] |
| SFE | Green onion | Acetamiprid Clothianidin Dinotefuran Imidacloprid Thiacloprid Thiamethoxam | - | - | 70–120 | [125] |
| QuEChERS | Mandarin Potato Green Pepper Hulled rice Soybean | Triflumezopyrim | - | - | 89.7–104.3 | [126] |
| QuEChERS | Vegetables | β-HCH, Υ-HCH Cypermethrin Profenophos 4-Nonylphenol p,p′-DDD, p,p′-DDTs | - | - | 69–114 | [127] |
| QuEChERS | Muscle chicken breast fillets | α-endosulfan Cypermethrin Endosulfan sulfate Permethrin DDT | - | - | 71.2–118.80 | [99] |
| DLLME + dSPE | Tomato Lettuce Carrot Celery | Hexaconazole Chlorpyrifos Diazinon Tebuconazole Diniconazole | 380–430 | 79–86 | 90–103 | [128] |
| DLLME + QuEChERS | Yogurt | OCPs OPPs Dinitroanilines Carbamates and pyrethroids Triazines Chloracetamides Dicarboximides Azoles | 5–16 | - | 70–120 | [129] |
| HF-LPME | Canned drinks | OPPs | - | - | 73.6–94.8 | [102] |
| HF-PLM | Rice | OCPs | 67.4–73.5% | 76.277–81.499 | 86.0–92.9 | [130] |
| CSDF-ME + UAE | Apple Strawberry Cucumber Tomato | OPPs | 21–205% | - | 83.0–108.0 | [131] |
| CSDF-ME | Juice | OPPs | 102–380 µg L−1 | 17–51 | 83–105 | [110] |
| CSDF-ME | Grape juice | OPPs | 510–960 | 25.5–48.0% | 90–110 | [112] |
| Detector | Food Matrix | Pesticide | LOD | LOQ | Ref. |
|---|---|---|---|---|---|
| 1. LC-MS/MS 2. GC-MS/MS | Honey | Carbendazim Thiabendazole Azoxystrobin Chlorpyrifos Imidacloprid | 10.0001–0.0004 mg/kg 0.001–0.004 mg/kg | 0.0002–0.0008 mg/kg 0.002–0.008 mg/kg | [156] |
| LC-MS/MS | Vine leaves | 512 pesticides | - | - | [142] |
| LC-MS/MS | Mandarins | 440 pesticides | <0.01 mg kg−1 | - | [143] |
| LC-MS/MS | Pistachio | 112 pesticides | 0.003 mg/kg | 0.01 mg/kg | [157] |
| LC-Q-TOF/MS | Mango | 345 pesticides | - | 0.5 to 20 µg/kg | [158] |
| HPLC | Fruit juice and white wine | Carbofuran Carbaryl Isoprocarb Diethofencarb | 0.3 µg/L | - | [159] |
| GC | Soybean | No residues of the target pesticides were detected | - | - | [107] |
| GC | Fruits and vegetables | Mancozeb | 0.003 kg−1 | 0.01 mg kg−1 | [73] |
| GC-MS | Oat flour | Triadimenol Flutriafol λ-cyhalothrin Difenoconazole Azoxystrobin | 1.7–12.9 µg kg−1 | 5.73–43.0 µg kg−1 | [145] |
| GC-MS | Spinach | 108 pesticides | 0.005–0.01 µg/g | 0.01–0.025 µg/g | [160] |
| GC-MS | Rice | 15 pesticides | 0.10–1.46 µg kg−1 | 0.390–4.85 µg kg−1 | [161] |
| GC-FID | Tomato | Spiromesifen | 0.0015 µg mL−1 | 0.006 µg mL−1 | [162] |
| GC-ECD | Milk | OCPs | 3.7 to 4.8 µg L−1 | 12–16 µg L−1 | [163] |
| GC-ECD | Melon Cucumber | Pyraclostrobin Difenoconazole Dimethomorph Azoxystrobin | - | 0.01–0.05 mg/L | [164] |
| GC-FPD | Beet | 35 pesticides | 0.0047–0.0261 mg/kg | 0.0143–0.0790 mg/kg | [146] |
| GC-MS/MS LC-MS/MS | Vegetables | 80 pesticides | 0.0004–0.0023 mg kg−1 | 0.0008–0.0047 mg kg−1 | [165] |
| GC-MS/MS | Lime Lemon | 45 pesticides | 1.56–25.23 ng/mL | 4.72–76.47 ng/mL | [166] |
| GC-MS/MS | Seafood | 44 pesticides | 2–3 ng/g | 7–10 ng/g | [167] |
| GC-MS/MS | Rice | Triazophos Dichlorvos Chlorpyrifos Malathion | 3.4–5.4 µg/kg | 20 µg/kg | [168] |
| HPLC-MS/MS | Rice | Acetamiprid Parathion Profenofos Bixafen | 10 µg/kg | - | [169] |
| HPLC-FL/UV GC-MS/ECD/NPD | Tomatoes | 180 pesticides | 5–10 µg/kg | 10–20 µg/kg | [170] |
| HPLC-HRMS | Grapes | 92 pesticides | 4.88–120.16 µg L−1 | 14.86–308.01 µg L−1 | [171] |
| UHPLC-MS/MS GC-MS/MS | Kumquat fruits | 16 insecticides 7 fungicides 5 acaricides 2 plant growth modulators | - | - | [172] |
| UHPLC-MS/MS | Fruits and vegetables | Fluindapyr + 5 metabolites | 0.0001–0.0002 mg/L | 0.0003–0.0006 mg/L | [115] |
| UHPLC-MS/MS | Apples | Pyraclostrobin | - | 0.01 mg kg−1 | [173] |
| UHPLC-MS/MS | Dates | Carbamates | 0.01–0.005 µg kg−1 | 0.003–0.04 µg kg−1 | [174] |
| SFC-MS/MS | Rice Wheat Maize | 9 pesticides | 0.01–42.9 µg/kg | 0.4–101.8 µg/kg | [48] |
| SFC-MS/MS | Jujube Peach Grape Pear | Metconazole | 4.30–95.9 ng/kg | 10.5–143.2 ng/kg | [175] |
| SFC-IM-Q-TOF/MS | Yam Potato | 20 pesticides | 0.1–8.8 ng/mL | 0.8–29.4 ng/mL | [152] |
| LC-HRMS/MS | Cider | 18 pesticides | - | 0.2 µg L−1 | [176] |
| LC-HRMS | Cereals and Grains | 730 pesticides | - | 5–20 µg/kg | [148] |
| 1. GC-MS 2. HPLC-UV | Cereal (wheat, rice, corn) | 323 pesticides | 1. 0.0025–0.005 mg kg−1 2. 0.003–0.027 mg kg−1 | - | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botnaru, A.A.; Lupu, A.; Morariu, P.C.; Nedelcu, A.H.; Morariu, B.A.; Di Gioia, M.L.; Lupu, V.V.; Dragostin, O.M.; Caba, I.-C.; Anton, E.; et al. Innovative Analytical Approaches for Food Pesticide Residue Detection: Towards One Health-Oriented Risk Monitoring. J. Xenobiot. 2025, 15, 151. https://doi.org/10.3390/jox15050151
Botnaru AA, Lupu A, Morariu PC, Nedelcu AH, Morariu BA, Di Gioia ML, Lupu VV, Dragostin OM, Caba I-C, Anton E, et al. Innovative Analytical Approaches for Food Pesticide Residue Detection: Towards One Health-Oriented Risk Monitoring. Journal of Xenobiotics. 2025; 15(5):151. https://doi.org/10.3390/jox15050151
Chicago/Turabian StyleBotnaru, Alexandra Andreea, Ancuta Lupu, Paula Cristina Morariu, Alin Horatiu Nedelcu, Branco Adrian Morariu, Maria Luisa Di Gioia, Vasile Valeriu Lupu, Oana Maria Dragostin, Ioana-Cezara Caba, Emil Anton, and et al. 2025. "Innovative Analytical Approaches for Food Pesticide Residue Detection: Towards One Health-Oriented Risk Monitoring" Journal of Xenobiotics 15, no. 5: 151. https://doi.org/10.3390/jox15050151
APA StyleBotnaru, A. A., Lupu, A., Morariu, P. C., Nedelcu, A. H., Morariu, B. A., Di Gioia, M. L., Lupu, V. V., Dragostin, O. M., Caba, I.-C., Anton, E., Vieriu, M., & Morariu, I. D. (2025). Innovative Analytical Approaches for Food Pesticide Residue Detection: Towards One Health-Oriented Risk Monitoring. Journal of Xenobiotics, 15(5), 151. https://doi.org/10.3390/jox15050151