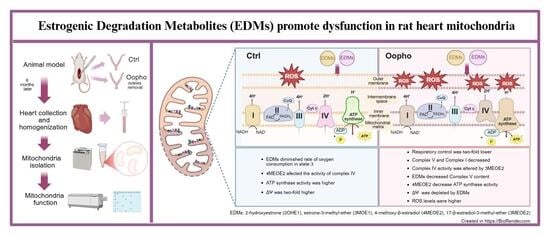
Estrogen Degradation Metabolites: Some Effects on Heart Mitochondria
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Isolation of Heart Mitochondria
2.4. EDM Incubation
2.5. Oxygen Consumption and Membrane Potential Measurements
2.6. Complex IV Activity
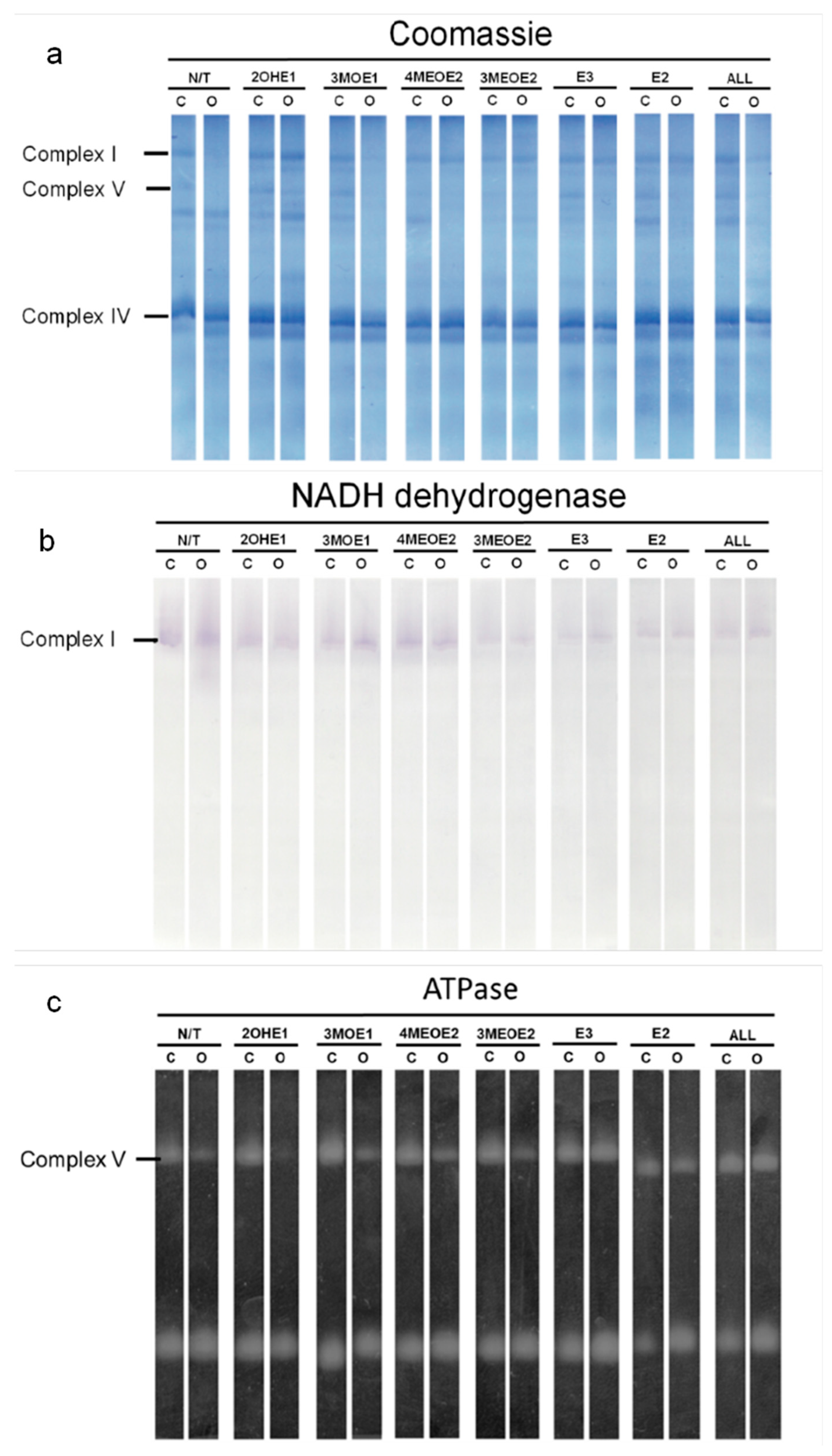
2.7. High-Resolution Clear-Native Polyacrylamide Gel Electrophoresis (hrCN-PAGE) and In-Gel Enzymatic Activities
2.8. Complex I (NADH-Ubiquinone Oxidoreductase) Activity
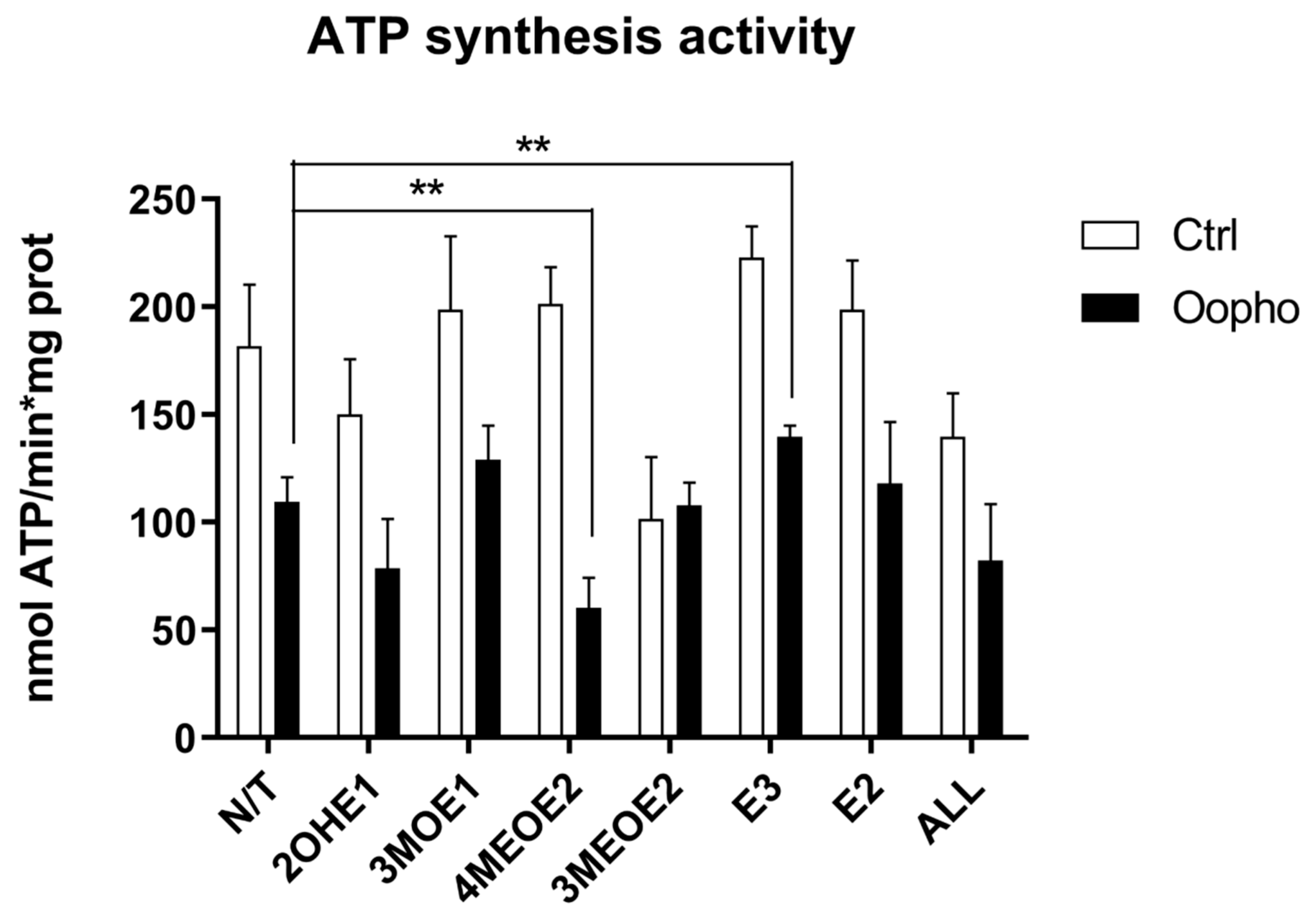
2.9. Complex V (ATP Synthesis) Activity
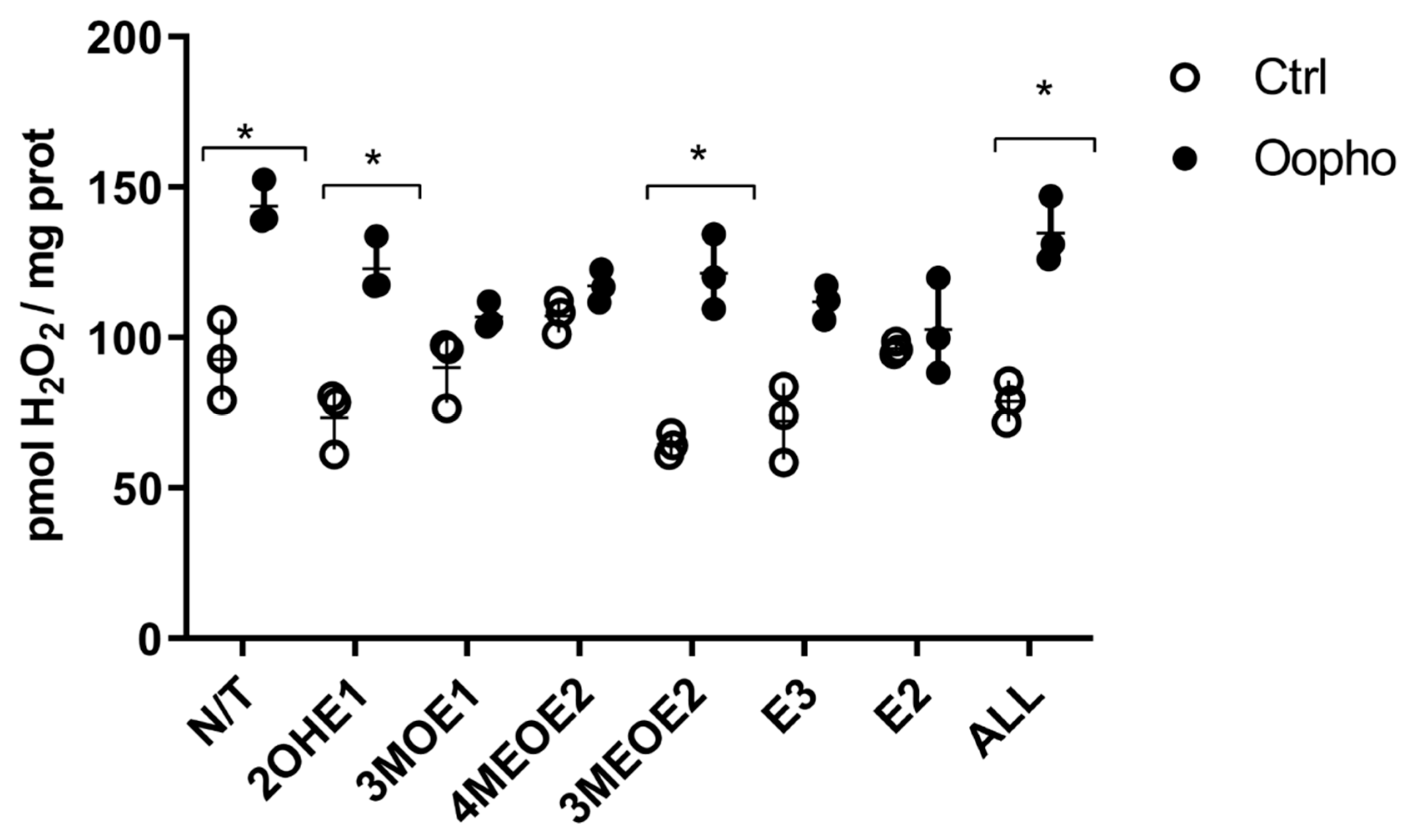
2.10. Reactive Oxygen Species (ROS)
2.11. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutiérrez-Lara, E.J.; Sánchez-López, A.; Murbartián, J.; Acosta-Cota, S.J.; Centurión, D. Effect of chronic administration of 17β-estradiol on the vasopressor responses induced by the sympathetic nervous system in insulin resistance rats. Steroids 2022, 188, 109132. [Google Scholar] [CrossRef]
- Visniauskas, B.; Kilanowski-Doroh, I.; Ogola, B.O.; Mcnally, A.B.; Horton, A.C.; Sugi, A.I.; Lindsey, S.H. Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases. J. Hum. Hypertens. 2022, 37, 609–618. [Google Scholar] [CrossRef]
- Dubey, R.K.; Jackson, E.K. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am. J. Physiol. Renal Physiol. 2001, 280, F365–F388. [Google Scholar] [CrossRef]
- Naftolin, F.; Friedenthal, J.; Nachtigall, R.; Nachtigall, L. Cardiovascular health and the menopausal woman: The role of estrogen and when to begin and end hormone treatment. F1000Research 2019, 8, 1576. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase—A Brief Overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Song, H.; Zhu, Z.; Wang, J.; Wang, R.; Du, M.; Fu, Y.; Yuan, J.; Tan, R. Decoding the enigmatic estrogen paradox in pulmonary hypertension: Delving into estrogen metabolites and metabolic enzymes. Cell. Mol. Biol. Lett. 2024, 29, 155. [Google Scholar] [CrossRef]
- Zhu, B. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef]
- Roy, D.; Singh, K. Estrogen-Induced Genetic Alterations and Their Role in Carcinogenicity. Curr. Genom. 2004, 5, 245–257. [Google Scholar] [CrossRef]
- Embrechts, J.; Lemière, F.; Van Dongen, W.; Esmans, E.L.; Buytaert, P.; Van Marck, E.; Kockx, M.; Makar, A. Detection of estrogen DNA-adducts in human breast tumor tissue and healthy tissue by combined nano LC-nano ES tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 482–491. [Google Scholar] [CrossRef]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, reviews3003-1. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef]
- Roy, D.; Liehr, J.G. Estrogen, DNA damage and mutations. Mutat. Res. 1999, 424, 107–115. [Google Scholar] [CrossRef]
- Musial, C.; Knap, N.; Zaucha, R.; Bastian, P.; Barone, G.; Bosco, G.L.; Lo-Celso, F.; Konieczna, L.; Belka, M.; Bączek, T.; et al. Induction of 2-hydroxycatecholestrogens O-methylation: A missing puzzle piece in diagnostics and treatment of lung cancer. Redox Biol. 2022, 55, 102395. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.L.; Rogan, E.G. Depurinating estrogen-DNA adducts, generators of cancer initiation: Their minimization leads to cancer prevention. Clin. Transl. Med. 2016, 5, 12. [Google Scholar] [CrossRef]
- Lira-Silva, E.; del Valle Mondragón, L.; Pérez-Torres, I.; Posadas-Sánchez, R.; Gómez, F.J.R.; Posadas-Romero, C.; Vargas-Barrón, J.; Pavón, N. Possible implication of estrogenic compounds on heart disease in menopausal women. Biomed. Pharmacother. 2023, 162, 114649. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.W.; Yang, L.; Pruthi, S.; Ingle, J.N.; Sandhu, N.; Rogan, E.G.; Cavalieri, E.L. Urine Biomarkers of Risk in the Molecular Etiology of Breast Cancer. Breast Cancer Basic Clin. Res. 2009, 3, BCBCR.S2112. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; López-Grueso, R.; Pallardó, F.V.; Viña, J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim. Biophys. Acta 2010, 1802, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Felty, Q.; Roy, D. Estrogen, mitochondria, and growth of cancer and non-cancer cells. J. Carcinog. 2005, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Klinge, C.M. Estrogenic Control of Mitochondrial Function and Biogenesis. J. Cell. Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogens regulate life and death in mitochondria. J. Bioenerg. Biomembr. 2017, 49, 307–324. [Google Scholar] [CrossRef]
- Sanchez, M.I.G.L.; Shearwood, A.M.J.; Chia, T.; Davies, S.M.K.; Rackham, O.; Filipovska, A. Estrogen-mediated regulation of mitochondrial gene expression. Mol. Endocrinol. 2015, 29, 14–27. [Google Scholar] [CrossRef]
- Razmara, A.; Sunday, L.; Stirone, C.; Xiao, B.W.; Krause, D.N.; Duckles, S.P.; Procaccio, V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008, 325, 782–790. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Yang, S.H.; Sarkar, S.N.; Pearce, V. Estrogen Actions on Mitochondria-Physiological and Pathological Implications. Mol. Cell. Endocrinol. 2008, 290, 51–59. [Google Scholar] [CrossRef]
- Yager, J.D.; Chen, J.Q. Mitochondrial estrogen receptors—New insights into specific functions. Trends Endocrinol. Metab. 2007, 18, 89–91. [Google Scholar] [CrossRef]
- Pavón, N.; Martínez-Abundis, E.; Hernández, L.; Gallardo-Pérez, J.C.; Alvarez-Delgado, C.; Cerbón, M.; Pérez-Torres, I.; Aranda, A.; Chávez, E. Sexual hormones: Effects on cardiac and mitochondrial activity after ischemia-reperfusion in adult rats. Gender difference. J. Steroid. Biochem. Mol. Biol. 2012, 132, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Pavón, N.; Cabrera-Orefice, A.; Gallardo-Pérez, J.C.; Uribe-Alvarez, C.; Rivero-Segura, N.A.; Vazquez-Martínez, E.R.; Cerbón, M.; Martinez-Abundis, E.; Torres-Narvaez, J.C.; Martínez-Memije, R.; et al. In female rat heart mitochondria, oophorectomy results in loss of oxidative phosphorylation. J. Endocrinol. 2017, 232, 221–235. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Moreira, P.I.; Custódio, J.B.A.; Nunes, E.; Oliveira, P.J.; Moreno, A.; Seia, R.; Oliveira, C.R.; Santos, M.S. Mitochondria from distinct tissues are differently affected by 17β-estradiol and tamoxifen. J. Steroid Biochem. Mol. Biol. 2011, 123, 8–16. [Google Scholar] [CrossRef]
- Uribe, S.; Ramirez, J.; Pena, A. Effects of beta-pinene on yeast membrane functions. J. Bacteriol. 1985, 161, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Karas, M.; Schägger, H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell. Proteom. 2007, 6, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Zerbetto, E.; Vergani, L.; Dabbeni-Sala, F. Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis 1997, 18, 2059–2064. [Google Scholar] [CrossRef]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Cortés-Hernández, P.; Vázquez-Memije, M.E.; García, J.J. ATP6 homoplasmic mutations inhibit and destabilize the human F1F0-ATP synthase without preventing enzyme assembly and oligomerization. J. Biol. Chem. 2007, 282, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Castillo, S.; Cabrera-Orefice, A.; Vázquez-Acevedo, M.; González-Halphen, D.; Uribe-Carvajal, S. During the stationary growth phase, Yarrowia lipolytica prevents the overproduction of reactive oxygen species by activating an uncoupled mitochondrial respiratory pathway. Biochim. Biophys. Acta 2011, 1817, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wittig, I.; Braun, H.P.; Schägger, H. Blue native PAGE. Nat. Protoc. 2006, 1, 418–428. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Estrogen and mitochondria function in cardiorenal metabolic syndrome. Prog. Mol. Biol. Transl. Sci. 2014, 127, 229–249. [Google Scholar] [CrossRef]
- Lancaster, T.S.; Jefferson, S.J.; Hunter, J.C.; Lopez, V.; Van Eyk, J.E.; Lakatta, E.G.; Korzick, D.H. Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiol. Genom. 2012, 44, 957–969. [Google Scholar] [CrossRef]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef]
- Nilsen, J.; Irwin, R.W.; Gallaher, T.K.; Brinton, R.D. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007, 27, 14069–14077. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.W.; Yao, J.; To, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J. Neuroendocrinol. 2012, 24, 236–248. [Google Scholar] [CrossRef]
- Stirone, C.; Duckles, S.P.; Krause, D.N.; Procaccio, V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol. Pharmacol. 2005, 68, 959–965. [Google Scholar] [CrossRef]
- Gustafsson, Å.B.; Gottlieb, R.A. Heart mitochondria: Gates of life and death. Cardiovasc. Res. 2008, 77, 334–343. [Google Scholar] [CrossRef]
- Griffiths, E.J. Mitochondria and heart disease. Adv. Exp. Med. Biol. 2012, 942, 249–267. [Google Scholar] [CrossRef]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Jobe, S.O.; Ramadoss, J.; Wargin, A.J.; Magness, R.R. Estradiol-17β and its Cytochrome P450- and Catechol-O-Methyltransferase–Derived Metabolites Selectively Stimulate Production of Prostacyclin in Uterine Artery Endothelial Cells. Hypertension 2013, 61, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Palou, A.; Picó, C.; Bonet, M.L.; Oliver, P. The uncoupling protein, thermogenin. Int. J. Biochem. Cell Biol. 1998, 30, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Liehr, J.G. Genotoxic effects of estrogens. Mutat. Res. 1990, 238, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008, 29, 463–475. [Google Scholar] [CrossRef]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Khalil, R.A. Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women. Int. J. Mol. Sci. 2025, 26, 5078. [Google Scholar] [CrossRef]
- Hogervorst, E.; Craig, J.; O’Donnell, E. Cognition and mental health in menopause: A review. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 69–84. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; He, H.-M.; Katzenellenbogen, J.A.; Lin, C.M.; Hamel, E. Synthesis, Antitubulin and Antimitotic Activity, and Cytotoxicity of Analogs of 2-Methoxyestradiol, an Endogenous Mammalian Metabolite of Estradiol That Inhibits Tubulin Polymerization by Binding to the Colchicine Binding Site. J. Med. Chem. 1995, 38, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Auborn, K.J.; Woodworth, C.; Dipaolo, J.A.; Bradlow, H.L. The interaction between HPV infection and estrogen metabolism in cervical carcinogenesis. Int. J. Cancer 1991, 49, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. The role of estrogen in the initiation of breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 89–96. [Google Scholar] [CrossRef]
- Samavat, H.; Kurzer, M.S. Estrogen Metabolism and Breast Cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef]
- Santen, R.J.; Yue, W.; Wang, J.-P. Estrogen metabolites and breast cancer. Steroids 2015, 99, 61–66. [Google Scholar] [CrossRef]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant Synthesis, Metabolism, and Plasma Accumulation of Circulating Estrogens and Estrogen Metabolites in Preeclampsia Implications for Vascular Dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Chen, W.; Cui, Y.; Zheng, S.; Huang, J.; Li, P.; Simoncini, T.; Zhang, Y.; Fu, X. 2-Methoxyestradiol Induces Vasodilation by Stimulating NO Release via PPARγ/PI3K/Akt Pathway. PLoS ONE 2015, 10, e0118902. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Jackson, G.M. Induction and Inhibition of Uterine Vasodilation by Catechol Estrogen in Oophorectomized, Nonpregnant Ewes. Endocrinology 1982, 110, 1333–1339. [Google Scholar] [CrossRef] [PubMed]



| Control | Oopho | |
|---|---|---|
| N/T | 6.71 ± 0.99 | 2.88 ± 0.58 |
| 2-hydroxyestrone (2OHE1) | 5.55 ± 1.05 | 0.67 ± 0.17 ** |
| Estrone-3-methyl-ether (3MOE1) | 3.20 ± 0.72 * | 0.51 ± 0.36 ** |
| 4-Methoxy-β-estradiol (4MEOE2) | 2.61 ± 0.025 ** | 0.71 ± 0.082 ** |
| 17-β-estradiol-3-methyl-ether (3MEOE2) | 2.62 ± 0.30 ** | 0.76 ± 0.11 ** |
| Estriol (E3) | 4.49 ± 0.67 * | 1.42 ± 0.16 * |
| 17-β-estradiol (E2) | 5.05 ± 1.11 | 1.17 ± 0.52 * |
| ALL | 2.24 ± 0.69 | 1.01 ± 0.61 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe-Alvarez, C.; Lira-Silva, E.; Morales-García, L.; Chiquete-Felix, N.; Roldán-Gómez, F.J.; Vargas-Barrón, J.; García-Trejo, J.J.; Silva-Palacios, A.; Uribe-Carvajal, S.; Pavón, N. Estrogen Degradation Metabolites: Some Effects on Heart Mitochondria. J. Xenobiot. 2025, 15, 170. https://doi.org/10.3390/jox15050170
Uribe-Alvarez C, Lira-Silva E, Morales-García L, Chiquete-Felix N, Roldán-Gómez FJ, Vargas-Barrón J, García-Trejo JJ, Silva-Palacios A, Uribe-Carvajal S, Pavón N. Estrogen Degradation Metabolites: Some Effects on Heart Mitochondria. Journal of Xenobiotics. 2025; 15(5):170. https://doi.org/10.3390/jox15050170
Chicago/Turabian StyleUribe-Alvarez, Cristina, Elizabeth Lira-Silva, Lilia Morales-García, Natalia Chiquete-Felix, Francisco Javier Roldán-Gómez, Jesús Vargas-Barrón, José J. García-Trejo, Alejandro Silva-Palacios, Salvador Uribe-Carvajal, and Natalia Pavón. 2025. "Estrogen Degradation Metabolites: Some Effects on Heart Mitochondria" Journal of Xenobiotics 15, no. 5: 170. https://doi.org/10.3390/jox15050170
APA StyleUribe-Alvarez, C., Lira-Silva, E., Morales-García, L., Chiquete-Felix, N., Roldán-Gómez, F. J., Vargas-Barrón, J., García-Trejo, J. J., Silva-Palacios, A., Uribe-Carvajal, S., & Pavón, N. (2025). Estrogen Degradation Metabolites: Some Effects on Heart Mitochondria. Journal of Xenobiotics, 15(5), 170. https://doi.org/10.3390/jox15050170