Prioritization and Sensitivity of Pesticide Risks from Root and Tuber Vegetables
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Pesticide Extraction and Analysis
2.3. Quality Assurance/Quality Control
2.4. Health Risk Assessment
2.4.1. Prioritization of Pesticides Using Risk Ranking
2.4.2. Chronic and Acute Risk Assessment
2.5. Monte Carlo Risk Simulation
3. Results and Discussion
3.1. Method Validation
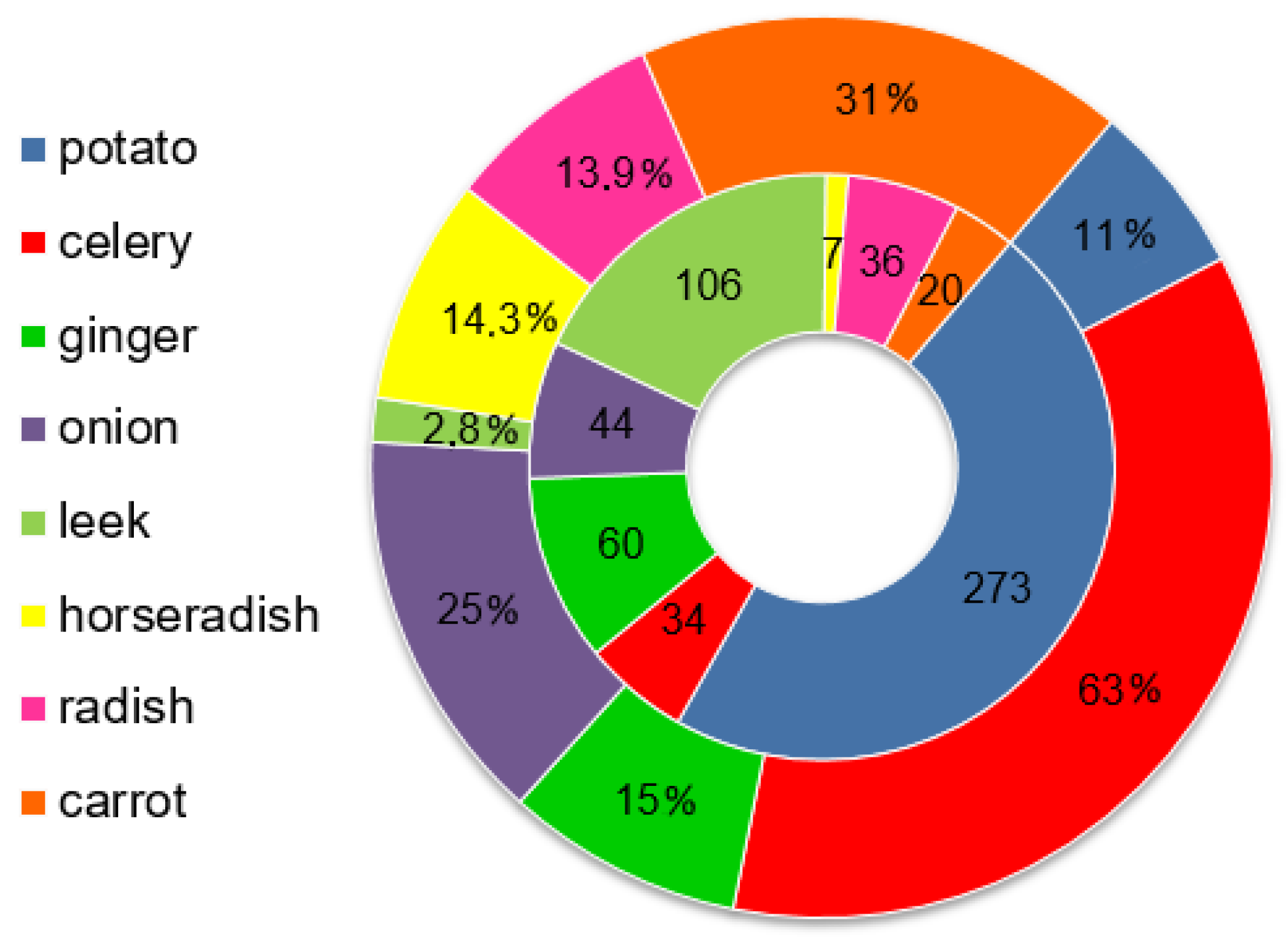
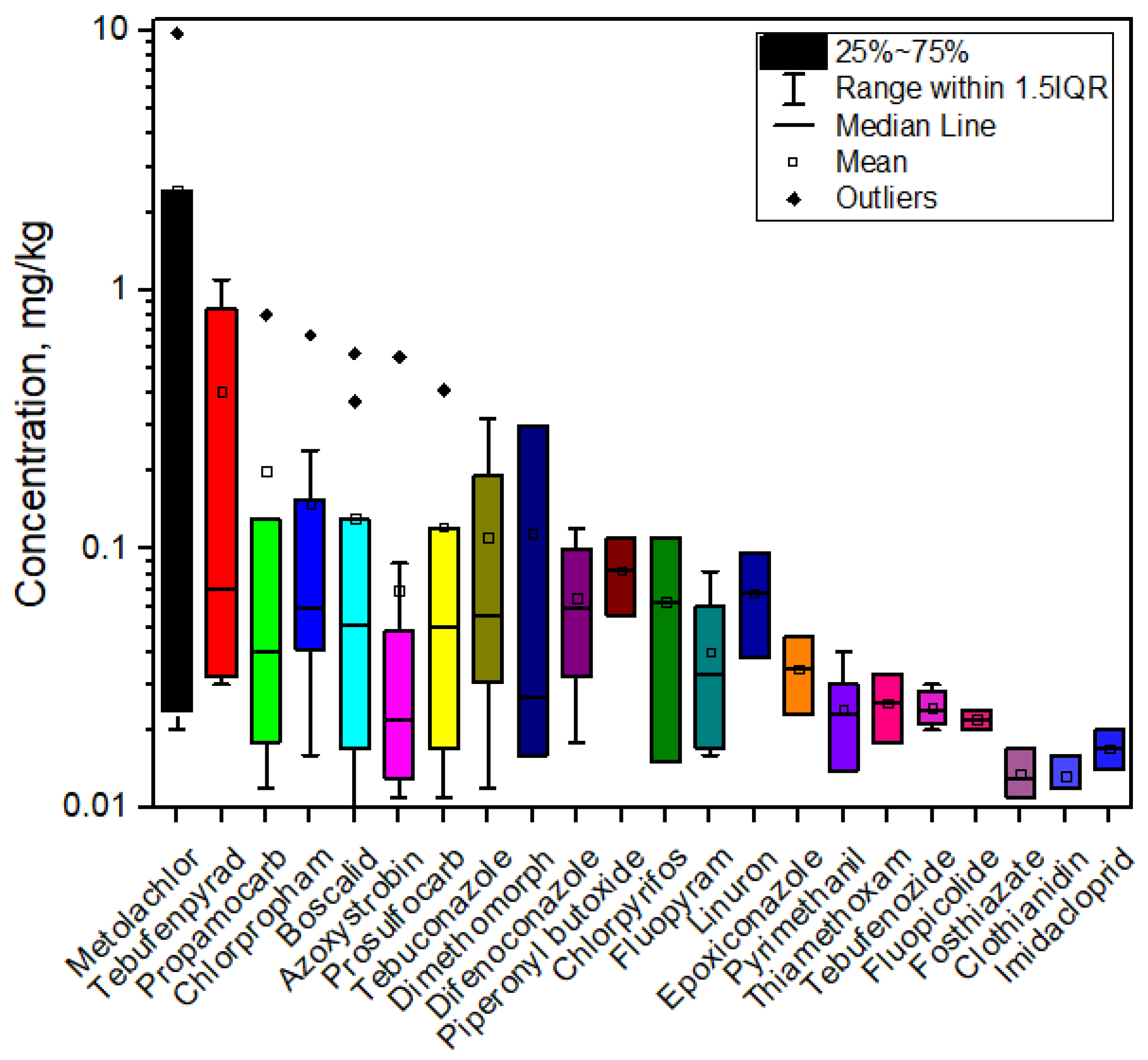
3.2. Pesticide Distribution
3.3. Cluster Analysis of Pesticide Residues
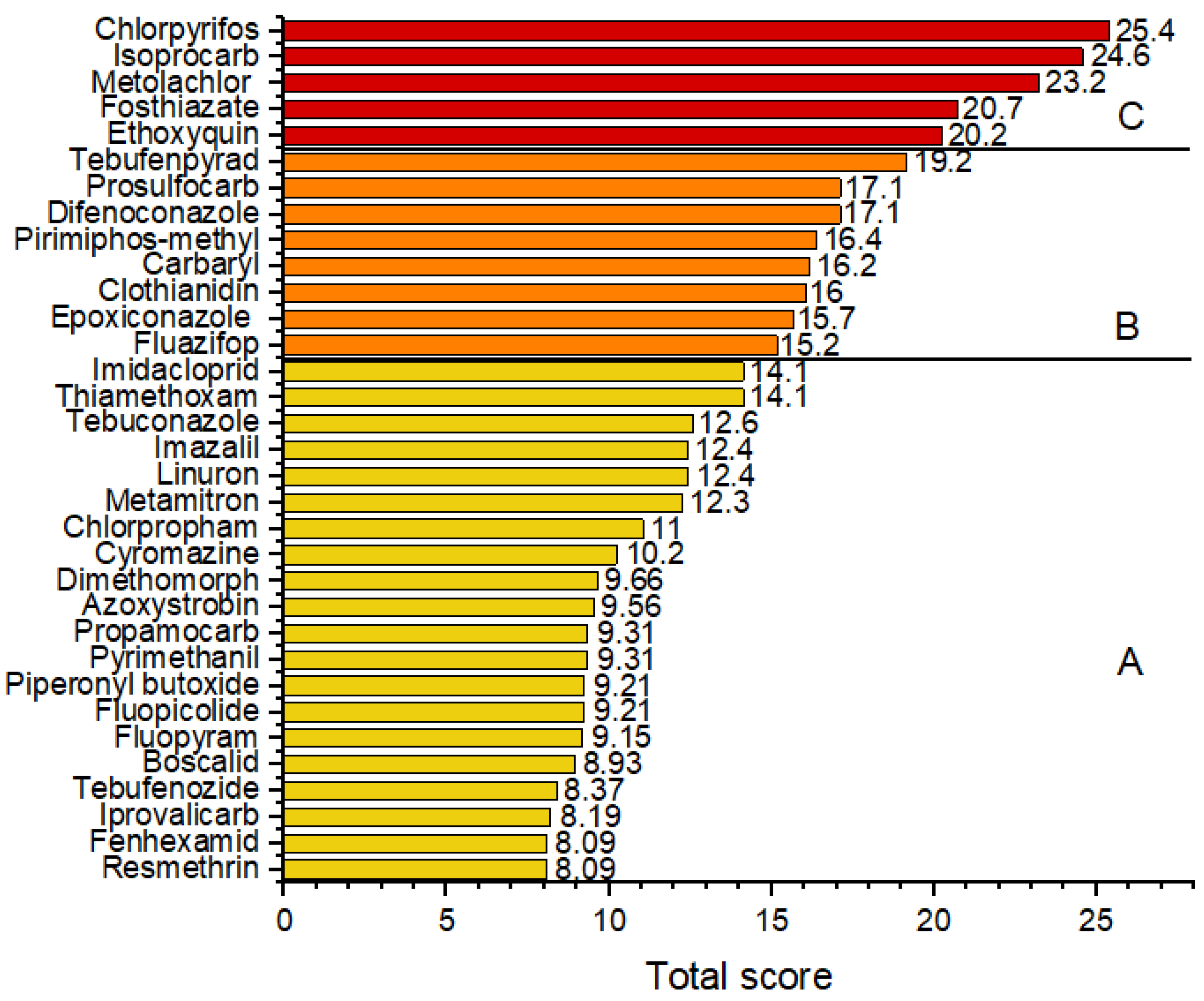
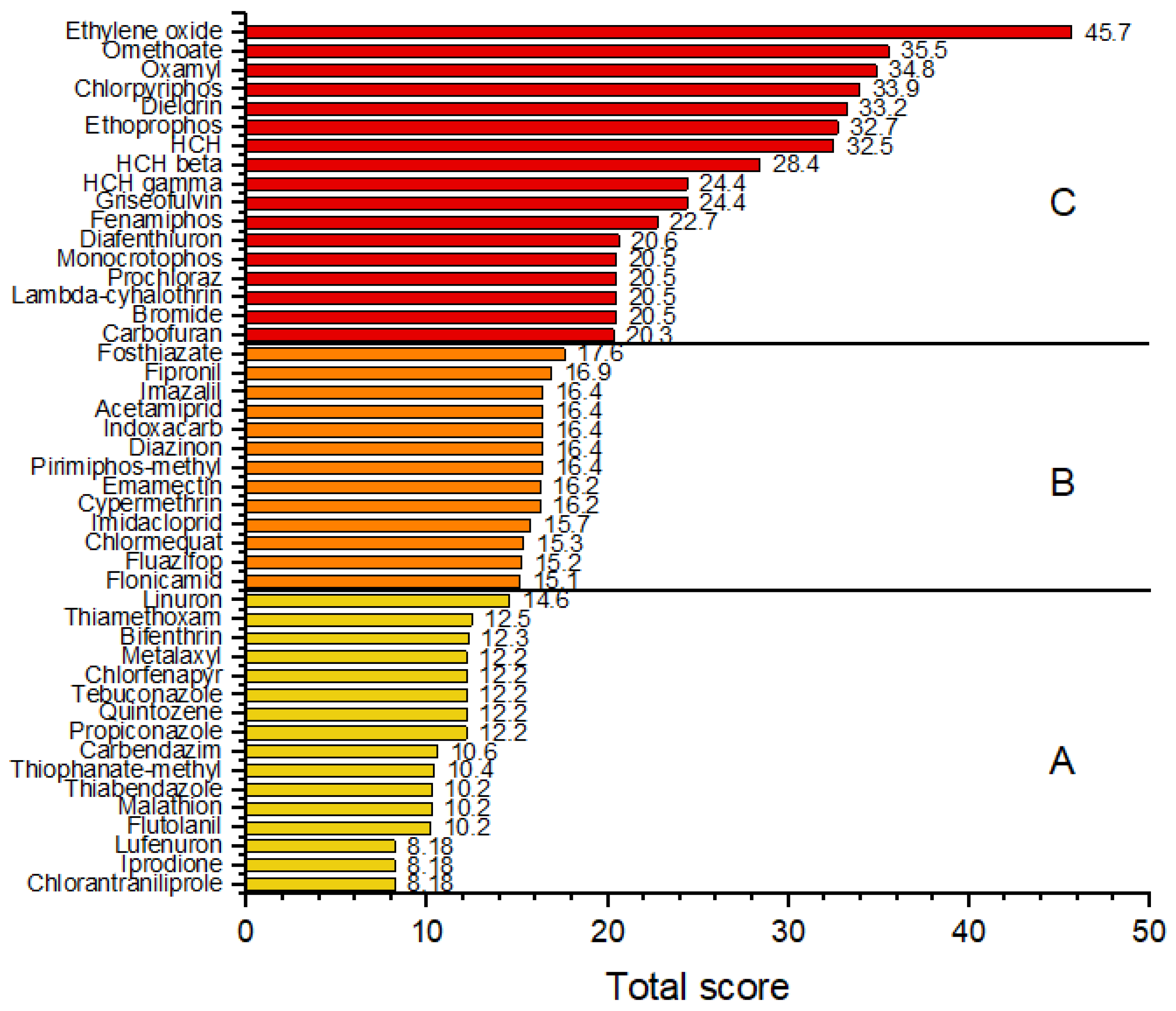
3.4. Risk Ranking
3.5. Acute and Chronic Risks of Pesticide Residues in Root Vegetables and Onions
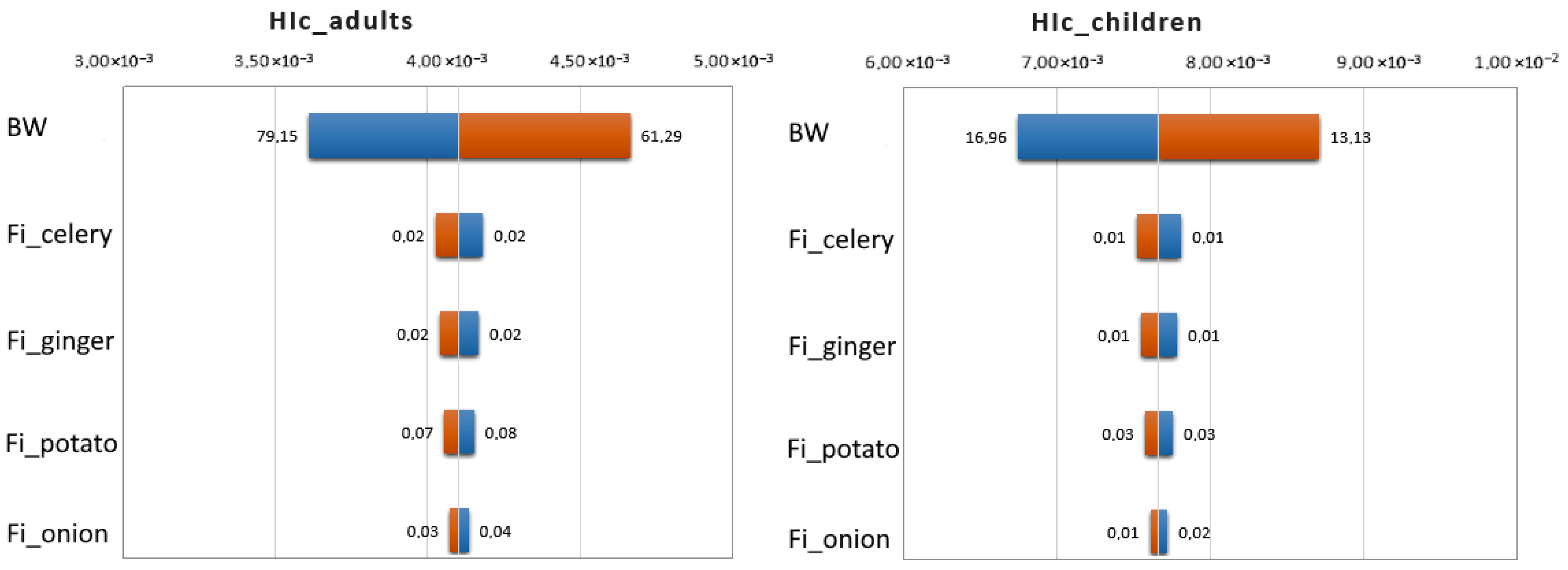
3.6. Monte Carlo Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to Pesticides and the Associated Human Health Effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Ma, C.; Wei, D.; Liu, P.; Fan, K.; Nie, L.; Song, Y.; Wang, M.; Wang, L.; Xu, Q.; Wang, J.; et al. Pesticide Residues in Commonly Consumed Vegetables in Henan Province of China in 2020. Front. Public Health 2022, 10, 901485. [Google Scholar] [CrossRef]
- Chandra, R.; Sharpanabharathi, N.; Prusty, B.A.K.; Azeez, P.A.; Kurakalva, R.M. Organochlorine Pesticide Residues in Plants and Their Possible Ecotoxicological and Agri Food Impacts. Sci. Rep. 2021, 11, 17841. [Google Scholar] [CrossRef] [PubMed]
- Horská, T.; Kocourek, F.; Stará, J.; Holý, K.; Mráz, P.; Krátký, F.; Kocourek, V.; Hajšlová, J. Evaluation of Pesticide Residue Dynamics in Lettuce, Onion, Leek, Carrot and Parsley. Foods 2020, 9, 680. [Google Scholar] [CrossRef]
- Sookhtanlou, M.; Allahyari, M.S.; Surujlal, J. Health Risk of Potato Farmers Exposed to Overuse of Chemical Pesticides in Iran. Saf. Health Work 2022, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Danek, M.; Fang, X.; Tang, J.; Plonka, J.; Barchanska, H. Simultaneous Determination of Pesticides and Their Degradation Products in Potatoes by MSPD-LC-MS/MS. J. Food Compos. Anal. 2021, 104, 104129. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, C.S.; Banerjee, B.D. Xeno-Estrogenic Pesticides and the Risk of Related Human Cancers. J. Xenobiotics 2022, 12, 344–355. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Chiu, Y.H.; Wang, Y.X.; Hart, J.E.; Bhupathiraju, S.N.; Mínguez-Alarcón, L.; Ding, M.; Willett, W.C.; Laden, F.; Chavarro, J.E. Intake of Fruits and Vegetables According to Pesticide Residue Status in Relation to All-Cause and Disease-Specific Mortality: Results from Three Prospective Cohort Studies. Environ. Int. 2022, 159, 107024. [Google Scholar] [CrossRef]
- Fandiño-Del-Rio, M.; Tore, G.; Peng, R.D.; Meeker, J.D.; Matsui, E.C.; Quirós-Alcalá, L. Characterization of Pesticide Exposures and Their Associations with Asthma Morbidity in a Predominantly Low-Income Urban Pediatric Cohort in Baltimore City. Environ. Res. 2024, 263, 120096. [Google Scholar] [CrossRef]
- de Rodrigues, M.B.; de Carvalho, D.S.; Chong-Silva, D.C.; Urrutia-Pereira, M.; de Albuquerque, G.S.C.; Cieslak, F.; Chong-Neto, H.J. Association between Exposure to Pesticides and Allergic Diseases in Children and Adolescents: A Systematic Review with Meta-Analysis. J. Pediatr. (Rio. J.) 2022, 98, 551–564. [Google Scholar] [CrossRef]
- Albadrani, M.S.; Aljassim, M.T.; El-Tokhy, A.I. Pesticide Exposure and Spontaneous Abortion Risk: A Comprehensive Systematic Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2024, 284, 117000. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Dmochowska-Ślęzak, K.; Grembecka, M. Root Vegetables—Composition, Health Effects, and Contaminants. Int. J. Environ. Res. Public Health 2022, 19, 15531. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ferdous, F.; Rayhan, M.I. Pesticide Knowledge and Attitude among the Potato Growing Farmers of Bangladesh and Determinant Factors. Front. Public Health 2024, 12, 1408096. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, Z.; Yan, J.; Xie, J. Nutritional Components, Phytochemical Compositions, Biological Properties, and Potential Food Applications of Ginger (Zingiber Officinale): A Comprehensive Review. J. Food Compos. Anal. 2024, 128, 106057. [Google Scholar] [CrossRef]
- Nićetin, M.; Pezo, L.; Pergal, M.; Lončar, B.; Filipović, V.; Knežević, V.; Demir, H.; Jelena, F.; Manojlović, D. Celery Root Phenols Content, Antioxidant Capacities and Their Correlations after Osmotic Dehydration in Molasses. Foods 2022, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Górska-Warsewicz, H.; Rejman, K.; Kaczorowska, J.; Laskowski, W. Vegetables, Potatoes and Their Products as Sources of Energy and Nutrients to the Average Diet in Poland. Int. J. Environ. Res. Public Health 2021, 18, 3217. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Rasheed, A.; Ahmad Khan, A.; Khan, R.; Ahmad, M.; Bashir, R.; Hassan Mohamed, M. Exploring the Health Benefits and Utility of Carrots and Carrot Pomace: A Systematic Review. Int. J. Food Prop. 2024, 27, 180–193. [Google Scholar] [CrossRef]
- Dong, C.; Hu, J. Residue Levels of Four Pesticides from Two Commercial Formulations on Gingers and Their Dietary Intake Risk Assessment under Open Field Conditions. J. Food Compos. Anal. 2024, 128, 106016. [Google Scholar] [CrossRef]
- Kang, L.; Liu, H.; Zhao, D.; Pan, C.; Wang, C. Pesticide Residue Behavior and Risk Assessment in Celery after Se Nanoparticles Application. Foods 2021, 10, 1987. [Google Scholar] [CrossRef]
- Rybacki, P.; Przygodziński, P.; Blecharczyk, A.; Kowalik, I.; Osuch, A.; Osuch, E. Strip Spraying Technology for Precise Herbicide Application in Carrot Field. Open Chem. 2022, 20, 287–296. [Google Scholar] [CrossRef]
- Cui, K.; Wang, J.; Ma, G.; Guan, S.; Liang, J.; Fang, L.; Ding, R.; Li, T.; Dong, Z.; Wu, X.; et al. Residue Levels, Processing Factors and Risk Assessment of Pesticides in Ginger from Market to Table. J. Hazard. Mater. 2024, 470, 134268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhuang, M.; Xiao, P.; Wang, K.; Song, J.; Liu, H.; Zhao, J.; Chu, Z. Pesticide Residues and Dietary Risk Assessment in Radishes in Shandong. J. Food Sci. 2022, 87, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- European Commission Pesticide Residues in Food—Maximum Residue Levels (MRLs). Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin. Off. J. Eur. Union 2025, 10, 1–21. [Google Scholar]
- P, S.; Thasale, R.; Kumar, D.; Mehta, T.G.; Limbachiya, R. Human Health Risk Assessment of Pesticide Residues in Vegetable and Fruit Samples in Gujarat State, India. Heliyon 2022, 8, e10876. [Google Scholar] [CrossRef]
- Lučić, M.; Momčilović, M.; Marković, J.; Jović, M.; Smičiklas, I.; Onjia, A. Monte Carlo Simulation of Health Risk from Cadmium, Lead, and Nickel in Cigarettes. Toxicol. Environ. Chem. 2023, 105, 92–110. [Google Scholar] [CrossRef]
- Miletić, A.; Lučić, M.; Onjia, A. Exposure Factors in Health Risk Assessment of Heavy Metal(Loid)s in Soil and Sediment. Metals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Radulović, J.; Lučić, M.; Nešić, A.; Onjia, A. Multivariate Assessment and Risk Ranking of Pesticide Residues in Citrus Fruits. Foods 2023, 12, 2454. [Google Scholar] [CrossRef]
- Maleki, N.S.; Shakerkhatibi, M.; Dolatkhah, M.; Safari, G.H. Cumulative Health Risk Assessment of Pesticide Residues in Apple Products in the Northwest of Iran Using Monte Carlo Simulation. Food Addit. Contam.-Part A 2023, 40, 992–1010. [Google Scholar] [CrossRef]
- Mahdavi, V.; Eslami, Z.; Gordan, H.; Ramezani, S.; Peivasteh-roudsari, L.; Ma’mani, L.; Mousavi Khaneghah, A. Pesticide Residues in Green-House Cucumber, Cantaloupe, and Melon Samples from Iran: A Risk Assessment by Monte Carlo Simulation. Environ. Res. 2023, 206, 112563. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Sun, J.; Zhang, G. Pesticide Residues in Vegetables from Gansu Province, China and Risk Assessment by Monte Carlo Simulation. Food Addit. Contam. Part B Surveill. 2024, 17, 251–260. [Google Scholar] [CrossRef]
- EN 15662:2008; Foods of Plant Origin—Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE—QuEChERS-Method. European Committee for Standardization (CEN): Brussels, Belgium, 2008.
- European Commission Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. SANTE/11312/2021 Rev. 2. Implemented by 01/01/2024. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 1 June 2025).
- Radulović, J.; Lučić, M.; Onjia, A. GC-MS/MS and LC-MS/MS Analysis Followed by Risk Ranking of Mepiquat and Pyrethroids in Coffee. J. Food Compos. Anal. 2024, 129, 106100. [Google Scholar] [CrossRef]
- Veterinary Residues Committee. Annual Report on Surveillance for Veterinary Residues in Food in the UK 2007; Veterinary Residues Committee: Addlestone, UK, 2007. [Google Scholar]
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification, 2019th ed.; WHO: Geneva, Switzerland, 2020; ISBN 978-92-4-000566-2. [Google Scholar]
- EPA. Pesticide Fact Sheet: Epoxiconazole; EPA: Washington, DC, USA, 2003; p. 19. [Google Scholar]
- EPA. Fact Sheet for Ethoxyquin; EPA: Washington, DC, USA, 2004. [Google Scholar]
- EPA. Pesticide Fact Sheet Fosthiazate; EPA: Washington, DC, USA, 2004. [Google Scholar]
- Alexander, J.; Autrup, H.; Bard, D.; Carere, A.; Guido Costa, L.; Cravedi, J.-P.; Di Domenico, A.; Fanelli, R.; Fink-Gremmels, J.; Gilbert, J.; et al. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the Commission Related to Gamma-HCH and Other Hexachlorcyclohexanes as Undesirable Substances in Animal Feed. EFSA J. 2005, 4, 365. [Google Scholar] [CrossRef]
- European Commission (EC) EU Pesticides Database. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 26 May 2025).
- AERU Agriculture and Environment Research Unit at the University of Hertfordshire: PPDB—Pesticide Properties Database. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed on 3 March 2025).
- JMPR; WHO. Inventory of Evaluations Performed by the Joint Meeting on Pesticide Residues (JMPR). Available online: https://apps.who.int/pesticide-residues-jmpr-database/Home/Search (accessed on 3 March 2025).
- Statistical Office of the Republic of Serbia. Household Budget Survey; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2023. [Google Scholar]
- WHO; FAO. International Estimated Short-Term Intake (IESTI). Available online: https://cdn.who.int/media/docs/default-source/food-safety/gems-food/guidance-iesti-2014.pdf?sfvrsn=9b24629a_2 (accessed on 10 August 2023).
- EFSA. Peer Review of the Pesticide Risk Assessment of the Active Substance Pyrimethanil. EFSA J. 2024, 22, e8998. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO. Pesticide Residues in Food 2003; FAO: Rome, Italy, 2004. [Google Scholar]
- EFSA. Statement on the Available Outcomes of the Human Health Assessment in the Context of the Pesticides Peer Review of the Active Substance Chlorpyrifos. EFSA J. 2019, 17, 5809. [Google Scholar] [CrossRef] [PubMed]
- Lučić, M.; Miletić, A.; Savić, A.; Lević, S.; Sredović Ignjatović, I.; Onjia, A. Dietary Intake and Health Risk Assessment of Essential and Toxic Elements in Pepper (Capsicum Annuum). J. Food Compos. Anal. 2022, 111, 104598. [Google Scholar] [CrossRef]
- Yu, J.J.; Bong, L.J.; Panthawong, A.; Chareonviriyaphap, T.; Liu, W.T.; Neoh, K.B. Effects of Piperonyl Butoxide Synergism and Cuticular Thickening on the Contact Irritancy Response of Field Aedes Aegypti (Diptera: Culicidae) to Deltamethrin. Pest Manag. Sci. 2021, 77, 5557–5565. [Google Scholar] [CrossRef]
- EFSA. Panel Safety and Efficacy of Ethoxyquin 6-ethoxy-1 2-dihydro-2 2 4-trimethylquinoline for All Animal.Pdf. EFSA J. 2015, 13, 4272. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, S.; Chen, Z.; Du, H.; Zhu, Q.; Dong, Z.; Li, H. Risk Assessment of Pesticide Residues in Dietary Intake of Celery in China. Regul. Toxicol. Pharmacol. 2015, 73, 578–586. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Średnicka-Tober, D.; Golba, J.; Nowacka, A.; Hołodyńska-Kulas, A.; Kopczyńska, K.; Góralska-Walczak, R.; Gnusowski, B. Evaluation of Pesticide Residues Occurrence in Random Samples of Organic Fruits and Vegetables Marketed in Poland. Foods 2022, 11, 1963. [Google Scholar] [CrossRef]
- Jirata, U.; Asere, T.G.; Balcha, Y.B.; Gure, A. Levels of Organochlorine Pesticides in Onion and Tomato Samples from Selected Towns of Jimma Zone, Ethiopia. Heliyon 2024, 10, e35033. [Google Scholar] [CrossRef]
- Chu, N.; Shu, X.; Meng, X.; Zhang, X.; Yang, J.; Li, B. Determination and Dietary Exposure Assessment of 79 Pesticide Residues in Chinese Onion (Allium fistulosum L.). CYTA-J. Food 2023, 21, 41–48. [Google Scholar] [CrossRef]
- EFSA. The 2020 European Union Report on Pesticide Residues in Food. Available online: https://multimedia.efsa.europa.eu/pesticides-report-2020/commodity/potatoes/ (accessed on 21 June 2025).
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union Report on Pesticide Residues in Food. EFSA J. 2023, 21, 7939. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; Miron, I.; et al. Reasoned Opinion on the Setting of Temporary Maximum Residue Levels for Chlorpropham in Potatoes. EFSA J. 2020, 18, e06061. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2022 European Union Report on Pesticide Residues in Food. EFSA J. 2024, 22, e8753. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Medina Pastor, P. The 2020 European Union Report on Pesticide Residues in Food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- Onjia, A.; Huang, X.; Trujillo González, J.M.; Egbueri, J.C. Chemometric Approach to Distribution, Source Apportionment, Ecological and Health Risk of Trace Pollutants. Front. Environ. Sci. 2022, 10, 1107465. [Google Scholar] [CrossRef]
- Sayed, A.; Chys, M.; De Rop, J.; Goeteyn, L.; Spanoghe, P.; Sampers, I. Pesticide Residues in (Treated) Wastewater and Products of Belgian Vegetable- and Potato Processing Companies. Chemosphere 2021, 280, 130619. [Google Scholar] [CrossRef]
- European Commission (EC) RASFF—Rapid Alert System for Food and Feed. Available online: https://food.ec.europa.eu/food-safety/rasff_en (accessed on 21 June 2025).
- Gkountouras, D.; Boti, V.; Albanis, T. Pesticides and Transformation Products Footprint in Greek Market Basket Vegetables: Comprehensive Screening by HRMS and Health Risk Assessment. Sci. Total Environ. 2024, 953, 176085. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Evaluation of QuEChERS Sample Preparation and Liquid Chromatography-Triple-Quadrupole Mass Spectrometry Method for the Determination of 109 Pesticide Residues in Tomatoes. Food Chem. 2015, 176, 319–332. [Google Scholar] [CrossRef]
- Golge, O.; Hepsag, F.; Kabak, B. Health Risk Assessment of Selected Pesticide Residues in Green Pepper and Cucumber. Food Chem. Toxicol. 2018, 121, 51–64. [Google Scholar] [CrossRef]
- Terfe, A.; Mekonen, S.; Jemal, T. Pesticide Residues and Effect of Household Processing in Commonly Consumed Vegetables in Jimma Zone, Southwest Ethiopia. J. Environ. Public Health 2023, 2023, 7503426. [Google Scholar] [CrossRef]
- Phopin, K.; Wanwimolruk, S.; Norkaew, C.; Buddhaprom, J.; Isarankura-Na-ayudhya, C. Boiling, Blanching, and Stir-Frying Markedly Reduce Pesticide Residues in Vegetables. Foods 2022, 11, 1463. [Google Scholar] [CrossRef]
- Cho, M.; Kim, M.; Im, J.; Seo, C.; Moon, Y.S.; Im, M.H. Variation of Boscalid Residues in Welsh Onion during Cooking Processes. Food Sci. Biotechnol. 2025, 34, 1351–1358. [Google Scholar] [CrossRef]
- Jensen, B.H.; Petersen, A.; Petersen, P.B.; Christensen, T.; Fagt, S.; Trolle, E.; Poulsen, M.E.; Hinge Andersen, J. Cumulative Dietary Risk Assessment of Pesticides in Food for the Danish Population for the Period 2012–2017. Food Chem. Toxicol. 2022, 168, 113359. [Google Scholar] [CrossRef]



| Pesticide | ME % | R2 | LOD µg/kg | R # % | RSD # % | R ## % | RSD ## % |
|---|---|---|---|---|---|---|---|
| Celery | |||||||
| Pyrimethanil | −27.4 | 0.9936 | 1.2 | 106 | 2.64 | 92.5 | 9.31 |
| Boscalid | 9.30 | 0.9955 | 0.9 | 94.7 | 5.75 | 110 | 3.95 |
| Tebuconazole | 15.5 | 0.9962 | 1.5 | 115 | 9.51 | 93.3 | 5.78 |
| Metolachlor | −10.3 | 0.9992 | 2.7 | 111 | 7.34 | 105 | 11.1 |
| Azoxystrobin | −13.2 | 0.9940 | 1.4 | 101 | 1.80 | 97.8 | 8.46 |
| Fluopyram | 11.3 | 0.9988 | 1.2 | 87.7 | 2.45 | 93.1 | 9.74 |
| Difenoconazole | 16.8 | 0.9939 | 2.9 | 89.5 | 11.2 | 113 | 10.1 |
| Prosulfocarb | −33.5 | 0.9943 | 1.8 | 99.7 | 3.12 | 105 | 11.5 |
| Linuron | −10.5 | 0.9937 | 1.5 | 104.3 | 7.78 | 95.1 | 7.35 |
| Carbaryl | 12.4 | 0.9953 | 1.4 | 114 | 8.20 | 94.2 | 3.65 |
| Resmethrin | −28.4 | 0.9982 | 1.9 | 93.2 | 11.3 | 102 | 7.12 |
| Potato | |||||||
| Pyrimethanil | 11.5 | 0.9991 | 1.6 | 102 | 2.43 | 94.3 | 4.95 |
| Metolachlor | −27.1 | 0.9963 | 2.5 | 109 | 4.01 | 103 | 6.01 |
| Azoxystrobin | 27.3 | 0.9987 | 1.3 | 98.3 | 3.98 | 95.4 | 4.75 |
| Fluopyram | −15.2 | 0.9974 | 2.3 | 90.1 | 3.40 | 97.2 | 5.20 |
| Chlorpropham | 35.0 | 0.9991 | 2.5 | 88.0 | 3.33 | 114 | 6.62 |
| Clothianidin | 45.2 | 0.9989 | 1.0 | 94.1 | 12.4 | 93.9 | 10.0 |
| Fluopicolide | −44.1 | 0.9948 | 3.1 | 113 | 4.98 | 113 | 3.94 |
| Fosthiazate | 17.6 | 0.9935 | 2.2 | 95.2 | 10.2 | 101 | 5.51 |
| Fluazifop | 38.3 | 0.9953 | 1.4 | 97.9 | 13.7 | 97.2 | 3.20 |
| Epoxiconazole | 16.2 | 0.9972 | 1.2 | 103 | 6.31 | 92.9 | 7.70 |
| Dimethomorph | 31.4 | 0.9990 | 2.8 | 111 | 4.05 | 101 | 3.90 |
| Thiamethoxam | 32.2 | 0.9937 | 3.0 | 85.9 | 6.72 | 93.3 | 5.19 |
| Piperonyl butoxide | 48.3 | 9.9986 | 1.4 | 90.2 | 2.90 | 94.0 | 4.20 |
| Imidacloprid | 55.8 | 0.9935 | 0.8 | 91.8 | 11.0 | 110 | 7.32 |
| Cyromazine | −17.3 | 0.9974 | 1.9 | 86.5 | 7.83 | 105 | 11.9 |
| Horseradish | |||||||
| Metamitron | −18.4 | 0.9990 | 1.1 | 89.8 | 3.38 | 92.8 | 8.15 |
| Ginger | |||||||
| Metolachlor | −24.5 | 0.9981 | 2.7 | 112 | 5.15 | 110 | 12.4 |
| Tebufenpyrad | −55.6 | 0.9935 | 1.1 | 95.1 | 14.3 | 107 | 5.52 |
| Tebufenozide | −23.1 | 0.9985 | 1.5 | 105 | 3.50 | 111 | 7.17 |
| Iprovalicarb | −29.9 | 0.9949 | 2.7 | 110 | 7.91 | 97.3 | 3.85 |
| Pirimiphos-methyl | 22.8 | 0.9951 | 1.3 | 93.7 | 10.5 | 93.0 | 5.19 |
| Onion | |||||||
| Fluopicolide | −7.5 | 0.9958 | 2.6 | 91.2 | 5.91 | 104 | 4.91 |
| Dimethomorph | −8.8 | 0.9977 | 1.3 | 85.3 | 3.89 | 94.9 | 6.30 |
| Piperonyl butoxide | 13.5 | 0.9964 | 3.1 | 99.1 | 1.99 | 98.3 | 3.73 |
| Imidacloprid | −13.3 | 0.9986 | 0.9 | 103 | 6.01 | 105 | 3.79 |
| Cyromazine | −15.6 | 0.9974 | 3.2 | 94.7 | 7.20 | 104 | 11.9 |
| Fenhexamid | −11.2 | 0.9943 | 2.3 | 112 | 3.34 | 111 | 9.91 |
| Ethoxyquin | 12.2 | 0.9983 | 0.9 | 87.4 | 5.07 | 103 | 5.54 |
| Imazalil | 25.1 | 0.9949 | 3.4 | 114 | 9.63 | 93.5 | 4.11 |
| Chlorpyrifos | 21.1 | 0.9986 | 2.5 | 95.7 | 13.4 | 113 | 11.0 |
| Propamocarb | −7.0 | 0.9940 | 3.0 | 92.1 | 2.95 | 98.1 | 9.78 |
| Radish | |||||||
| Boscalid | −12.2 | 0.9973 | 1.2 | 84.7 | 3.96 | 97.2 | 12.8 |
| Azoxystrobin | −14.5 | 0.9951 | 1.7 | 98.3 | 7.05 | 110 | 3.20 |
| Thiamethoxam | −11.1 | 0.9959 | 2.9 | 104 | 8.70 | 112 | 6.86 |
| Chlorpyrifos | 14.8 | 0.9968 | 2.3 | 93.5 | 4.12 | 94.2 | 7.33 |
| Propamocarb | −8.7 | 0.9973 | 1.6 | 107 | 5.95 | 112 | 11.5 |
| Carrot | |||||||
| Boscalid | −15.9 | 0.9984 | 1.1 | 93.9 | 8.14 | 98.0 | 5.60 |
| Tebuconazole | −33.5 | 0.9975 | 1.7 | 95.1 | 4.83 | 107 | 4.77 |
| Azoxystrobin | −6.4 | 0.9961 | 1.2 | 105 | 6.24 | 113 | 5.95 |
| Prosulfocarb | 18.1 | 0.9992 | 2.0 | 111 | 5.13 | 105 | 8.48 |
| Isoprocarb | 11.0 | 0.9957 | 2.8 | 89.0 | 11.6 | 94.9 | 2.85 |
| Potatoes | Onion | Celery | Radish | Ginger | Carrot | Leek | Horseradish |
|---|---|---|---|---|---|---|---|
| PYM | PYM | PYM | BOS | MTL | BOS | BOS | MTT |
| MTL | BOS | BOS | AZX | TBP | TEB | TEB | |
| AZX | AZX | TEB | TMX | TBZ | AZX | AZX | |
| FPM | FPM | MTL | CPF | IPV | PSC | PBO | |
| CHP | FPC | AZX | MTT | PPM | ISP | ||
| CLT | DMM | FPM | PPC | ||||
| FPC | IMI | DFZ | |||||
| FST | FHX | PSC | |||||
| FLP | ETQ | LIN | |||||
| EPX | IMZ | CRB | |||||
| DMM | CPF | RMT | |||||
| TMX | |||||||
| PBO | |||||||
| IMI | |||||||
| CYR | |||||||
| PPC |
| Acute Risk for Adults | |||
| Vegetable | HIa Min | HIa Max | HIa Mean |
| Celery | 2.96 × 10−4 | 2.19 × 10−1 | 2.87 × 10−2 |
| Ginger | 4.25 × 10−4 | 5.11 × 10−1 | 2.86 × 10−1 |
| Potato | 1.93 × 10−4 | 1.23 × 10−1 | 1.30 × 10−2 |
| Onion and leek | 1.82 × 10−4 | 1.21 × 10−1 | 1.84 × 10−2 |
| Radish | 1.66 × 10−4 | 9.11 × 10−2 | 2.32 × 10−2 |
| Carrot | 2.32 × 10−3 | 5.46 × 10−3 | 3.89 × 10−3 |
| All root vegetables | 1.66 × 10−4 | 5.11 × 10−1 | 5.04 × 10−2 |
| Acute Risk for Children | |||
| Vegetable | HIa Min | HIa Max | HIa Mean |
| Celery | 8.52 × 10−4 | 6.31 × 10−1 | 8.26 × 10−2 |
| Ginger | 6.23 × 10−5 | 7.47 × 10−2 | 2.41 × 10−2 |
| Potato | 4.59 × 10−4 | 2.92 × 10−1 | 3.09 × 10−2 |
| Onion and leek | 3.26 × 10−4 | 2.16 × 10−1 | 3.50 × 10−2 |
| Radish | 9.72 × 10−5 | 2.83 × 10−1 | 7.14 × 10−2 |
| Carrot | 7.98 × 10−3 | 1.88 × 10−2 | 1.34 × 10−2 |
| All root vegetables | 6.23 × 10−5 | 6.31 × 10−1 | 4.49 × 10−2 |
| Chronic Risk for Adults | |||
| Vegetable | HIc Min | HIc Max | HIc Mean |
| Celery | 1.96 × 10−5 | 5.90 × 10−2 | 5.73 × 10−3 |
| Ginger | 7.52 × 10−5 | 3.89 × 10−2 | 1.08 × 10−2 |
| Potato | 4.68 × 10−5 | 1.51 × 10−2 | 2.47 × 10−3 |
| Onion and leek | 5.19 × 10−5 | 2.40 × 10−2 | 3.31 × 10−3 |
| Radish | 4.51 × 10−5 | 3.63 × 10−3 | 7.42 × 10−4 |
| Carrot | 1.80 × 10−5 | 6.38 × 10−3 | 1.38 × 10−3 |
| All root vegetables | 1.80 × 10−5 | 5.90 × 10−2 | 4.08 × 10−3 |
| Chronic Risk for Children | |||
| Vegetable | HIc Min | HIc Max | HIc Mean |
| Celery | 3.66 × 10−5 | 1.10 × 10−1 | 1.07 × 10−2 |
| Ginger | 1.40 × 10−4 | 7.27 × 10−2 | 2.02 × 10−2 |
| Potato | 8.73 × 10−5 | 2.83 × 10−2 | 4.62 × 10−3 |
| Onion and leek | 9.68 × 10−5 | 4.48 × 10−2 | 6.18 × 10−3 |
| Radish | 8.42 × 10−5 | 6.77 × 10−3 | 1.39 × 10−3 |
| Carrot | 3.36 × 10−5 | 1.19 × 10−2 | 2.57 × 10−3 |
| All root vegetables | 3.36 × 10−5 | 1.10 × 10−1 | 7.62 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lučić, M.; Onjia, A. Prioritization and Sensitivity of Pesticide Risks from Root and Tuber Vegetables. J. Xenobiot. 2025, 15, 125. https://doi.org/10.3390/jox15040125
Lučić M, Onjia A. Prioritization and Sensitivity of Pesticide Risks from Root and Tuber Vegetables. Journal of Xenobiotics. 2025; 15(4):125. https://doi.org/10.3390/jox15040125
Chicago/Turabian StyleLučić, Milica, and Antonije Onjia. 2025. "Prioritization and Sensitivity of Pesticide Risks from Root and Tuber Vegetables" Journal of Xenobiotics 15, no. 4: 125. https://doi.org/10.3390/jox15040125
APA StyleLučić, M., & Onjia, A. (2025). Prioritization and Sensitivity of Pesticide Risks from Root and Tuber Vegetables. Journal of Xenobiotics, 15(4), 125. https://doi.org/10.3390/jox15040125







