Methyl Jasmonate and Ammonium Bicarbonate: Distinct and Synergistic Impacts on Indoor Cannabis Production Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Media and Baseline Fertility
2.3. Treatment Application
2.4. Data Collection
2.4.1. Growth and Chlorophyll Traits
2.4.2. Yield Traits
2.4.3. Trichome Density Counting
2.4.4. Cannabinoid Analysis
2.5. Statistical Analysis
3. Results
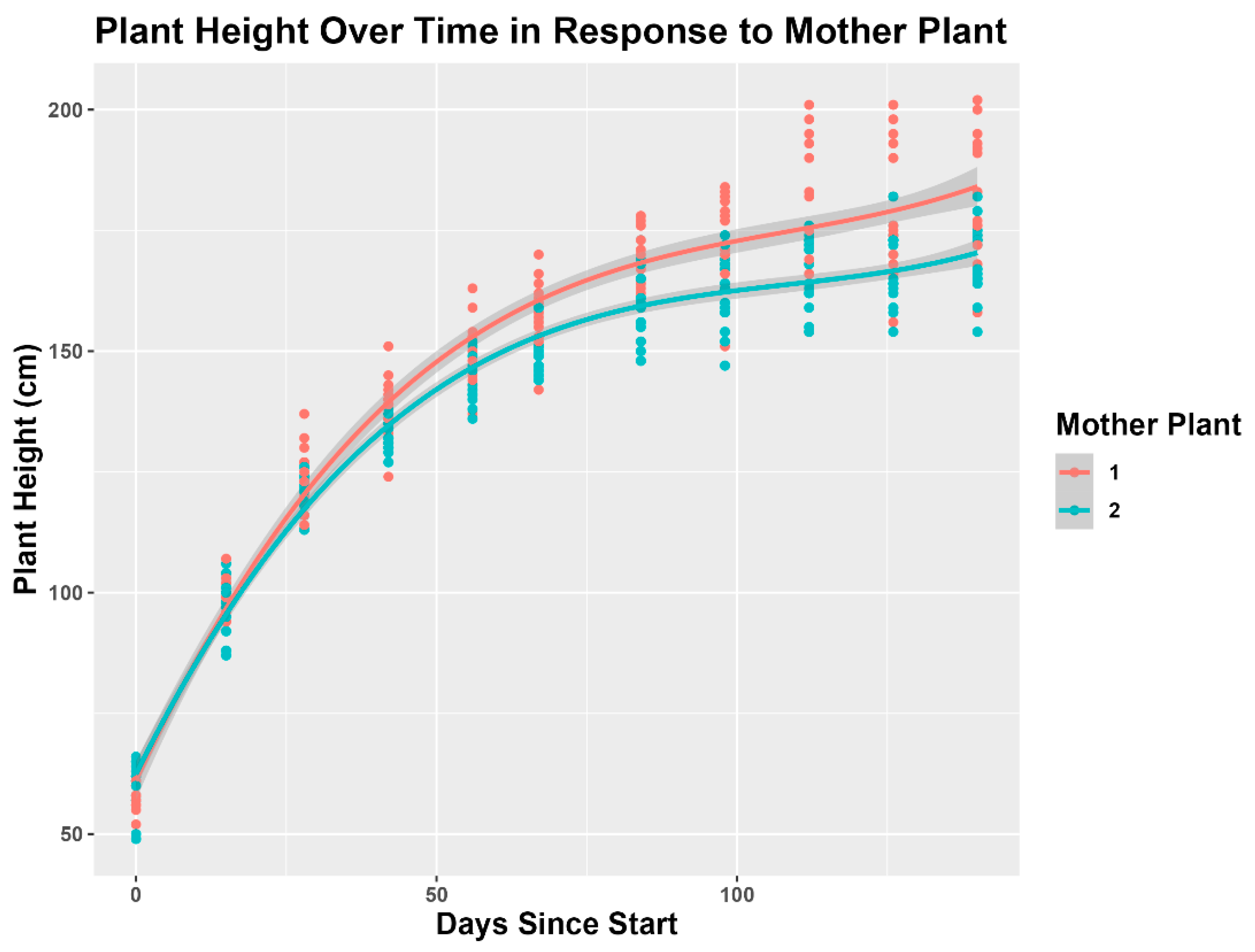
3.1. Plant Height
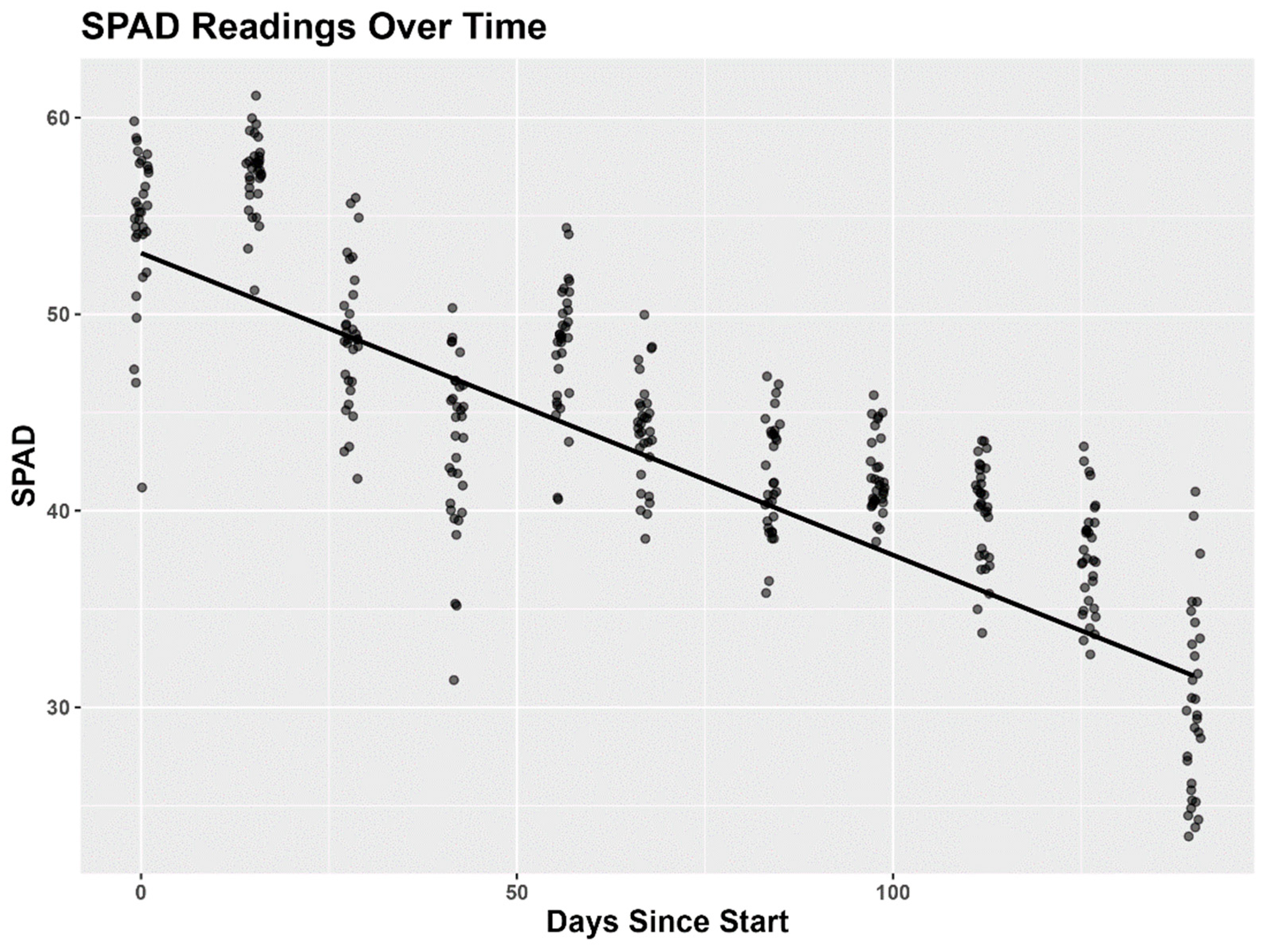
3.2. Soil Plant Analysis Development (SPAD) Chlorophyll Meter
3.3. Fresh and Dry Total Biomass
3.4. Bucked Biomass
3.5. Trichome Density
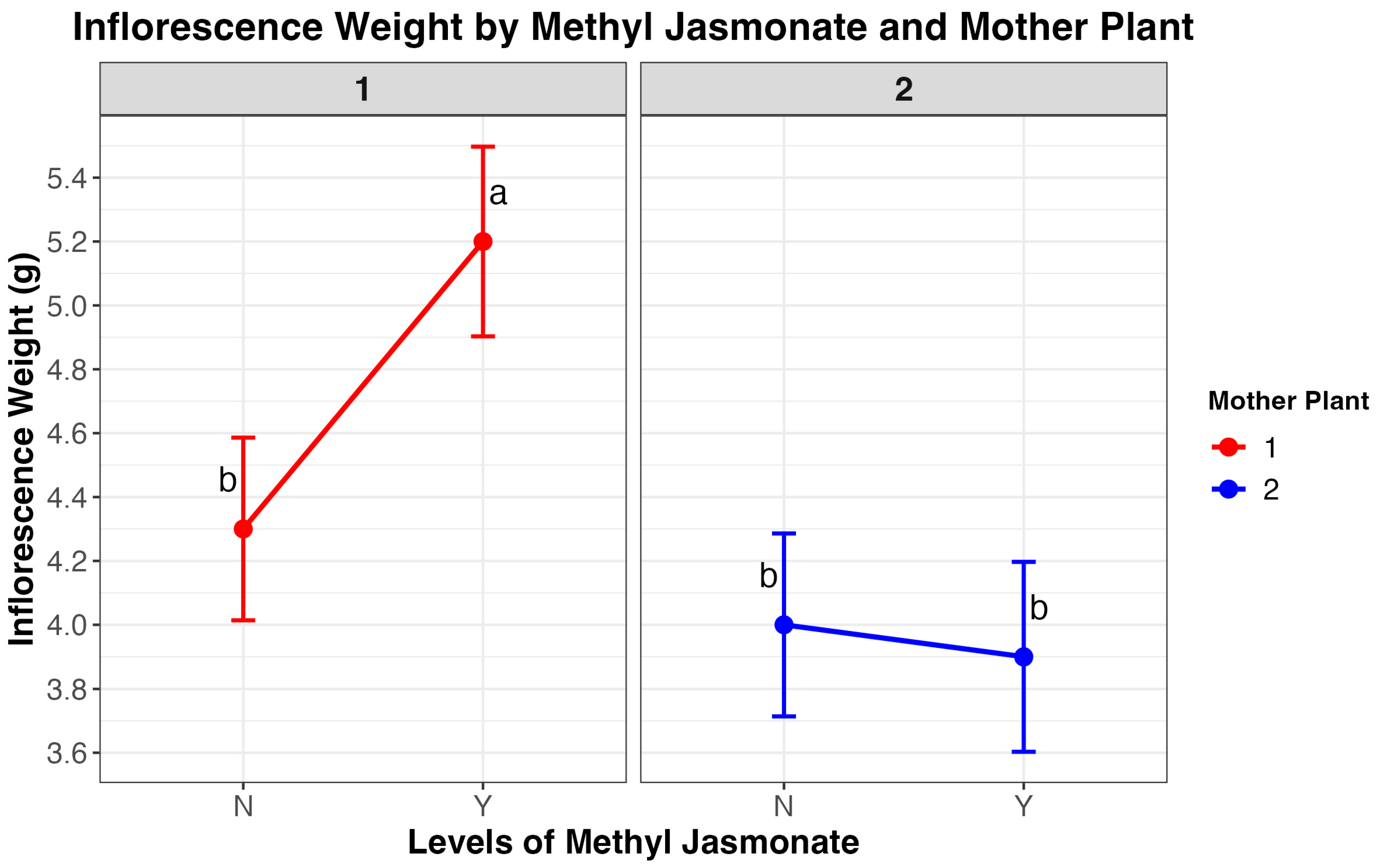
3.6. Inflorescence Biomass
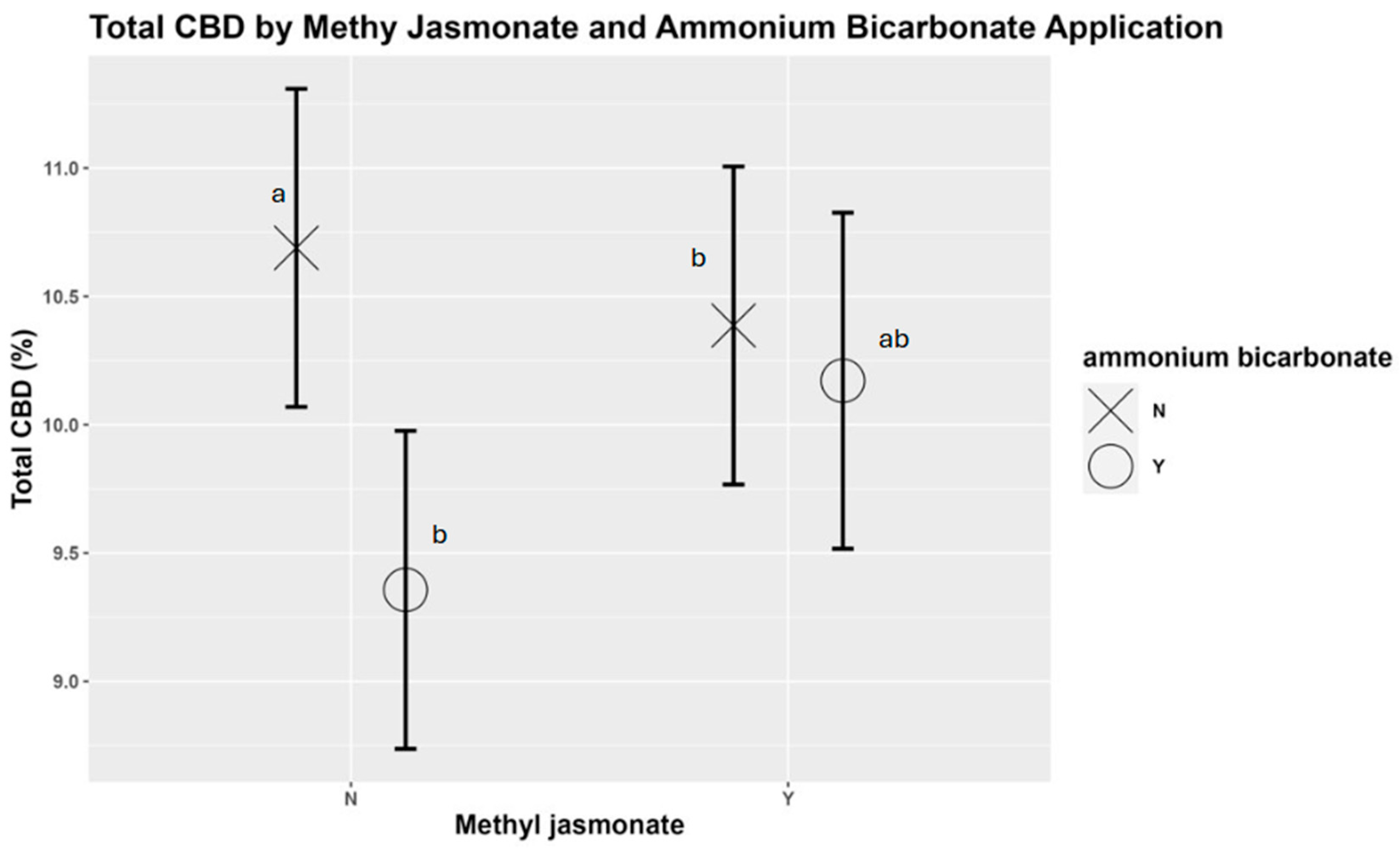
3.7. Cannabinoid Content (CBD and THC)
4. Discussion
4.1. Plant Height
4.2. Soil Plant Analysis Development (SPAD) Meter
4.3. Fresh and Dry Total Biomass
4.4. Bucked Biomass
4.5. Trichome Density
4.6. Inflorescence Weight
4.7. Cannabinoid Content (CBD and THC)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaminski, K.P.; Hoeng, J.; Goffman, F.; Schlage, W.K.; Latino, D. Opportunities, challenges, and scientific progress in hemp crops. Molecules 2024, 29, 2397. [Google Scholar] [CrossRef] [PubMed]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A comprehensive review on Cannabis sativa ethnobotany, phytochemistry, molecular docking and biological activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Panday, D.; Acharya, B.S.; Bhusal, N.; Afshar, R.K.; Smith, A.; Ghalehgolabbehbahani, A. Precision nitrogen management for optimal yield and cannabinoid profile in CBD hemp agronomy. Agrosyst. Geosci. Environ. 2024, 8, e70028. [Google Scholar] [CrossRef]
- Williams, A.; Brym, Z.; Chen, C.; Collins, A.; Crawford, J.; Darby, H.; Dedecker, J.; Ellison, S.; Fike, J.; Gage, K.; et al. Comparing agronomic performance of industrial hemp varieties for suitable production in the United States. Agron. J. 2025, 117, e70006. [Google Scholar] [CrossRef]
- Doumar, H.; El Mostafi, H.; Elhessni, A.; Touhami, M.E.; Mesfioui, A. Exploring the diversity of cannabis cannabinoid and non-cannabinoid compounds and their roles in Alzheimer’s disease: A review. IBRO Neurosci. Rep. 2025, 18, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Ruberto, G.; Cristino, L. Recent Research on Cannabis sativa L.: Phytochemistry, new matrices, cultivation techniques, and recent updates on its brain-related effects (2018–2023). Molecules 2023, 28, 3387. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef]
- Johnson, H.B. Plant pubescence: An ecological perspective. Bot. Rev. 1975, 41, 233–258. [Google Scholar] [CrossRef]
- Stratmann, J.W.; Bequette, C.J. Hairless but no longer clueless: Understanding glandular trichome development. J. Exp. Bot. 2016, 67, 5285–5287. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New Phytol. 2020, 225, 2251–2266. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.J. Secreting glandular trichomes: More than just hairs. Plant Physiol. 1991, 96, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis glandular trichomes: A cellular metabolite factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Kostina, E.; Wulff, A.; Julkunen-Tiitto, R. Growth, structure, stomatal responses and secondary metabolites of birch seedlings (Betula pendula) under elevated UV-B radiation in the field. Trees 2001, 15, 483–491. [Google Scholar] [CrossRef]
- Olfa, B.; Imen, T.; Mohamed, C.; Ayachi M, B.N. Essential oil and trichome density from Origanum majorana L. shoots affected by leaf age and salinity. Biosci. J. (Online) 2016, 2016, 238–245. [Google Scholar] [CrossRef]
- Prozherina, N.; Freiwald, V.; Rousi, M.; Oksanen, E. Interactive effect of springtime frost and elevated ozone on early growth, foliar injuries and leaf structure of birch (Betula pendula). New Phytol. 2003, 159, 623–636. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Sharma, S.; Alberti, T.; De Sarandy Raposo, R.; Anterola, A.M.; Weber, J.; Diatta, A.A.; Da Cunha Leme Filho, J.F. The effects of water-deficit stress on Cannabis sativa L. development and production of secondary metabolites: A review. Horticulturae 2025, 11, 646. [Google Scholar] [CrossRef]
- Brian Traw, M.; Dawson, T.E. Reduced performance of two specialist herbivores (Lepidoptera: Pieridae, Coleoptera: Chrysomelidae) on new leaves of damaged black mustard plants. Environ. Entomol. 2002, 31, 714–722. [Google Scholar] [CrossRef]
- Dalin, P.; Ågren, J.; Björkman, C.; Huttunen, P.; Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–105. [Google Scholar]
- Acosta, I.F.; Farmer, E.E. Jasmonates. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2010; Volume 8, p. e0129. [Google Scholar]
- Cheong, J.J.; Choi, Y.D. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Apicella, P.V.; Sands, L.B.; Ma, Y.; Berkowitz, G.A. Delineating genetic regulation of cannabinoid biosynthesis during female flower development in Cannabis sativa. Plant Direct 2022, 6, e412. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of changes in leaf properties mediated by methyl jasmonate (MeJA) on foliar absorption of Zn, Mn and Fe. Ann. Bot. 2017, 120, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil. Sci. 2018, 181, 409–418. [Google Scholar] [CrossRef]
- Li, C.-K.; Chen, R.-Y. Ammonium bicarbonate used as a nitrogen fertilizer in China. Fertil. Res. 1980, 1, 125–136. [Google Scholar] [CrossRef]
- Ahmadi, F.; Kallinger, D.; Starzinger, A.; Lackner, M. Hemp (Cannabis sativa L.) Cultivation: Chemical fertilizers or organic technologies, a comprehensive review. Nitrogen 2024, 5, 624–654. [Google Scholar] [CrossRef]
- Ryu, B.-R.; Kim, C.-H.; Kwon, T.-H.; Han, J.-H.; Gim, G.-J.; Jahirul Islam, M.; Azad, M.O.K.; Hafizur Rahman, M.; Soyel Rana, M.; Lim, J.-D.; et al. Industrial hemp clone selection method under LED smart farm condition based on CBD production per cubic meter. Agronomy 2022, 12, 1809. [Google Scholar] [CrossRef]
- Da Cunha Leme Filho, J.F.; Schuchman, S.; Creager, K.E.; Saunders, G.; Diatta, A.A.; Didaran, F.; Boren, A.C.; Gage, K.L. Implementation of a standardized cloning and propagation protocol for optimizing Cannabis sativa L. cultivation. J. Agric. Sci. 2024, 17, 5. [Google Scholar] [CrossRef]
- Garrido, J.; Rico, S.; Corral, C.; Sanchez, C.; Vidal, N.; Martinez-Quesada, J.J.; Ferreiro-Vera, C. Exogenous application of stress-related signaling molecules affect growth and cannabinoid accumulation in medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2022, 13, 1082554. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models usinglme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) to nitrogen supply under long photoperiod. Front. Plant Sci. 2020, 11, 572293. [Google Scholar] [CrossRef]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L., 2nd; Pearson, B.; Kjelgren, R.; Brym, Z. Response of essential oil hemp (Cannabis sativa L.) growth, biomass, and cannabinoid profiles to varying fertigation rates. PLoS ONE 2021, 16, e0252985. [Google Scholar] [CrossRef] [PubMed]
- Kandel, B.P. Spad value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 475. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Chen, L.H.; Xu, M.; Cheng, Z.; Yang, L.T. Effects of nitrogen deficiency on the photosynthesis, chlorophyll a fluorescence, antioxidant system, and sulfur compounds in Oryza sativa. Int. J. Mol. Sci. 2024, 25, 10409. [Google Scholar] [CrossRef]
- Hamblin, J.; Stefanova, K.; Angessa, T.T. Variation in chlorophyll content per unit leaf area in spring wheat and implications for selection in segregating material. PLoS ONE 2014, 9, e92529. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, V.; Kishore, K. Leaf senescence: An overview. Indian. J. Plant Physiol. 2016, 21, 225–238. [Google Scholar] [CrossRef]
- Danish, S.; Sana, S.; Hussain, M.B.; Dawar, K.; Almoallim, H.S.; Ansari, M.J.; Hareem, M.; Datta, R. Effect of methyl jasmonate and GA3 on canola (Brassica napus L.) growth, antioxidants activity, and nutrient concentration cultivated in salt-affected soils. BMC Plant Biol. 2024, 24, 363. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X. The MYB transcription factor superfamily of Arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef]
- Ocaña, J.; Walter, B.; Schellenbaum, P. Stable MSAP markers for the distinction of Vitis vinifera cv Pinot noir clones. Mol. Biotechnol. 2013, 55, 236–248. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis lighting: Decreasing blue photon fraction increases yield but efficacy is more important for cost effective production of cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef]
- Welling, M.T.; Deseo, M.A.; O’Brien, M.; Clifton, J.; Bacic, A.; Doblin, M.S. Metabolomic analysis of methyl jasmonate treatment on phytocannabinoid production in Cannabis sativa. Front. Plant Sci. 2023, 14, 1110144. [Google Scholar] [CrossRef]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert: Glandular trichomes as sensors for insect herbivores. New Phytol. 2009, 184, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Vom Endt, D.; Kijne, J.W.; Memelink, J. Transcription factors controlling plant secondary metabolism: What regulates the regulators? Phytochemistry 2002, 61, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Handa, A.K. Hormonal regulation of tomato fruit development: A molecular perspective. J. Plant Growth Regul. 2005, 24, 67–82. [Google Scholar] [CrossRef]
- Dilena, E.; Hunt, I.; Close, D.C. Optimal nitrogen rates and clonal effects on cannabinoid yields of medicinal cannabis. Sci. Rep. 2025, 15, 12341. [Google Scholar] [CrossRef]
- Massuela, D.C.; Munz, S.; Hartung, J.; Nkebiwe, P.M.; Graeff-Hönninger, S. Cannabis hunger games: Nutrient stress induction in flowering stage—Impact of organic and mineral fertilizer levels on biomass, cannabidiol (CBD) yield and nutrient use efficiency. Front. Plant Sci. 2023, 14, 1233232. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. The highs and lows of P supply in medical cannabis: Effects on cannabinoids, the ionome, and morpho-physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- James, M.S.; Vann, M.C.; Suchoff, D.H.; McGinnis, M.; Whipker, B.E.; Edmisten, K.L.; Gatiboni, L.C. Hemp yield and cannabinoid concentrations under variable nitrogen and potassium fertilizer rates. Crop Sci. 2023, 63, 1555–1565. [Google Scholar] [CrossRef]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × Environment interactions of industrial hemp cultivars highlight diverse responses to environmental factors. Agrosyst. Geosci. Environ. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Goyal, S.S.; Huffaker, R.C. Nitrogen toxicity in plants. In Nitrogen in Crop Production; Amer Society of Agronomy: Madison, WI, USA, 1984; pp. 97–118. [Google Scholar] [CrossRef]
- Da Cunha Leme Filho, J.F.; Schuchman, S.; De Sarandy Raposo, R.; Diatta, A.A.; Didaran, F.; Sharma, S.; Walters, A.; Gage, K.L. The role of jasmonates in modulating growth, trichome density, and cannabinoid accumulation in Cannabis sativa L. Int. J. Plant Biol. 2025, 16, 68. [Google Scholar] [CrossRef]



| Factor | Treatments |
|---|---|
| Methyl jasmonate (MeJA) |
|
| Ammonium bicarbonate (AB) |
|
| “Cherry Wine” mother plant (clones from two distinct “Cherry Wine” stock plants) |
|
| Treatment | Timing | Rate |
|---|---|---|
| Methyl jasmonate (MeJA) |
| 1 mM MeJA + 0.1% Tween-20 |
| Ammonium bicarbonate (AB) |
| 0.5 g per plant |
| Source | Sum of Squares | Mean Squares | Numerator df | Denominator df | F | p |
|---|---|---|---|---|---|---|
| Methyl jasmonate | 9.78 | 9.78 | 1 | 30 | 0.40 | 0.53 |
| Ammonium bicarbonate | 6.85 | 6.85 | 1 | 30 | 0.28 | NS |
| Mother plant | 0.00 | 0.00 | 1 | 30 | 0.00 | 0.99 |
| Days since start | 65,743.09 | 65,743.09 | 1 | 310 | 2674.77 | *** |
| Days since start 2 | 16,516.71 | 16,516.71 | 1 | 310 | 671.99 | *** |
| Days since start 3 | 7040.93 | 7040.93 | 1 | 310 | 286.46 | *** |
| Ammonium bicarbonate × days since start | 165.84 | 165.84 | 1 | 310 | 6.75 | ** |
| Ammonium bicarbonate × methyl jasmonate | 0.10 | 0.10 | 1 | 30 | 0.00 | NS |
| Ammonium bicarbonate × mother plant | 4.37 | 4.37 | 1 | 21 | 0.18 | NS |
| Days since start × methyl jasmonate | 9.39 | 9.39 | 1 | 310 | 0.38 | NS |
| Days since start × mother plant | 1719.74 | 1719.74 | 1 | 310 | 69.97 | *** |
| Methyl jasmonate × mother plant | 2.87 | 2.87 | 1 | 21 | 0.12 | NS |
| Ammonium bicarbonate × days since start × methyl jasmonate | 1191.47 | 1191.47 | 1 | 310 | 48.48 | *** |
| Ammonium bicarbonate × methyl jasmonate × mother plant | 6.36 | 6.36 | 1 | 21 | 0.26 | *** |
| Source | Sum of Squares | Mean Squares | Numerator df | Denominator df | F | p |
|---|---|---|---|---|---|---|
| Methyl jasmonate | 0.00 | 0.00 | 1 | 24 | 0.73 | NS |
| Ammonium bicarbonate | 0.00 | 0.00 | 1 | 24 | 0.01 | NS |
| Mother plant | 0.02 | 0.02 | 1 | 24 | 11.05 | ** |
| Source | Sum of Squares | Mean Squares | Numerator df | Denominator df | F | p |
|---|---|---|---|---|---|---|
| Methyl jasmonate | 228.94 | 228.94 | 1 | 27 | 20.14 | *** |
| Ammonium bicarbonate | 6.89 | 6.89 | 1 | 27 | 0.61 | NS |
| Mother plant | 15.81 | 15.81 | 1 | 27 | 1.39 | NS |
| Source | Sum of Squares | Mean Squares | Numerator df | Denominator df | F | p |
|---|---|---|---|---|---|---|
| Methyl jasmonate | 0.50 | 0.50 | 1 | 23 | 0.92 | NS |
| Ammonium bicarbonate | 4.61 | 4.61 | 1 | 23 | 8.43 | ** |
| Mother plant | 5.28 | 5.28 | 1 | 23 | 9.66 | ** |
| Ammonium bicarbonate × methyl jasmonate | 2.40 | 2.40 | 1 | 23 | 4.39 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Cunha Leme Filho, J.F.; Schuchman, S.; Shikanai, A.; Sharma, S.; Alberti, T.; Diatta, A.A.; Walters, A.; Gage, K.L. Methyl Jasmonate and Ammonium Bicarbonate: Distinct and Synergistic Impacts on Indoor Cannabis Production Dynamics. Int. J. Plant Biol. 2025, 16, 78. https://doi.org/10.3390/ijpb16030078
Da Cunha Leme Filho JF, Schuchman S, Shikanai A, Sharma S, Alberti T, Diatta AA, Walters A, Gage KL. Methyl Jasmonate and Ammonium Bicarbonate: Distinct and Synergistic Impacts on Indoor Cannabis Production Dynamics. International Journal of Plant Biology. 2025; 16(3):78. https://doi.org/10.3390/ijpb16030078
Chicago/Turabian StyleDa Cunha Leme Filho, Jose F., Spencer Schuchman, Avery Shikanai, Shiksha Sharma, Thais Alberti, Andre A. Diatta, Alan Walters, and Karla L. Gage. 2025. "Methyl Jasmonate and Ammonium Bicarbonate: Distinct and Synergistic Impacts on Indoor Cannabis Production Dynamics" International Journal of Plant Biology 16, no. 3: 78. https://doi.org/10.3390/ijpb16030078
APA StyleDa Cunha Leme Filho, J. F., Schuchman, S., Shikanai, A., Sharma, S., Alberti, T., Diatta, A. A., Walters, A., & Gage, K. L. (2025). Methyl Jasmonate and Ammonium Bicarbonate: Distinct and Synergistic Impacts on Indoor Cannabis Production Dynamics. International Journal of Plant Biology, 16(3), 78. https://doi.org/10.3390/ijpb16030078







