Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production
Abstract
1. Introduction
2. Materials and Methods
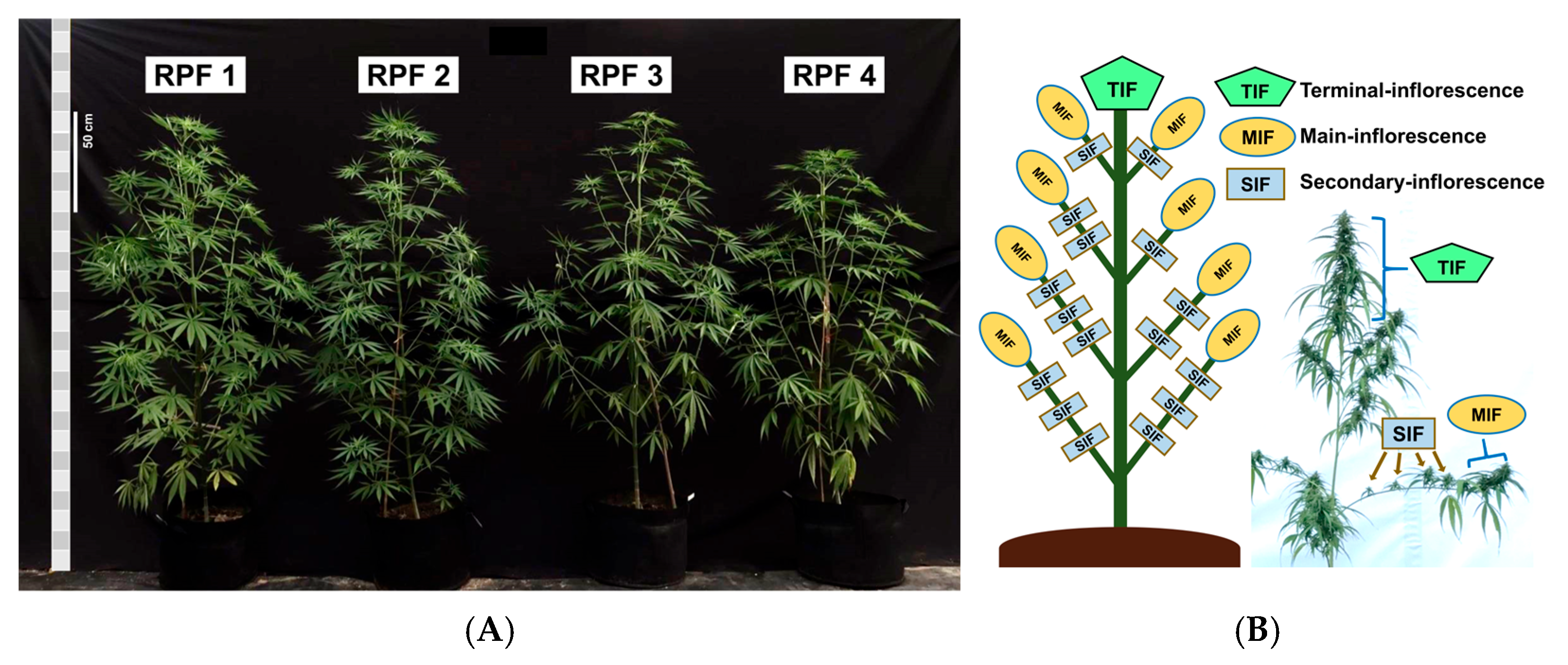
2.1. Plant Material and Experimental Design
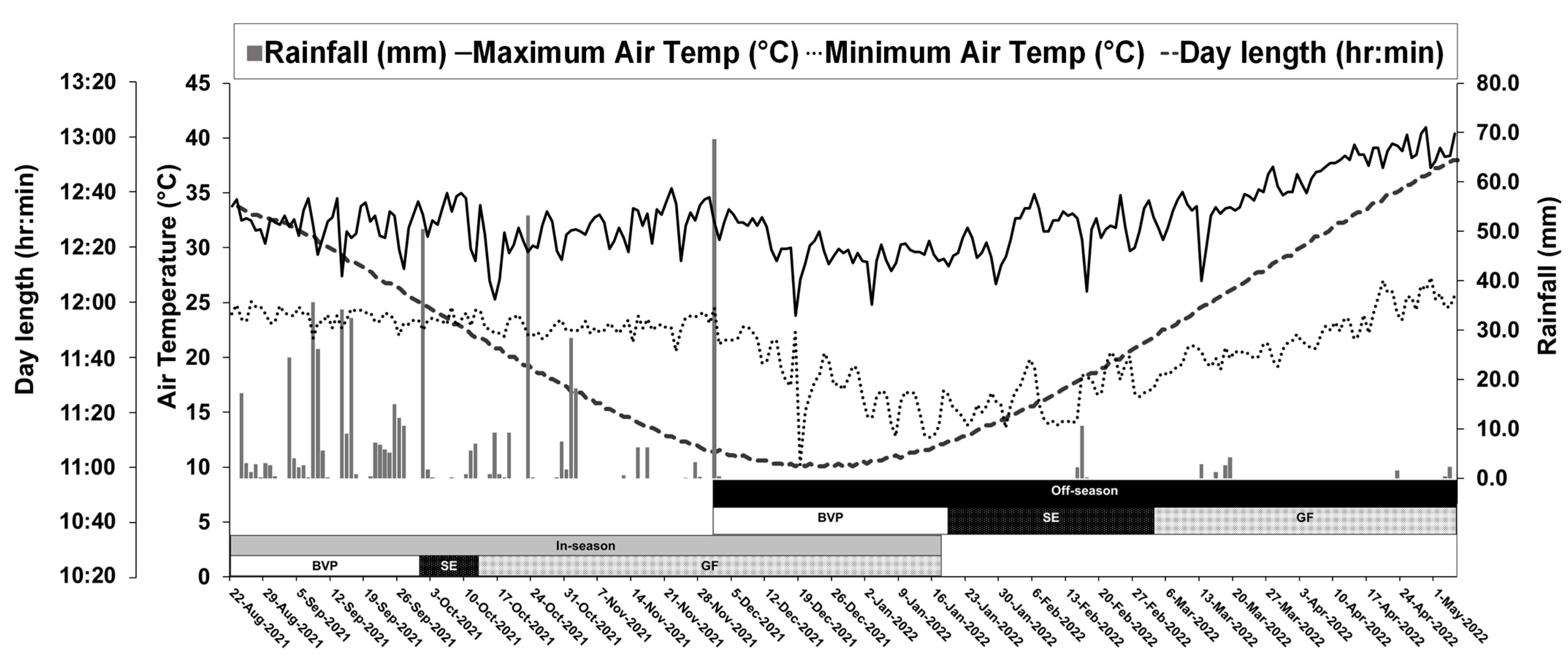
2.2. Growth Conditions
2.3. Sex Expression Determination
2.4. Yield and Yield Component Determination
2.5. Statistical Analysis
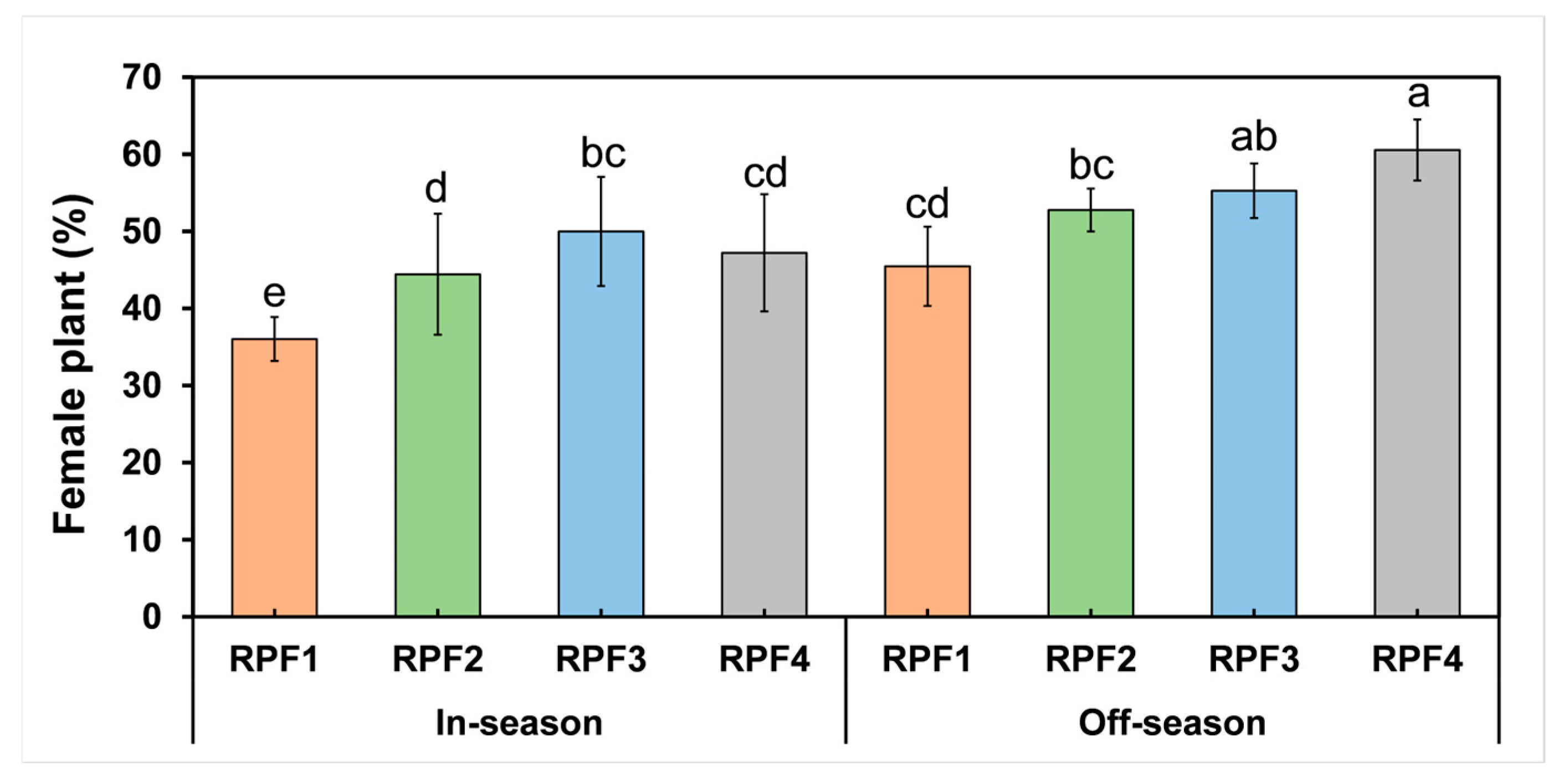
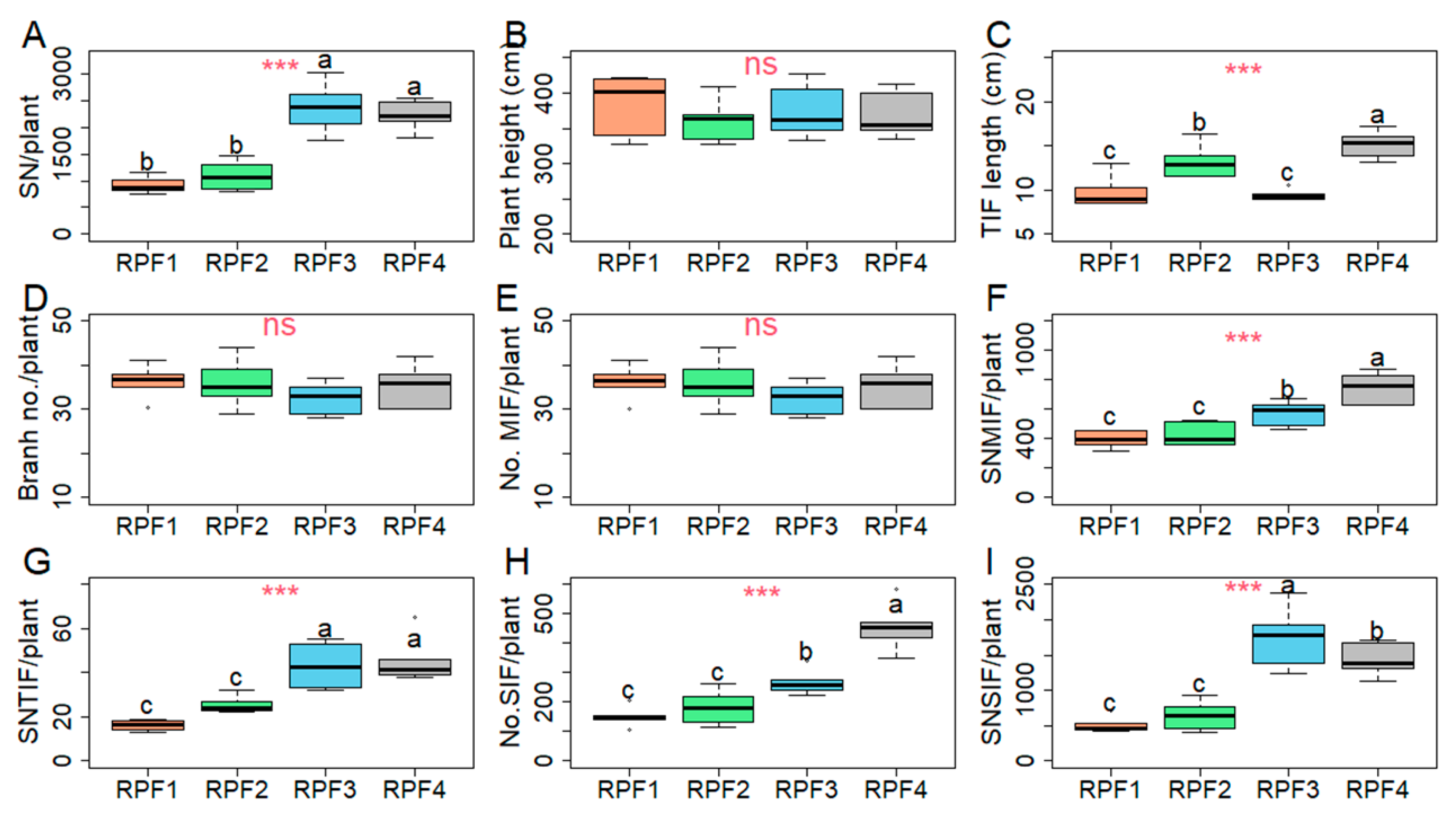
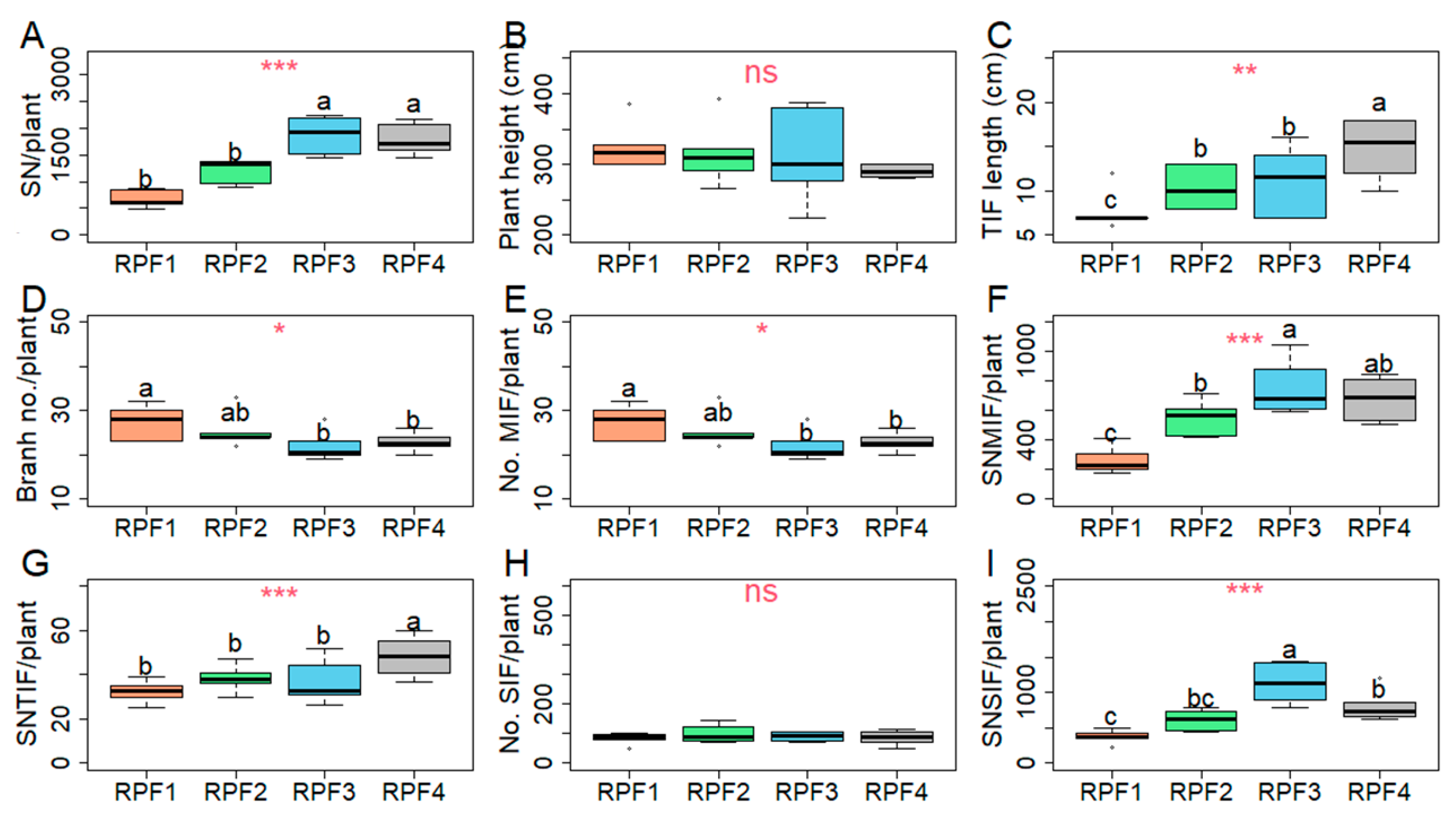
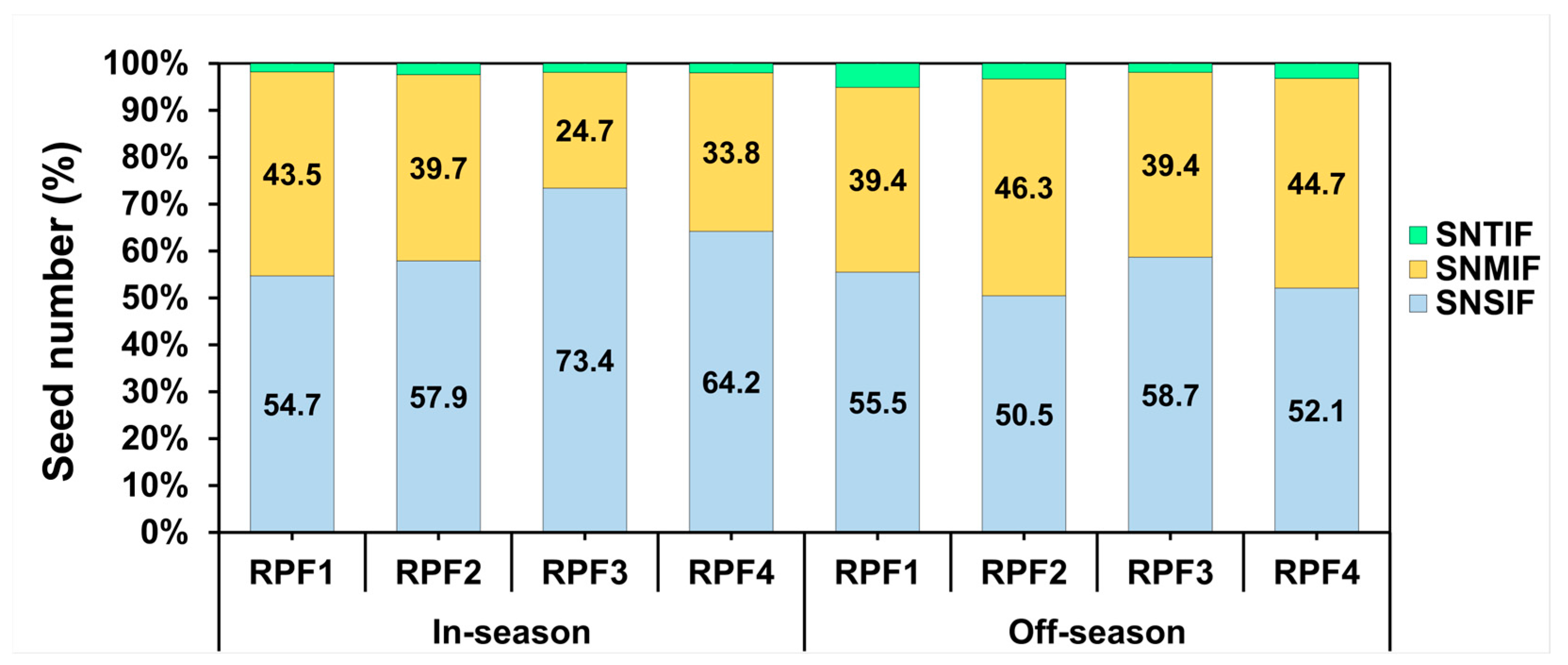
3. Results
3.1. Growth Conditions
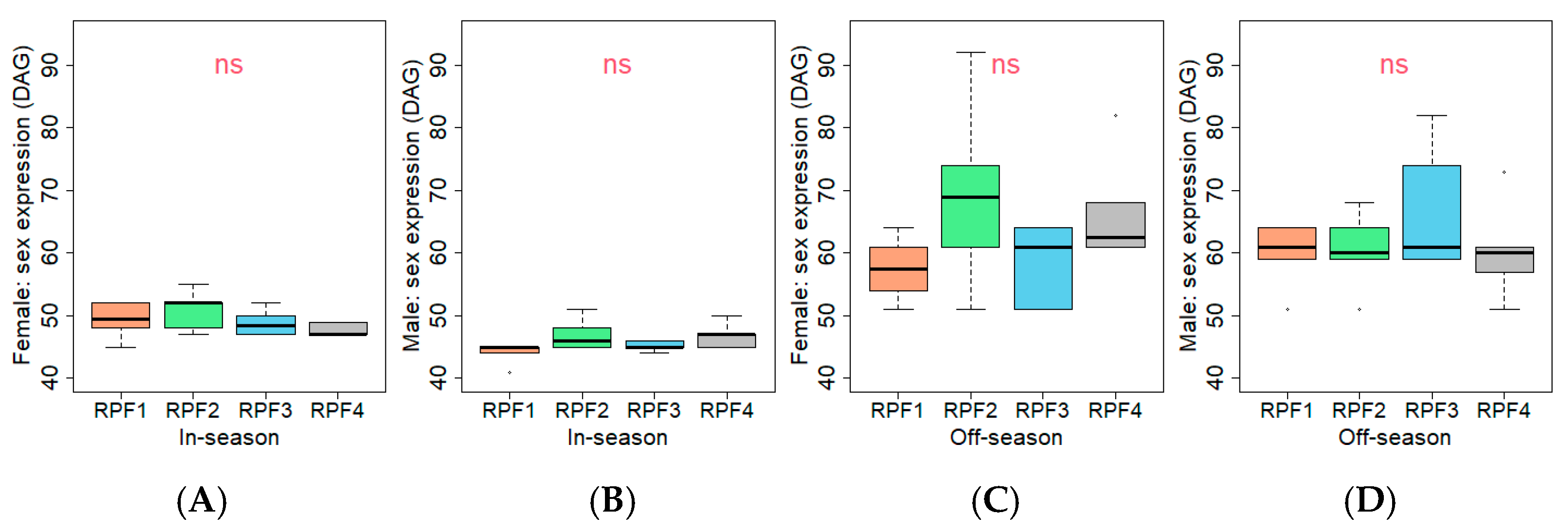
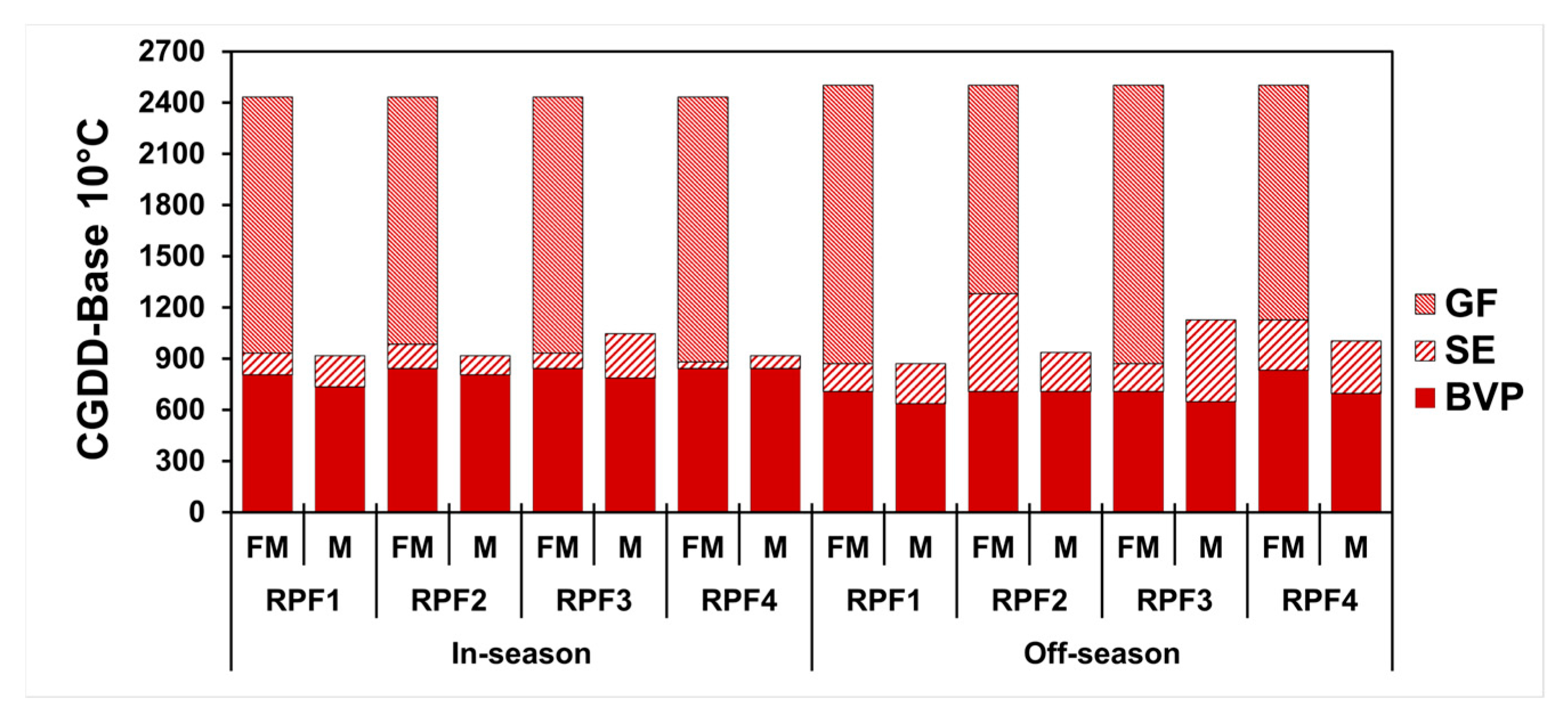
3.2. Hemp Development
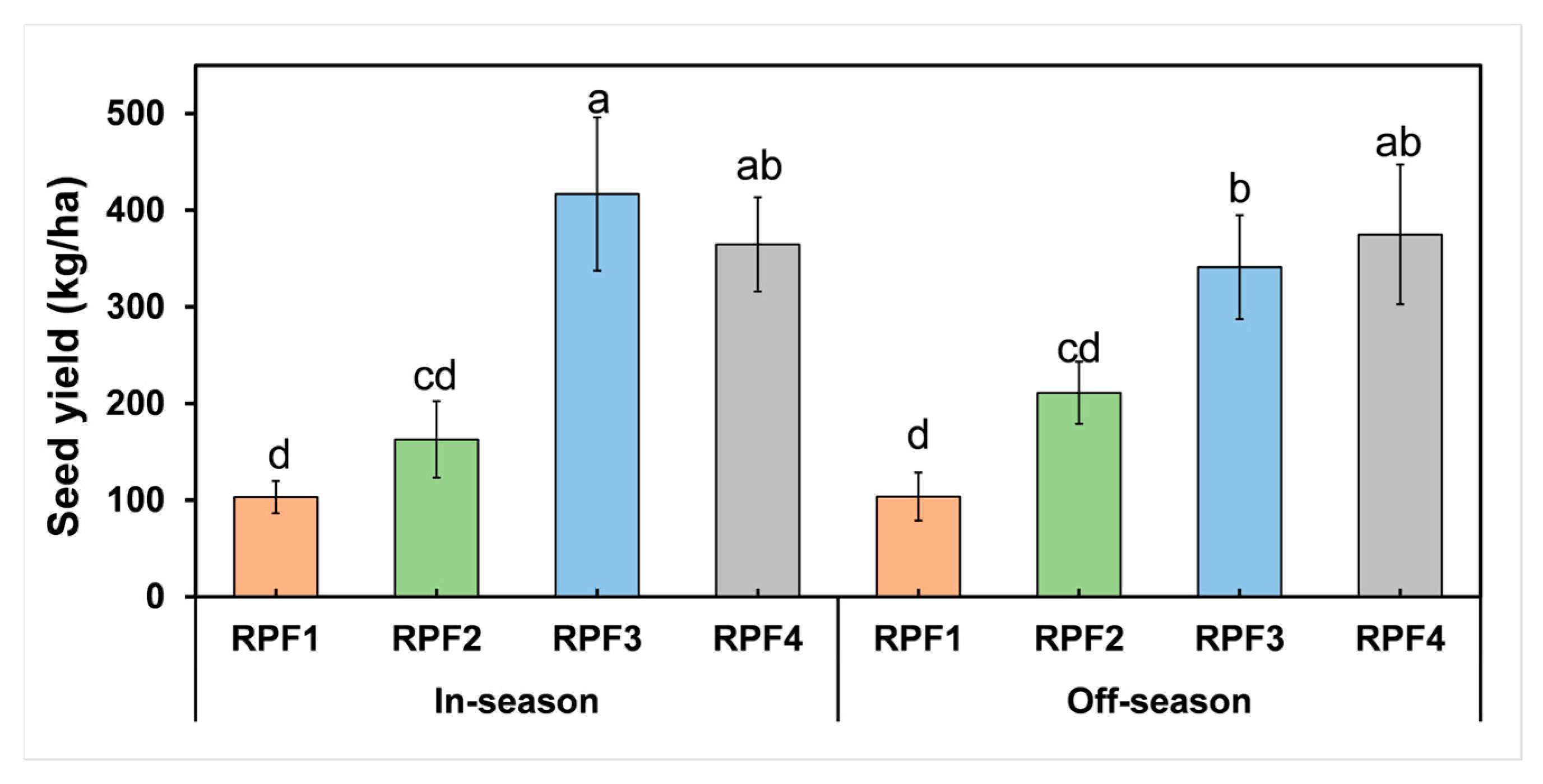
3.3. Hemp Seed Yield Productivity
3.4. Yield Component Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic Characterization of Hempseed (Cannabis sativa L.). J. Proteom. 2016, 147, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. A Comparative Study of the Structural and Functional Properties of Isolated Hemp Seed (Cannabis sativa L.) Albumin and Globulin Fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil Content, Tocopherol Composition and Fatty Acid Patterns of the Seeds of 51 Cannabis sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed Composition of Ten Industrial Hemp Cultivars Approved for Production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Fawzi Mahomoodally, M. A Comparative Study of Seed Yield and Oil Composition of Four Cultivars of Hemp (Cannabis sativa L.) Grown from Three Regions in Northern Iran. Ind. Crops Prod. 2020, 152, 112397. [Google Scholar] [CrossRef]
- Montserrat-de La Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical Analysis of Minor Bioactive Components and Cannabidiolic Acid in Commercial Hemp Seed Oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a Nutritional Resource: An Overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Pratap Singh, A.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants Help Favorably Regulate the Kinetics of Lipid Peroxidation, Polyunsaturated Fatty Acids Degradation and Acidic Cannabinoids Decarboxylation in Hempseed Oil. Sci. Rep. 2020, 10, 10567. [Google Scholar] [CrossRef]
- Abu Ghazal, T.S.; Subih, H.S.; Obeidat, B.S.; Awawdeh, M.S. Hemp Seed Oil Effects on Female Rats Fed a High-Fat Diet and Modulating Adiponectin, Leptin, and Lipid Profile. Agriculture 2023, 13, 449. [Google Scholar] [CrossRef]
- Gong, M.; Lu, H.; Li, L.; Feng, M.; Zou, Z. Integration of Transcriptomics and Metabonomics Revealed the Protective Effects of Hemp Seed Oil against Methionine–Choline-Deficient Diet-Induced Non-Alcoholic Steatohepatitis in Mice. Food. Funct. 2023, 14, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Q.; Zhou, Y.; Fan, P. CLG from Hemp Seed Inhibits LPS-Stimulated Neuroinflammation in BV2 Microglia by Regulating NF-κB and Nrf-2 Pathways. ACS Omega 2019, 4, 16517–16523. [Google Scholar] [CrossRef] [PubMed]
- Opyd, P.M.; Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Dietary Hemp Seeds More Effectively Attenuate Disorders in Genetically Obese Rats than Their Lipid Fraction. J. Nutr. 2020, 150, 1425–1433. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A. The Effect of Hemp (Cannabis sativa L.) Seeds and Hemp Seed Oil on Vascular Dysfunction in Obese Male Zucker Rats. Nutrients 2021, 13, 2575. [Google Scholar] [CrossRef]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive Quality of Romanian Hemp Varieties (Cannabis sativa L.) with Special Focus on Oil and Metal Contents of Seeds. Chem. Cent. J. 2012, 6, 122. [Google Scholar] [CrossRef]
- Rusu, I.E.; Marc, R.A.; Mureşan, C.C.; Mureşan, A.E.; Mureşan, V.; Pop, C.R.; Chiş, M.S.; Man, S.M.; Filip, M.R.; Onica, B.-M.; et al. Hemp (Cannabis sativa L.) Flour-Based Wheat Bread as Fortified Bakery Product. Plants 2021, 10, 1558. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Žydelis, R.; Ruzgas, R.; Bakšinskaitė, A.; Tilvikienė, V. The Seeds of Industrial Hemp (Cannabis sativa L.) a Source of Minerals and Biologically Active Compounds. J. Nat. Fibers 2022, 19, 13025–13039. [Google Scholar] [CrossRef]
- Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.Ł.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules 2021, 26, 7699. [Google Scholar] [CrossRef]
- Krungsri Research Hemp: A New Economic Crop, Opportunities and Challenges. Available online: https://www.krungsri.com/th/research/research-intelligence/hemp-2021 (accessed on 21 July 2021).
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant. Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef]
- Sankari, H.S. Comparison of Bast Fibre Yield and Mechanical Fibre Properties of Hemp (Cannabis sativa L.) Cultivars. Ind. Crops Prod. 2000, 11, 73–84. [Google Scholar] [CrossRef]
- Musio, S.; Müssig, J.; Amaducci, S. Optimizing Hemp Fiber Production for High Performance Composite Applications. Front. Plant. Sci. 2018, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, W.; Van Delden, S.H.; Van Dam, J.E.G.; Pereira Marinho, J.P.; Struik, P.C.; Stomph, T.J. Plant Weight Determines Secondary Fibre Development in Fibre Hemp (Cannabis sativa L.). Ind. Crops Prod. 2019, 139, 111493. [Google Scholar] [CrossRef]
- Campiglia, E.; Radicetti, E.; Mancinelli, R. Plant Density and Nitrogen Fertilization Affect Agronomic Performance of Industrial Hemp (Cannabis sativa L.) in Mediterranean Environment. Ind. Crops Prod. 2017, 100, 246–254. [Google Scholar] [CrossRef]
- Faux, A.-M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The Relationship of Stem and Seed Yields to Flowering Phenology and Sex Expression in Monoecious Hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing Hemp (Cannabis sativa L.) Cultivars for Dual-Purpose Production under Contrasting Environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Layko, I.; Mishchenko, S. Relationship between oil content and quantitative characteristics of hemp seeds. Bast Tech. Crops 2019, 7, 34–41. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. Review of Flowering Control in Industrial Hemp. J. Nat. Fibers 2012, 9, 23–36. [Google Scholar] [CrossRef]
- Lisson, S.N.; Mendham, N.J.; Carberry, P.S. Development of a Hemp (Cannabis sativa L.) Simulation Model 2. The Flowering Response of Two Hemp Cultivars to Photoperiod. Aust. J. Exp. Agric. 2000, 40, 413. [Google Scholar] [CrossRef]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic Flowering Response of Essential Oil, Grain, and Fiber Hemp (Cannabis sativa L.) Cultivars. Front. Plant Sci. 2021, 12, 694153. [Google Scholar] [CrossRef]
- Garfinkel, A.R.; Wilkerson, D.G.; Chen, H.; Smart, L.B.; Rojas, B.M.; Getty, B.A.; Michael, T.P.; Crawford, S. Genetic Mapping of SNP Markers and Candidate Genes Associated with Day-Neutral Flowering in Cannabis sativa L. bioRxiv 2023. [Google Scholar] [CrossRef]
- McMillan, C. Experimental Hybridization of Xanthium strumarium (Compositae) from Asia and America. I. Responses of F1 Hybrids to Photoperiod and Temperature. Am. J. Bot. 1975, 62, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends. Plant. Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Rahn, B.; Pearson, B.J.; Trigiano, R.N.; Gray, D.J. The Derivation of Modern Cannabis Varieties. Crit. Rev. Plant. Sci. 2016, 35, 328–348. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cattivello, C. The Performance and Potentiality of Monoecious Hemp (Cannabis sativa L.) Cultivars as a Multipurpose Crop. Agronomy 2018, 8, 162. [Google Scholar] [CrossRef]
- Ferfuia, C.; Zuliani, F.; Danuso, F.; Piani, B.; Cattivello, C.; Dorigo, G.; Baldini, M. Performance and Stability of Different Monoecious Hemp Cultivars in a Multi-Environments Trial in North-Eastern Italy. Agronomy 2021, 11, 1424. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing Time and Prediction of Flowering of Different Hemp (Cannabis sativa L.) Genotypes in Southern Europe. Ind. Crops Prod. 2012, 37, 20–33. [Google Scholar] [CrossRef]
- Flajšman, M.; Ačko, D.K. Influence of Edaphoclimatic Conditions on Stem Production and Stem Morphological Characteristics of 10 European Hemp (Cannabis sativa L.) Varieties. Acta Agric. Slov. 2020, 115, 399–407. [Google Scholar] [CrossRef]
- Department of Agriculture Announcement Advertisement for Registration Application of New Plant Variety Under the Plant Variety Protection Act B.E. 1999. Available online: https://www.doa.go.th/pvp/wp-content/uploads/2020/11/AnnoDOA_Public52.pdf (accessed on 7 July 2021).
- Pinmanee, S. New Hemp Varieties of Thailand. Available online: https://www.hrdi.or.th/Articles/Detail/18 (accessed on 3 May 2021).
- Nanakorn, W. Hemp (Cannabis) Fundamentals: Biology and Cultivation Techniques; Botanical Society of Thailand: Chiangmai, Thailand, 2021; Volume 1. [Google Scholar]
- Kaveeta, L.; Promratrak, K.; Nanakorn, M.; Papun, Y.; Suwanwong, S.; Thantiviwat, S.; Nanakorn, W. Morphological and Anatomical Characters of Hemp; Kasetsart University: Bangkok, Thailand, 2004; pp. 576–583. [Google Scholar]
- Sengloung, T.; Kaveeta, L.; Nanakorn, W. Effect of Sowing Date on Growth and Development of Thai Hemp (Cannabis sativa L.). Agric. Nat. Resour. 2009, 43, 423–431. [Google Scholar]
- Suriyong, S.; Vearasilp, S.; Krittigamas, N.; Pinmanee, S.; Punyalue, A. Effect of Seed Maturity on Seed Physiological Quality, Oil Content and Fatty Acid Composition of Hemp Seed. CMUJ Nat. Sci. 2012, 11, 351–358. [Google Scholar]
- Lu, G.; Zhang, F.; Zheng, P.; Cheng, Y.; Liu, F.-I.; Fu, G.; Zhang, X. Relationship Among Yield Components and Selection Criteria for Yield Improvement in Early Rapeseed (Brassica napus L.). Agric. Sci. China 2011, 10, 997–1003. [Google Scholar] [CrossRef]
- Amasino, R.M. Control of Flowering Time in Plants. Curr. Opin. Genet. Dev. 1996, 6, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Opik, H.; Rolfe, S.A.; Willis, A.J. The Physiology of Flowering Plants; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Klose, C.; Nagy, F.; Schäfer, E. Thermal Reversion of Plant Phytochromes. Mol. Plant 2020, 13, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, S.; Colauzzi, M.; Bellocchi, G.; Venturi, G. Modelling Post-Emergent Hemp Phenology (Cannabis sativa L.): Theory and Evaluation. Eur. J. Agron. 2008, 28, 90–102. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Bellocchi, G.; Cosentino, S.L.; Pahkala, K.; Stomph, T.J.; Westerhuis, W.; Zatta, A.; Venturi, G. Evaluation of a Phenological Model for Strategic Decisions for Hemp (Cannabis sativa L.) Biomass Production across European Sites. Ind. Crops Prod. 2012, 37, 100–110. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Zuliani, F.; Danuso, F. Suitability Assessment of Different Hemp (Cannabis sativa L.) Varieties to the Cultivation Environment. Ind. Crops Prod. 2020, 143, 111860. [Google Scholar] [CrossRef]
- Schaffner, J.H. Influence of Environment on Sexual Expression in Hemp. Bot. Gaz. 1921, 71, 197–219. [Google Scholar] [CrossRef][Green Version]
- Ming, R.; Bendahmane, A.; Renner, S.S. Sex Chromosomes in Land Plants. Annu. Rev. Plant Biol. 2011, 62, 485–514. [Google Scholar] [CrossRef]
- Patten, M.M. Evolution: Various Routes to Sex Determination. Curr. Biol. 2022, 32, R416–R418. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. The Experimental Modification of Sex Expression in Flowering Plants. Biol. Rev. 1957, 32, 38–90. [Google Scholar] [CrossRef]
- Song, S.; Huang, H.; Liu, H.; Sun, G.; Chen, R. Low Temperature during Seedling Stage Promotes Female Flower Determination but Not Yield of Chieh-Qua. Hortic. Environ. Biotechnol. 2012, 53, 343–348. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D.; Janick, J.; Whipkey, A. Hemp: A New Crop with New Uses for North America; ASHA Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Schaffner, J.H. The Influence of Relative Length of Daylight on the Reversal of Sex in Hemp. Ecology 1923, 4, 323–334. [Google Scholar] [CrossRef]
- Small, E.; Antle, T. A Preliminary Study of Pollen Dispersal in Cannabis sativa in Relation to Wind Direction. J. Ind. Hemp 2003, 8, 37–50. [Google Scholar] [CrossRef]
- Thain, S.C.; Vandenbussche, F.; Laarhoven, L.J.J.; Dowson-Day, M.J.; Wang, Z.-Y.; Tobin, E.M.; Harren, F.J.M.; Millar, A.J.; Van Der Straeten, D. Circadian Rhythms of Ethylene Emission in Arabidopsis. Plant Physiol. 2004, 136, 3751–3761. [Google Scholar] [CrossRef]
- Galoch, E. The Hormonal Control of Sex Differentiation in Dioecious Plants of Hemp (Cannabis sativa). The Influence of Plant Growth Regulators on Sex Expression in Male and Female Plants. Acta Soc. Bot. Pol. 1978, 47, 153–162. [Google Scholar] [CrossRef]
- Freeman, D.C.; Harper, K.T.; Charnov, E.L. Sex Change in Plants: Old and New Observations and New Hypotheses. Oecologia 1980, 47, 222–232. [Google Scholar] [CrossRef]
- Tambal, H.A.A.; Erskine, W.; Baalbaki, R.; Zaiter, H. Relationship of Flower and Pod Numbers Per Inflorescence with Seed Yield in Lentil. Exp. Agric. 2000, 36, 369–378. [Google Scholar] [CrossRef]
- Strelin, M.M.; Aizen, M.A. The Interplay between Ovule Number, Pollination and Resources as Determinants of Seed Set in a Modular Plant. PeerJ 2018, 6, e5384. [Google Scholar] [CrossRef]
- Shi, W.; Muthurajan, R.; Rahman, H.; Selvam, J.; Peng, S.; Zou, Y.; Jagadish, K.S.V. Source-Sink Dynamics and Proteomic Reprogramming under Elevated Night Temperature and Their Impact on Rice Yield and Grain Quality. New Phytol. 2013, 197, 825–837. [Google Scholar] [CrossRef]
- McNairn, R.B. Phloem Translocation and Heat-Induced Callose Formation in Field-Grown Gossypium hirsutum L. Plant Physiol. 1972, 50, 366–370. [Google Scholar] [CrossRef][Green Version]
- Sita, K.; Sehgal, A.; Bhandari, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Impact of Heat Stress during Seed Filling on Seed Quality and Seed Yield in Lentil (Lens culinaris Medikus) Genotypes. J. Sci. Food Agric. 2018, 98, 5134–5141. [Google Scholar] [CrossRef] [PubMed]
- Nasehzadeh, M.; Ellis, R.H. Wheat Seed Weight and Quality Differ Temporally in Sensitivity to Warm or Cool Conditions during Seed Development and Maturation. Ann. Bot. 2017, 120, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Thawonkit, T.; Insalud, N.; Dangtungee, R.; Bhuyar, P. Integrating Sustainable Cultivation Practices and Advanced Extraction Methods for Improved Cannabis Yield and Cannabinoid Production. Int. J. Plant Biol. 2025, 16, 38. [Google Scholar] [CrossRef]
- Ferfuia, C.; Fantin, N.; Piani, B.; Zuliani, F.; Baldini, M. Seed Growth and Oil Accumulation in Two Different Varieties of Industrial Hemp (Cannabis sativa L.). Ind. Crops Prod. 2024, 216, 118723. [Google Scholar] [CrossRef]
- Gambín, B.L.; Borrás, L. Resource Distribution and the Trade-off between Seed Number and Seed Weight: A Comparison across Crop Species. Ann. Appl. Biol. 2010, 156, 91–102. [Google Scholar] [CrossRef]
| Season | Cultivar | Seed Yield (g/Plant) | 100-Seed Weight (g) |
|---|---|---|---|
| In-season | RPF1 | 28.6 ± 4.6 cd | 3.13 ± 0.04 e |
| RPF2 | 37.6 ± 9.1 c | 3.43 ± 0.06 bcd | |
| RPF3 | 83.4 ± 15.8 a | 3.52 ± 0.07 ab | |
| RPF4 | 81.6 ± 10.9 a | 3.67 ± 0.11 a | |
| Off-season | RPF1 | 22.6 ± 5.4 d | 3.43 ± 0.05 bcd |
| RPF2 | 40.0 ± 6.1 c | 3.32 ± 0.17 cd | |
| RPF3 | 61.7 ± 9.7 b | 3.31 ± 0.16 d | |
| RPF4 | 61.9 ± 11.9 b | 3.48 ± 0.27 bc | |
| F-test | *** | *** | |
| C.V. (%) | 18.90 | 4.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongplew, P.; Kangsopa, J.; Hermhuk, S.; Tongkoom, K.; Bhuyar, P.; Insalud, N. Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production. Int. J. Plant Biol. 2025, 16, 67. https://doi.org/10.3390/ijpb16020067
Thongplew P, Kangsopa J, Hermhuk S, Tongkoom K, Bhuyar P, Insalud N. Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production. International Journal of Plant Biology. 2025; 16(2):67. https://doi.org/10.3390/ijpb16020067
Chicago/Turabian StyleThongplew, Pheeraphan, Jakkrapong Kangsopa, Sutheera Hermhuk, Krittiya Tongkoom, Prakash Bhuyar, and Nednapa Insalud. 2025. "Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production" International Journal of Plant Biology 16, no. 2: 67. https://doi.org/10.3390/ijpb16020067
APA StyleThongplew, P., Kangsopa, J., Hermhuk, S., Tongkoom, K., Bhuyar, P., & Insalud, N. (2025). Sex Expression and Seed Yield Stability in Thai Hemp (Cannabis sativa L.): Seasonal Effects on Dioecious Cultivars for Optimized Seed Production. International Journal of Plant Biology, 16(2), 67. https://doi.org/10.3390/ijpb16020067









