Abstract
Andean grassland ecosystems in Peru are characterized by diverse plant species adapted to environmental factors including weather, soil type, elevation, slope orientation, and soil moisture. This study evaluated the floristic composition, alpha diversity, indicator species, and suitable species for Andean livestock in the Sillapata micro-watershed, Junín region, Peru, across rainy and dry seasons. Data collection involved 100 m linear transects, and analyses included floristic composition and dissimilarity, Shannon-Wiener (H′) and Simpson (D) diversity indices, and the identification of indicator and suitable species using QGIS vs 3.28.14 and R software vs 4.3.2. Results revealed a total of 130 species classified into 74 genera and 23 families, with Asteraceae and Poaceae as the dominant families, exhibiting variations in richness and dissimilarity between control points and seasonal periods. Alpha diversity (H′) ranged from 2.07 to 3.1867, while Simpson’s index (D) ranged from 0.7644 to 0.9234. Six indicator species were identified, along with 11 families containing suitable species, predominantly Poaceae (38–60%), Cyperaceae (11–15%), and Asteraceae (3–9%). The findings indicate that the studied ecosystem exhibits a heterogeneous floristic composition with medium to low and variable diversity, influenced by seasonal climatic changes and the current grassland management regime, which involves rotational grazing with cattle adapted to high-altitude conditions.
1. Introduction
High-altitude ecosystems in the Peruvian Andes support a diverse array of plant species adapted to specific environmental conditions, including weather, soil type, elevation, slope orientation, and soil moisture [1,2,3]. This floristic diversity underpins the ecological balance and the provision of key ecosystem services, such as water regulation, soil conservation, biodiversity maintenance, and climate change mitigation [1,4,5].
Andean grasslands are shaped by a combination of climatic and soil factors, grazing animals, and fire regimes [6,7], with plant species exhibiting varied responses to. grazing intensity [3]. The Sillapata micro-watershed, a part of the Shullcas sub-basin, is representative of these Andean grassland ecosystems and is utilized for grazing various livestock, including cattle, sheep, alpacas, and llamas. Investigating the impact of climate variability (i.e., rainy and dry seasons) and grazing practices on the dynamics of this ecosystem is crucial for understanding its function, productivity, and the well-being of local communities [6].
This study aims to evaluate the floristic composition, diversity, and the presence of indicator species to assess the ecological condition of the grassland ecosystem and to provide a scientific basis for conservation and rangeland management strategies.
The assessment of ecological condition necessitates an understanding of grassland degradation, encompassing its drivers (e.g., overgrazing, land-use change), processes (e.g., declines in plant growth and primary production), and consequences (e.g., reductions in productivity and species richness) [2,7]. In this research, we examine floristic composition and alpha diversity, measured using the Shannon-Wiener index [8,9], with consideration of the influence of rainfall patterns on vegetation development and species diversity [10]. Indicator species, defined as those with distinct morphological traits and high indicative value for ecosystem health [11,12], were identified based on their sensitivity to environmental changes and specific habitat requirements [11,13].
Furthermore, we analyzed the suitability of plant species for livestock, categorizing them as suitable, poorly consumed, or unsuitable [13,14], and highlighted the ecological and economic importance of the Poaceae family [15,16,17,18]. While traditional field methods remain prevalent in grassland ecosystem assessments, the effectiveness of GIS technology and remote sensing for accurate plant species identification warrants further investigation [3,16,19,20]. Sustainable grazing management is essential for the conservation of suitable forage species, as appropriate grazing regimes can maintain biodiversity and ecosystem services [21].
This study focuses on determining the variation in floristic diversity, ecological indicator species, and suitable livestock forage species along an altitudinal gradient within the Sillapata micro-watershed of the Acopalca community in the Junín region of Peru. We employed Shannon-Wiener and Simpson indices and identified indicator and suitable species across nine sites during both rainy and dry periods [22,23,24].
2. Materials and Methods
2.1. Study Location
The study was carried out in 2024 within the Sillapata micro-watershed, a 672-hectare area within the Shullcas sub-basin. The micro-watershed is located in the territory of the Acopalca Peasant Community, in the province of Huancayo, department of Junín, Peru. Nine monitoring points were established across the study area based on differences in vegetation cover, determined by the variation in dominant species observed in the field (Figure 1). The surrounding areas have similar varying vegetation cover and are under a moderately rotational grazing regime with cattle and sheep, in other cases with alpacas and sheep.
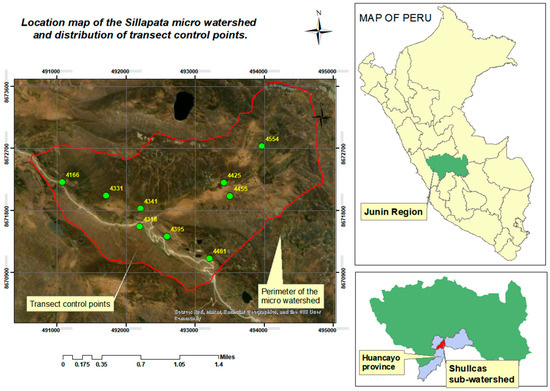
Figure 1.
Map of the location of the study area in relation to the provincial, regional and national area.
The region exhibits two well-defined rainfall patterns: a rainy period, occurring between November and April, with rainfall ranging from 800 to 1150 mm, and a dry period, from May to October, with minimal rainfall (0–20 mm). Temperature fluctuations in the area include daily maxima from 12 to 21 °C and nighttime/early morning minima from −10 to 4 °C. The local population consists of pastoral families who depend on grazing mixed herds of cattle, sheep, alpacas, and llamas as the primary economic driver of their social and economic well-being.
2.2. Data Collection Techniques
Nine control points were established based on a spatial analysis of the micro-watershed using QGIS 3.28.14 software, according to differences in vegetation cover [22], and marked with wooden stakes for evaluations during rainy and dry seasons [22,24]. Transect starting points were georeferenced using a Garmin GPS 64s.
An initial survey identified control points and collected natural grass taxa. These taxa were identified by specialists from the “Andean Ecosystem” research group at the Universidad Nacional del Centro del Perú and compared with certified specimens in the group’s laboratory. Floristic presence was recorded along 100 m linear transects (using a cm-graduated winch), noting species whose leaves or stems contacted the measurement mark, or the leaf projection for tall plants.
Plant botanical identity, soil, mulch, rock, or moss (if no species was present) were recorded on field cards, along with control point, site name, and other details. Suitable species for Andean livestock were identified by compiling a list of suitable and unsuitable species from previous studies in the Peruvian Andes. A matrix was created to show the relationship between suitable species and livestock type [25].
2.3. Data Processing Techniques
Vascular floristic richness was counted, and data were organized into a double-entry matrix for each control point. After cleaning the data, it was integrated into a larger matrix for analysis using Rstudio software vs 4.3.2. Specific richness was determined by counting families, genera, and species. Dominant species were identified at each control point. Multivariate dissimilarity analysis [26] of richness was performed using the INDVAL method used by Dufrene & Legendre, 1997 [27].
Indicator species were identified using statistical analysis of ordered data [22]. An indicator value for each species was determined based on specificity (habitat exclusivity) and fidelity (habitat occurrence frequency), using the vegan package and the vegdist function in R with the Bray-Curtis method [27]. Indicator species had an indicator value >90% and a significance of p < 0.05.
Data matrices were tested for normality using an accumulation curve.
To calculate the dissimilarity, the Bray-Curtis distance was used in the following equation, widely used in ecology for abundance or cover data:
where: BCij is the sum of the lowest values (see example below) only for those species common to both sites, Si, Sj are the total number of specimens counted at both sites [27,28].
The Shannon-Wiener index (H′) was calculated using the equation:
where S = number of species, ln = natural logarithm, Pi = proportion of individuals of species i (calculated as ni/N), ni = number of individuals of species i, N = total number of individuals [28].
H′ = −Σ (Pi ∗ ln(Pi))
Simpson’s index (D) was calculated using the equation:
where pi = proportional abundance of species i (ni/total individuals) [29].
D = Σ (pi2)
Indicator species were also identified using the INDVAL method in R, with the equation:
where: VISij = indicator value of a species at a site, Specificityij = proportion of the j-th site, Fidelityij = proportion of individuals of the i-th species [30].
VISij = Specificityij ∗ Fidelityij ∗ 100
Indicator species were determined by an indicator value > 90% and significance at p < 0.05. Dominant and indicator species were mapped using free GIS software.
3. Results
3.1. Variation Dynamics of Floristic Diversity According to Altitudinal Gradient in the Sillapata Micro-Watershed in the Community of Acopalca
3.1.1. Species Richness and Composition
The study area comprises 130 species classified into 74 genera and 23 families. The most represented families (Figure 2) were Asteraceae (23% of genera, 19% of species) and Poaceae (20% of genera, 31% of species), indicating the dominance of grasses. Cyperaceae was the third most prevalent family (9% of genera and species). Cariophyllaceae had 8% of the genera but only 2% of the species. Gentianaceae and Umbelliferae also showed notable representation, with 6% and 4% of the species, respectively. The remaining families each accounted for less than 2% of both genera and species.
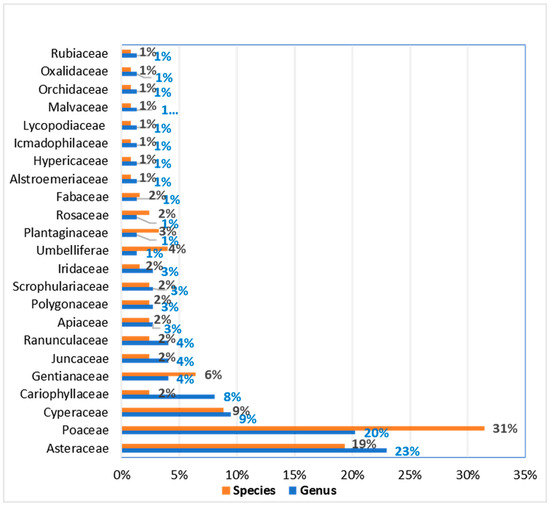
Figure 2.
Distribution of species richness grouped according to genus and families.
Two species with the highest abundance at each control point for the two seasonal periods were located on the map (Figure 3).
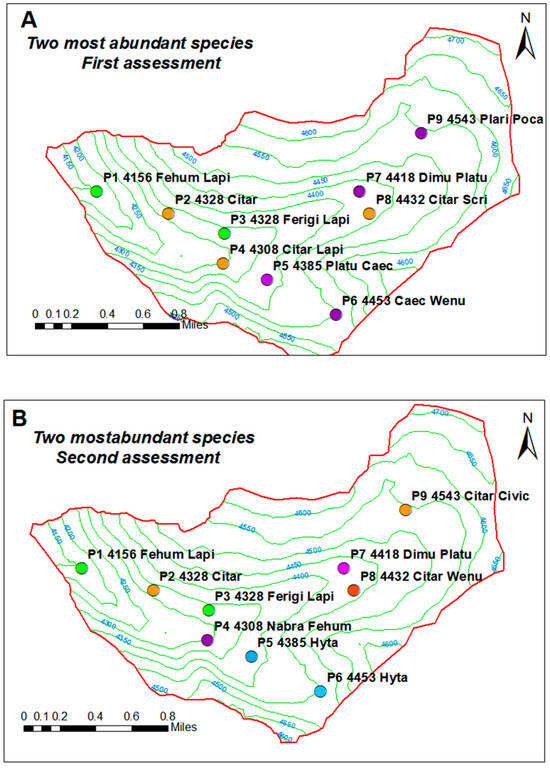
Figure 3.
Distribution of the two most abundant species present at each control point, according to the period of assessment. Where Fehum: Festuca humilior, Lapi: Lachemilla pinnata, Citar: Cinnagrostis tarmensis, Ferigi: Festuca rigidifolia, Nabra: Nassella brachyphylla, Hyta: Hypochoeris taraxacoides, Wenu: Werneria nubigena, Dimu: Distichia muscoide, Platu: Plantago tubulosa, Caec: Carex ecuadorica, Sciri: Sirpus rigidus, Plari: Plantago rigidus, Civic: Cinnagrostis vicunarum.
Figure 3 illustrates the two dominant plant species identified at each control point, demonstrating variations in species dominance associated with differences in altitude, soil type, and soil moisture. Where Fehum: Festuca humilior, Lapi: Lachemilla pinnata, Citar: Cinnagrostis tarmensis, Ferigi: Festuca rigidifolia, Nabra: Nassella brachyphylla, Hyta: Hypochoeris taraxacoides, Wenu: Werneria nubigena, Dimu: Distichia muscoide, Platu: Plantago tubulosa, Caec: Carex ecuadorica, Sciri: Sirpus rigidus, Plari: Plantago rigidus, Civic: Cinnagrostis vicunarum.
Figure 3 shows the two dominant species at each control point, which vary according to altitude, soil type, and soil moisture.
During the rainy season (Figure 3A), nine sampling points were identified, each with distinct plant associations determined by soil depth, water saturation, and slope; for instance, P1 was dominated by Festuca humilior and Lachemilla pinnata in oversaturated deep soils, while P7 featured Distichia muscoides and Plantago tubulosa, reflecting wetland characteristics. In the dry season (Figure 3B), points P1–P3, P7, and P8 maintained the same dominant species, but significant changes were observed in P4, P5, P6, and P9, such as the replacement of Cinnagrostis tarmensis and Lachemilla pinnata by Nassella brachyphylla and Festuca humilior in P4 due to heavy grazing, and the dominance of Hypochaeris taraxacoides in P5 and P6, favored by its semi-erect growth habit during the rainy season.
Details on location, elevation, and other characteristics are in Appendix A Table A4.
The multivariate analysis of richness dissimilarity between control points (Table 1) showed significant differences between sites (dissimilarity > 0.6). In the rainy season, P7 differed notably from P3, P4, and P8, and P9 from P3, P5, and P8 (dissimilarity > 0.9). In the dry season, P7 remained distinct from P4 and P5, and P8 from P6 and P8. Dissimilarity values varied between the two seasons, with some increasing and others decreasing.

Table 1.
Wealth dissimilarity values between control points, in two seasonal periods.
3.1.2. Shannon-Wiener (H′) and Simpson Diversity Index (D)
During the rainy season, the Shannon-Wiener index (H′) ranged from 2.0762 at the highest control point (P9) to 2.9497 at P6. Notable variation was observed at P5 (2.7841) and P6, followed by a decline at P7 (2.3137). At the remaining points (P1, P2, P3, P4, and P8), H′ values ranged between 2.1741 and 2.2722, reflecting medium diversity levels (i.e., values between 2 and 3.5).
In the dry season, H′ values increased, ranging from 2.2102 at P7 to 3.1837 at P6. However, this increase was not uniform; H′ at P7 decreased by 0.10 (Figure 4C) in the permanent wetland conditions, while values at other points, such as P2 and P8, increased more substantially. Numerical details are in Appendix A Table A3.
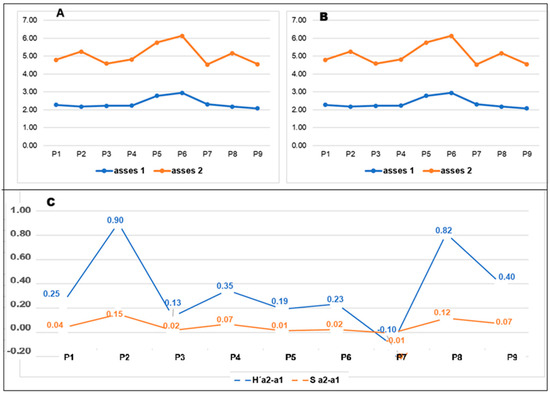
Figure 4.
Comparison of the Shannon-Wiener index in two periods, rainy and dry seasons.
In the first evaluation, Simpson’s diversity index (D) varied from 0.7644 at P2 to 0.9234 at P6, indicating greater heterogeneity. Lower values (0.7644–0.8417″) were observed at P1, P2, P3, P4, P7, P8, and P9, while higher values occurred at P5 (0.9150) and P6 (0.9234). In the second evaluation, D values increased, except for P7, which decreased by 0.1 (Figure 4C).
Consistent with H′ results, Simpson’s index (D) values closer to 1 indicate lower diversity, aligning with the presence of 2 dominant species across the evaluated transects (Table A3 of the Appendix A).
3.2. Presence of Indicator Species of the Grassland Ecosystem According to Altitudinal Gradient in the Sillapata Micro-Watershed in the Community of Acopalca
Species within the 9 control points (Figure 5) were classified for both evaluations. Both graphs show a similar structure (3, 2, and 4 sites), but control point groupings differ between the rainy and dry seasons. Points in the first group tended to shift to the third or fourth group, reflecting changes in floristic composition between the two periods.

Figure 5.
Hierarchical ranking of control points according to the presence of indicator species. P1 to P9 correspond to the location of the control points. The grouping into three groups marked in red indicates the similarity of the control points according to the presence of indicator species.
During the rainy season (Table 2), indicator species were identified. Lachemilla pinnata was the indicator species for the first hierarchical grouping (P4, P3, and P1 in Figure 5 and Figure 6), represented by a dark green buffer on the map. Azorella crenata and Phylloscirpus rigidus were indicator species for the second grouping (P2 and P8), represented by a light green buffer. The third grouping (P5, P6, P7, and P9) had no significant indicator species (p-value > 0.05).

Table 2.
Presence of indicator species in two seasonal periods: rainy and dry.

Figure 6.
Altitudinal distribution of indicator species by hierarchical classification. Where Lapi: Lachemilla pinnata, Gebe: Gentiana bellidifolia, Wepi: Werneria Pygmaea.
In the dry season, 6 indicator species were identified. Deschampsia eminens, Cinnagrostis densiflora, Eleocharis albibracteata, and Lachemilla pinnata were indicator species for the first hierarchical grouping (P5). Gentianella bellidofolia and Werneria pygmaea were indicator species for the third hierarchical grouping (P2, P4, P8, and P9, represented by green). P1 and P3 had no indicator species
3.3. Variation Dynamics of Suitable Species for Andean Livestock According to Altitudinal Gradient in the Sillapata Micro-Watershed in the Community of Acopalca
Above 3800 m in the Sillapata micro-watershed, the grassland ecosystem supports grazing by 4 domestic livestock species: cattle, sheep, alpacas, and llamas, along with wild vicuñas. Fifty-five suitable species (SS) for grazing have been identified. Less suitable species (LSE) were omitted, as they are considered complementary when SS are depleted or absent. Figure 7 shows that alpacas and sheep benefit from the largest number of forage species, while vicuñas, as wild species, rely on the fewest.
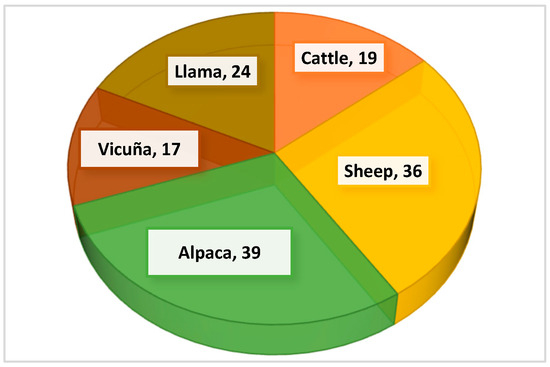
Figure 7.
Number of suitable species identified for each animal species.
Eleven families with suitable species (SS) were identified (Figure 7). Of these families, 39% are preferred by alpacas, 36% by sheep, followed by llamas and cattle. Vicuñas prefer only 17% of the suitable species. Not all species are suitable for all livestock (Table 3); preference varies with plant morphology and the animal’s anatomy and physiology. Llamas and sheep consume the greatest number of unique species, while cattle, sheep, and alpacas share 3–4 pasture species. Poaceae has the highest species representation (38% in the first evaluation, 62% in the second), followed by Cyperaceae (15% and 11%, respectively) and a third family (13% and 5%). The remaining families each represent less than 4%.

Table 3.
List of suitable species specified for each livestock type.
Suitable species were ranked according to the preference of the three most abundant domestic livestock types (Figure 8). Rankings were compared for K = 2 and K = 3, and the graph generated by K3 was used to provide a more comprehensive interpretation. During the rainy season, the best grazing areas for cattle were sites P3 and P7 (first group), followed by P5 and P9 (second group), and then P2, P8, P4, P6, and P1 (third group). In the dry season, the ranking changed: P1, P6, P4, and P5 (first group); P2, P8, and P9 (second group); and P3 and P7 (third group).
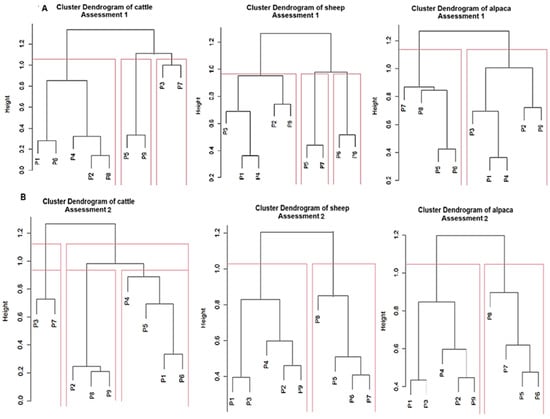
Figure 8.
Diagram of the hierarchical grouping of suitable grassland species by livestock type in seasonal rainy and dry periods. P1 to P9correspond to the control points.
For sheep, the rainy season assessment identified three groups of suitable species (SS): the first (P6 and P8), the second (P5 and P7), and the third (P1 and P4-P3). The second evaluation grouped the sites into two clusters: the first (P5, P6, P7, and P8) and the second (P1, P3, P4, P2, and P9). For alpacas, two groups were optimal in both evaluations. The first evaluation’s groups were: P2, P5, P1, P4, and P3 (first group) and P5, P6, P7, and P8, and P2, P9, P4, P1 and P3 (second group). The hierarchical grouping differed between evaluations due to an apparent inversion in SS presence between the rainy and dry seasons.
4. Discussion
This study demonstrates that Andean grassland ecosystems support communities with complex species richness and composition (Figure 1 and Figure 2), varying with geographic area, altitude, climatic conditions, grassland management, and soil type.
The identification of 23 families, 74 genera, and 130 species (Figure 2) does not define a unique richness for Andean grasslands. Compared to other studies (51 families, 21 genera, and 78 species in dry grassland in Junín; 81 species, 52 genera, 22 families in Tanta Lima; 22 families, 59 genera, and 83 species in Reserva Paisajística Nor-Yauyos; 32 families with 172 species in Ica; 14 families of vascular plants, 33 genera, and 41 species in Junín’s wetland; and 61 vascular species in Puno) [31,32,33,34,35,36], our findings simply confirm that each site’s species composition is adapted to its specific environmental conditions, climate, and anthropogenic influences. However, the study site’s current species composition appears stable and healthy.
The presence of the sole dominant perennial plant Cinnagrostis tarmensis at P2 in both assessments was likely determined by the inceptisol soil type, the slow decomposition of organic matter, and the steep, rocky slopes at high elevations with intense solar radiation—conditions also found at P8. Additionally, its resilience allows it to thrive in cold environments with well-drained soils, some solar exposure, and moderate levels of disturbance [35,36].
The shift in dominant species at P4 between evaluations (Figure 3) resulted from changes in dominant cover. During the dry season evaluation, this site had been grazed, with lingering effects from the rainy season. Conversely, during the rainy season evaluation, the area was rested, allowing erect perennials like Nassella brachyphylla and Festuca humilior to regain vigor (i.e., growth height and canopy cover). Consequently, the annual Plantago tubulosa and the perennial, prostrate Carex ecuadorica (cushion species) were overshadowed [36]. Dominant species changes at other sites also stemmed from the temporary appearance of species like Hypochaeris taraxacoides due to rainy season effects.
The dissimilarity in species richness among the 9 control points (Table 1) reflects differences in species composition, influenced by factors such as soil conditions and heterogeneous moisture levels across microhabitats defined by topography, elevation, slope orientation, and inclination [1,3,26]. Although distance and elevation differences between control points were relatively small, the observed increases and decreases in dissimilarity result from richness changes driven by the factors mentioned above.
Alpha diversity, measured by the Shannon-Wiener index (H′), ranged from 2.0762 to 3.1837 across both evaluations, indicating medium diversity. The slight increase in H′ during the dry season evaluation (ranging from −0.10 to 0.9) suggests that the rainy season promoted the presence of temporary species [22,27,37]. Simpson’s diversity index (D) ranged from 0 to 1, with values closer to 1 indicating lower diversity [38,39]. This aligns with P2’s single dominant species in both evaluations. Simpson’s index, based on the probability of two randomly selected individuals belonging to the same species, complements the H′ results.
These shifts likely result from rainfall variations between the two distinct Andean seasons, coupled with temperature fluctuations and grazing effects on plant community composition due to livestock feed preferences. Our H′ (2.55–2.90) and D (0.93–0.89) values are similar to those reported for Peruvian grasslands above 4000 m in Junín, suggesting that these grasslands typically have 1 or 2 dominant species, influenced by natural conditions and grazing. Similar results were obtained by Yaranga et al., (2018) in research carried out in other areas of Junín, as well as the D index from 05 to 0.71 in Aguada Blanca in Arequipa [40,41,42].
To analyze indicator species (Figure 5), control points were hierarchically clustered using the Bray-Curtis dissimilarity. The similarity in graphic structure reflects the floristic composition of each control point, which varied between evaluations due to site-specific characteristics and the influence of rainfall and temperature [2,5,13,40]. Consequently, the number of indicator species changed from 4 (typically prostrate and permanent) during the rainy season to 6 during the dry season, including four temporary species: Eleocharis albibracteata, Lachemilla pinnata, Gentianella bellidofolia, and Werneria pygmaea (Table 2). This dynamic indicates that indicator species in these grasslands are not fixed but change over time [26], findings on the varying indicator species status of grazed and ungrazed plants.
Figure 5 and Figure 6 show no clear relationship between indicator species and altitude or control point specificity during the rainy season evaluation. For instance, species in the green group 1 are separated by more than 200 m in elevation, and those in the orange group 3 are similarly dispersed [24,39,41]. This suggests niche sharing as a survival strategy, with species seeking soil nutrients and competing for resources for vegetative growth [24,39]. This interpretation also explains the changes observed in indicator species between evaluations.
Of the 23 plant families recorded in the assessed high-Andean grassland ecosystem, only 11 included species suitable for herbivorous livestock. Figure 7 and Table 3 summarize the forage species identified for the four main native Andean herbivores. The Poaceae family was the most dominant, corroborating previous findings [14], which highlight the ecological prominence of grasses in high-altitude ecosystems due to their physiological and morphological adaptations to cold, radiation-intense environments.
The cluster diagrams in Figure 8 illustrate that each of the three main livestock types in the micro-basin exhibits specific plant species preferences. Consequently, site similarity varies depending on the richness and composition of suitable species, which, in turn, is influenced by habitat conditions. The heterogeneity in suitable species composition results in distinct clusters reflecting livestock-specific preferences and differences between evaluations. These findings are crucial for informing rotational and complementary grazing management strategies to enhance ecosystem sustainability under mixed grazing regimes [42,43,44]. Changes in site clustering between evaluations are related to changes in site richness between the rainy and dry seasons, along with the grazing regime.
5. Conclusions
Andean grassland ecosystems exhibit a diverse and complex floristic community, composed of 23 families, 79 genera and 172 species, which show variations in their spatial distribution among control points. This variation was not strongly affected by altitude, as initially proposed, but was influenced by other conditions, such as humidity, soil type, slope orientation, precipitation, relief and grazing regime, which proved to be the most visible factors in the experience. Regarding alpha diversity, the Andean grasslands showed values within the medium range, which is in agreement with the results of studies carried out under similar conditions. On the other hand, the seasonal change from rainy to dry also showed changes in the richness of each site, with a greater number of temporary species in the rainy season, which also determined the presence of indicator species that were not unique to each site. Regarding the suitable species (D) for livestock, these corresponded to less than 50% of the dominant families, such as Poaceae and Cyperaceae, which shows that very few natural grass species were preferred by each Andean domestic species. This evidences the existence of a specificity in the natural grasses that support the diet of each type of livestock
Author Contributions
R.M.Y. contributed to conceptualization, methodology and original draft manuscript, F.C.C. data validation, E.M.M. contributed to data collection. J.A.O. contributed to data análisis. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the four authors’ own financial resources, and the use of laboratory and field equipment available to the “Andean ecosystem” research group.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to express their profound gratitude to the anonymous reviewers’ comments and all individuals have consented for improving the Manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
List of vascular species identified in the Sillapata micro-watershed.
Table A1.
List of vascular species identified in the Sillapata micro-watershed.
| Clave | Especie |
|---|---|
| Acacre | Acaulimalva crenata (A.W.Hill) Krapov |
| Acaen | Acaulimalva engleriana (Ulbr.) Krapov. |
| Acpu | Aciachne pulvinata Benth. |
| Agbre | Agrostis breviculmis Hitchc. |
| Agtol | Agrostis tolucensis Kunth. |
| Alpig | Alstroemeria pygmaea Herb. |
| Anhans | Anatherostipa hans-meyeri (Pilg.) Peñail. |
| Anodo | Anthoxanthum odoratum L. |
| Arac | Arenaria acaulis Montesinos & Kool |
| Aredi | Arenaria digyma Willd. ex D.F.K.Schltdl |
| Arad | Aristida adscensionis L. |
| Aren | Aristida enodis Hack. |
| Azbi | Azorella biloba (Schltdl.) Wedd. LC. |
| Azcom | Azorella compacta Phil. |
| Azcre | Azorella crenata (Ruiz & Pav.) Pers. |
| Azma | Azorella madreporica Clos. |
| Azmul | Azorella multifida (Ruiz & Pav.) Pers |
| Bacae | Baccharis caespitosa (Lam.) Pers |
| Badi | Bartsia diffusa Benth. |
| Bape | Bartsia pedicularoides Benth. |
| Bepip | Belloa piptolepis (Wedd.) S.E.Freire, Chemisquy, Anderb. & Urtubey by Asteraceae |
| Brola | Bromus catarthicus Vahl. |
| Brola | Bromus lanatus Kunth. |
| Casag | Caltha sagittata Cav. |
| Caram | Cardionema ramosissimum (Weinm.) A.Nelson & J.F.Macbr., 1913 |
| Caec | Carex ecuadorica Kük. |
| Caheb | Carex hebetata Boott |
| Caman | Carex mandoniana Boeck. |
| Cedan | Cerastium danguyi J.F. |
| Ceimb | Cerastium imbricatum Kunth vel aff. |
| Cicur | Cinnagrostis curvula Wedd. |
| Ciden | Cinnagrostis densiflora (J. Presl) P.M. Peterson, Soreng, Romasch. & Barberá |
| Cifus | Cinnagrostis fuscata (J. Presl) P.M. Peterson, Soreng, Romasch. & Barberá |
| Cijam | Cinnagrostis jamesonii (Steud.) P.M. Peterson, Soreng, Romasch. & Barberá vel aff. |
| Cirig | Cinnagrostis rigesens (J.Presl) P.M.Peterson, Soreng, Romasch. & Barbe |
| Ciri | Cinnagrostis rigida (Kunth) P.M.Peterson, Soreng, Romasch. & Barberá |
| Cispi | Cinnagrostis spicigera (J. Presl) P.M. Peterson, Soreng, Romasch. & Barberá |
| Citar | Cinnagrostis tarmensis (Pilg.) P.M.Peterson, Soreng, Romasch. & Barberá |
| Civic | Cinnagrostis vicunarum (Wedd.) P.M. Peterson, Soreng, Romasch. & Barberá |
| Codes | Conyza deserticola Phil. |
| Cuaise | Cuatrecasasiella isernii (Cuatrec.) H.Rob. |
| Cyses | Cyperus seslerioides Kunth. |
| Desem | Deschampsia eminens (J. Presl) Saarela |
| Dimu | Distichia muscoides Nees & Meyen. |
| Elal | Eleocharis albibracteata Nees & Meyen ex Kunth vel aff. |
| Fehua | Festuca huamachucensis Infantes |
| Fehum | Festuca humilior Nees & Meyen |
| Ferige | Festuca rigescens (J. Presl) Kunth |
| Ferigi | Festuca rigidifolia Tovar |
| Gacor | Galium corymbosum Ruiz & Pav. |
| Gapur | Gamochaeta purpurea (L.) Cabrera |
| Gaspi | Gamochaeta spicata (Klatt) Cabrera. |
| Gecas | Gentiana aff. Casapaltensis Ball. |
| Gehir | Gentiana hirculus (Griseb.) Fabris |
| Gepo | Gentiana potamophylla L. |
| Gebe | Gentianella bellidifolia (Hook.fil.) Holub |
| Gencar | Gentianella carneorubra (Gilg) |
| Gein | Gentianella incurva (Hook.) Fabris. |
| Gesan | Gentianella sandien (@luanasandien) |
| Geses | Geranium sessiliflorum Cav. |
| Gnado | Gnapahalium dombeyanum DC. |
| Gnapur | Gnaphaliium purpurium L. |
| Gnaspi | Gnaphalium spicatum Mill. |
| Halum | Halenia umbellata (Ruiz & Pav.) Gilg |
| Homut | Hordeum muticum J. Presl |
| Hysil | Hypericum silenoides Juss |
| Hyta | Hypochaeris taraxacoides Ball. |
| Isset | Isolepis setacea (L.) R. Br. |
| Justi | Juncus stipulatus Nees & Meyen |
| Kospi | Koeleria spicata (L.) Barberá, Quintanar, Soreng & P.M. Peterson |
| Ladi | Lachemilla diplophylla (Diels) Rothm. |
| Lapi | Lachemilla pinnata Ruiz & Pav |
| Laaph | Lachenilla aphanoides (Mutis ex L.fil.) Rothm |
| Lian | Lilaeopsis andina A.W.Hill. |
| Lilma | Lilaeopsis macloviana (Gand.) A.W. Hill |
| Lusub | Luciliocline subspicata (Wedd.) Hieron |
| Lulon | Luciocline longifolia (Cuatrec. & Aristeg.) M.O.Dillon & Sagást |
| Luan | Lupinus ananeanus Ulbr. |
| Luch | Lupinus chlorolepis C.P. Sm. |
| Lura | Luzula racemosa Desv. |
| Lycra | Lycopodium crassum Humb. & Bonpl |
| Mistri | Misbrookea strigosissima (A.Gray) V.A.Funk |
| Mnlon | Mniodes longifolia (Cuatrec. & Aristeg.) S.E.Freire |
| Muvo | Muehlenbeckia volcanica (Benth.) Endl.(Mullaka, Machi machi) |
| Muli | Muhlenbergia ligularis (Hack.) Hitchc |
| Mygym | Myrosmodes gymnandra Rchb.f. |
| Nabra | Nassella brachyphylla (Hitchc.) Barkworth |
| Name | Nassella meyeniana (Trin. & Rupr.) Parodi |
| Namu | Nassella mucronata (Kunth) R.W.Pohl |
| Nesp | Neobartsia sp. (Cabrera & Botta) Uribe-Convers & Tank |
| Nopin | Nototriche pinnata (Cav.) Hill. Hill, |
| Oltri | Olsynium trinerve (Baker) R.A. Rodr. & Martic. |
| Orein | Oreithales integrifolia (DC.) Schltdl. |
| Orean | Oreomyrrhis andicola (Kunth) Hook |
| Orlim | Oritrophium limnophilum (Sch.Bip.) Cuatrec |
| Oxde | Oxalis debilis corymbosa Kunth. |
| Paov | Paranephelius ovatus Wedd. |
| Paan | Paronychia andina A. Gray |
| Papil | Paspalum pilgerianum Chase. |
| Papy | Paspalum pygmaeum Hack. |
| Phyac | Phylloscirpus acaulis (Phil.) Goetgh. & D.A.Simpson |
| Phybo | Phylloscirpus boliviensis (Barros) Dhooge & Goetgh. |
| Phyri | Phylloscirpus rigidus (Phil.) |
| Plalam | Plantago lamprophylla Pilg. |
| Plari | Plantago rigida Kunth. |
| Plase | Plantago sericea Ruiz & Pav |
| Platu | Plantago tubulosa Decne. |
| Poae | Poa aequigluma Tovar |
| Poan | Poa annua L. |
| Poca | Poa candamoana Pilg. |
| Pogym | Poa gymnantha Pilg. |
| Pokur | Poa kurtzii R.E. Fr. vel aff. |
| Pomar | Poa marshallii Tovar |
| Poper | Poa perligulata Pilg. |
| Pospi | Poa spicigera Tovar vel aff. |
| Psevi | Pseudognaphalium viravira (Molina) Anderb. |
| Pymo | Pycnophyllum molle J. Rémy |
| Rapra | Ranunculus praemorsus Kunth |
| Ronu | Rockhausenia nubigena (Kunth) D.J.N. Hind |
| Ruac | Rumex acetocella L. |
| Ruma | Rumex maritimus. |
| Scri | Scirpus rigidus (Steud.) Boeckeler |
| Siman | Silene mandonii (Rohrb.) Bocquet |
| Sismi | Sisyrinchium micranthum Cav. |
| Tamve | Thamnolia vermicularis (Sw.) Ach. ex Schaer) |
| Wenu | Werneria nubigena Kunth. |
| Wepi | Werneria pinnatifida J.Rémy |
| Wepy | Werneria pygmaea Gillies ex Hook |
| Wevi | Werneria villosa A.Gray |
| Zamut | Zameioscirpus muticus Dhooge & Goetgh. |

Table A2.
Classification of species richness according to genus and family.
Table A2.
Classification of species richness according to genus and family.
| Family | Genus | No Species | Familia | Genero | No Species |
|---|---|---|---|---|---|
| Alstroemeriaceae | Alstroemeria | 1 | Geraniaceae | Geranium | 1 |
| Apiaceae | Lilaeopsis | 2 | Hypericaeae | Hypericum | 1 |
| Oreomyrrhis | 1 | Icmadophilaceae | Thamnolia | 1 | |
| Asteraceae | Werneria | 4 | Iridaceae | Olsynium | 1 |
| Plantago | 4 | Sisyrinchium | 1 | ||
| Gnaphalium | 3 | Juncaceae | Distichia | 1 | |
| Acaulimalva | 2 | Juncus | 1 | ||
| Gamochaeta | 2 | Luzula | 1 | ||
| Luciliocline | 2 | Lycopodiaceae | Lycopodium | 1 | |
| Baccharis | 1 | Malvaceae | Nototriche | 1 | |
| Belloa | 1 | Ochidaceae | Myrosmodes | 1 | |
| Conyza | 1 | Oxalidaceae | Oxalis | 1 | |
| Cuatrecasasiella | 1 | Poaceae | Cinnagrostis | 11 | |
| Hypochaeris | 1 | Poa | 8 | ||
| Misbrookea | 1 | Festuca | 3 | ||
| Mniodes | 1 | Nassella | 3 | ||
| Oritrophium | 1 | Agrostis | 2 | ||
| Paranephelius | 1 | Aristida | 2 | ||
| Pseudognaphalium | 1 | Bromus | 2 | ||
| Rockhausenia | 1 | Paspalum | 2 | ||
| Caryophyllaceae | Arenaria | 2 | Aciachne | 1 | |
| Cerastium | 2 | Anatherostipa | 1 | ||
| Cardionema | 1 | Anthoxantum | 1 | ||
| Paronychia | 1 | Deschampsia | 1 | ||
| Pycnophyllum | 1 | Hordeum | 1 | ||
| Silene | 1 | Koeleria | 1 | ||
| Cyperaceae | Carex | 3 | Muhlenbergia | 1 | |
| Phylloscirpus | 3 | Polygonaceae | Rumex | 2 | |
| Cyperus | 1 | Muehlenbeckia | 1 | ||
| Elocharis | 1 | Ranunculaceae | Caltha | 1 | |
| Isolepis | 1 | Oreithales | 1 | ||
| Scirpus | 1 | Ranunculus | 1 | ||
| Zameioscirpus | 1 | Rosaceae | Lachemilla | 3 | |
| Fabaceae | Lupinus | 2 | Rubiaceae | Galium | 1 |
| Gentianaceae | Gentianella | 4 | Scrophulariaceae | Bartsia | 2 |
| Gentiana | 3 | Neobartsia | 1 | ||
| Halenia | 1 | Umbelliferae | Azorella | 5 |

Table A3.
Shannon-Wiener and Simpson diversity values in two periods, rainy and dry seasons.
Table A3.
Shannon-Wiener and Simpson diversity values in two periods, rainy and dry seasons.
| Control Points | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
|---|---|---|---|---|---|---|---|---|---|
| First assessment | |||||||||
| Shanon | 2.272 | 2.177 | 2.223 | 2.230 | 2.784 | 2.950 | 2.314 | 2.174 | 2.076 |
| Simpson | 0.841 | 0.764 | 0.845 | 0.833 | 0.915 | 0.923 | 0.842 | 0.802 | 0.825 |
| Second assessment | |||||||||
| Shanon | 2.519 | 3.079 | 2.353 | 2.576 | 2.976 | 3.184 | 2.210 | 2.990 | 2.477 |
| Simpson | 0.881 | 0.931 | 0.861 | 0.902 | 0.928 | 0.947 | 0.835 | 0.920 | 0.896 |

Table A4.
Table of attributes of the first and second evaluation.
Table A4.
Table of attributes of the first and second evaluation.
| A | Point | Ord. | E | N | Z | de1 | de1n | de2 | de2n | Ind_sp |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 1 | 491,069 | 8,672,207 | 4156 | Fehum | 27 | Lapi | 21 | Lapi | |
| P2 | 2 | 491,709 | 8,672,011 | 4328 | Citar | 42 | 0 | Azcre | ||
| P3 | 1 | 492,206 | 8,671,828 | 4328 | Ferigi | 28 | Lapi | 18 | Lapi | |
| P4 | 1 | 492,195 | 8,671,565 | 4308 | Citar | 26 | Lapi | 23 | Lapi | |
| P5 | 3 | 492,588 | 8,671,419 | 4385 | Platu | 15 | Caec | 14 | ||
| P6 | 3 | 493,206 | 8,671,106 | 4453 | Caec | 15 | Wenu | 13 | ||
| P7 | 3 | 493,415 | 8,672,203 | 4418 | Dimu | 29 | Platu | 17 | ||
| P8 | 2 | 493,505 | 8,672,010 | 4432 | Citar | 39 | Scri | 13 | Welam | |
| P9 | 3 | 493,964 | 8,672,728 | 4543 | Plari | 34 | Poca | 13 | ||
| B | Point | Ord. | E * | N * | Z | de1 | de1n | de2 | de2n | Ind_sp |
| P1 | 2 | 491,069 | 8,672,207 | 4156 | Fehum | 23 | Lapi | 17 | ||
| P2 | 3 | 491,709 | 8,672,011 | 4328 | Citar | 18 | 0 | Lapi | ||
| P3 | 2 | 492,206 | 8,671,828 | 4328 | Ferigi | 27 | Lapi | 18 | ||
| P4 | 3 | 492,195 | 8,671,565 | 4308 | Nabra | 14 | Fehum | 13 | Gebe | |
| P5 | 1 | 492,588 | 8,671,419 | 4385 | Hyta | 18 | 0 | Lapi, | ||
| P6 | 1 | 493,206 | 8,671,106 | 4453 | Hyta | 10 | 0 | Lapi | ||
| P7 | 1 | 493,415 | 8,672,203 | 4418 | Dimu | 29 | Platu | 14 | Lapi | |
| P8 | 3 | 493,505 | 8,672,010 | 4432 | Citar | 18 | Wenu | 11 | Wepy | |
| P9 | 3 | 493,964 | 8,672,728 | 4543 | Citar | 17 | Civic | 16 | Gebe |
* E and N correspond to the geographical coordinates, Z correspond to altitude in meters, de1 corresponds to the first dominant species, and de1n to the number of shares; de2, and de2n correspond to the second dominant species, both in the first and second evaluation, Ind_sp are the indicator species.

Figure A1.
Panoramic view of the Sillapata micro water-shed study area, overlooking the Huaytapallana snow-capped mountain in the Shullcas sub-basin.
References
- Török, P.; Helm, A.; Kiehl, K.; Buisson, E.; Valkó, O. Beyond the species pool: Modification of species dispersal, establishment, and assembly by habitat restoration. Restor. Ecol. 2018, 26, S65–S72. [Google Scholar] [CrossRef]
- Torchelsen, F.P.; Cordero, R.L.; Overbeck, G.E. Conservation of species-rich subtropical grasslands: Traditional management vs. legal conservation requirements in primary and secondary grasslands. Acta Bot. Bras. 2020, 34, 342–351. [Google Scholar] [CrossRef]
- Chu, T.J.; Shih, Y.J.; Shih, C.H.; Wang, J.Q.; Huang, L.M.; Tsai, S.C. Developing a Model to Select Indicator Species Based on Individual Species’ Contributions to Biodiversity. Appl. Sci. 2022, 12, 6748. [Google Scholar] [CrossRef]
- Scotton, M. Mountain grassland restoration: Effects of sowing rate, climate and soil on plant density and cover. Sci. Total Environ. 2019, 651, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Bartha, S.; Házi, J.; Purger, D.; Zimmermann, Z.; Szabó, G.; Guller, Z.E.; Csathó, A.I.; Csete, S. Beta Diversity Is Better—Microhabitat Diversity and Multiplet Diversity Offer Novel Insights into Plant Coexistence in Grassland Restoration. Diversity 2024, 16, 769. [Google Scholar] [CrossRef]
- Yu, J.; Wan, L.; Liu, G.; Ma Ke Cheng, H.; Shen, Y.; Liu, Y.; Su, X. Managing grassland systems to optimise livestock Production. In Improving Grassland and Pasture Management in Temperate Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 209–238. [Google Scholar] [CrossRef]
- Tiscornia, G.; Jaurena, M.; Baethgen, W. Factores impulsores, procesos y consecuencias de la degradación de pastizales nativos: Perspectivas a partir de una revisión bibliográfica y una encuesta en pastizales del Río de la Plata. Agronomía 2019, 9, 239. [Google Scholar] [CrossRef]
- Yaranga, R.M.; Pizarro, E.S.; Cano, D.; Chanamé, F.C.; Orellana, J.A. Composition, Diversity, and Value of Ecological Importance in Andean Grassland Ecosystems according to the Altitudinal Gradient in the Huacracocha Micro-Watershed, Peru. Ann. Res. Rev. Biol. 2023, 38, 43–56. [Google Scholar] [CrossRef]
- Basavegowda, D.H.; Schleip, I.; Mosebach, P.; Weltzien, C. Deep learning-based detection of indicator species for monitoring biodiversity in semi-natural grasslands. Environ. Sci. Ecotechnol. 2024, 21, 100419. [Google Scholar] [CrossRef]
- Gao, X. Interactive Effects of Grazing and Climate on Grassland Vegetation Diversity in Arid and Semi-Arid Regions; Authorea, Inc.: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.-O.; Glimskär, A.; Nilsson, S.; Tälle, M. Site factors are more important than management for indicator species in semi-natural grasslands in southern Sweden. Plant Ecol. 2020, 221, 577–594. [Google Scholar] [CrossRef]
- Legendre, P. Indicator Species: Computation. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2024; pp. 533–538. [Google Scholar] [CrossRef]
- Descombes, P.; Walthert, L.; Baltensweiler, A.; Meuli, R.G.; Karger, D.N.; Ginzler, C.; Zurell, D.; Zimmermann, N.E. Spatial modelling of ecological indicator values improves predictions of plant distributions in complex landscapes. Ecography 2020, 43, 1448–1463. [Google Scholar] [CrossRef]
- Flores, E. Cambio climático: Pastizales altoandinos y seguridad alimentaria. Revista de Glaciares y Ecosistemas de Montaña. 2016. Available online: https://scholar.google.es/scholar?hl=es&as_sdt=0%2C5&q=Pastizales+altoandinos+y+seguridad+alimentaria&btnG= (accessed on 12 March 2024).
- Carla, J.C. Restauración Ecológica de Praderas Altoandinas para la Mejora de las Pasturas Naturales en el Sector Apas, Huancaya, Yauyos. Bachelor’s Thesis, Facultad de Ingeniería Ambiental, Universidad Católica Sedes Sapianteae, Lima, Peru, 2020; p. 154. Available online: https://hdl.handle.net/20.500.14095/807 (accessed on 12 March 2024).
- Onofre, C.I. Diagnóstico de la Condición Ambiental del Bofedal Moyobamba a Través de un Estudio Agrostológico, Distrito de Canchayllo, Provincia de Jauja. Bachelor’s Thesis, Facultad de Ingeniería Agraria, Universidad Católica Sedes Sapianteae, Lima, Peru, 2020; p. 117. Available online: https://hdl.handle.net/20.500.14095/859 (accessed on 12 March 2024).
- Trillo, S.F. Autoecología de Festuca Dolichophylla-Festuca Humilior, y Repuesta a la Adición de NPK en la Puna Peruana. Ph.D. Thesis, UNALM, Lima, Peru, 2021; 108p. [Google Scholar]
- Jorge, J.B.; Galarza, P.V. Evaluación del Estado de Conservación del Bofedal Sector Moya en el Santuario Histórico de Chacamarca, Junín. Bachelor’s Thesis, Universidad Católica Sedes Sapientiae, Facultad de Ingeniería Agraria, Lima, Peru, 2020; 59p. Available online: https://hdl.handle.net/20.500.14095/989 (accessed on 5 February 2024).
- Fernandes, G.W.; Bahia, T.d.O.; Almeida, H.A.; Conceição, A.A.; Loureiro, C.G.; Luz, G.R.; Neves, A.C.; Oki, Y.; Pereira, G.C.; Pirani, J.R.; et al. Floristic and functional identity of rupestrian grasslands as a subsidy for environmental restoration and policy. Ecol. Complex. 2020, 43, 100833. Available online: https://www.sciencedirect.com/science/article/pii/S1476945X19300807 (accessed on 18 January 2024). [CrossRef]
- Capuñay, K. Composición de la Dieta de las Vicuñas (Vicugna vicugna) Usando Técnicas de Microhistología en Heces. repositorio.lamolina.edu.pe. 2022. Available online: https://repositorio.lamolina.edu.pe/handle/20.500.12996/5588 (accessed on 5 December 2023).
- Lan, D.; Eldridge, D.J.; Morgan, J.W.; Witt, G.B. Un marco para predecir los efectos del pastoreo de ganado y la exclusión del pastoreo sobre los valores de conservación en los ecosistemas naturales de Australia. Rev. Aust. Botánica 2007, 55, 401–415. [Google Scholar] [CrossRef]
- Al-Bakre, D.A. Diversity of Indicator and Dominant Plant Species along Elevation Gradients in Prince Mohammad Bin Salman Nature Reserve, KSA. Diversity 2023, 15, 1081. [Google Scholar] [CrossRef]
- Ati, G.; Shucad, J.; Vaca, M.; Chamorro, H.; Guallpa, M.; Horna, S.; Lara, N.; Cushquicullma, D. Classification, composition and floristic diversity in natural grasslands to different strata in the protected area ichubamba yasepan, through geostatistical analysis. J. Namib. Stud. Hist. Politics Cult. 2023, 33, 2006–2025. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, W.; Wang, J.; Ferreira, C.S.S. Precipitation drives the floristic composition and diversity of temperate grasslands in China. Glob. Ecol. Conserv. 2021, 32, e01933. [Google Scholar] [CrossRef]
- Yaranga, R.; Orellana, J.; Pizarro, S. Species of the Poaceae family suitable for Andean livestock farming in the Peruvian Andes reported in GBIF and local studies. Glob. J. Ecol. 2024, 9, 057–065. [Google Scholar] [CrossRef]
- Jaurena, M.; Bentancur, O.; Ayala, W.; Rivas, M. Especies indicadoras y estructura de praderas naturales de basalto con cargas contrastantes de ovinos. Agrociencia 2011, 15, 103–114. [Google Scholar] [CrossRef]
- Okxanen, J. Multivariate Análisis of Ecologycal Communities in R: Vegan Tutorial. Community Disimilarities. 2015. Available online: https://john-quensen.com/wp-content/uploads/2018/10/Oksanen-Jari-vegantutor.pdf (accessed on 13 June 2024).
- Manzanilla, G.; Mata, J.; Treviño, E.; Aguirre, A.; Alanís, E.; Yerena, J. Diversidad, estructura y composición florística de bosques templados del sur de Nuevo León. Revista Mexicana de Ciencias Forestales 2020, 11, 94–123. [Google Scholar] [CrossRef]
- Moreno, E.C. Métodos para medirla biodiversidad. In M&T—Manuales y Tesis; SEA: Zaragoza, Spain, 2001; Volume 1, 84p. [Google Scholar]
- De Cáceres, M.; Legendre, P.; Moretti, P. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Renzo, A.; Erika, R. Diversidad Florística de los Bofedales en el Distrito de Tanta—Yauyos—Lima. Facultad de Ing. Forestal y del Ambiente, Universidad Nacional del Centro del Perú, Huancayo, Peru, 2022. Available online: http://hdl.handle.net/20.500.12894/9448 (accessed on 8 December 2023).
- Montesinos-Tubée, D.B.; Cleef, A.M.; Sýkora, K.V. The subnival vegetation of Moquegua, South Peru: Chasmophytes, grasslands and cushion communities. Ecologies 2021, 2, 71–111. [Google Scholar] [CrossRef]
- Yaranga, R. High-Andean wetland of Peru: Floristic diversity, primary net aerial productivity, ecological condition, and carrying capacity. Sci. Agropecu. 2020, 11, 213–221. [Google Scholar] [CrossRef]
- Palomino, F.P.E. Insights of Andean High Plains (Altiplano) tussock Grasslands of Cummo Huacullani, Puno, Peru. opensiuc.lib.siu.edu. 2022. Available online: https://opensiuc.lib.siu.edu/dissertations/2070/ (accessed on 8 December 2023).
- Intagri, S.C. (sf) Clasificación del Suelo: WRB y Soil Taxonomy. Available online: https://www.intagri.com/articulos/suelos/clasificacion-del-suelo-WRB-y-soil-taxonomy (accessed on 15 January 2024).
- Oscar, T. Las Gramíneas (Poaceae) del Perú. Monografías del Real Jardín Botánico. In RUIZIA; Real Jardín Botánico, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1993; Volume 13, p. 481. [Google Scholar]
- Dicovskiy Riobóo, L.M.; Pedroza Pacheco, M.E. Éxito académico en los universitarios. Caso de estudio: Ingeniería Agroindustrial de la UNI Sede regional del norte, Nicaragua. Rev. Ciencia Tecnol. Higo 2018, 8, 33–42. [Google Scholar] [CrossRef]
- Liang, M.W.; Liang, C.; Hautier, Y.; Wilcox, K.R.; Wang, S. Review for “Grazing-induced biodiversity loss impairs grassland ecosystem stability at multiple scales”. Ecol. Lett. 2021, 24, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, R. Evaluación de la diversidad florística y restauración dirigida en pastizales abandonados luego del cultivo de Lepidium meyenii—Junín. Ingenieer Thesis, Facultad de Zootecnia, Universidad de Nacional del Centro del Perú, Huancayo, Peru, 2018; 124p. Available online: https://repositorio.uncp.edu.pe/handle/20.500.12894/4396 (accessed on 12 March 2024).
- Servicio Nacional Forestal y de Fauna Silvestre—SERFOR. Guía para la Identificación de Especies de pastos con Palatabilidad para Vicuñas. 2022. Available online: https://repositorio.serfor.gob.pe/handle/SERFOR/932 (accessed on 10 March 2024).
- Zapana Landaeta, J.C. Evaluación de pastizales naturales y determinación de la carga animal actual en la comunidad chila, puno. Perú. Rev. Investig. 2020, 8, 1286–1296. [Google Scholar] [CrossRef]
- Quinteros-Gómez, Y.; Macedo-Bedoya, J.; Santos-Linares, V.; Angeles-Alvarez, F.; Gómez-Ticerán, D.; Campos-De la Cruz, J.; Solis Sarmiento, J.; Salinas-Inga, A.; Valencia-Saavedra, Z. Floristic Diversity and Distribution Pattern along an Altitudinal Gradient in the Central Andes: A Case Study of Cajatambo, Peru. Plants 2024, 13, 3328. [Google Scholar] [CrossRef]
- Yaranga, R.; Custodio, M.; Chanamé, F.; Pantoja, R. Diversidad florística de pastizales según formación vegetal en la subcuenca del río Shullcas, Junín, Perú. Sci. Agropecu. 2018, 9, 511–517. [Google Scholar] [CrossRef]
- Peso, G. Evaluación de la diversidad florística y generación de estrategias de conservación de los pastizales en la Reserva de Salinas de Aguada Blanca, Departamento de Arequipa. Bachelor’s Thesis, Universidad Católica de Santa María, Arequipa, Peru, 2024; p. 110. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).