The Influence of Plant Growth Regulators (PGRs) on Physical and Chemical Characteristics of Hops (Humulus lupulus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study I—The Effect of PGR Active Ingredients and Concentrations on Hop Cone Yield
2.1.1. Plant Material, Experiment Site, and Management
2.1.2. PGR Treatments
2.1.3. Hops Harvest and Data Collection
2.2. Study II—The Effect of Gibberellic Acid and Ethephon on Hop Growth, Yield, Acid Concentration, and VOC Composition
2.2.1. PGR Treatments
2.2.2. Hops Harvest and Data Collection
2.2.3. Alpha and Beta Acids
2.2.4. Hop Storage Index (HSI)
2.2.5. Essential Oil of Volatile Compounds
Essential Oil Extraction
Gas Chromatography—Mass Spectrometer (GC-MS) Analysis of the Essential Oil
Identification
Compound Response
2.3. Plant Growth Environmental Conditions
2.4. Experiment Design and Statistics
3. Results and Discussion
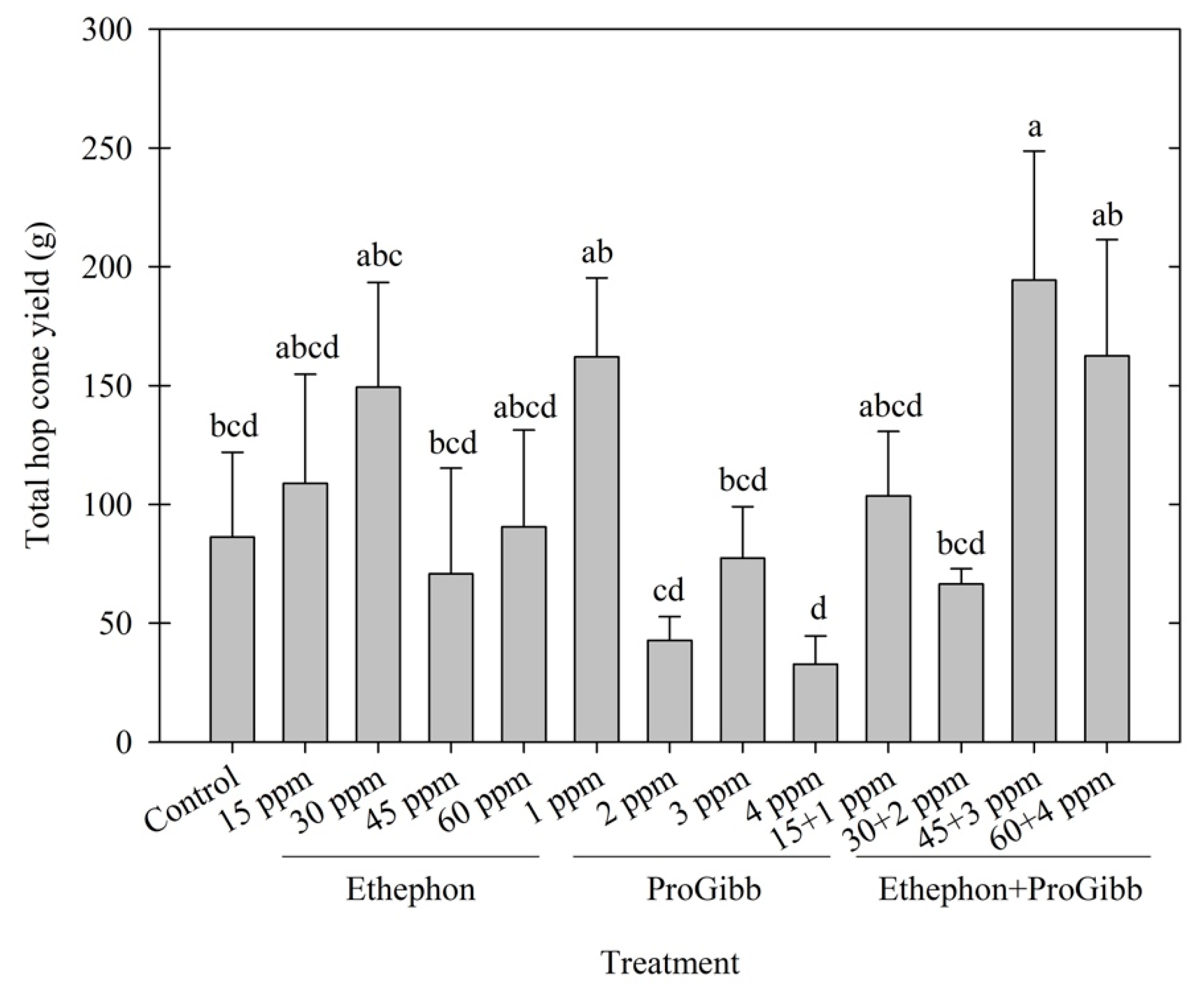
3.1. Cone Yield
3.2. Alpha and Beta Acids
3.3. Hop Storage Index
3.4. Volatiles
3.5. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudura, E.; Coldea, T. Hop-Derived Prenylflavonoids and Their Importance in Brewing Technology—A Review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2015, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Natsume, S.; Takagi, H.; Shiraishi, A.; Murata, J.; Toyonaga, H.; Patzak, J.; Takagi, M.; Yaegashi, H.; Uemura, A.; Mitsuoka, C.; et al. The Draft Genome of Hop (Humulus lupulus), an Essence for Brewing. Plant Cell Physiol. 2015, 56, 428–441. [Google Scholar] [CrossRef]
- McAdam, E.L.; Freeman, J.S.; Whittock, S.P.; Buck, E.J.; Jakse, J.; Cerenak, A.; Javornik, B.; Kilian, A.; Wang, C.H.; Andersen, D.; et al. Quantitative Trait Loci in Hop (Humulus lupulus L.) Reveal Complex Genetic Architecture Underlying Variation in Sex, Yield, and Cone Chemistry. BMC Genom. 2013, 14, 360. [Google Scholar] [CrossRef]
- da Rosa Almeida, A.; Maciel, M.V.D.O.B.; Cardoso Gasparini Gandolpho, B.; Machado, M.H.; Teixeira, G.L.; Bertoldi, F.C.; Noronha, C.M.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Brazilian Grown Cascade Hop (Humulus lupulus L.): LC-ESI-MS-MS and GC-MS Analysis of Chemical Composition and Antioxidant Activity of Extracts and Essential Oils. J. Am. Soc. Brew. Chem. 2021, 79, 156–166. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Świeca, M.; Cichocka, J.; Gawlik-Dziki, U. The Phenolic Content and Antioxidant Activity of the Aqueous and Hydroalcoholic Extracts of Hops and Their Pellets. J. Inst. Brew. 2013, 119, 103–110. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Koziol, A.; Stryjewska, A.; Librowski, T.; Salat, K.; Gawel, M.; Moniczewski, A.; Lochynski, S. An Overview of the Pharmacological Properties and Potential Applications of Natural Monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Hair Styling Clay for Men—Matte Strong Hold Molding Pomade for Beard and Hair—Natural Ingredients Barley, Hops, Yeast—Paraben Free—Water Based for Smooth Finish. Available online: https://www.amazon.com/Hair-Styling-Clay-Men-Ingredients/dp/B075TT8RRK (accessed on 9 July 2024).
- Hop to Bed. Available online: https://www.avenabotanicals.com/products/hop-to-bed?variant=1076800956¤cy=USD&utm_medium=product_sync&utm_source=google&utm_content=sag_organic&utm_campaign=sag_organic&gad_source=1&gclid=CjwKCAjwkJm0BhBxEiwAwT1AXNEWCuXEnBvrJGLhH44FjKrl5VurpQX4qXDlGcI9i40km_lB6utqoxoCziAQAvD_BwE (accessed on 9 July 2024).
- Sierra Nevada Hop Splash Sparkling Hop-Infused Water. Available online: https://sierranevada.com/family/non-alcoholic?gad_source=1&gclid=CjwKCAjwkJm0BhBxEiwAwT1AXOsXDZaPvpoaS-9f4UnWSQTn5VwLusXyo5Mz0A14OJIREdDi1rkxHxoCpEkQAvD_BwE (accessed on 9 July 2024).
- Greenbar Distillery Fruitlab Hops Liqueur. Available online: https://www.frootbat.com/product/4895562/Greenbar-Distillery-Fruitlab-Hops-Liqueur-750ml-Bottle/United-States?gad_source=1&gclid=CjwKCAjwkJm0BhBxEiwAwT1AXGhYN2yzJajYPJUcq-eKxow_YnC9G8y1VjEazkxzqH_oSHUB6EhZoRoCMDUQAvD_BwE (accessed on 9 July 2024).
- Harbour, K.B. A free exhaustive literature review on hops (Humulus lupulus L.). J. Am. Soc. Brew. Chem. 2023, 81, 504. [Google Scholar]
- Aberl, A.; Coelhan, M. Determination of volatile compounds in different hop varieties by headspace-trap GC/MS in comparison with conventional hop essential oil analysis. J. Agric. Food Chem. 2012, 60, 2785–2792. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Harris, J. Odour intensities of hop oil components. J. Sci. Food Agric. 1966, 17, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, G.B.; Van Engel, E.L. Hop aroma component profile and the aroma unit. J. Am. Soc. Brew. Chem. 1992, 50, 77–81. [Google Scholar] [CrossRef]
- Steinhaus, M.; Wilhelm, W.; Schieberle, P. Comparison of the most odour-active volatiles in different hop varieties by application of a comparative aroma extract dilution analysis. Eur. Food Res. Technol. 2007, 226, 45–55. [Google Scholar] [CrossRef]
- Steinhaus, M.; Schieberle, P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. Variety Spalter Select) based on GC-olfactometry and odor dilution techniques. J. Agric. Food Chem. 2000, 48, 1776–1783. [Google Scholar] [CrossRef]
- Dresel, M.; Vogt, C.; Dunkel, A.; Hofmann, T. Quantitation of key tastants and reconstitution of the taste of hops (Humulus lupulus L.) cv. Cascade based on its bitter compounds, organic acids, and minerals. J. Agric. Food Chem. 2013, 61, 2034–2043. [Google Scholar]
- Foster, A.; Gahr, A. Characterization of Hop Oils. Brew. Sci. 2013, 66, 93–103. [Google Scholar]
- Goiris, K.; De Ridder, M.; De Rouck, G.; Boeykens, A.; Van Opstaele, F.; Aerts, G.; de Cooman, L.; De Keukeleire, D. The Oxygenated Sesquiterpenoid Fraction of Hops in Relation to the Spicy Hop Character of Beer. J. Inst. Brew. 2002, 108, 86–93. [Google Scholar] [CrossRef]
- Inui, T.; Tsuchiya, F.; Ishimaru, M.; Oka, K.; Komura, H. Different beers with different hops: Relevant compounds for their aroma characteristics. J. Agric. Food Chem. 2013, 61, 4758–4764. [Google Scholar] [CrossRef]
- National Beer Sales and Production Data. Available online: https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed on 30 August 2024).
- DamjanoviĆ, K.; Varga, I. World Beer Production and Hop Use. Res. J. Agric. Sci. 2021, 53, 78–84. [Google Scholar]
- Fritsch, H.T.; Schieberle, P. Identification based on quantitative measurements and aroma recombination of the character impact odorants in a Bavarian Pilsner-type beer. J. Agric. Food Chem. 2005, 53, 7544–7551. [Google Scholar] [CrossRef]
- Lermusieau, G.; Bulens, M.; Collin, S. Use of GC-olfactometry to identify the hop aromatic compounds in beer. J. Agric. Food Chem. 2001, 49, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Wanikawa, A.; Kono, K.; Shibata, K. Comparison of the odor-active compounds in unhopped beer and beers hopped with different hop varieties. J. Agric. Food Chem. 2006, 54, 8855–8861. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L. Disentangling photoperiod from hop vernalization and dormancy for global production and speed breeding. Sci. Rep. 2019, 9, 16003. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.M.; Babar, A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef]
- Runkle, E. Understanding how PGRs work. Greenh. Prod. News 2017, 27, 54. [Google Scholar]
- Runkle, E. Environment and PGR interactions. Greenh. Prod. News 2015, 25, 74. [Google Scholar]
- PGR Active Ingredients. Available online: https://gpnmag.com/article/pgr-active-ingredients/ (accessed on 2 October 2024).
- Bergmann, B.A.; Dole, J.M.; McCall, I. Gibberellic acid shows promise for promoting flower stem length in four field-grown cut flowers. HortTechnology 2016, 26, 287–292. [Google Scholar] [CrossRef]
- Zheng, C.; Kwame Acheampong, A.; Shi, Z.; Halaly, T.; Kamiya, Y.; Ophir, R.; Galbraith, D.W.; Or, E. Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 2018, 69, 1635–1648. [Google Scholar] [CrossRef]
- Runkle, E. Using chlormequat chloride with success. Greenh. Prod. News 2014, 24, 42. [Google Scholar]
- Latimer, J.G.; Freeborn, J. New uses of PGRs in ornamentals: Configure (6-BA) increases branching of herbaceous perennials. Proc. Plant Growth Regul. Soc. Am. 2009, 36, 88–93. [Google Scholar]
- Li, Y.; Zhang, D.; Xing, L.; Zhang, S.; Zhao, C.; Han, M. Effect of exogenous 6-benzylaminopurine (6-BA) on branch type, floral induction and initiation, and related gene expression in ‘Fuji’ apple (Malus domestica Borkh). Plant Growth Regul. 2016, 79, 65–70. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Brooks, S.N.; Likens, S.T. Gibberellin A3-induced growth responses of Fuggle hops (Humulus lupulus L.). Crop Sci. 1964, 4, 310–313. [Google Scholar] [CrossRef]
- Thomas, G.G.; Goldwin, G.K. Late applications of hormone mixtures to increase the yield of seedless hops (Humulus lupulus L.). J. Hortic. Sci. 1976, 51, 515–523. [Google Scholar] [CrossRef]
- Rowland, N.C. Effects of Plant Growth Regulators on Growth and Reproduction of Humulus lupulus. Master’s Thesis, Tennessee State University, Nashville, TN, USA, 2011. [Google Scholar]
- Pearson, B.J. Growth and strobile yield among 20 hops (Humulus lupulus) cultivars utilizing a traditional tall-trellis production system in Florida. HortScience 2018, 53, S138. [Google Scholar]
- Pearson, B.J.; Smith, R.M. Effect of Humulus lupulus cultivar on first-year growth and strobile yield utilizing a tall-trellis production system in Florida, United States. In Proceedings of the International Symposium on Tropical and Temperate Horticulture (ISTTH2016), Cairns, QLD, Australia, 20–25 November 2016; Volume 1205, pp. 497–504. [Google Scholar]
- ASBC Methods of Analysis. α- and β-Acids in Hops and Hop Pellets by Spectrophotometry and by Conductometric Titration. Methods Hops-6. Am. Soc. Brew. Chem. 2008. [Google Scholar]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadawallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of two extraction techniques, solid-phase microextraction versus continuous liquid–liquid extraction/solvent-assisted flavor evaporation, for the analysis of flavor compounds in Gueuze Lambic beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef]
- Kook, J.R.; Kim, E.J.; Choi, D.G.; Guak, S. Chemical thinning of ‘Hongro’ apple with ammonium thiosulfate and MaxCel. Hortic. Environ. Biotechnol. 2009, 50, 79–83. [Google Scholar]
- Szot, I.; Lipa, T.; Dobrzanski, B., Jr.; Kaplan, M.; Baryla, P. The influence of Floredux, MaxCel and Brevis on the yield of apple trees cv. Sampion. Acta Agrophysica 2018, 25, 4. [Google Scholar] [CrossRef]
- Greene, D.W.; Krupa, J.; Vezina, M. Effect of MaxCel on fruit set, fruit size, and fruit characteristics of Summerland McIntosh apples, 2005 results. Fruit Notes 2006, 72, 12–15. [Google Scholar]
- Jamal Uddin, A.F.M.; Hossan, M.J.; Islam, M.S.; Ahsan, M.K.; Mehraj, H. Strawberry growth and yield responses to gibberellic acid concentrations. J. Exp. Biosci. 2012, 3, 51–56. [Google Scholar]
- Bauerle, W.L. Gibberellin A3 induced flowering intensification in Humulus lupulus L.: Synchronizing vegetative phase change and photoperiod induction. Sci. Hortic. 2022, 302, 111183. [Google Scholar] [CrossRef]
- Bauerle, W.L. Separate and combined effects of supplemental CO2, gibberellic acid, and light on hop quality and yield. Plants 2024, 13, 1670. [Google Scholar] [CrossRef]
- Currey, C.J.; Erwin, J.E. Foliar applications of plant growth regulators affect stem elongation and branching of 11 kalanchoe species. HortTechnology 2012, 22, 338–344. [Google Scholar] [CrossRef]
- Faust, J.E.; Lewis, K.P. The effects of ethephon on cutting yield of 23 selected annual cultivars. Acta Hortic. 2005, 683, 141–144. [Google Scholar] [CrossRef]
- Watanabe, H.; Hase, S.; Saigusa, M. Effects of the combined application of ethephon and gibberellin on growth of rice (Oryza sativa L.) seedlings. Plant Prod. Sci. 2007, 10, 468–472. [Google Scholar] [CrossRef]
- Nance, M.R.; Setzer, W.N. Volatile components of aroma hops (Humulus lupulus L.) commonly used in beer brewing. J. Brew. Distill. 2011, 2, 16–22. [Google Scholar]
- Hieronymus, S. For the Love of Hops: The Practical Guide to Aroma, Bitterness, and the Culture of Hops; Brewers Publications, a Division of the Brewers Association: Boulder, CO, USA, 2012. [Google Scholar]
- Zattler, F.; Chrometzka, P. The influence of gibberellic acid on the flower and cone development in hop (Humulus lupulus L.). Theor. Appl. Genet. 1968, 38, 213–218. [Google Scholar] [CrossRef]
- Cascade Technical Specifications. Available online: https://www.yakimachief.com/cascade.html (accessed on 28 June 2024).
- Cardenas-Pinto, S.B.; Wendrick, N.; Pearson, B.J.; Thompson-Witrick, K. The chemical, physical, and sensory evaluation of greenhouse-cultivated Florida hops. In Proceedings of the World Brewing Congress, Minnesota, MN, USA, 17–20 August 2024. [Google Scholar]
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756. [Google Scholar] [CrossRef]
- Pollach, G.; Hein, W.; Beddie, D. Application of hop beta-acids and rosin acids in the sugar industry. Zuckerindustrie 2002, 127, 921–930. [Google Scholar]
- Understanding the importance of the Hop Storage Index. Available online: https://www.canr.msu.edu/news/understanding-the-importance-of-the-hop-storage-index (accessed on 9 July 2024).
- Nickerson, G.B.; Likens, S.T. Hop storage index. J. Am. Soc. Brew. Chem. 1979, 37, 184–187. [Google Scholar] [CrossRef]
- Cocuzza, S.; Lutz, A.; Müller-Auffermann, K. Influence of picking date on the initial hop storage index of freshly harvested hops. Tech. Q. Master Brew. Assoc. Am. 2013, 2, 66–71. [Google Scholar] [CrossRef]
- Lermusieau, G.; Collin, S. Varietal discrimination of hop pellets. II. Comparison between fresh and aged samples. J. Am. Soc. Brew. Chem. 2001, 59, 39–43. [Google Scholar] [CrossRef]
- McMillan, J.; Zunkel, M.; Insa, A.M.; Schönberger, C. The relevance of Hop Storage Index (HSI) for hop usage. Brew. Sci. Yearbook 2023 2024, 76, 147. [Google Scholar]
- Rettberg, N.; Biendl, M.; Garbe, L.A. Hop aroma and hoppy beer flavor: Chemical backgrounds and analytical tools—A review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Perpète, P.; Mélotte, L.; Dupire, S.; Collin, S. Varietal discrimination of hop pellets by essential oil analysis I. Comparison of fresh samples. J. Am. Soc. Brew. Chem. 1998, 56, 104–108. [Google Scholar] [CrossRef]
- Sharp, D.C.; Qian, Y.; Shellhammer, G.; Shellhammer, T.H. Contributions of select hopping regimes to the terpenoid content and hop aroma profile of ale and lager beers. J. Am. Soc. Brew. Chem. 2017, 75, 93–100. [Google Scholar] [CrossRef]
- Schmiderer, C.; Grausgruber-Gröger, S.; Grassi, P.; Steinborn, R.; Novak, J. Influence of gibberellin and daminozide on the expression of terpene synthases and on monoterpenes in common sage (Salvia officinalis). J. Plant Physiol. 2010, 167, 779–786. [Google Scholar] [CrossRef]
- Bose, S.K.; Yadav, R.K.; Mishra, S.; Sangwan, R.S.; Singh, A.K.; Mishra, B.; Srivastava, A.K.; Sangwan, N.S. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. [Google Scholar] [CrossRef]
- Rabiei, B.; Bahador, S.; Kordrostami, M. The expression of monoterpene synthase genes and their respective end products are affected by gibberellic acid in Thymus vulgaris. J. Plant Physiol. 2018, 230, 101–108. [Google Scholar] [CrossRef]
- Schieberle, P. Primary odorants of pale lager beer. Z. Lebensm. Unters. Forsch. 1991, 193, 558–565. [Google Scholar] [CrossRef]
- Féchir, M.; Weaver, G.; Roy, C.; Shellhammer, T.H. Exploring the regional identity of Cascade and Mosaic® hops grown at different locations in Oregon and Washington. J. Am. Soc. Brew. Chem. 2023, 81, 480–492. [Google Scholar] [CrossRef]
- Elsharif, S.A.; Buettner, A. Structure–odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives. J. Agric. Food Chem. 2016, 66, 2324–2333. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C. Flavor chemistry of beer: Part II: Flavor and threshold of 239 aroma volatiles. Tech. Q. Master Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Elsharif, S.A.; Banerjee, A.; Buettner, A. Structure-odor relationships of linalool, linalyl acetate, and their corresponding oxygenated derivatives. Front. Chem. 2015, 3, 163755. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Rechcigl, J.; Bollin, S.; Deng, Z.; Agehara, S. Hop (Humulus lupulus L.) phenology, growth, and yield under subtropical climatic conditions: Effects of cultivars and crop management. Aust. J. Crop Sci. 2021, 15, 764–772. [Google Scholar] [CrossRef]
- Fortuna, G.C.; Neves, C.S.; Campos, O.P.; Gomes, J.A.O.; Silva, J.C.R.L.; Souza, A.A.; de Funari, C.S.; Marques, M.O.M.; Bonfim, F.P.G. Hop tropicalization: Chemical compositions of varieties grown under organic and conventional systems in subtropical conditions. Horticulturae 2023, 9, 855. [Google Scholar] [CrossRef]
- Loussert, P. Analysis of climate change scenarios and associated impact on the development of hop production in the South-West of France. In Proceedings of the Scientific-Technical Commission of the International Hop Growers’ Convention, Ljubljana, Slovenia, 25–29 June 2023; Weihrauch, F., Ed.; Scientific-Technical Commission of the International Hop Growers’ Convention: Ljubljana, Slovenia, 2023; pp. 99–103. [Google Scholar]
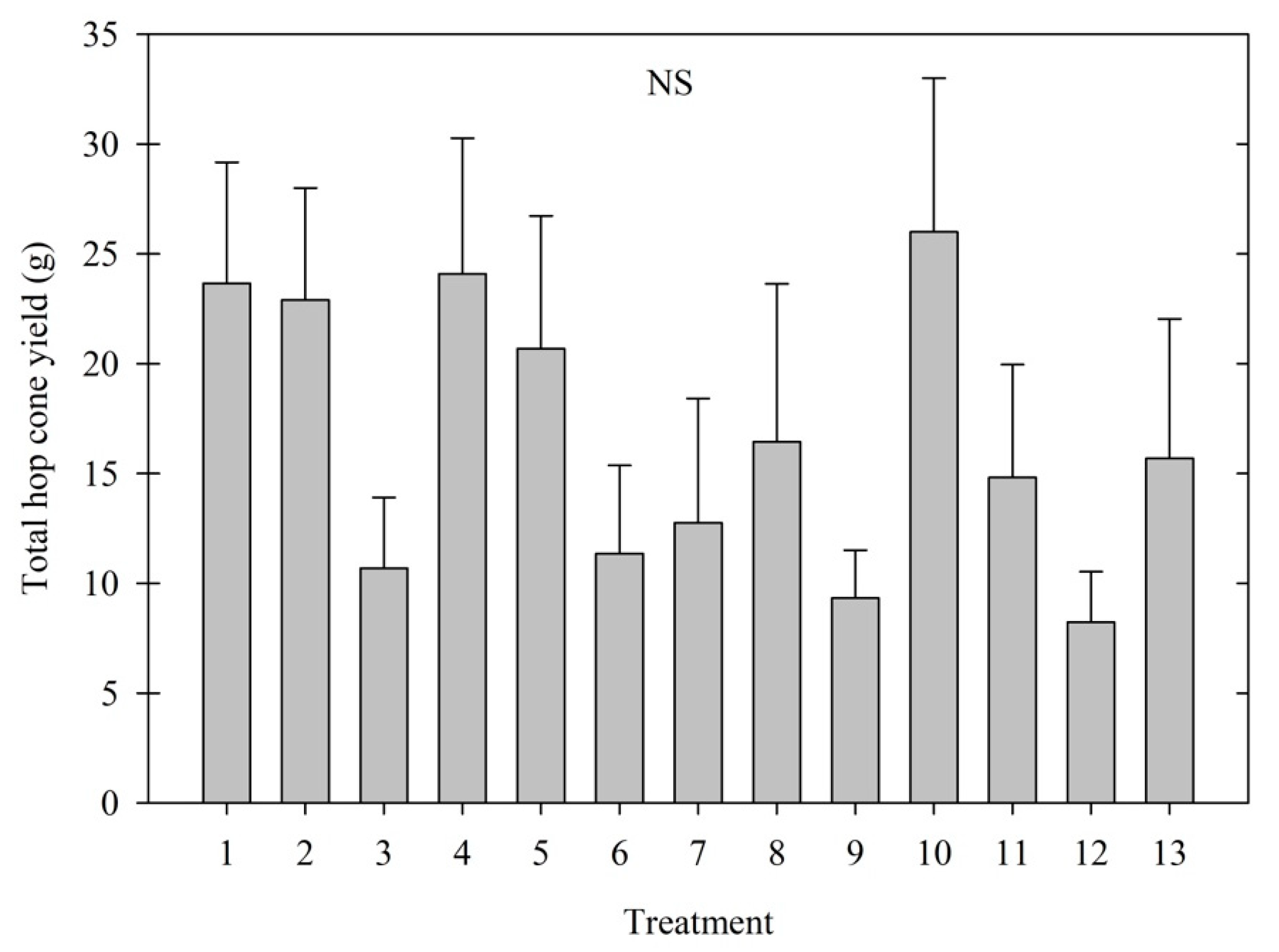

| Treatment | Name | Active Ingredient | Application Rate | Application Method | Bine Height When Applied (m) |
|---|---|---|---|---|---|
| 1 | Control (Water) | NA | NA | Spray | 1.5–2.4 |
| 2 | ProGibb | Gibberellic acid | 4 mg/L (ppm) | Spray | 0.3–1.2 |
| 3 | ProGibb | Gibberellic acid | 4 mg/L (ppm) | Spray | 1.5–2.4 |
| 4 | ProGibb | Gibberellic acid | 7 mg/L (ppm) | Spray | 0.3–1.2 |
| 5 | ProGibb | Gibberellic acid | 7 mg/L (ppm) | Spray | 1.5–2.4 |
| 6 | MaxCel | 6-Benzyladenine | 50 mg/L (ppm) | Spray | 1.5–2.4 |
| 7 | MaxCel | 6-Benzyladenine | 250 mg/L (ppm) | Spray | 1.5–2.4 |
| 8 | Cycocel | Chlormequat-chloride | 600 mg/L (ppm) | Drench | 1.5–2.4 |
| 9 | Cycocel | Chlormequat-chloride | 1200 mg/L (ppm) | Drench | 1.5–2.4 |
| 10 | Ethephon | Ethephon phosphonic acid | 50 mg/L (ppm) | Spray | 1.5–2.4 |
| 11 | Ethephon | Ethephon phosphonic acid | 200 mg/L (ppm) | Spray | 1.5–2.4 |
| 12 | Cycocel + Ethephon | Chlormequat-chloride + Ethephon phosphonic acid | 600 + 50 mg/L (ppm) | Drench + Spray | 1.5–2.4 |
| 13 | Cycocel + Ethephon | Chlormequat-chloride + Ethephon phosphonic acid | 1200 + 200 mg/L (ppm) | Drench + Spray | 1.5–2.4 |
| Treatment | Name | Active Ingredient | Application Rate |
|---|---|---|---|
| 1 | Control (Water) | NA | NA |
| 2 | Ethephon | Ethephon phosphonic acid | 15 mg/L (ppm) |
| 3 | Ethephon | Ethephon phosphonic acid | 30 mg/L (ppm) |
| 4 | Ethephon | Ethephon phosphonic acid | 45 mg/L (ppm) |
| 5 | Ethephon | Ethephon phosphonic acid | 60 mg/L (ppm) |
| 6 | ProGibb | Gibberellic acid | 1 mg/L (ppm) |
| 7 | ProGibb | Gibberellic acid | 2 mg/L (ppm) |
| 8 | ProGibb | Gibberellic acid | 3 mg/L (ppm) |
| 9 | ProGibb | Gibberellic acid | 4 mg/L (ppm) |
| 10 | Ethephon + ProGibb | Ethephon phosphonic acid + Gibberellic acid | 15 + 1 mg/L (ppm) |
| 11 | Ethephon + ProGibb | Ethephon phosphonic acid + Gibberellic acid | 30 + 2 mg/L (ppm) |
| 12 | Ethephon + ProGibb | Ethephon phosphonic acid + Gibberellic acid | 45 + 3 mg/L (ppm) |
| 13 | Ethephon + ProGibb | Ethephon phosphonic acid + Gibberellic acid | 60 + 4 mg/L (ppm) |
| Air Temperature (°C, 60 cm) | Air Temperature (°C, 2 m) | Air Temperature (°C, 10 m) | Soil Temperature (°C, −10 cm) | RH (%, 2 m) | Solar Radiation (w/m2, 2 m) | Wind Speed (mph, 10 m) | ||
|---|---|---|---|---|---|---|---|---|
| Study I | May 2019 | 26.1 ± 0.3 | 25.9 ± 0.3 | 25.6 ± 0.3 | 30.1 ± 0.4 | 73.2 ± 1.3 | 238.2 ± 10.7 | 5.5 ± 0.2 |
| June 2019 | 27.3 ± 0.3 | 27.1 ± 0.3 | 26.7 ± 0.3 | 30.4 ± 0.4 | 81.6 ± 1.3 | 205.7 ± 9.7 | 5.2 ± 0.2 | |
| July 2019 | 27.2 ± 0.2 | 26.9 ± 0.2 | 26.6 ± 0.2 | 30.9 ± 0.2 | 83.8 ± 0.9 | 195.8 ± 10.4 | 4.4 ± 0.2 | |
| August 2019 | 27.7 ± 0.2 | 27.5 ± 0.2 | 27.0 ± 0.2 | 30.9 ± 0.2 | 84.8 ± 0.8 | 234.2 ± 10.4 | 4.6 ± 0.2 | |
| Study II | May 2021 | 25.2 ± 0.3 | 24.9 ± 0.3 | 24.5 ± 0.3 | 30.1 ± 0.2 | 67.9 ± 1.4 | 286.1 ± 8.5 | 6.4 ± 0.3 |
| June 2021 | 27.0 ± 0.3 | 26.6 ± 0.3 | 26.2 ± 0.3 | 30.4 ± 0.3 | 79.5 ± 1.5 | 220.5 ± 11.9 | 5.3 ± 0.2 | |
| July 2021 | 27.1 ± 0.2 | 26.7 ± 0.2 | 26.5 ± 0.2 | 30.2 ± 0.3 | 84.4 ± 1.0 | 224.1 ± 12.6 | 4.7 ± 0.2 | |
| August 2021 | 27.9 ± 0.2 | 27.5 ± 0.2 | 27.1 ± 0.2 | 30.9 ± 0.2 | 82.2 ± 0.9 | 214.1 ± 8.7 | 4.7 ± 0.2 | |
| September 2021 | 26.3 ± 0.2 | 26.2 ± 0.2 | 25.4 ± 0.1 | 29.9 ± 0.1 | 81.7 ± 1.3 | 240.3 ± 19.3 | 4.5 ± 0.2 |
| PGR Treatment | Alpha % | Beta % | Hop Storage Index |
|---|---|---|---|
| Commercial Standard | 4.0–9.0 [57] | 5.5–9.0 [57] | --- |
| Control (Water) | 3.28 (0.21) G | 1.59 (0.06) G | 0.23 (0.02) A |
| Ethephon 15 mg/L | 3.73 (0.16) F | 1.88 (0.09) F | 0.02 (0.01) D |
| Ethephon 30 mg/L | 3.24 (0.30) G | 2.06 (0.12) E | 0.16 (0.01) B |
| Ethephon 45 mg/L | 2.07 (0.09) H | 0.87 (0.14) H | 0.07 (0.06) D |
| Ethephon 60 mg/L | 2.15 (0.02) H | 0.87 (0.06) H | 0.14 (0.05) B |
| ProGibb 1 mg/L | 3.84 (0.16) F | 1.89 (0.04) F | 0.17 (0.01) B |
| ProGibb 2 mg/L | 3.92 (0.12) EF | 1.69 (0.02) G | 0.14 (0.01) B |
| ProGibb 3 mg/L | 4.08 (0.01) E | 2.28 (0.01) D | 0.16 (0.01) B |
| ProGibb 4 mg/L | 5.44 (0.04) B | 2.32 (0.01) D | 0.17 (0.01) B |
| Ethephon 15 mg/L + ProGibb 1 mg/L | 6.09 (0.02) A | 2.90 (0.01) A | 0.17 (0.01) B |
| Ethephon 30 mg/L + ProGibb 2 mg/L | 5.00 (0.11) C | 2.64 (0.08) B | 0.15 (0.01) B |
| Ethephon 45 mg/L + ProGibb 3 mg/L | 4.12 (0.17) E | 2.48 (0.09) C | 0.15 (0.01) B |
| Ethephon 60 mg/L + ProGibb 4 mg/L | 4.51 (0.01) D | 1.98 (0.01) EF | 0.09 (0.01) CD |
| Approximate Concentration (mg/L) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | ||||||||||||||
| Compound | LRI Value | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Acids | ||||||||||||||
| Hexadecanoic acid | 1930 | 1.45 (0.41) | 0.32 (0.55) | ------- | ------- | 8.15 (1.52) | 2.30 (1.09) | 4.07 (2.58) | ------- | 2.43 (4.21) | 1.95 (1.39) | ------- | 6.40 (9.06) | ------- |
| Alcohols | ||||||||||||||
| 3-Hexenol | 840 | 0.85 (0.10) | 0.50 (0.03) | 0.88 (0.01) | 0.53 (0.08) | ------- | ------- | ------- | ------- | ------- | 0.31 (0.44) | 1.97 (0.11) | 1.97 (0.11) | ------- |
| Octanol | 972 | ------- | 0.15 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 0.09 (0.12) | 2.93 (0.10) | ------- | ------- |
| 1-Octen-3-ol | 976 | 3.02 (2.64) | 0.41 (0.04) | 0.97 (0.01) | 0.43 (0.02) | 1.75 (0.24) | ------- | 2.99 (0.10) | 0.58 (0.08) | 3.88 (0.12) | 0.63 (0.05) | 8.99 (0.18) | 2.00 (0.07) | 4.53 (0.50) |
| Benzyl alcohol | 1031 | 0.23 (0.01) | 0.13 (0.07) | ------- | 0.43 (0.10) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 0.99 (0.14) | ------- |
| 2-Nonanol | 1095 | ------- | ------- | 0.24 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 0.35 (0.60) | ------- | ------- |
| Subtotal | 4.02 BC (2.47) | 1.21 CD (0.15) | 2.12 BCD (0.01) | 1.39 CBD (0.20) | 1.75 CBD (0.24) | ------- D | 2.99 BCD (0.10) | 0.58 CD (0.08) | 3.88 BC (0.12) | 1.02 BCD (0.51) | 14.58 A (1.61) | 4.96 B (0.18) | 4.53 BC (0.50) | |
| Aldehyde | ||||||||||||||
| E–2-Pentenal | 789 | ------- | 0.28 (0.02) | 0.61 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- |
| 2-Hexenal | 838 | 0.99 (0.09) | 0.32 (0.02) | 0.82 (0.01) | 0.12 (0.21) | ------- | ------- | 3.02 (0.43) | ------- | 2.34 (1.74) | 0.47 (0.03) | 10.57 (0.60) | 0.51 (0.06) | 6.68 (0.34) |
| Benzenecarboxaldehyde | 954 | 0.40 (0.05) | 0.23 (0.01) | 0.36 (0.01) | 0.13 (0.23) | ------- | ------- | 2.10 (0.25) | 0.35 (0.06) | 2.28 (0.28) | 0.34 (0.06) | 3.55 (0.03) | 2.16 (0.29) | 0.73 (1.03) |
| 2,4-Heptadienal | 997 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 4.28 (2.11) | ------- | ------- |
| Nonanal | 1096 | 0.63 (0.04) | 0.28 (0.23) | ------- | ------- | ------- | ------- | 4.45 (0.35) | ------- | 6.69 (0.54) | 0.35 (0.06) | 18.10 (0.75) | ------- | 10.02 (1.40) |
| Subtotal | 2.02 DE (0.13) | 1.10 DE (0.19) | 1.78 D (0.01) | 0.25 E (0.44) | ------- | ------- | 9.56 C (0.54) | 0.35 DE (0.06) | 9.77 C (2.6) | 1.16 DE (0.09) | 36.50 A (2.12) | 2.67 D (0.35) | 17.43 B (0.04) | |
| Esters | ||||||||||||||
| Methyl hexanoate | 921 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 2.62 (0.14) | ------- | ------- |
| Methyl heptanoate | 1023 | ------- | ------- | ------- | ------- | ------- | ------- | 0.68 (0.02) | ------- | 2.58 (0.18) | ------- | 8.09 (0.29) | ------- | 3.91 (0.16) |
| Methyl octanoate | 1118 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 1.62 (0.34) | ------- | 3.28 (0.13) | ------- | ------- |
| Methyl 4-decenoate | 1293 | 0.99 (0.05) | 0.16 (0.01) | 0.59 (0.41) | ------- | ------- | ------- | 2.54 (0.58) | ------- | 6.95 (2.69) | ------- | 18.20 (0.78) | ------- | 10.78 (0.57) |
| Methyl geranoate | 1304 | ------- | ------- | 0.77 (0.01) | ------- | ------- | ------- | 1.19 (0.73) | ------- | ------- | ------- | 12.94 (0.47) | ------- | 6.32 (0.62) |
| Nerol acetate | 1363 | ------- | 0.15 (0.02) | 2.08 (0.01) | 1.13 (0.21) | ------- | ------- | ------- | 0.56 (0.12) | 12.88 (5.86) | ------- | 18.60 (0.83) | ------- | 8.80 (0.95) |
| Linalyl 3-methylbutanoate | 1460 | 2.15 (0.70) | ------- | 1.92 (0.01) | ------- | ------- | ------- | ------- | ------- | 5.51 (1.57) | ------- | 6.61 (0.01) | ------- | ------- |
| Geranyl isobutyrate | 1501 | ------- | ------- | 1.57 (0.01) | 0.61 (0.16) | ------- | ------- | ------- | ------- | 4.74 (0.56) | ------- | 7.86 (2.66) | ------- | ------- |
| 5E,7Z-Dodecadienyl acetate | 1659 | 7.41 (2.00) | 1.78 (0.33) | 3.28 (0.06) | 4.62 (0.31) | ------- | 3.30 (0.75) | 2.34 (0.70) | 4.05 (0.95) | 5.30 (2.19) | 1.56 (0.01) | 10.56 (0.4) | ------- | 4.21 ± 5.96 |
| Subtotal | 10.55 C (2.75) | 2.09 C (0.34) | 8.88 C (1.05) | 6.16 C (0.32) | ------- | 3.30 C (0.75) | 7.31 C (1.93) | 1.24 C (0.29) | 34.40 B (6.71) | 1.56 C (0.01) | 78.64 A (5.41) | ------- | 34.02 B (8.25) | |
| Ketone | ||||||||||||||
| 2-Nonanone | 1084 | 0.22 (0.02) | ------- | 0.22 (0.01) | ------- | ------- | ------- | 0.89 (0.03) | ------- | 1.97 (0.03) | ------- | 8.89 (0.46) | ------- | 2.39 (3.38) |
| 2-Decanone | 1152 | ------- | ------- | 0.26 (0.01) | ------- | 1.11 (0.01) | ------- | ------- | ------- | ------- | ------- | 5.62 (0.11) | ------- | ------- |
| 2-Undecanone | 1280 | 1.57 (0.17) | 0.35 (0.05) | 1.59 (0.01) | ------- | ------- | 0.96 (0.08) | 4.26 (1.14) | 0.41 (0.02) | 7.45 (2.58) | 0.41 (0.04) | 24.68 (0.86) | 0.87 (0.08) | 15.91 (1.26) |
| 2-Dodecanone | 1343 | 0.27 (0.07) | ------- | 0.38 (0.01) | ------- | ------- | ------- | 0.56 (0.08) | ------- | ------- | ------- | 3.92 (0.48) | ------- | ------- |
| β -Ionone | 1474 | 0.32 (0.28) | ------- | 0.5 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 2.35 (0.86) | ------- | ------- |
| 2-Tridecanone | 1489 | 1.52 (0.37) | ------- | 2.44 (0.01) | 0.70 (0.07) | 2.82 (0.01) | 1.02 (0.21) | 3.49 (1.07) | 0.82 (0.13) | 4.89 (2.70) | ------- | 19.36 (1.08) | 1.49 (0.15) | 5.42 (7.67) |
| Subtotal | 3.20 D (0.45) | 0.35 D (0.05) | 5.37 CD (0.01) | 0.70CD (0.07) | 3.93 CD (0.01) | 1.98 D (0.29) | 7.85 CD (3.49) | 1.23 D (0.11) | 14.00 C (4.35) | 0.41 D (0.04) | 58.82 A (11.53) | 2.36 D (0.07) | 23.72 B (9.78) | |
| Monoterpene | ||||||||||||||
| β-pinene | 977 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 0.94 (0.40) | ------- | 1.13 (0.15) | ------- | ------- |
| Myrcene | 990 | ------- | ------- | ------- | ------- | ------- | ------- | 11.94 (1.95) | ------- | 62.44 (3.23) | ------- | 98.90 (4.73) | ------- | 55.15 (0.43) |
| Neral | 1233 | 0.20 (0.01) | ------- | 0.41 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 1.62 (0.06) | ------- | ------- |
| Citral | 1259 | 0.40 (0.06) | 0.12 (0.01) | 0.94 (0.01) | 0.47 (0.18) | ------- | ------- | ------- | 0.41 (0.01) | 2.88 (0.87) | 0.21 (0.05) | 5.61 (0.14) | 0.92 (0.04) | ------- |
| Subtotal | 0.53 E (0.12) | 0.12 E (0.01) | 1.22 E (0.31) | 0.47 E (0.18) | ------- | ------- | 11.94 D (1.95) | 0.41 E (0.01) | 65.94 B (3.25) | 0.21 E (0.05) | 107.26 A (5.04) | 0.92 E (0.04) | 55.15 C (0.43) | |
| Sequiterpenes | ||||||||||||||
| (E)-β caryophyllene | 1412 | 0.31 (0.12) | ------- | ------- | ------- | ------- | ------- | 1.76 (0.21) | ------- | 9.96 (3.35) | ------- | 29.90 (1.76) | ------- | 15.64 (1.66) |
| γ-Muurolene | 1422 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 2.08 (0.11) | ------- | 0.74 (1.05) |
| β-Famesene | 1443 | ------- | ------- | ------- | ------- | ------- | ------- | 0.86 (0.22) | ------- | 5.99 (2.89) | ------- | 18.34 (0.94) | ------- | 8.72 (1.65) |
| α-Humulene | 1452 | 0.75 (0.23) | ------- | 0.34 (0.32) | 1.21 (1.37) | ------- | 0.91 (0.44) | 4.06 (1.00) | 0.35 (0.20) | 16.62 (7.30) | 0.60 (0.25) | 61.10 (3.84) | ------- | 34.23 (3.30) |
| Epicubenol | 1639 | 0.74 (0.13) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 5.40 (2.26) | ------- | 6.40 (0.80) |
| 1-Epicadinol | 1646 | 1.49 (0.45) | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 6.82 (1.96) | ------- | 14.09 (0.28) | ------- | 9.22 (1.45) |
| Subtotal | 2.79 C (1.26) | ------- | 0.34 C (0.32) | 1.21 C (1.37) | ------- | 0.91 C (0.44) | 6.10 C (0.44) | 0.35 C (0.20) | 37.12 B (17.60) | 0.60 C (0.25) | 126.20 A (3.21) | ------- | 45.56 B (0.79) | |
| Terepene alcohol | ||||||||||||||
| Linalool | 1092 | 2.79 (0.17) | 0.91 (0.09) | 4.89 (0.31) | 2.93 (0.42) | 6.54 (0.48) | 2.28 (0.42) | 2.71 (0.29) | 1.64 (0.03) | 17.57 (1.47) | 1.47 (0.02) | 35.06 (1.90) | 6.27 (0.67) | 13.77 (1.34) |
| Ipsdienol | 1139 | ------- | ------- | 0.60 (0.01) | ------- | ------- | ------- | ------- | ------- | ------- | 0.24 (0.04) | 4.84 (0.28) | ------- | ------- |
| 4-Terpinenol | 1185 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 3.31 (0.35) | ------- | ------- |
| α-Terpineol | 1194 | 1.08 (0.08) | 0.64 (0.05) | 0.97 (0.01) | 0.56 (0.08) | 2.18 (0.50) | 1.04 (0.41) | 2.30 (0.41) | 0.66 (0.09) | 3.51 (0.67) | 0.76 (0.18) | 9.00 (1.47) | 5.80 (0.81) | 4.91 (0.14) |
| Geraniol | 1243 | 0.73 (0.11) | 0.22 (0.06) | 2.95 (0.41) | 1.58 (0.42) | 2.46 (0.59) | ------- | ------- | 0.45 (0.10) | 5.39 (4.29) | 0.48 (0.05) | 12.80 (0.33) | 1.80 (0.16) | 5.08 (0.02) |
| α-Selinene | 1494 | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | ------- | 13.09 (1.24) | ------- | ------- |
| Subtotal | 4.35 EF (0.43) | 1.70 F (0.26) | 9.21 D (1.25) | 5.07 E (0.81) | 10.36 CD (1.16) | 3.32 EF (0.83) | 5.02 E (0.70) | 2.75 EF (0.01) | 26.47 B (5.39) | 2.95 EF (0.11) | 77.00 A (3.07) | 13.87 C (1.33) | 23.77 B (1.45) | |
| Epoxide | ||||||||||||||
| Caryophyllene oxide | 1588 | 1.56 (0.42) | 0.48 (0.14) | 1.75 (0.01) | ------- | 2.32 (0.28) | 1.25 (0.59) | 1.90 (0.44) | 0.77 (0.15) | 4.81 (1.55) | 0.75 (0.70) | 12.69 (0.75) | 1.11 (0.71) | 10.31 (1.39) |
| Humulene epoxide II | 1616 | 2.98 (1.11) | ------- | 3.30 (0.01) | ------- | 4.84 (0.13) | ------- | 3.42 (1.43) | 1.44 (0.12) | 6.11 (3.76) | ------- | 22.23 (2.41) | ------- | 19.91 (2.98) |
| Caryophyllene oxide II | 1663 | 6.84 (1.82) | 1.96 (0.30) | 13.41 (1.51) | 4.37 (0.35) | 12.53 (0.80) | 3.79 (0.97) | 7.83 (2.73) | 2.87 (0.86) | 16.19 (10.48) | 1.40 (0.24) | 41.50 (2.83) | 6.30 (1.46) | 30.19 (5.23) |
| Subtotal | 10.38 CD (3.46) | 2.45 D (0.16) | 16.78 BCD (1.41) | 4.37 D (0.35) | 19.70 BC (0.65) | 4.62 D (1.50) | 12.02 CD (4.55) | 5.08 CD (1.13) | 25.51 B (17.12) | 2.05 D (0.46) | 69.00 A (14.70) | 7.41 CD (0.75) | 60.41 A (9.60) | |
| Overall Total | 39.29 D (4.54) | 7.02 D (2.21) | 42.91 CD (7.63) | 17.01 D (2.21) | 32.56 D (13.84) | 14.05 D (5.22) | 63.94 CD (12.72) | 12.00 D (1.57) | 216.75 B (47.90) | 11.91 D (1.86) | 567.66 A (21.02) | 39.23 D (9.15) | 264.6 BC (9.70) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wendrick, N.A.; Campbell, S.M.; Gazaleh, J.E.; Huo, H.; Thompson-Witrick, K.A.; Pearson, B.J. The Influence of Plant Growth Regulators (PGRs) on Physical and Chemical Characteristics of Hops (Humulus lupulus L.). Int. J. Plant Biol. 2025, 16, 79. https://doi.org/10.3390/ijpb16030079
Zhang M, Wendrick NA, Campbell SM, Gazaleh JE, Huo H, Thompson-Witrick KA, Pearson BJ. The Influence of Plant Growth Regulators (PGRs) on Physical and Chemical Characteristics of Hops (Humulus lupulus L.). International Journal of Plant Biology. 2025; 16(3):79. https://doi.org/10.3390/ijpb16030079
Chicago/Turabian StyleZhang, Mengzi, Nicholas A. Wendrick, Sean M. Campbell, Jacob E. Gazaleh, Heqiang Huo, Katherine A. Thompson-Witrick, and Brian J. Pearson. 2025. "The Influence of Plant Growth Regulators (PGRs) on Physical and Chemical Characteristics of Hops (Humulus lupulus L.)" International Journal of Plant Biology 16, no. 3: 79. https://doi.org/10.3390/ijpb16030079
APA StyleZhang, M., Wendrick, N. A., Campbell, S. M., Gazaleh, J. E., Huo, H., Thompson-Witrick, K. A., & Pearson, B. J. (2025). The Influence of Plant Growth Regulators (PGRs) on Physical and Chemical Characteristics of Hops (Humulus lupulus L.). International Journal of Plant Biology, 16(3), 79. https://doi.org/10.3390/ijpb16030079







