Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Screening and Data Extraction
2.2.4. Quality Assessment and Bias Evaluation
2.3. Data Synthesis
3. Results
3.1. Variation in Maternal-Microbiota-Targeted Interventions and Their Impact on Neonatal Immunity
| Authors | Country | Dominant Microbiota Influence | Key Finding | Allergy Risk Association (A True/False Indicator Showing Whether the Research in That Country Found a Direct Link Between Maternal Microbiota Composition and Allergy Risk in Infants) |
|---|---|---|---|---|
| Friedman. [45], Soderborg et al. [46], Beckers et al. [47] | USA | Gut microbiota | Maternal obesity extensively reprograms the infant’s gut microbiota, potentially affecting immune responses. | True |
| Li X et al. [48] | China | Gut and vaginal microbiota | Maternal diet is linked to microbial diversity, with potential implications for neonatal immunity. | True |
| Furuhjelm et al. [49], Hansen et al. [50] | Denmark | Gut microbiota | Fish oil administration in women during pregnancy showed a reduced risk of allergies in the children. | False |
| Butler et al. [51], Butler É. et al. [52] | New Zealand | Vaginal microbiota | Vaginal seeding (the process of exposing babies delivered by C-section to maternal vaginal microbiota) may restore microbial transmission and promote immune development. | True |
| Shahzad et al. [53] | Pakistan | Gut microbiota | Maternal diet and hygiene practices have a sizable impact on the maternal microbiota and neonatal health. | True |
| Huang et al. [54], Jiang et al. [55], Bertelsen et al. [56] | Finland | Gut microbiota | Pregnancy supplementation with probiotics leads to modifications of the maternal microbiota composition, which may benefit neonatal immunity. | False |
| Yang et al. [65], Ferretti et al. [66] Notarbartolo et al. [67] | United Kingdom | Gut and breast milk microbiota | The composition of the maternal gut microbiota is associated with vitamin D levels in the early gestational period, which may be of importance for neonatal immune function. | True |
| Dierikx et al. [64] | The Netherlands | Gut microbiota | Maternal antibiotic timing (hospitalization pre-cesarean incision vs. post-cord clamping) influences neonatal gut microbiota development. | True |
| Fransson et al. [59], Dunn et al. [60] | Sweden | Gut microbiota | Maternal skin microbiota colonization induces gut microbiota composition and primes immune systems. | False |
| Hatmal et al. [57], Zamanillo et al. [58] | Spain | Breast milk microbiota | Maternal food sources modulate the expression profile of immune-related miRNAs secreted in breast milk. | False |
| Chen et al. [61], Zijlmans et al. [62], Hechler et al. [63] | Germany | Gut microbiota | Maternal psychological stress during pregnancy is associated with changes in gut microbiota diversity that may undermine neonatal immunity. | True |
3.2. Quantitative Summary of the Findings on the Influence of the Maternal Microbiota and Allergic Disease Endpoints
3.3. Microbiota Interventions and Allergy Risk Reduction: A Quantitative Summary
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RCTs | Randomized controlled trials |
| GRADE | Grading of Recommendations Assessment, Development, and Evaluation |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | Medical subject heading |
| CS | Cesarean section |
| VMT | Vaginal microbiota transfer |
| ASD | Autism spectrum disorder |
| GDM | Gestational diabetes mellitus |
| PCOS | Polycystic ovary syndrome |
| BMI | Body mass index |
| HMOs | Human milk oligosaccharides |
Appendix A
| |
| The maternal microbiota also influence neonatal immunity and susceptibility to early childhood allergies [1]. During pregnancy, the maternal microbiota become significantly altered in both composition and functional capacity, as seen in the gut, vaginal, oral, and skin microbiota, mediated mainly by hormonal, immunological, and metabolic adaptations, promoting fetal development and immune programming [2]. Parental side factors, including the mode of delivery, mother’s diet, antibiotic use, and breastfeeding patterns, are supplementary determinants of the early microbial habitat of the neonate, which can contribute towards immune maturation and confer protection against allergic diseases, including asthma, eczema, and food allergies [3]. The development of neonatal immunity is a highly dynamic process that depends upon early exposure to the microbiota to establish tolerance and regulate inflammatory pathways [4]. Reduced maternal microbiota diversity due to cesarean section, antibiotic exposure, or dysbiosis has been implicated in increased risk of immune dysregulation and allergic sensitization in infancy and early childhood [5]. Recent studies have proposed that maternal-microbiota-directed intervention strategies, including the use of probiotics and dietary changes during pregnancy, may promote beneficial microbial transmission and lead to improved neonatal immune health [6]. This systematic review examines the impact of the maternal microbiota profile on the immune development of neonates and the subsequent risk of allergy in early childhood. Our goals are to assess the impact of the maternal microbiota on immune responses that influence the risk of subsequent allergic disease, identify microbial factors linked with allergic predisposition or protection, and outline opportunities for therapeutic translation to improve maternal, at-birth, and, potentially, lifelong health. Despite significant research activity, however, our understanding of maternal microbiome–immune interactions is limited, and there is no consensus on what microbiota-modulating interventions are effective. By collating existing evidence, this study seeks to further clinically relevant knowledge regarding microbiota-driven immune programming and provide evidence to guide interventions to protect against allergy in early life. | |
| |
| The objective of this systematic review is to evaluate the association of the maternal microbiota composition with neonatal immunity and the risk of early childhood allergies. This review emphasizes the role of maternal microbial populations (including gut, vaginal, oral, skin, and breast milk microbiota) and their effects on immune programming in the offspring. It considers important contributing factors such as the mode of delivery, maternal antibiotic use, breastfeeding, diet, and stress, examining their influences on neonatal immune responses and allergic predisposition. | |
| |
| To evaluate the extent and patterns of the influence of the maternal microbiota on neonatal immune development and early childhood allergy risk. To investigate the factors shaping maternal microbiota composition, including the mode of delivery, antibiotic use, diet, breastfeeding, and maternal stress, as well as their impacts on neonatal immune programming.
| |
| |
| Population or participants and conditions of interest | The review will comprise pregnant individuals of all demographic and geographic characteristics. The summary of the main NEUHADS study and the condition of interest was the maternal microbiota composition’s impact on neonatal immunity and early childhood allergy risk. Intervention studies focusing on maternal microbial communities (gut, vaginal, oral, skin, and breast milk microbiota) and the microbiota’s functions in the immune programming of neonates will also be considered in the review. This will also include research exploring factors including the mode of delivery, antibiotic use, maternal diet, and breastfeeding, as well as maternal stress, which may shape variation in maternal microbiota and how this may lead to differences in infant immune, allergy, and related outcomes. |
| Interventions or exposures |
|
| Comparisons or control groups | Standard Care: Comparison of neonatal immune responses and allergy risk in infants born to mothers with diverse, healthy microbiota versus those with microbiota alterations (e.g., due to antibiotic use, diet, or stress). |
| Outcomes of interest | Primary Outcomes:
|
| Setting |
|
| Inclusion criteria | Studies that investigate the impact of the maternal microbiota composition on neonatal immune development and the risk of early childhood allergies. Studies involving pregnant individuals and their neonates, evaluating microbiota-related factors such as maternal gut, vaginal, oral, skin, and breast milk microbiota. Studies reporting quantitative data on outcomes of interest, including immune markers, microbial diversity, allergy incidence, or long-term health implications.
|
| Exclusion criteria |
|
| |
| Electronic databases |
|
| Keywords | (Maternal Microbiota OR Pregnancy Microbiota OR Gut Microbiota in Pregnancy OR Vaginal Microbiota OR Neonatal Immunity OR Immune Development in Infants OR Early-Life Immune Programming OR Allergy Risk OR Childhood Allergies OR Atopic Disease Development OR Breast Milk Microbiota OR Mode of Delivery OR Maternal Antibiotic Use OR Probiotic Supplementation in Pregnancy) |
| |
| Details of methods, number of reviewers, how agreements are reached and disagreements are dealt with, etc. | Three main reviewers and a fourth to resolve any disagreements. Any outstanding disagreements on an article are resolved by the supervisor. |
| Quality assessment tools or checklists used with references or URLs | The protocol will define the method of literature critique/appraisal use, and the STROBE tool will be used for the relevant content and methodology used in the each of the papers to be reviewed. |
| Narrative synthesis details of what and how the synthesis will be performed | Narrative synthesis will be performed alongside any meta-analysis and will be carried out using a framework that consists of four elements. First, a theory is developed on how the intervention works, why, and for whom. Second, a preliminary synthesis of findings of the included studies is developed. Third, relationships within and between the studies are explored. Fourth, the robustness of the synthesis is assessed. |
| Meta-analysis details of what and how analysis and testing will be performed. If no meta-analysis is to be conducted, please give a reason. | Although a meta-analysis is planned, this will only become apparent when we see what data are extracted and made available from the systematic review. It is necessary to consider how heterogeneity will be explored. |
| Grading evidence system used, if any, such as GRADE | GRADE will be used for evidence assessment. |
| |
| Additional material summaries, tables, flowcharts, etc., to be included in the final paper | A flowchart of the whole process—protocol, data extraction, tables, and forest plots of studies—will be included in the final review. |
Appendix B
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1; lines 2 to 3 |
| ABSTRACT | |||
| Abstract | 2 | See PRISMA 2020 for the abstract checklist. | Page 1; lines 18 to 34 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 1 and 2; lines 39 to 57 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Page 2; lines 57 to 63 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 2 and 3; lines 85 to 100 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 2; lines 64 to 76 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Page 2; lines 77 to 83 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | Page 3; lines 100 to 110 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and, if applicable, details of the automation tools used in the process. | Page 3; lines 100 to 110 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, and analyses), and, if not, the methods used to decide which results to collect. | ND |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | ND | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study, whether they worked independently, and, if applicable, details of automation tools used in the process. | Page 3; lines 113 to 123 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of the results. | ND |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing them against the planned groups for each synthesis (item #5)). | Page 3 and 4; lines 124 to 142 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as the handling of missing summary statistics or data conversions. | Page 3 and 4; lines 124 to 142 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Page 3 and 4; lines 124 to 142 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If a meta-analysis was performed, describe the model(s), the method(s) to identify the presence and extent of statistical heterogeneity, and the software package(s) used. | Page 3 and 4; lines 124 to 142 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | ND | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | ND | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | Page 3; lines 114 to 123 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | ND |
| RESULTS | |||
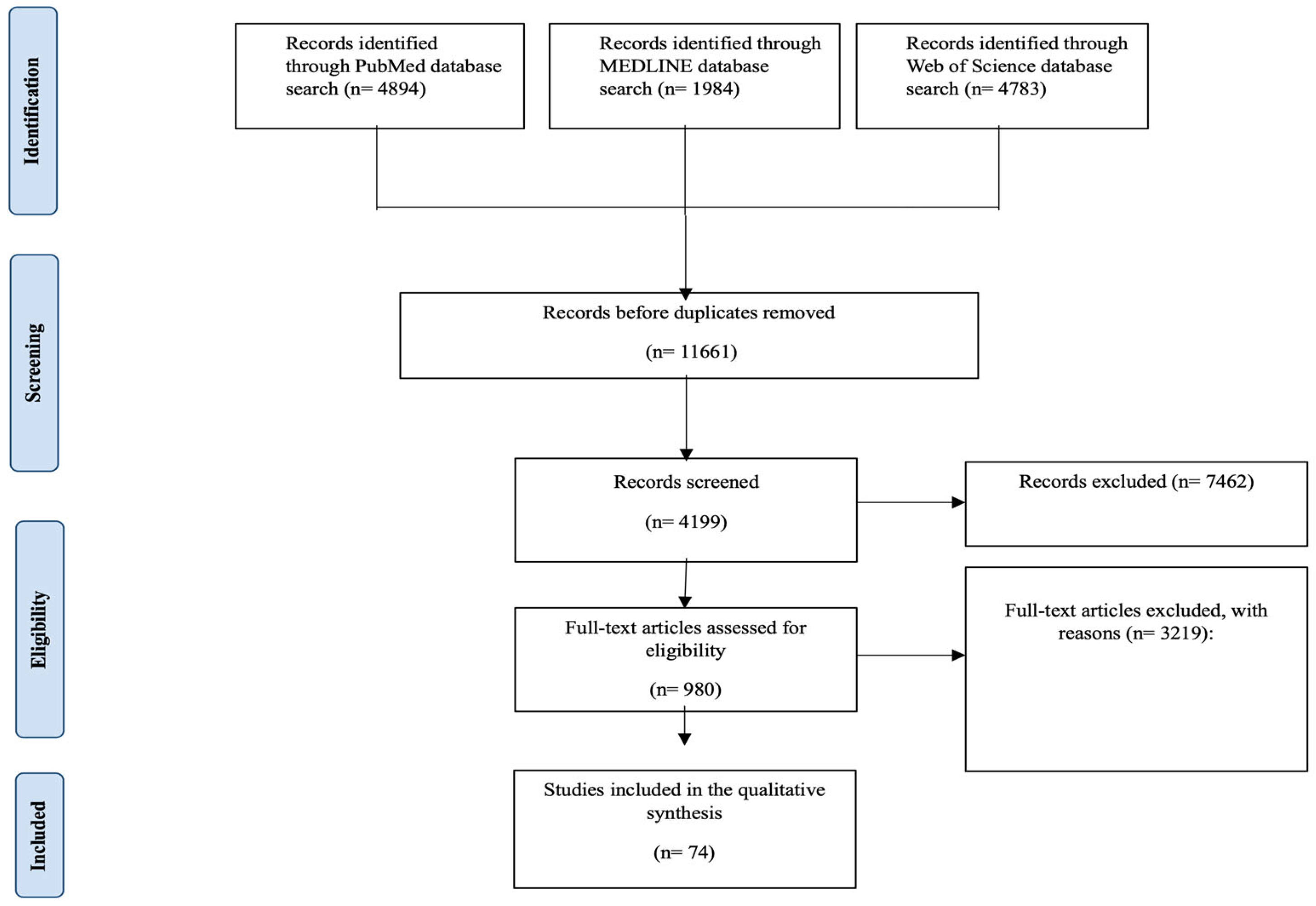
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 4; lines 144 to 152 |
| 16b | Cite studies that might appear to meet the inclusion criteria but were excluded, and explain why they were excluded. | Page 4; lines 144 to 152 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 5 and 6; lines 182 to 186 |
| Risk of bias in studies | 18 | Present assessments of the risk of bias for each included study. | Page 3; lines 113 to 123 |
| Results of individual studies | 19 | For all outcomes, present for each study (a) the summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Page 5 to 7; lines 166 to 169 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Page 7; lines 188 to 231 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was performed, present each summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | ND | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | ND | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | ND | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | ND |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | ND |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 10; lines 238 to 247 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 10; lines 248 to 283 | |
| 23c | Discuss any limitations of the review processes used. | Page 11; lines 285 to 306 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Page 11; lines 307 to 321 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Page 2; lines 64 to 67 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Page 2; lines 64 to 67 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Page 2; lines 64 to 67 | |
| Support | 25 | Describe sources of financial or non-financial support for the review and the role of the funders or sponsors in the review. | Page 12; line 341 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 12; line 351 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found; template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | ND |
Appendix C
| Selection | Comparability | Outcome | Quality Score | Risk of Bias (0–3: High, 4–6: Moderate, 7–9: Low) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Article | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | ||
| Nyangahu, 2019 [1] | (A) truly representative | (A) derived from the same population | * | * | One factor controlled (maternal diet) | * | Yes | * | Moderate Quality Study (6) | Moderate Risk |
| Edwards, 2017 [2] | (A) truly representative | (A) derived from the same population | ** | ** | Two factors controlled (maternal diet and antibiotic use) | ** | Yes | ** | Good Quality Study (8) | Low Risk |
| Jeong, 2021 [3] | (B) somewhat representative | (A) derived from the same population | * | * | One factor controlled (mode of delivery) | * | Yes | ** | Good Quality Study (7) | Low Risk |
| Grech, 2021 [5] | (A) truly representative | (A) derived from the same population | ** | ** | Two factors controlled (breastfeeding and antibiotic exposure) | * | Yes | * | Good Quality Study (7) | Low Risk |
| DuPont, 2023 [6] | (B) somewhat representative | (B) from a different but comparable population | * | * | One factor controlled (maternal gut microbiota) | ** | Yes | ** | Moderate Quality Study (6) | Moderate Risk |
| Sinha, 2024 [17] | (A) truly representative | (A) derived from the same population | ** | ** | Two factors controlled (maternal antibiotic use and delivery mode) | ** | Yes | ** | Good Quality Study (8) | Low Risk |
| Aparicio, 2023 [19] | (A) truly representative | (A) derived from the same population | * | * | One factor controlled (maternal vitamin D levels) | ** | Yes | * | Good Quality Study (7) | Low Risk |
| Halkjær, 2024 [26] | (B) somewhat representative | (B) from different but comparable population | * | * | One factor controlled | ** | Yes | ** | Moderate Quality Study (6) | Moderate Risk |
| Bertelsen, 2013 [56] | (A) truly representative | (A) derived from the same population | ** | ** | Two factors controlled (maternal probiotic supplementation and breastfeeding) | ** | Yes | ** | Good Quality Study (8) | Low Risk |
Appendix D
| Selection | Comparability | Outcome | Quality Score | Risk of Bias (0–3: High, 4–6: Moderate, 7–9: Low) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | |||
| Jeong, 2022 [3] | ** | * | ** | ** | * | ** | ** | Good Quality Study (8) | Low Risk |
| Nunez, 2021 [4] | ** | * | ** | ** | ** | ** | ** | Good Quality Study (8) | Low Risk |
| Moher, 2009 [7] | * | * | ** | * | * | ** | ** | Good Quality Study (8) | Low Risk |
| Brozek, 2020 [8] | ** | ** | ** | * | ** | ** | ** | Good Quality Study (8) | Low Risk |
| Stang, 2010 [9] | ** | * | ** | ** | ** | ** | ** | Good Quality Study (9) | Low Risk |
| Higgins, 2011 [10] | ** | ** | ** | * | ** | ** | ** | Good Quality Study (9) | Low Risk |
| Wilson, 2021 [11] | * | * | ** | * | * | ** | ** | Moderate Quality Study (6) | Moderate Risk |
| Zhou, 2023 [12] | ** | ** | ** | * | ** | ** | ** | Good Quality Study (9) | Low Risk |
Appendix E
| Articles | Bias Arising from the Randomization Process | Bias Due to Deviations from Intended Interventions | Blinding of Participants and Personnel | Bias Due to Missing Outcome Data | Bias in Measurement of the Outcome | Bias in Selection of the Reported Result | Overall RoB |
|---|---|---|---|---|---|---|---|
| Jones, 2024 [13] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Embleton, 2023 [15] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Mueller, 2023 [16] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Mokkala, 2021 [20] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Sugino, 2022 [21] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Bisgaard, 2023 [41] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Wan, 2023 [43] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Sun, 2022 [44] | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
Appendix F
| Articles | Microbiota Factor | Outcomes Assessed | Effect Size |
|---|---|---|---|
| Wilson et al. (2021) [11] | Vaginal microbial transfer | Microbiome restoration in CS infants | RR 0.58 |
| Zhou et al. (2023) [12] | Vaginal microbiota transfer | Neurodevelopment and microbiome | RR 0.67 |
| Mueller et al. (2023) [16] | Vaginal seeding at birth | Microbial engraftment in neonates | OR 1.8 |
| Liu et al. (2022) [29] | Vaginal seeding post-CS | Allergy and microbiota composition | OR 0.65 |
| Carpén et al. (2022) [40] | Maternal intestinal flora transfer | Neonatal gut colonization | RR 2.84 |
| Dierikx et al. (2021) [64] | Antibiotics timing during CS | Initial microbial colonization | OR 1.5 |
| Fransson et al. (2022) [59] | CS-associated microbiota patterns | Microbiota variation and delivery | RR 5.3 |
| Linnér et al. (2020) [30] | Skin-to-skin microbiota transfer | Preterm infant microbiota shifts | RR 0.13 |
| Dominguez-Bello et al. (2020) [5] | Vertical transmission via skin contact | Maternal-offspring microbial overlap | OR 3.8 |
| Hamidi et al. (2023) [31] | Antibiotic prophylaxis at CS | Gut microbiome composition in neonates | RR 0.72 |
| Sinha et al. (2024) [17] | Microbiota reshaping in CS context | Immune and microbiota modulation | OR 4.1 |
| Chen et al. (2024) [61] | Vaginal seeding for CS-born infants | Correction of abnormal microbiota | OR 0.3 |
| Butler et al. (2020) [52] | Perception of vaginal seeding | Behavioral study on delivery options | OR 5.2 |
| Butler et al. (2021) [51] | Diet and vaginal microbiota at delivery | Microbiota shift via delivery route | RR 2.94 |
| Sun et al. (2022) [44] | Impact of birth mode on microbiome | Microbiome shaping by delivery mode | RR 0.70 |
| Maqsood et al. (2022) [37] | Antibiotic use in mothers affects breast milk microbiota | Breast milk microbiome composition | RR 1.7 |
| Gajecka et al. (2023) [27] | Maternal antibiotics and neonatal microbiome shifts | Neonatal microbiota composition | OR 2.1 |
| DuPont et al. (2023) [6] | Antibiotic-induced dysbiosis and microbiome restoration | Reversal of maternal/infant dysbiosis | RR 4.10 |
| Edwards et al. (2017) [2] | Antibiotic exposure influences maternal gut microbiota | Maternal gut microbiota variation | OR 0.9 |
| Zijlmans et al. (2015) [62] | Perinatal antibiotic exposure and infant microbiota diversity | Reduced microbial diversity in neonates | RR 0.14 |
| Embleton et al. (2023) [15] | Exclusive human milk diet in preterm infants | Gut microbiome composition in preterm infants | RR 3.25 |
| Yeruva et al. (2023) [23] | Human milk miRNAs and microbiota modulation | Infant gut microbiota and growth | RR 1.7 |
| Zamanillo et al. (2019) [58] | Breast milk microRNA influence on infant BMI and microbiota | Infant BMI and gut microbial changes | RR 2.3 |
| Notarbartolo et al. (2022) [67] | Breast milk microbiota composition | Microbiota diversity and child health outcomes | OR 1.46 |
| Davis et al. (2022) [69] | Breastfeeding effects on gut microbiome and immunity | Early immune development and gut colonization | OR 1.64 |
| Sillner et al. (2021) [70] | Breastfeeding vs. formula feeding metabolite profiles | Microbial and metabolic profiles over time | OR 4.07 |
| Ferretti et al. (2018) [66] | Mother-to-infant microbial transfer via breastfeeding | Infant gut colonization patterns | RR 2.78 |
| Gilley et al. (2022) [14] | Breastfeeding and maternal obesity influencing microbiota | Infant gut microbiota variation in obese mothers | OR 2.92 |
| Ji et al. (2023) [71] | Probiotics, prebiotics, and breastfeeding synergy | Health and gut microbiome improvements | RR 0.77 |
| Bertelsen et al. (2013) [56] | Breastfeeding and probiotic milk reducing allergic diseases | Reduced allergic disease incidence in infants | RR 0.65 |
References
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The maternal gut microbiome during pregnancy. MCN Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2021, 65, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.; Réot, L.; Menu, E. Neonatal immune system ontogeny: The role of maternal microbiota and associated factors. How Might Non-Hum. Primate Model Enlighten Path? Vaccines 2021, 9, 584. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- DuPont, H.; Salge, M. The importance of a healthy microbiome in pregnancy and infancy and microbiota treatment to reverse dysbiosis for improved health. Antibiotics 2023, 12, 1617. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Brozek, J.L.; Canelo-Aybar, C.; Akl, E.A.; Bowen, J.M.; Bucher, J.; Chiu, W.A.; Cronin, M.; Djulbegovic, B.; Falavigna, M.; Guyatt, G.H.; et al. GRADE Guidelines 30: The GRADE approach to assessing the certainty of modeled evidence—An overview in the context of health decision-making. J. Clin. Epidemiol. 2020, 129, 138–150. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Wilson, B.C.; Butler, É.M.; Grigg, C.P.; Derraik, J.G.B.; Chiavaroli, V.; Walker, N.; Thampi, S.; Creagh, C.; Reynolds, A.J.; Vatanen, T.; et al. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. eBioMedicine 2021, 69, 103443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qiu, W.; Wang, J.; Zhao, A.; Zhou, C.; Sun, T.; Xiong, Z.; Cao, P.; Shen, W.; Chen, J.; et al. Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of cesarean-born infants: A blinded randomized controlled trial. Cell Host Microbe 2023, 31, 1232–1247.e5. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Reinke, S.N.; Mousavi-Derazmahalleh, M.; Garssen, J.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Keelan, J.; Prescott, S.L.; Palmer, D.J.; et al. Maternal prebiotic supplementation during pregnancy and lactation modifies the microbiome and short chain fatty acid profile of both mother and infant. Clin. Nutr. 2024, 43, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17, e12921. [Google Scholar] [CrossRef]
- Embleton, N.D.; Sproat, T.; Uthaya, S.; Young, G.R.; Garg, S.; Vasu, V.; Masi, A.C.; Beck, L.; Modi, N.; Stewart, C.J.; et al. Effect of an exclusive human milk diet on the gut microbiome in preterm infants. JAMA Netw. Open 2023, 6, e231165. [Google Scholar] [CrossRef]
- Mueller, N.T.; Differding, M.K.; Sun, H.; Wang, J.; Levy, S.; Deopujari, V.; Appel, L.J.; Blaser, M.J.; Kundu, T.; Shah, A.A.; et al. Maternal Bacterial Engraftment in Multiple Body Sites of Cesarean Section Born Neonates after Vaginal Seeding—A Randomized Controlled Trial. mBio 2023, 14, e00491-23. [Google Scholar] [CrossRef]
- Sinha, T.; Prins, J.R.; Fernández-Pato, A.; Kruk, M.; Dierikx, T.; De Meij, T.; De Boer, M.; De Boer, J.F.; Scherjon, S.; Kurilshikov, A.; et al. Maternal antibiotic prophylaxis during cesarean section has a limited impact on the infant gut microbiome. Cell Host Microbe 2024, 32, 1444–1454.e6. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, Y.; Chen, X.; Li, S.; Mei, H.; Xiao, H.; Ma, X.; Liu, Z.; Li, R. Gut microbiome and serum amino acid metabolome alterations in autism spectrum disorder. Sci. Rep. 2024, 14, 4037. [Google Scholar] [CrossRef]
- Aparicio, A.; Gold, D.R.; Weiss, S.T.; Litonjua, A.A.; Lee-Sarwar, K.; Liu, Y.Y. Association of Vitamin D Level and Maternal Gut Microbiome during Pregnancy: Findings from a Randomized Controlled Trial of Antenatal Vitamin D Supplementation. Nutrients 2023, 15, 2059. [Google Scholar] [CrossRef]
- Mokkala, K.; Paulin, N.; Houttu, N.; Koivuniemi, E.; Pellonperä, O.; Khan, S.; Pietilä, S.; Tertti, K.; Elo, L.L.; Laitinen, K. Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: A randomised, double-blind, placebo-controlled clinical trial. Gut 2021, 70, 309–318. [Google Scholar] [CrossRef]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A maternal higher-complex carbohydrate diet increases bifidobacteria and alters early life acquisition of the infant microbiome in women with gestational diabetes mellitus. Front. Endocrinol. 2022, 13, 921464. [Google Scholar] [CrossRef] [PubMed]
- Wasan, Y.; Baxter, J.A.B.; Spiegel-Feld, C.; Begum, K.; Rizvi, A.; Iqbal, J.; Hulst, J.; Bandsma, R.; Suleman, S.; Soofi, S.; et al. Elucidating the dynamics and impact of the gut microbiome on maternal nutritional status during pregnancy, effect on pregnancy outcomes and infant health in rural Pakistan: Study protocol for a prospective, longitudinal observational study. BMJ Open 2024, 14, e081629. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Mulakala, B.K.; Rajasundaram, D.; Gonzalez, S.; Cabrera-Rubio, R.; Martínez-Costa, C.; Collado, M.C. Human milk miRNAs associate to maternal dietary nutrients, milk microbiota, infant gut microbiota and growth. Clin. Nutr. 2023, 42, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Muhoozi, G.K.M.; Li, K.; Atukunda, P.; Skaare, A.B.; Willumsen, T.; Enersen, M.; Westerberg, A.C.; Morris, A.; Vieira, A.R.; Iversen, P.O.; et al. Child saliva microbiota and caries: A randomized controlled maternal education trial in rural Uganda. Sci. Rep. 2022, 12, 7857. [Google Scholar] [CrossRef]
- Juncker, H.G.; Jakobsen, R.R.; Naninck, E.F.G.; Davids, M.; Herrema, H.; Van Goudoever, J.B.; De Rooij, S.R.; Korosi, A. Maternal stress in the early postpartum period is associated with alterations in human milk microbiome composition. Brain Behav. Immun. 2025, 124, 74–84. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Danielsen, M.R.; De Knegt, V.E.; Andersen, L.O.; Stensvold, C.R.; Nielsen, H.V.; Mirsepasi-Lauridsen, H.C.; Krogfelt, K.A.; Cortes, D.; Petersen, A.M. Multi-strain probiotics during pregnancy in women with obesity influence infant gut microbiome development: Results from a randomized, double-blind placebo-controlled study. Gut Microbes 2024, 16, 2337968. [Google Scholar] [CrossRef]
- Gajecka, M.; Gutaj, P.; Jaskiewicz, K.; Rydzanicz, M.; Szczapa, T.; Kaminska, D.; Kosewski, G.; Przyslawski, J.; Ploski, R.; Wender-Ozegowska, E. Effects of maternal type 1 diabetes and confounding factors on neonatal microbiomes. Diabetologia 2023, 67, 312–326. [Google Scholar] [CrossRef]
- Ujvari, D.; Trouva, A.; Hirschberg, A.L.; Vanky, E. Maternal serum levels of prokineticin-1 related to pregnancy complications and metformin use in women with polycystic ovary syndrome: A post hoc analysis of two prospective, randomised, placebo-controlled trials. BMJ Open 2023, 13, e073619. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.T.; Zhou, S.J.; Zhou, H.H.; Xiong, Y.; Yang, J.; Zhou, Y.B.; Chen, D.J.; Liu, J.M. Effects of vaginal seeding on gut microbiota, body mass index, and allergy risks in infants born through cesarean delivery: A randomized clinical trial. Am. J. Obstet. Gynecol. MFM 2022, 5, 100793. [Google Scholar] [CrossRef]
- Linnér, A.; Westrup, B.; Lode-Kolz, K.; Klemming, S.; Lillieskold, S.; Pike, H.M.; Morgan, B.; Bergman, N.J.; Rettedal, S.; Jonas, W. Immediate parent-infant skin-to-skin study (IPISTOSS): Study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open 2020, 10, e038938. [Google Scholar] [CrossRef]
- Hamidi, M.; Cruz-Lebrón, A.; Sangwan, N.; Blatz, M.A.; Levine, A.D. Maternal vertical microbial transmission during Skin-to-Skin care. Adv. Neonatal Care 2023, 23, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Yang, M.; Jia, C.; He, Z.; Zhou, S.; Ruan, P.; Wang, Y.; Tang, C.; Pan, W.; et al. Time-restricted eating reveals a “younger” immune system and reshapes the intestinal microbiome in human. Redox Biol. 2024, 78, 103422. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Wells, J.C.K.; Wei, Z.; Bajaj-Elliott, M.; Nielsen, D.S.; Fewtrell, M.S. A Stress Reduction Intervention for Lactating Mothers Alters Maternal Gut, Breast Milk, and Infant Gut Microbiomes: Data from a Randomized Controlled Trial. Nutrients 2024, 16, 1074. [Google Scholar] [CrossRef] [PubMed]
- Nel, N.H.; Haddad, E.N.; Kerver, J.M.; Cassidy-Bushrow, A.E.; Comstock, S.S. Maternal Body Mass Index Associates with Prenatal Characteristics and Fecal Microbial Communities. Nutrients 2024, 16, 1881. [Google Scholar] [CrossRef]
- Stevens, A.J.; Heiwari, T.M.; Rich, F.J.; Bradley, H.A.; Gur, T.L.; Galley, J.D.; Kennedy, M.A.; Dixon, L.A.; Mulder, R.T.; Rucklidge, J.J. Randomised Control Trial Indicates Micronutrient Supplementation May Support a More Robust Maternal Microbiome for Women with Antenatal Depression During Pregnancy. Clin. Nutr. 2024, 43, 120–132. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, W.J.; Wu, F.; Zhang, X.Y.; Li, X.; Wei, J.; Chen, T.T.; Liu, Z.X. Individuality and generality of intratumoral microbiome in the three most prevalent gynecological malignancies: An observational study. Microbiol. Spectr. 2024, 12, e01004-24. [Google Scholar] [CrossRef]
- Maqsood, R.; Skidmore, P.T.; Holland, L.A.; Au, J.L.; Khan, A.K.; Wu, L.I.; Ma, N.; Begnel, E.R.; Chohan, B.H.; Adhiambo, J.; et al. Dynamic Changes in Breast Milk Microbiome in the Early Postpartum Period of Kenyan Women Living with HIV Are Influenced by Antibiotics but Not Antiretrovirals. Microbiol. Spectr. 2022, 10, e02080-21. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, L.; Wang, X.; Fan, W.; Zhao, J. Characterizing the supragingival microbiome of healthy pregnant women. Front. Cell. Infect. Microbiol. 2022, 12, 1016523. [Google Scholar] [CrossRef]
- Sánchez-Salguero, E.; Corona-Cervantes, K.; Guzmán-Aquino, H.A.; De La Borbolla-Cruz, M.F.; Contreras-Vargas, V.; Piña-Escobedo, A.; García-Mena, J.; Santos-Argumedo, L. Maternal IGA2 recognizes similar fractions of colostrum and fecal neonatal microbiota. Front. Immunol. 2021, 12, 712130. [Google Scholar] [CrossRef]
- Carpén, N.; Brodin, P.; De Vos, W.M.; Salonen, A.; Kolho, K.L.; Andersson, S.; Helve, O. Transplantation of maternal intestinal flora to the newborn after elective cesarean section (SECFLOR): Study protocol for a double blinded randomized controlled trial. BMC Pediatr. 2022, 22, 565. [Google Scholar] [CrossRef]
- Bisgaard, H.; Mikkelsen, M.; Rasmussen, M.A.; Sevelsted, A.; Schoos, A.M.M.; Brustad, N.; Eliasen, A.U.; Thorsen, J.; Chawes, B.; Gürdeniz, G.; et al. Atopic and non-atopic effects of fish oil supplementation during pregnancy. Thorax 2023, 78, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Church, J.A.; Edens, T.J.; Mutasa, K.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Ntozini, R.; Chasekwa, B.; Carr, L.; et al. The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine 2021, 39, 5391–5400. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; An, L.; Ren, Z.; Wang, S.; Yang, H.; Ma, J. Effects of galactooligosaccharides on maternal gut microbiota, glucose metabolism, lipid metabolism and inflammation in pregnancy: A randomized controlled pilot study. Front. Endocrinol. 2023, 14, 1034266. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yamada, P.; Paetow, A.; Chan, M.; Arslan, A.; Landberg, R.; Dominguez-Bello, M.G.; Young, B.K. A randomized controlled trial of the effects of whole grains versus refined grains diets on the microbiome in pregnancy. Sci. Rep. 2022, 12, 7509. [Google Scholar] [CrossRef]
- Friedman, J.E. The maternal microbiome: Cause or consequence of obesity risk in the next generation? Obesity 2017, 25, 497–498. [Google Scholar] [CrossRef]
- Soderborg, T.K.; Borengasser, S.J.; Barbour, L.A.; Friedman, J.E. Microbial transmission from mothers with obesity or diabetes to infants: An innovative opportunity to interrupt a vicious cycle. Diabetologia 2016, 59, 895–906. [Google Scholar] [CrossRef]
- Beckers, K.F.; Flanagan, J.P.; Sones, J.L. Microbiome and pregnancy: Focus on microbial dysbiosis coupled with maternal obesity. Int. J. Obes. 2023, 48, 439–448. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Nie, C.; Li, Q.; Sun, X.; Li, H.; Zhang, Y. Altered vaginal microbiome and relative co-abundance network in pregnant women with penicillin allergy. Allergy Asthma Clin. Immunol. 2020, 16, 79. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Larsson, J.; Fredriksson, M.; Böttcher, M.F.; Fälth-Magnusson, K.; Duchén, K. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 2009, 98, 1461–1467. [Google Scholar] [CrossRef]
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy Clin. Immunol. 2016, 139, 104–111.e4. [Google Scholar] [CrossRef]
- Butler, É.M.; Reynolds, A.J.; Derraik, J.G.B.; Wilson, B.C.; Cutfield, W.S.; Grigg, C.P. The views of pregnant women in New Zealand on vaginal seeding: A mixed-methods study. BMC Pregnancy Childbirth 2021, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Butler, É.M.; Chiavaroli, V.; Derraik, J.G.B.; Grigg, C.P.; Wilson, B.C.; Walker, N.; O’Sullivan, J.M.; Cutfield, W.S. Maternal bacteria to correct abnormal gut microbiota in babies born by C-section. Medicine 2020, 99, e21315. [Google Scholar] [CrossRef] [PubMed]
- Muhamamd, S.; Ismail, M.; Misselwitz, B.; Saidal, A.; Andrews, S.C.; Iqbal, K.; Akarsu, H.; Nabhanic, Z.A. Child health, nutrition and gut microbiota development during the first two years of life; study protocol of a prospective cohort study from the Khyber Pakhtunkhwa, Pakistan. F1000Research 2024, 13, 1336. [Google Scholar] [CrossRef]
- Huang, T.; Li, Z.; Tye, K.D.; Chan, S.N.; Tang, X.; Luo, H.; Wang, D.; Zhou, J.; Duan, X.; Xiao, X. Probiotic supplementation during pregnancy alters gut microbial networks of pregnant women and infants. Front. Microbiol. 2022, 13, 1042846. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, L.; Xia, J.; Cheng, L.; Chen, G.; Wang, J.; Raghavan, V. Probiotics supplementation during pregnancy or infancy on multiple food allergies and gut microbiota: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e25–e41. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Brantsæter, A.L.; Magnus, M.C.; Haugen, M.; Myhre, R.; Jacobsson, B.; Longnecker, M.P.; Meltzer, H.M.; London, S.J. Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J. Allergy Clin. Immunol. 2013, 133, 165–171.e8. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.I.; Olaimat, A.N.; Alshaer, W.; Hasan, H.; Albakri, K.A.; Alkhafaji, E.; Issa, N.N.; Al-Holy, M.A.; Abderrahman, S.M.; et al. Immunomodulatory properties of human breast milk: MicroRNA contents and potential epigenetic effects. Biomedicines 2022, 10, 1219. [Google Scholar] [CrossRef]
- Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef]
- Fransson, E.; Gudnadottir, U.; Hugerth, L.W.; Itzel, E.W.; Hamsten, M.; Boulund, F.; Pennhag, A.; Du, J.; Schuppe-Koistinen, I.; Brusselaers, N.; et al. Cohort profile: The Swedish Maternal Microbiome project (SweMaMi)—Assessing the dynamic associations between the microbiome and maternal and neonatal adverse events. BMJ Open 2022, 12, e065825. [Google Scholar] [CrossRef]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The maternal infant microbiome. MCN Am. J. Matern. Child Nurs. 2017, 42, 318–325. [Google Scholar] [CrossRef]
- Chen, H.J.; Bischoff, A.; Galley, J.D.; Peck, L.; Bailey, M.T.; Gur, T.L. Discrete role for maternal stress and gut microbes in shaping maternal and offspring immunity. Neurobiol. Stress 2022, 21, 100480. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, M.A.C.; Korpela, K.; Riksen-Walraven, J.M.; De Vos, W.M.; De Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Hechler, C.; Borewicz, K.; Beijers, R.; Saccenti, E.; Riksen-Walraven, M.; Smidt, H.; De Weerth, C. Association between Psychosocial Stress and Fecal Microbiota in Pregnant Women. Sci. Rep. 2019, 9, 4463. [Google Scholar] [CrossRef] [PubMed]
- Dierikx, T.; Berkhout, D.; Eck, A.; Tims, S.; Van Limbergen, J.; Visser, D.; De Boer, M.; De Boer, N.; Touw, D.; Benninga, M.; et al. Influence of timing of maternal antibiotic administration during caesarean section on infant microbial colonisation: A randomised controlled trial. Gut 2021, 71, 1803–1811. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Giuffrè, M.; Montante, C.; Corsello, G.; Carta, M. Composition of human breast milk microbiota and its role in children’s health. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 194. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The effects of delivery mode on the gut microbiota and health: State of art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Järvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol 2022, 150, 523–534. [Google Scholar] [CrossRef]
- Sillner, N.; Walker, A.; Lucio, M.; Maier, T.V.; Bazanella, M.; Rychlik, M.; Haller, D.; Schmitt-Kopplin, P. Longitudinal profiles of dietary and microbial metabolites in formula- and breastfed infants. Front. Mol. Biosci. 2021, 8, 660456. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Arguelles-Lopez, A.; Aguayo-Patrón, S.V.; De La Barca, A.M.C. Breastfeeding Shapes the Gut Microbiota and Its Structure Is Associated with Weight Gain Trajectories in Mexican Infants. Nutrients 2025, 17, 826. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal gut microbiota in the health of mothers and offspring: From the perspective of immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.D.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Mancini, A.; Palermo, A.; Inchingolo, A.M.; Dipalma, G. The Impact of cesarean section delivery on Intestinal microbiota: Mechanisms, Consequences, and Perspectives—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1055. [Google Scholar] [CrossRef]
| Authors | Country | Study Design | Patients (N) | Summary | Level of Evidence |
|---|---|---|---|---|---|
| Wilson et al. [11] | New Zealand | RCT | 47 | Maternal vaginal microbiota given orally did not affect early gut microbiota development in infants born via CS. | I |
| Zhou et al. [12] | China | RCT | 68 | VMT could provide both a safe and effective intervention to promote gut microbiota maturation and neurodevelopment in fecal-microbiota-transplantation-based infant studies of cesarean-born infants in the first 42 days of life. | I |
| Jones et al. [13] | Australia | RCT | 74 | Maternal prebiotic supplementation during pregnancy and lactation alters the maternal and infant gut microbiota toward healthier compositions with higher levels of beneficial bacteria. | I |
| Gilley et al. [14] | USA | Clinical Trial | 74 | The study found that maternal obesity is associated with alterations in infant gut microbiota and metabolism. | II |
| Embleton et al. [15] | United Kingdom | RCTs | 126 | These findings indicate that microbiota mechanisms may not be the primary mediators of the clinical benefits of human-milk-derived products. | I |
| Mueller et al. [16] | USA | RCT | 20 | Vaginal seeding (exposing CS-born neonates to maternal vaginal microbiota) enhanced the transfer of maternal intestinal microbiota to neonates. | I |
| Sinha et al. [17] | The Netherlands | RCT | 107 | Maternal antibiotic timing around cesarean (pre-incision vs. post-cord clamping) showed little impact on the child’s gut microbiota development. | I |
| Chang et al. [18] | China | Controlled Clinical Trial | 60 | Children with ASD have a different gut microbiota composition and eccentric metabolic status from that of neurotypical controls. | II |
| Aparicio et al. [19] | USA | RCT | 881 | Maternal gut microbiota composition is associated with vitamin D levels during early pregnancy. | I |
| Mokkala et al. [20] | Finland | RCT | 270 | Women with GDM had reduced gut microbiota flexibility, indicating that the gut bacteria of women with GDM did not switch as readily in response to dietary manipulations. | I |
| Sugino et al. [21] | USA | RCT | 34 | A complex-carbohydrate-rich maternal diet in pregnancy may modulate the gut microbiota transferable to mothers and their babies and may reduce the risk of developing obesity and related immune dysfunction in infants. | I |
| Wasan et al. [22] | Pakistan | Prospective Observational Cohort Study | 400 | Nutrition and diet have always been known to have an important role in keeping up a healthy state of the body, especially in the developing fetus and pregnancy. One such component of nutrition is the maternal gut microbiota, which influence the nutrition and pregnancy outcomes. | III |
| Yeruva et al. [23] | USA, Spain | Clinical Trial | 60 | Maternal dietary sources have a considerable effect on the expression profile of milk miRNAs. These miRNAs are related to maternal dietary nutrients, milk microbiota, infant gut microbiota, and infant growth and development. | II |
| Muhoozi et al. [24] | Uganda | RCT | 511 | Maternal education about nutrition, hygiene, and stimulation is associated with child salivary microbiota composition and a decreased prevalence of dental caries. | I |
| Juncker et al. [25] | The Netherlands, Denmark | Prospective Observational Cohort Study | 92 | The human milk microbiota are significantly altered in association with maternal stress during early postpartum. It has been proposed that these modifications may affect early gut colonization in infants with consequences for their future health and development. | III |
| Halkjær et al. [26] | Denmark | RCT | 50 | This study found that treatment with the multi-strain probiotic Vivomixx® in obese pregnant women did not induce significant changes in gut microbiota diversity or composition in their neonates until 9 months of age. | I |
| Gajecka et al. [27] | Poland | Observational Study | 92 | This study found that maternal glycemic dysregulation during the first trimester was case-specific and associated with the alteration of neonate ear-skin microbiota. | III |
| Ujvari et al. [28] | Norway | RCT | 264 | This study found that pregnant women with PCOS, metformin use, and hyperandrogenism levels were linked to lower levels of prokineticin-1, which is a protein associated with angiogenesis and immune regulation. | I |
| Liu et al. [29] | China | RCT | 120 | This study found that there was no appreciable effect of vaginal seeding on gut microbiota composition, body mass index, or allergy risk in infants in the first two years of life. | I |
| Linnér et al. [30] | Sweden, Norway | RCT | 150 | Neonatal colonization by maternal skin microbiota might influence gut microbiota colonization and prime immune systems. | I |
| Hamidi et al. [31] | USA | Observational Study | 25 | This study shows that prolonged STS care increases vertical microbial transmission from mother to infant, which enriches the preterm infant’s oral and intestinal microbiota. | III |
| Chen et al. [32] | China | Clinical Trial | 49 | This study indicates that promoting healthy maternal gut microbiota through practices could beneficially impact the microbial milieu/landscape communicated to the infant and, thereby, impact immune development and risk of allergic disease in the child. | II |
| Yu et al. [33] | United Kingdom, China, Denmark | RCT | 38 | This study indicates that stress-reducing interventions for lactating mothers could change maternal gut, breast milk, and infant gut microbiota. These changes could affect infant health outcomes, including weight gain. | I |
| Nel et al. [34] | USA | Observational Study | 84 | This study implies that maternal BMI and socioeconomic factors associated with it can modulate gut microbial composition during pregnancy, which may influence neonatal immunity and the risk of developing allergic disorders early in life. | III |
| Stevens et al. [35] | New Zealand | RCT | 33 | Micronutrient supplementation in the setting of pregnancy may be associated with increased diversity and stability of the maternal microbiota, which may translate into benefits for neonatal immunity and early childhood allergies. | I |
| Xiao et al. [36] | China | Observational Study | 90 | This study highlights the possibility that maternal microbiota composition modulates neonatal immunity, with implications for childhood allergy development, and that maternal microbial habitats could have a major impact on the offspring’s health. | III |
| Maqsood et al. [37] | Kenya | RCT | 49 | Antiretroviral therapy does not substantially change the breast milk microbiota; however, antibiotic use influences the composition of the breast milk microbiota. | I |
| Zhang et al. [38] | China | Observational Study | 40 | This study implies that the physiological changes that occur during pregnancy can modify the oral microbiota and may have important roles in neonatal and maternal health, such as in neonatal immunity or early-childhood atopy. | III |
| Sánchez-Salguero et al. [39] | Mexico | Observational Study | 99 | Maternal microbial transfer through colostrum may have immunological implications with potential long-term consequences for neonatal immunity and the risk of developing allergies in early childhood. | III |
| Carpén et al. [40] | Finland | RCT | 60 | This study indicates that the transport of maternal fecal microbiota to newborn infants may help to diagnose the effects of the maternal microbiota configuration on neonatal immunity and the role of early intervention in combatting childhood allergies. | I |
| Bisgaard et al. [41] | Denmark | RCT | 736 | This study investigated the impact of fish oil supplementation on neonatal immunity and allergy development in early childhood, which was significant, reducing the risk of non-atopic asthma by 73%. | I |
| Robertson et al. [42] | Zimbabwe | RCT | 158 | This study indicates that gut microbiota composition in early life may not be a major determinant of vaccine responses in low-income settings, though maternal and environmental factors can affect the maturation of neonatal immunity. | I |
| Wan et al. [43] | China | RCT | 52 | This study suggests that maternal diet can shape the microbiota and, as such, may carry downstream effects on the infant’s host immunity and what colonizes the newborn gut. | I |
| Sun et al. [44] | USA | RCT | 248 | The study shows that a maternal dietary intervention can shape the maternal microbiota (especially vaginal) and ultimately determine the microbial composition transferred between mother and infant. | I |
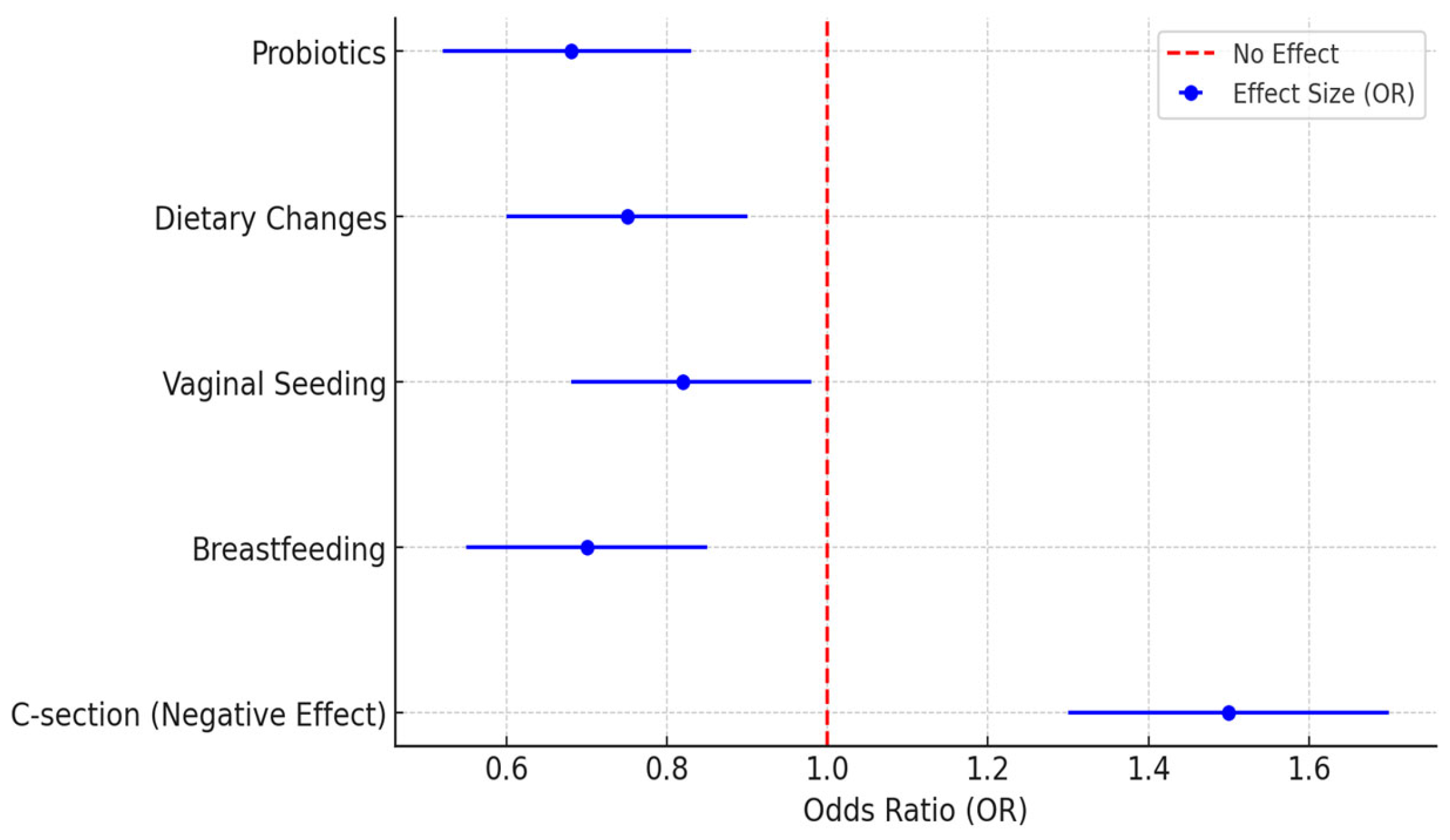
| Factor | Number of Studies | Effect Size (RR/OR) | Association with Allergy Risk |
|---|---|---|---|
| Mode of delivery (CS vs. vaginal birth) | 15 | OR = 1.5–2.2 | Increased allergy risk |
| Maternal antibiotic use | 10 | OR = 1.8–2.6 | Increased allergy risk |
| Breastfeeding (vs. formula feeding) | 12 | OR = 0.7–0.9 | Protective effect |
| Probiotic supplementation in pregnancy | 8 | RR = 0.6–0.8 | Reduced allergy risk |
| Maternal stress | 5 | OR = 1.9–2.4 | Increased allergy risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Jehani, A.N.; Shuaib, M.; Alsharif, A.; Alsubaie, K.A.; Khraisat, A.; Alsharif, A.; Altaf, M.; Almasry, R.H.; Kayali, A.M.; Abdallah, S.A. Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review. Pediatr. Rep. 2025, 17, 67. https://doi.org/10.3390/pediatric17030067
Al Jehani AN, Shuaib M, Alsharif A, Alsubaie KA, Khraisat A, Alsharif A, Altaf M, Almasry RH, Kayali AM, Abdallah SA. Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review. Pediatric Reports. 2025; 17(3):67. https://doi.org/10.3390/pediatric17030067
Chicago/Turabian StyleAl Jehani, Ayah Nabil, Manal Shuaib, Arwa Alsharif, Khlood Abdulaziz Alsubaie, Ayda Khraisat, Abdulaziz Alsharif, Manaf Altaf, Ruba H. Almasry, Amal Mohamed Kayali, and Shouq Abdin Abdallah. 2025. "Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review" Pediatric Reports 17, no. 3: 67. https://doi.org/10.3390/pediatric17030067
APA StyleAl Jehani, A. N., Shuaib, M., Alsharif, A., Alsubaie, K. A., Khraisat, A., Alsharif, A., Altaf, M., Almasry, R. H., Kayali, A. M., & Abdallah, S. A. (2025). Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review. Pediatric Reports, 17(3), 67. https://doi.org/10.3390/pediatric17030067





