Influence of Calcination Temperature on Photocatalyst Performances of Floral Bi2O3/TiO2 Composite
Abstract
:1. Introduction
2. Experiment
2.1. Reagent
2.2. Floral Bi2O3/TiO2 Preparation
2.3. Characterization
2.4. Photocatalytic Performance Test
3. Results and Discussion
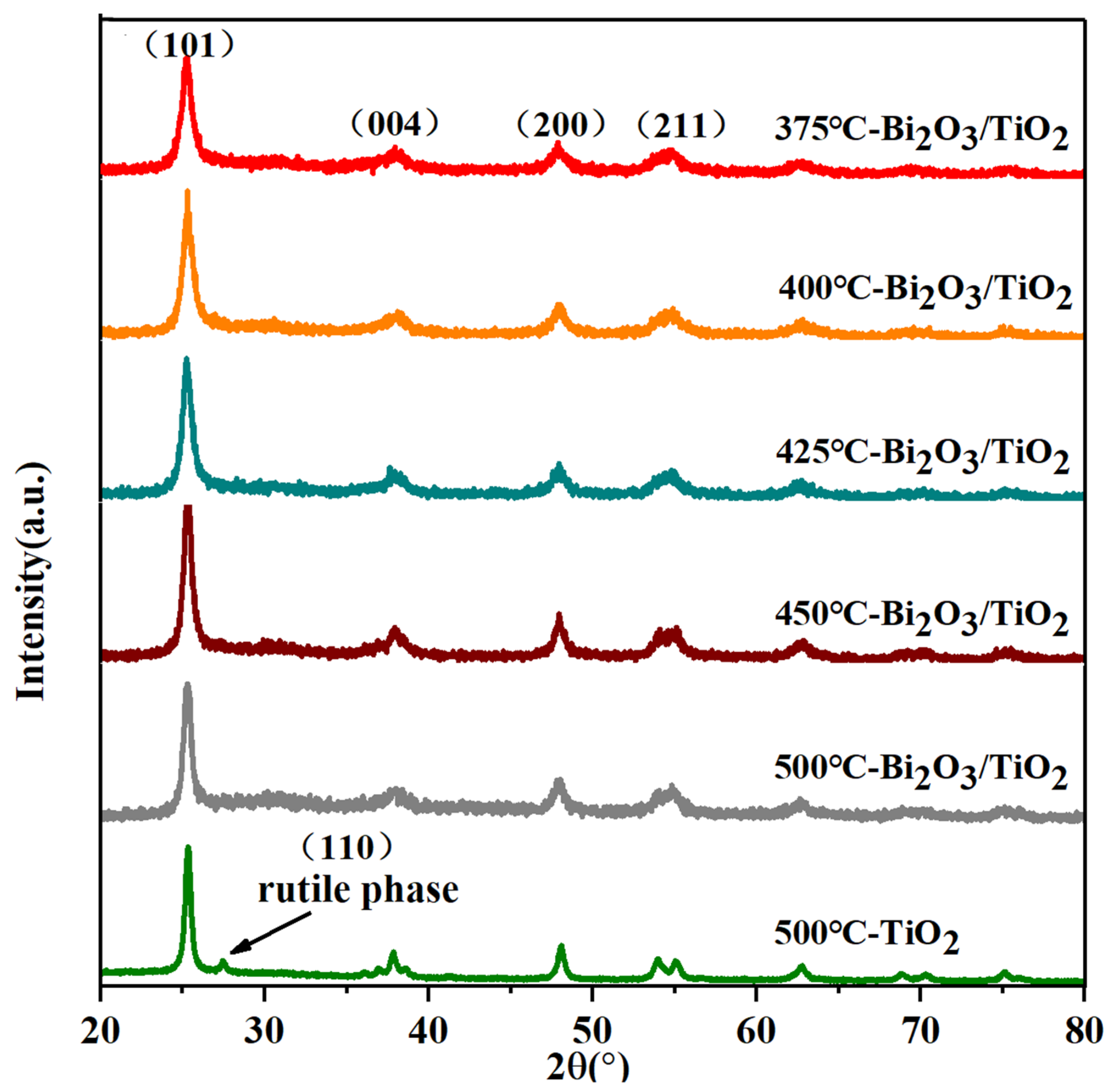
3.1. Crystal Structure Analysis
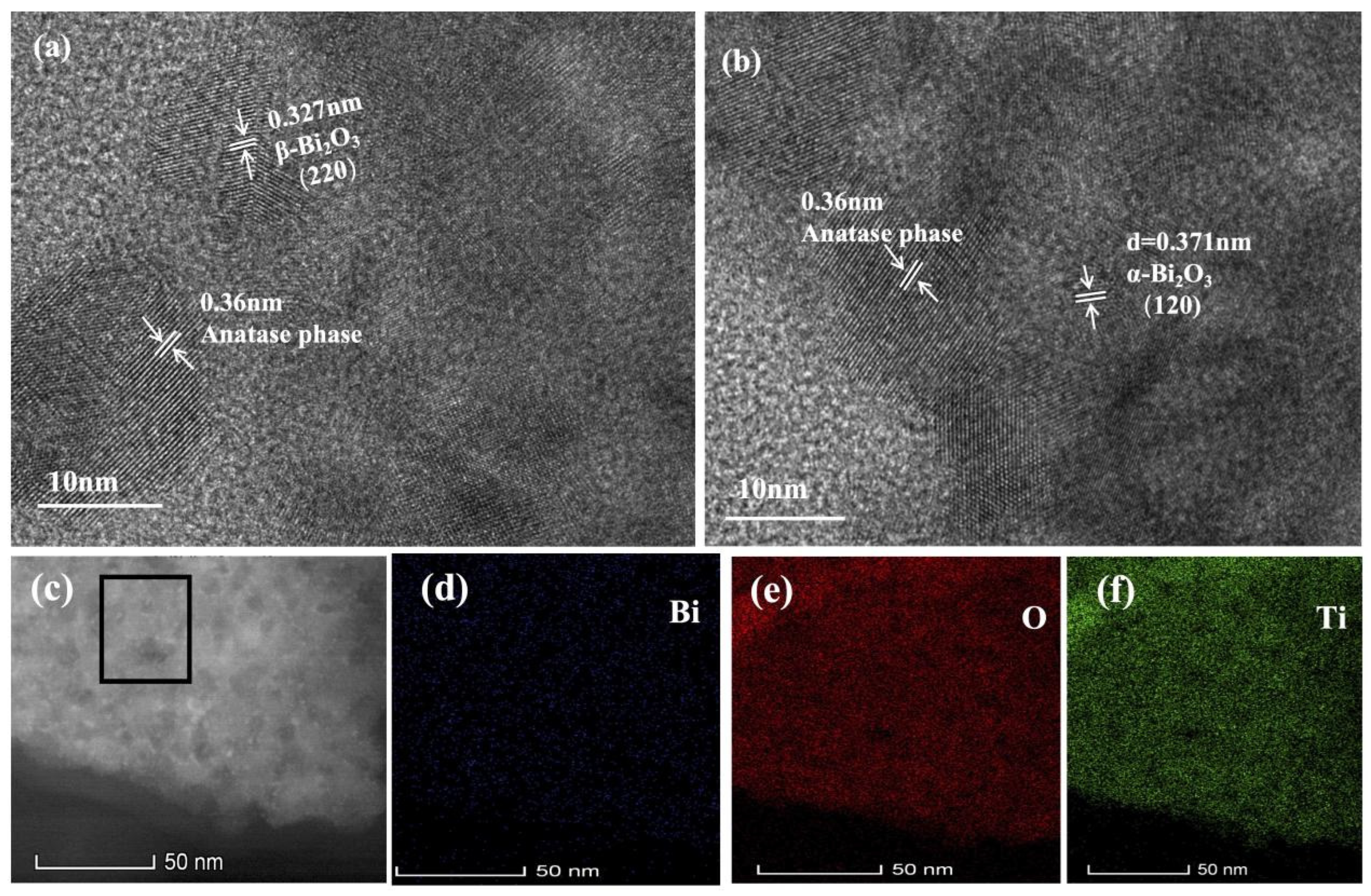
3.2. Micromorphology and Analysis
3.3. Analysis of Light Absorption Region and Band Gap
3.4. Analysis of Surface Elements
3.5. Analysis of the Specific Surface Area
3.6. Photoluminescence Spectroscopy Analysis
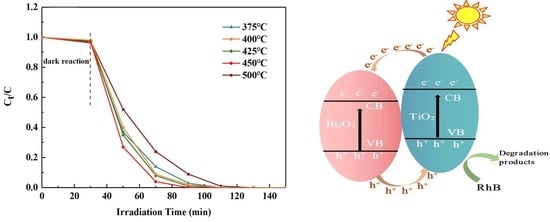
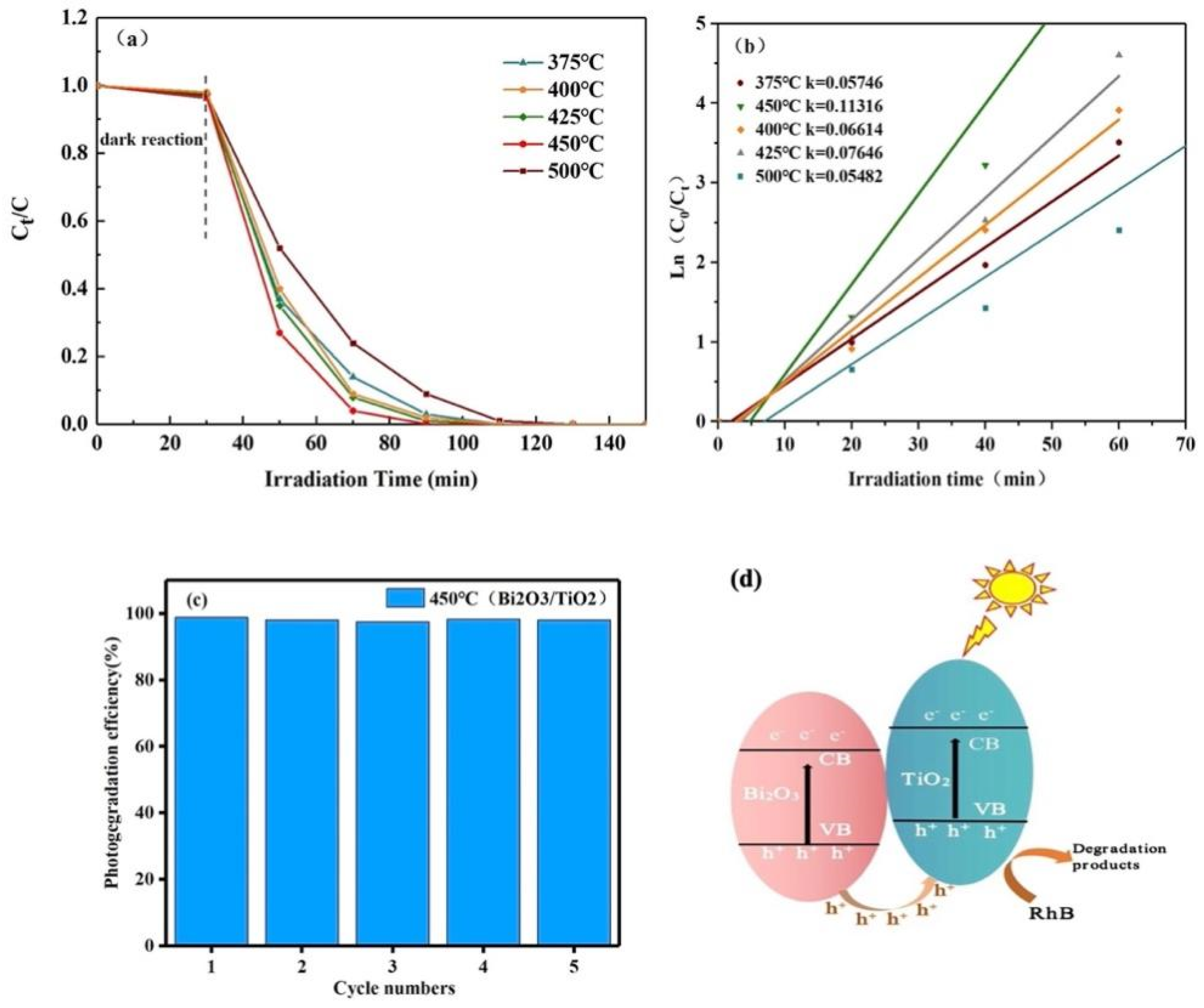
3.7. Photocatalytic Performance Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tang, Q.-Y.; Yang, M.-J.; Yang, S.-Y.; Xu, Y.-H. Enhanced photocatalytic degradation of glyphosate over 2D CoS/BiOBr heterojunctions under visible light irradiation. J. Hazard. Mater. 2020, 407, 124798. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dai, Z.; Feng, M.; Gu, M.; Xie, Y. Graphitic carbon nitride/ferroferric oxide/reduced graphene oxide nanocomposite as highly active visible light photocatalyst. Nano Res. 2022, 1–6. [Google Scholar] [CrossRef]
- Perumal, K.; Shanava, S.; Karthigeyan, A.; Ahamad, T.; Alshehri, S.M.; Murugakoothan, P. Hydrothermal assisted precipitation synthesis of highly stable g-C3N4/BiOBr/CdS photocatalyst with enhanced visible light photocatalytic degradation of tetracycline. Diam. Relat. Mater. 2020, 110, 108091. [Google Scholar] [CrossRef]
- Tong, H.; Ji, Y.; He, T.; He, R.; Chen, M.; Zeng, J.; Wu, D. Preparation and photocatalytic performance of UIO-66/La-MOF composite. Water Sci. Technol. 2022, 86, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Sujinnapram, S.; Wongrerkdee, S. Synergistic effects of structural, crystalline, and chemical defects on the photocatalytic performance of Y-doped ZnO for carbaryl degradation. J. Environ. Sci. 2022, 124, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Su, R.; Wang, Y.; Zhang, Y.; Gao, B.; Wang, Y.; Ma, D.; Li, Q. Visible light-driven chlorite activation process for enhanced sulfamethoxazole antibiotics degradation, antimicrobial resistance reduction and biotoxicity elimination. Chem. Eng. J. 2022. [Google Scholar] [CrossRef]
- Dong, J.; Yang, F.; Wang, Q.; Jiang, C.; Wang, H.; Cao, X.; Li, Z.; Wang, Z. Preparation of porous carbon@TiO2 composites for the adsorption/sonocatalytic degradation of organic dyes. J. Mol. Liq. 2022, 367, 120469. [Google Scholar] [CrossRef]
- Shi, W.; Sun, W.; Liu, Y.; Zhang, K.; Sun, H.; Lin, X.; Hong, Y.; Guo, F. A self-sufficient photo-Fenton system with coupling in-situ production H2O2 of ultrathin porous g-C3N4 nanosheets and amorphous FeOOH quantum dots. J. Hazard. Mater. 2022, 436, 129141. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Zhu, C.; Zhu, F.; Liu, P.; Wang, D.; Fang, G.; Chen, N.; Gao, S.; Zhou, D. Efficient chlorinated alkanes degradation in soil by combining alkali hydrolysis with thermally activated persulfate. J. Hazard. Mater. 2022, 438, 129571. [Google Scholar] [CrossRef]
- Fang, L.; Chu, L.; Wang, J.; Yang, Q. Treatment of polyacrylamide-containing wastewater by ionizing radiation: Efficient reduction of viscosity and degradation of polyacrylamide. Radiat. Phys. Chem. 2023, 202, 110547. [Google Scholar] [CrossRef]
- Wei, B.; Qi, H.; Zou, J.; Li, H.; Wang, J.; Xu, B.; Ma, H. Degradation mechanism of amylopectin under ultrasonic irradiation. Food Hydrocoll. 2020, 111, 106371. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Li, L.; Liu, J.; Ding, Y.; Zhao, T.; Zhang, Y. Atomic-scale simulations of the deoxynivalenol degradation induced by reactive oxygen plasma species. Food Res. Int. 2022, 162, 111939. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Zhao, Y.; Wang, H.; Chen, X.; Liu, K.; Zhang, K.; Wei, Q.; Wang, B. Study on preparation, photocatalytic performance and degradation mechanism of polymeric carbon nitride/Pt/nano-spherical MoS2 composite. J. Phys. Chem. Solids 2022, 166, 110700. [Google Scholar] [CrossRef]
- Kumar, G.; Dutta, R.K. Sunlight-induced enhanced photocatalytic reduction of chromium (VI) and photocatalytic degradation of methylene blue dye and ciprofloxacin antibiotic by Sn3O4/SnS2 nanocomposite. Environ. Sci. Pollut. Res. 2022, 29, 57758–57772. [Google Scholar] [CrossRef]
- Xiang, W.; Yuan, J.; Wu, Y.; Luo, H.; Xiao, C.; Zhong, N.; Zhao, M.; Zhong, D.; He, Y. Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants. Chin. Chem. Lett. 2022, 33, 3632–3640. [Google Scholar] [CrossRef]
- Gu, S.; Liu, X.; Wang, H.; Liu, Z.; Xing, H.; Yu, L. Preparation and characterization of TiO2 photocatalytic composites supported by blast furnace slag fibres for wastewater degradation. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Hu, L.; Chen, J.; Wei, Y.; Wang, M.; Xu, Y.; Wang, C.; Gao, P.; Liu, Y.; Liu, C.; Song, Y.; et al. Photocatalytic degradation effect and mechanism of Karenia mikimotoi by non-noble metal modified TiO2 loading onto copper metal organic framework (SNP-TiO2@Cu-MOF) under visible light. J. Hazard. Mater. 2023, 442, 130059. [Google Scholar] [CrossRef]
- Khairy, M.; Kamar, E.M.; Mousa, M.A. Photocatalytic activity of nano-sized Ag and Au metal-doped TiO2 embedded in rGO under visible light irradiation. Mater. Sci. Eng. B 2022, 286, 116023. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Ji, J.; Li, K.; Cao, J.; Feng, Q.; Huang, H. Effective regulation of surface bridging hydroxyls on TiO2 for superior photocatalytic activity via ozone treatment. Appl. Catal. B Environ. 2022, 304, 120952. [Google Scholar] [CrossRef]
- Gordanshekan, A.; Arabian, S.; Nazar, A.R.S.; Farhadian, M.; Tangestaninejad, S. A comprehensive comparison of green Bi2WO6/g-C3N4 and Bi2WO6/TiO2 S-scheme heterojunctions for photocatalytic adsorption/degradation of Cefixime: Artificial neural network, degradation pathway, and toxicity estimation. Chem. Eng. J. 2023, 451, 139067. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Luque, R. Design of TiO2/Ag3BiO3 n-n heterojunction for enhanced degradation of tetracycline hydrochloride under visible-light irradiation. Environ. Res. 2022, 215, 114315. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Chafiq, L.; Dalhatou, S.; Bonnet, P.; Nasr, M.; Gaillard, N.; Dikdim, J.M.D.; Monier, G.; Assadi, A.A.; Zeghioud, H. g-C3N4/TiO2 S-scheme heterojunction photocatalyst with enhanced photocatalytic Carbamazepine degradation and mineralization. J. Photochem. Photobiol. A Chem. 2022, 430, 113971. [Google Scholar] [CrossRef]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Zeng, Y.; Zhou, Y.; Zhao, J.; Li, S.; Liu, X.; Yu, Q.; et al. Light-driven photothermal catalysis for degradation of toluene on CuO/TiO2 Composite: Dominating photocatalysis and auxiliary thermalcatalysis. Appl. Surf. Sci. 2022, 601, 154144. [Google Scholar] [CrossRef]
- Hou, J.; Yang, H.; He, B.; Ma, J.; Lu, Y.; Wang, Q. High photocatalytic performance of hydrogen evolution and dye degradation enabled by CeO2 modified TiO2 nanotube arrays. Fuel 2022, 310, 122364. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Yang, L.; Fu, F.; Xu, H.; Fan, X. Photocatalytic performance of TiO2/Bi2WO6 photocatalysts with trace Fe3+ dopant for gaseous toluene decomposition. J. Environ. Chem. Eng. 2022, 10, 107708. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of -Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140–141, 433–443. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Lu, J.; Wang, Z.; Xu, B.; Qin, X.; Zhang, X.; Dai, Y. Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468–15475. [Google Scholar] [CrossRef]
- Gao, J.; Rao, S.; Yu, X.; Wang, L.; Xu, J.; Yang, J.; Liu, Q. Dimensional-matched two dimensional/two dimensional TiO2/Bi2O3 step-scheme heterojunction for boosted photocatalytic performance of sterilization and water splitting. J. Colloid Interface Sci. 2022, 628, 166–178. [Google Scholar] [CrossRef]
- Ke, T.; Shen, S.; Yang, K.; Lin, D. In situ fabrication of Bi2O3/C3N4/TiO2@C photocatalysts for visible-light photodegradation of sulfamethoxazole in water. Appl. Surf. Sci. 2022, 580, 152302. [Google Scholar] [CrossRef]
- Chen, J.; Tang, T.; Feng, W.; Liu, X.; Yin, Z.; Zhang, X.; Chen, J.; Cao, S. Large-Scale Synthesis of p−n Heterojunction Bi2O3/TiO2 Nanostructures as Photocatalysts for Removal of Antibiotics under Visible Light. ACS Appl. Nano Mater. 2022, 5, 1296–1307. [Google Scholar] [CrossRef]
- Ren, C.; Qiu, W.; Zhang, H.; He, Z.; Chen, Y. Degradation of benzene on TiO2/SiO2/Bi2O3 photocatalysts under UV and visible light. J. Mol. Catal. A Chem. 2015, 398, 215–222. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Opt. Int. J. Light Electron Opt. 2021, 241, 166934. [Google Scholar] [CrossRef]
- Li, L.; Tao, R.; Liu, Y.; Zhou, K.; Fan, X.; Han, Y.; Tang, L. Co3O4 nanoparticles/Bi2O3 nanosheets: One step synthesis, high-efficiency thermal catalytic performance, and catalytic mechanism research. Mol. Catal. 2022, 528, 112483. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, R.; Gao, M.; Kang, Q.; Ma, J. Morphology control of 3D hierarchical urchin-like hollow SiO2@TiO2 spheres for photocatalytic degradation: Influence of calcination temperature. J. Alloy. Compd. 2021, 853, 157202. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Tao, T.; Zhao, Y.; Wang, P.; Ding, Z.; Chen, M. Controlled synthesis of Bi2O3/TiO2 catalysts with mixed alcohols for the photocatalytic oxidation of HCHO. Environ. Technol. 2019, 40, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Sajjad, A.K.L.; Chen, F.; Zhang, J. Study on highly visible light active Bi2O3 loaded ordered mesoporous titania. Appl. Catal. B Environ. 2010, 94, 272–280. [Google Scholar] [CrossRef]
- Shen, J.; Wang, H.; Song, Y.; Zhou, Y.; Ye, N.; Fang, L.; Wang, L. Amorphous carbon coated TiO2 nanocrystals embedded in a carbonaceous matrix derived from polyvinylpyrrolidone decomposition for improved Li-storage performance. Chem. Eng. J. 2014, 240, 379–386. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bognar, B.; Horvath, M.; Schneider, G.; Kovacs, J.; Scarpellini, A.; Castelli, A.; Colombo, M.; Prato, M. Hydrothermal evolution of PF-co-doped TiO2 nanoparticles and their antibacterial activity against carbapenem-resistant Klebsiella pneumoniae. Appl. Catal. B Environ. 2018, 231, 115–122. [Google Scholar] [CrossRef]
- Korösi, L.; Pertics, B.; Schneider, G.; Bognár, B.; Kovács, J.; Meynen, V.; Scarpellini, A.; Pasquale, L.; Prato, M. Photocatalytic inactivation of plant pathogenic bacteria using TiO2 nanoparticles prepared hydrothermally. Nanomaterials 2020, 10, 1730. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O'Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Xiao, X.; Tu, S.; Zuo, X.; Nan, J. Synthesis of flower-like heterostructured -Bi2O3/Bi2O2CO3microspheres using Bi2O2CO3self-sacrifice precursor and itsvisible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B Environ. 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Xu, D.; Hai, Y.; Zhang, X.; Zhang, S.; He, R. Bi2O3 cocatalyst improving photocatalytic hydrogen evolution performance of TiO2. Appl. Surf. Sci. 2017, 400, 530–536. [Google Scholar] [CrossRef]
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-Khathami, N.D.; Mohamed, R.M. Co3O4 Nanoparticles Accommodated Mesoporous TiO2 framework as an Excellent Photocatalyst with Enhanced Photocatalytic Properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, R.; Li, H.; Xu, Z.; Dai, H.; Gao, H.; Yu, H.; Wang, Z.; Wang, Y.; Liu, Y.; et al. Boosting visible light photocatalysis in an Au@TiO2 yolk-in-shell nanohybrid. Appl. Catal. B Environ. 2022, 303, 120869. [Google Scholar] [CrossRef]
- Duan, X.; Yang, J.; Hu, G.; Yang, C.; Chen, Y.; Liu, Q.; Ren, S.; Li, J. Optimization of TiO2/ ZSM-5 photocatalysts: Energy band engineering by solid state diffusion method with calcination. J. Environ. Chem. Eng. 2021, 9, 105563. [Google Scholar] [CrossRef]
- Majhi, D.; Das, K.; Mishra, A.; Dhiman, R.; Mishra, B.G. One pot synthesis of CdS/BiOBr/Bi2O2CO3: A novel ternary double Zscheme heterostructure photocatalyst for efficient degradation of atrazine. Appl. Catal. B Environ. 2020, 260, 118222. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bognár, B.; Bouderias, S.; Castelli, A.; Scarpellini, A.; Pasquale, L.; Prato, M. Highly-efficient photocatalytic generation of superoxide radicals by phase-pure rutile TiO2 nanoparticles for azo dye removal. Appl. Surf. Sci. 2019, 493, 719–728. [Google Scholar] [CrossRef]
- Bina, B.; Fatehizadeh, A.; Taheri, E.; Heydari, M.; Darvishmotevalli, M.; Bazmeh, A. Atenolol removal from aqueous solutions using Bi2O3/TiO2 under UV-C and visible light irradiations. Int. J. Environ. Anal. Chem. 2022, 1–22. [Google Scholar] [CrossRef]



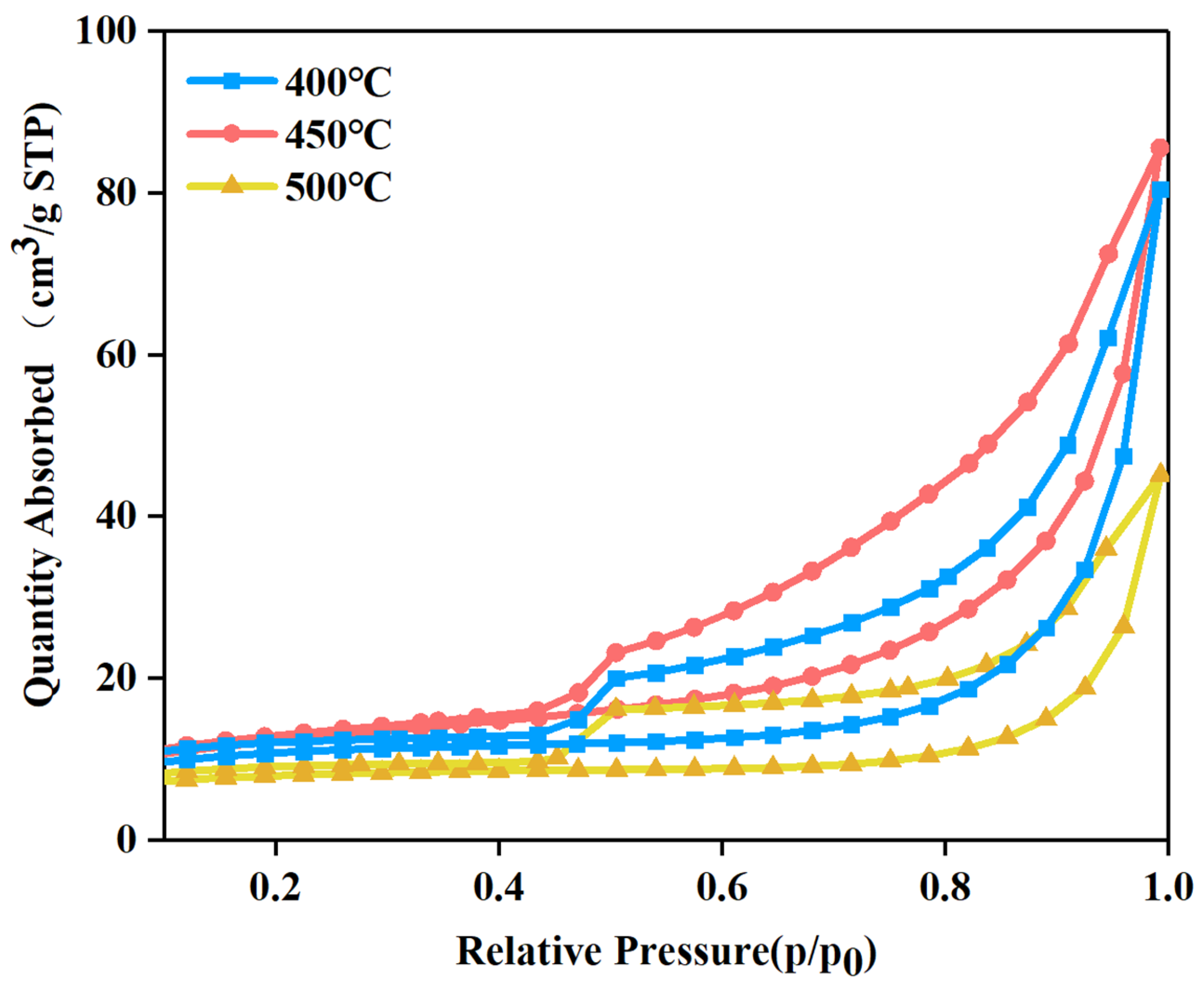

| Samples | Specific Surface Area (m²/g) | Mean Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| TiO2 | 9.1 | 10.5 | 0.1 |
| Bi2O3 | 3.2 | 6.2 | 0.01 |
| Bi2O3/TiO2 400 °C | 35.2 | 11.9 | 0.15 |
| Bi2O3/TiO2 450°C | 40.1 | 12.4 | 0.21 |
| Bi2O3/TiO2 500 °C | 25.7 | 11.1 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Influence of Calcination Temperature on Photocatalyst Performances of Floral Bi2O3/TiO2 Composite. Catalysts 2022, 12, 1635. https://doi.org/10.3390/catal12121635
Wang M, Li C, Liu B, Qin W, Xie Y. Influence of Calcination Temperature on Photocatalyst Performances of Floral Bi2O3/TiO2 Composite. Catalysts. 2022; 12(12):1635. https://doi.org/10.3390/catal12121635
Chicago/Turabian StyleWang, Mingjun, Che Li, Bingfang Liu, Wenzhen Qin, and Yu Xie. 2022. "Influence of Calcination Temperature on Photocatalyst Performances of Floral Bi2O3/TiO2 Composite" Catalysts 12, no. 12: 1635. https://doi.org/10.3390/catal12121635