Photocatalytically Active Semiconductor Cu3P Unites with Flocculent TiN for Efficient Removal of Sulfamethoxazole
Abstract
1. Introduction
2. Results
2.1. Characterization Analysis
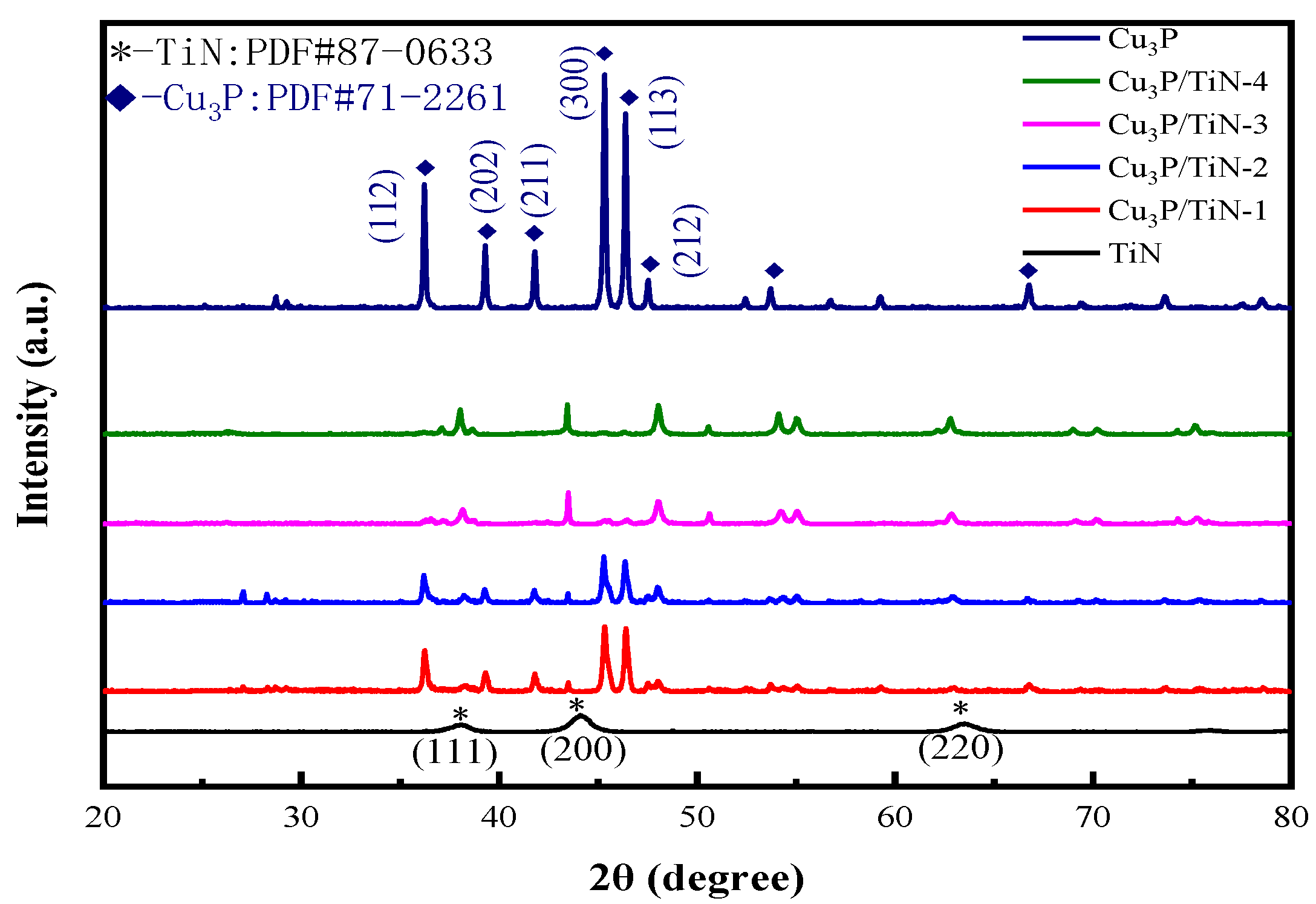
2.1.1. XRD Patterns
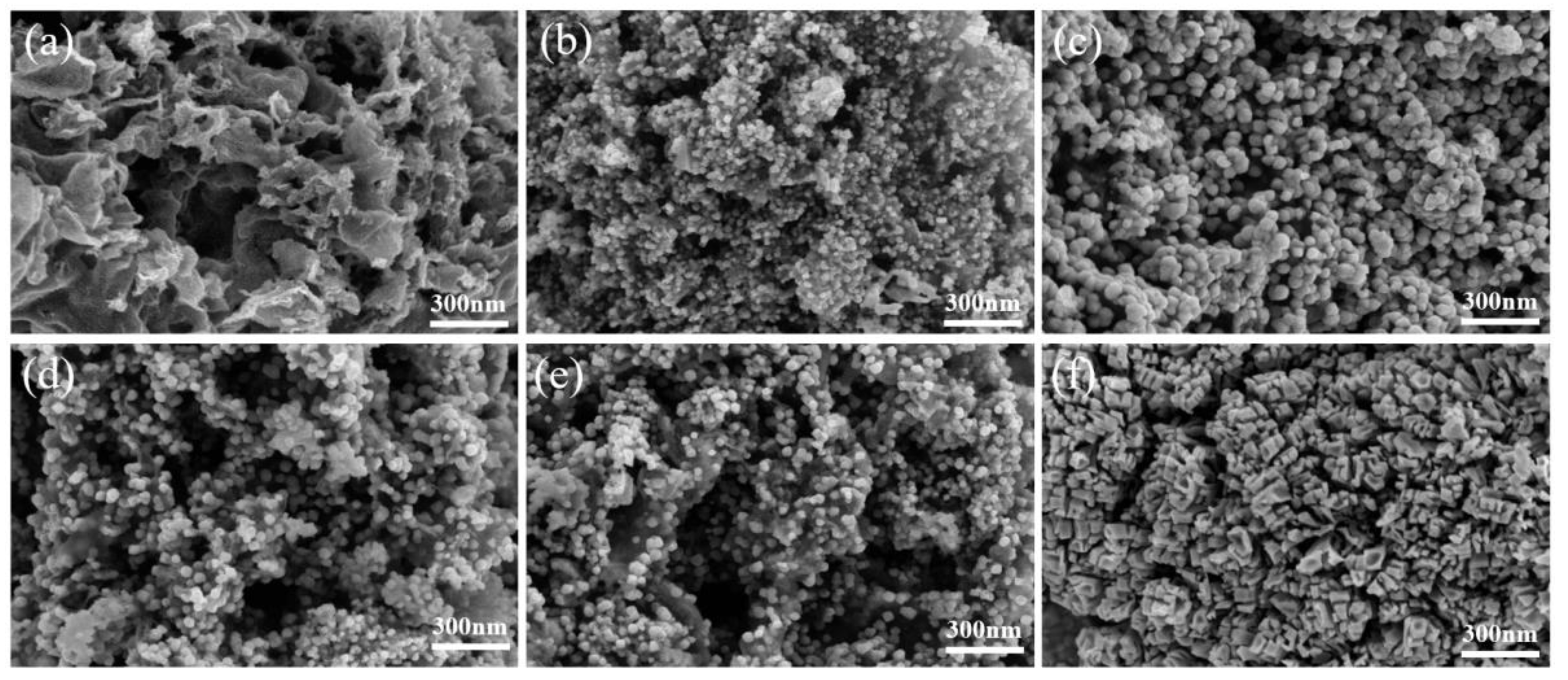
2.1.2. SEM Images
2.1.3. N2 Adsorption—Desorption Isotherms
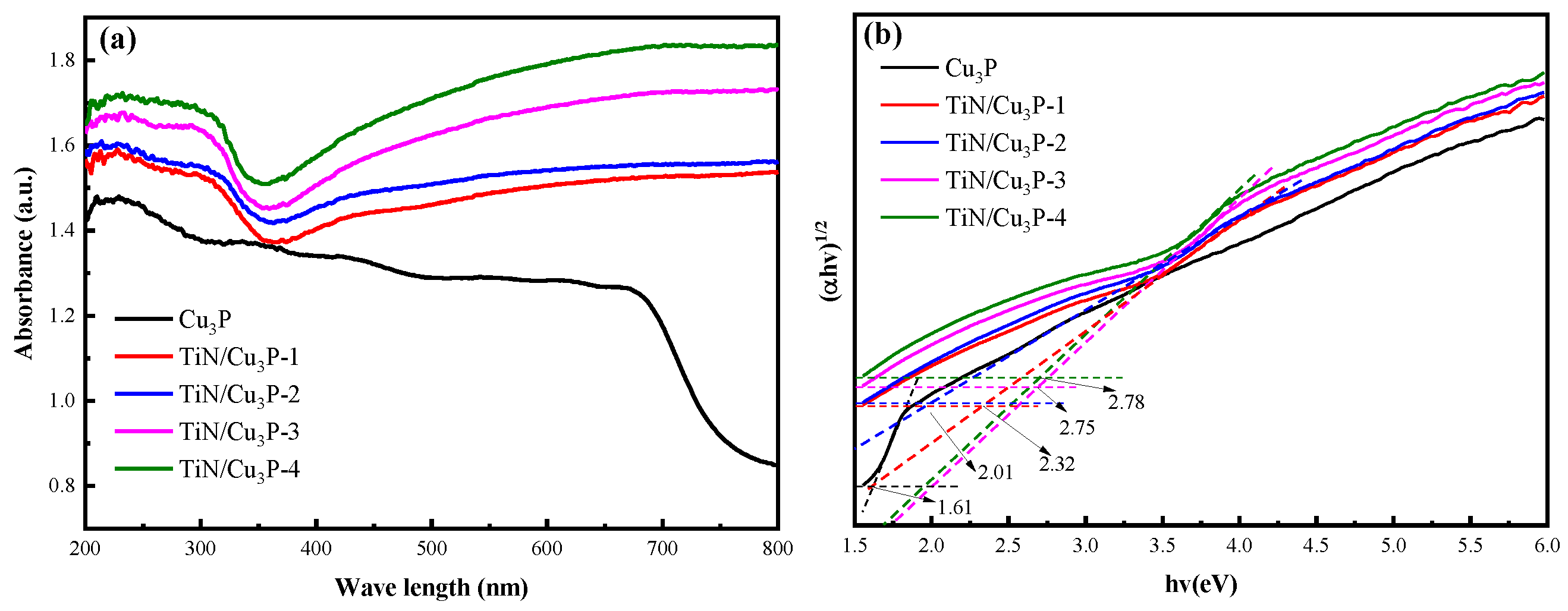
2.2. Optical Property
2.2.1. Map of DRS
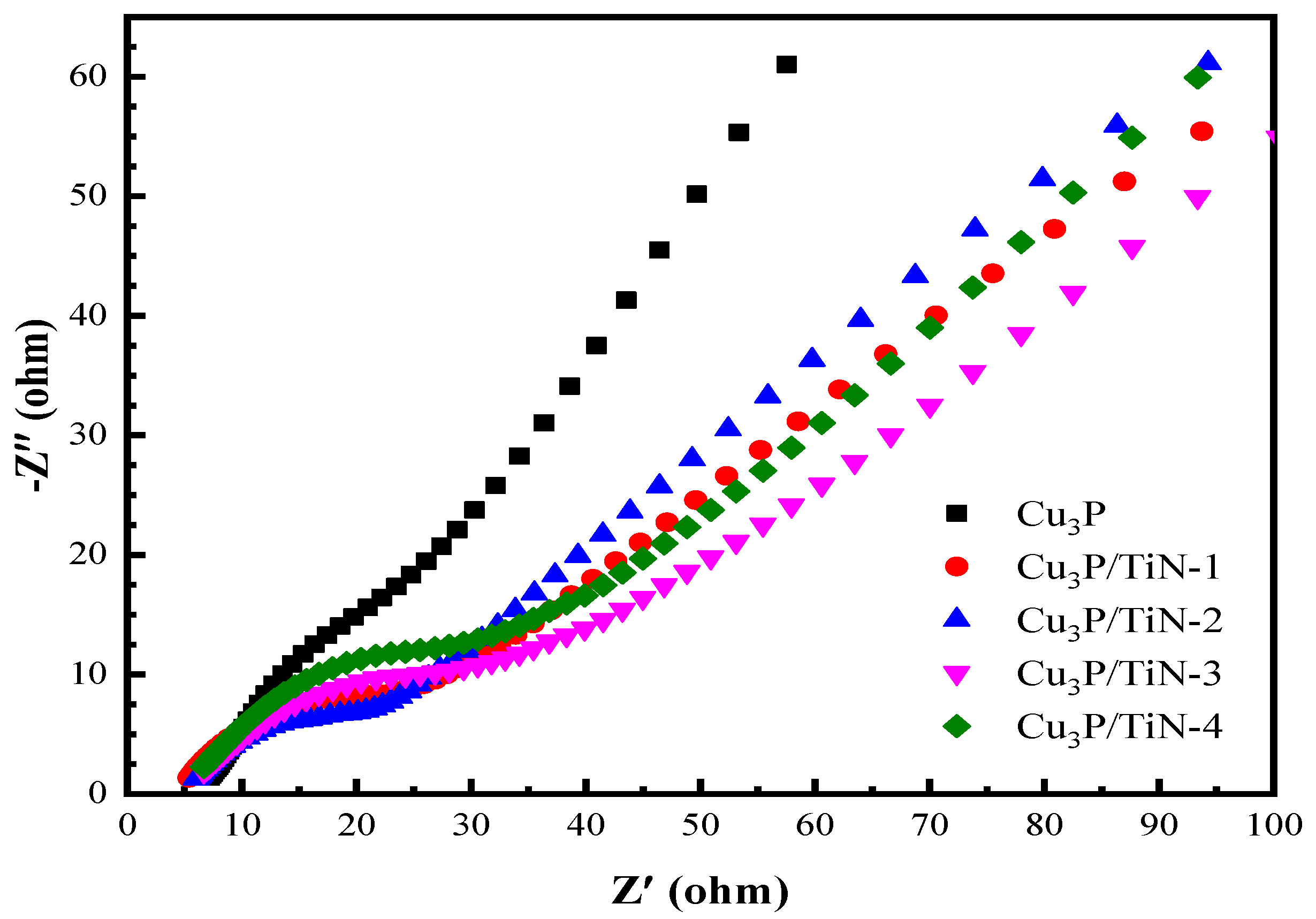
2.2.2. Electrochemical Impedance
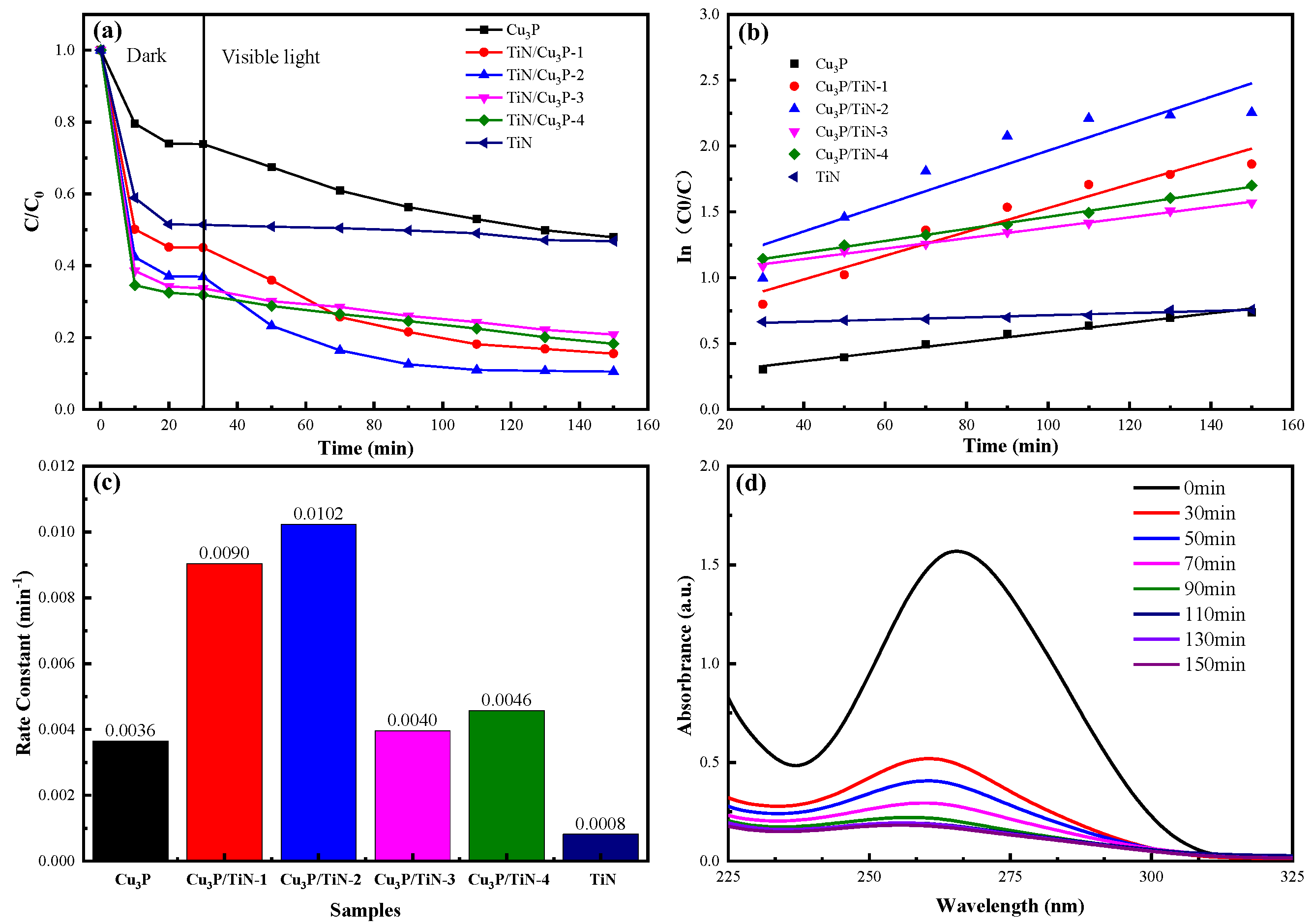
2.3. Performance of Photocatalysts
2.3.1. Activity of the Photocatalyst
2.3.2. Stability and Reusability
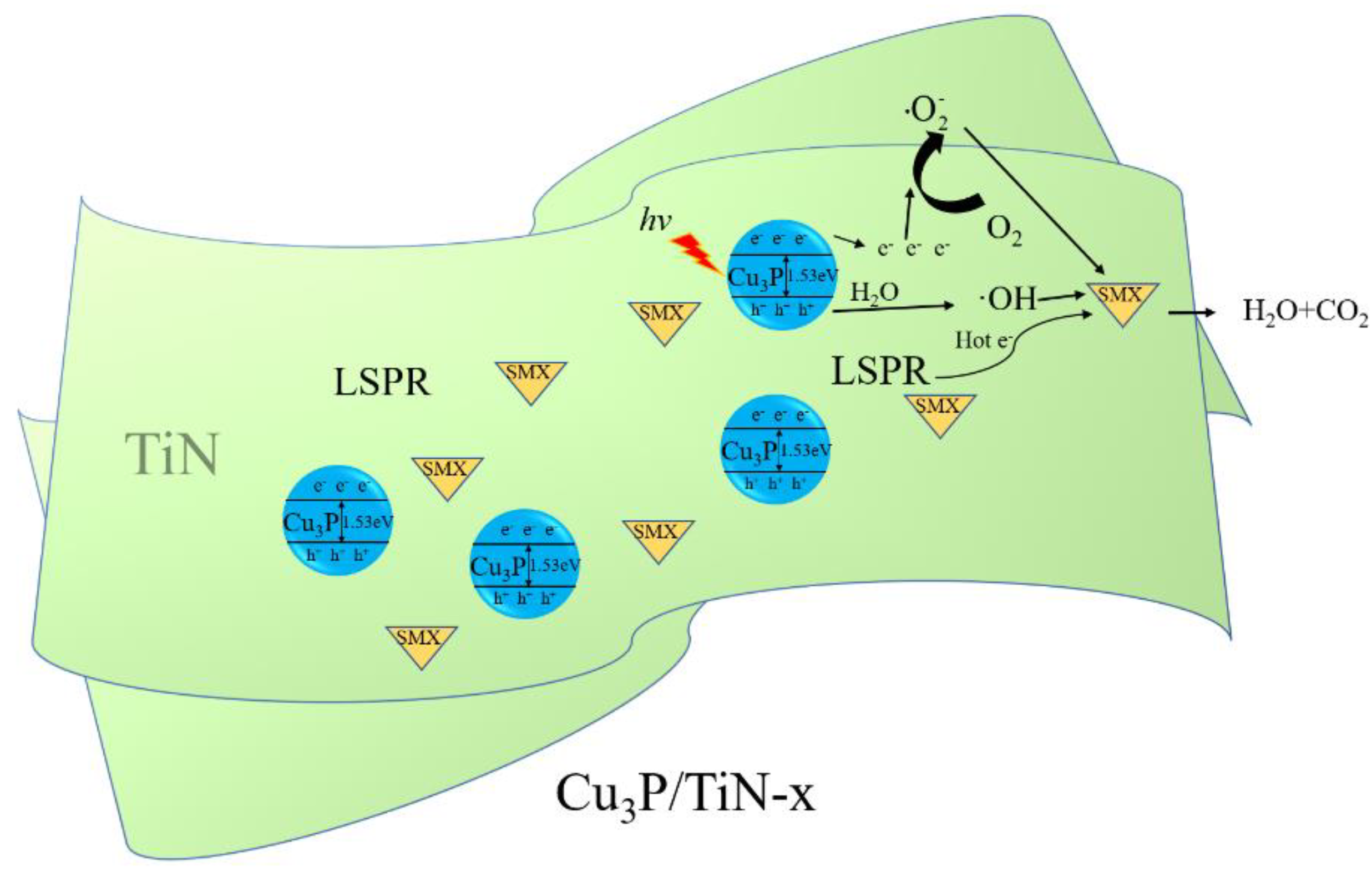
2.3.3. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Photocatalysts
3.3. Characterization Methods
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lai, C.; Xu, F.; Zhang, M.; Li, B.; Liu, S.; Yi, H.; Li, L.; Qin, L.; Liu, X.; Fu, Y.; et al. Facile synthesis of CeO2/carbonate doped Bi2O2CO3 Z—scheme heterojunction for improved visible—light photocatalytic performance: Photodegradation of tetracycline and photocatalytic mechanism. J. Colloid Interface Sci. 2021, 588, 283–294. [Google Scholar] [CrossRef]
- Wei, W.; Gong, H.; Sheng, L.; Wu, H.; Zhu, S.; Feng, L.; Li, X.; You, W. Highly efficient photocatalytic activity and mechanism of novel Er3+ and Tb3+ co—doped BiOBr/g—C3N5 towards sulfamethoxazole degradation. Ceram. Int. 2021, 47, 24062–24072. [Google Scholar] [CrossRef]
- Shi, H.; Cheng, X.; Peng, J.; Feng, H.; Yang, X.; Quan, L.; Jiang, L.; Tontiwachwuthikul, P. Structure–Activity Correlation Analyses of MEA + 3A1P/MAE Isomers with a Coordinative Effect Study. Ind. Eng. Chem. Res. 2022, 61, 3091–3103. [Google Scholar] [CrossRef]
- Govindaraj, T.; Mahendran, C.; Manikandan, V.S.; Archana, J.; Shkir, M.; Chandrasekaran, J. Fabrication of WO3 nanorods/RGO hybrid nanostructures for enhanced visible—light—driven photocatalytic degradation of Ciprofloxacin and Rhodamine B in an ecosystem. J. Alloys Compd. 2021, 868, 159091. [Google Scholar] [CrossRef]
- Shi, H.; Fu, J.; Wu, Q.; Huang, M.; Jiang, L.; Cui, M.; Idem, R.; Tontiwachwuthikul, P. Studies of the coordination effect of DEA—MEA blended amines (within 1 + 4 to 2 + 3 M) under heterogeneous catalysis by means of absorption and desorption parameters. Sep. Purif. Technol. 2020, 236, 116179. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth—based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res 2021, 199, 111360. [Google Scholar] [CrossRef]
- Shi, H.; Peng, J.; Cheng, X.; Yang, X.; Jin, J.; Hu, J. The CO2 desorption analysis of tri—solvent MEA+BEA+DEEA with several commercial solid acid catalysts. Int. J. Greenh. Gas Control 2022, 116, 103647. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Yan, Y.; Wang, W.; Ren, Y.; Li, X. Synthesis of highly porous g—C3N4 nanotubes for efficient photocatalytic degradation of sulfamethoxazole. Mater. Today Commun. 2021, 27, 102288. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Z.; He, W.; Lu, W.; Shi, H. Influence of thiourea modification on the NH3—SCR activity of CeO2: Simultaneous tuning morphology and surface acidity. J. Energy Inst. 2021, 98, 322–333. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Niu, J.; Chen, M.; Zhou, Y. Direct Z—scheme Ag3PO4/Bi4Ti3O12 heterojunction with enhanced photocatalytic performance for sulfamethoxazole degradation. Sep. Purif. Technol. 2020, 241, 116622. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Z.; Jin, J.; Lu, W.; Shi, H. Influence of CS2 pretreatment on the NH3—SCR activity of CeO2: Synergistic promotional effect of sulfation and reduction. J. Environ. Chem. Eng. 2021, 9, 106836. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, T.; Zuo, Y.; Wu, Q.; Zhang, Y.; Fan, Y.; Tontiwachwuthikul, P. Synthesis of Cu3P/SnO2 composites for degradation of tetracycline hydrochloride in wastewater. RSC Adv. 2021, 11, 33471–33480. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, W.; Li, J.; Huang, L.; Lu, W. The synergistic promotional effect of W doping and sulfate modification on the NH3—SCR activity of CeO2 catalyst. Mol. Catal. 2022, 522, 112250. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Smart pathways for the photocatalytic degradation of sulfamethoxazole drug using F—Pd co—doped TiO2 nanocomposites. Appl. Catal. B Environ. 2020, 267, 118716. [Google Scholar] [CrossRef]
- Xiong, Z.-B.; Li, Z.-Z.; Li, C.-X.; Wang, W.; Lu, W.; Du, Y.-P.; Tian, S.-L. Green synthesis of Tungsten—doped CeO2 catalyst for selective catalytic reduction of NO with NH3 using starch bio—template. Appl. Surf. Sci. 2021, 536, 147719. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Z.; Yang, Q.; Zhou, F.; Lu, W.; Shi, H. Influence of copper doping on the low—medium NH3—SCR activity of magnetic W/Fe2O3 catalyst: Its synergetic effect of glucose dosage. J. Environ. Chem. Eng. 2022, 10, 108694. [Google Scholar] [CrossRef]
- Shi, H. Synthesis of SnO2 Hollow Microspheres with Efficient Photocatalytic Activity for Tetracycline Hydrochloride. Int. J. Electrochem. Sci. 2020, 15, 1539–1547. [Google Scholar] [CrossRef]
- Zuo, Y.; Huang, M.; Sheng, W.; Xu, Q.; Tao, W.; Li, Z. Highly stable and methanol tolerant oxygen reduction reaction electrocatalyst Co/CoO/SnO@N—C nanocubes by one—step introduction of functional components. Int. J. Hydrogen Energy 2022, 47, 917–927. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, B.; Huang, C.; Ma, C.; Song, X.; Xu, Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions. J. Mater. Chem. 2012, 22, 4050–4055. [Google Scholar] [CrossRef]
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple—homojunction gradient nitrogen doped TiO2 for photocatalytic degradation of sulfamethoxazole, degradation mechanism, and toxicity assessment. Chem. Eng. J. 2021, 422, 130507. [Google Scholar] [CrossRef]
- Hua, S.; Qu, D.; An, L.; Jiang, W.; Wen, Y.; Wang, X.; Sun, Z. Highly efficient p—type Cu3P/n—type g—C3N4 photocatalyst through Z—scheme charge transfer route. Appl. Catal. B Environ. 2019, 240, 253–261. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, L.; Liu, X.; Sun, X.; Wang, J.; Du, H. Special Z—scheme Cu3P/TiO2 hetero—junction for efficient photocatalytic hydrogen evolution from water. J. Alloys Compd. 2022, 894, 162331. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, M.; Li, R.; Chen, Y. Novel Cu3P/g—C3N4 p—n heterojunction photocatalysts for solar hydrogen generation. Sci. China Mater. 2018, 61, 861–868. [Google Scholar] [CrossRef]
- Cheng, Z.; Qi, W.; Pang, C.H.; Thomas, T.; Wu, T.; Liu, S.; Yang, M. Recent Advances in Transition Metal Nitride-Based Materials for Photocatalytic Applications. Adv. Funct. Mater. 2021, 31, 2100553. [Google Scholar] [CrossRef]
- Liu, S.; Qi, W.; Adimi, S.; Guo, H.; Weng, B.; Attfield, J.P.; Yang, M. Titanium Nitride—Supported Platinum with Metal—Support Interaction for Boosting Photocatalytic H2 Evolution of Indium Sulfide. ACS Appl. Mater. Interfaces 2021, 13, 7238–7247. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Qi, W.; Kuang, W.; Adimi, S.; Guo, H.; Thomas, T.; Liu, S.; Wang, Z.; Yang, M. Chromium—titanium nitride as an efficient co—catalyst for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 15774–15781. [Google Scholar] [CrossRef]
- Qin, L.; Huang, D.; Xu, P.; Zeng, G.; Lai, C.; Fu, Y.; Yi, H.; Li, B.; Zhang, C.; Cheng, M.; et al. In—situ deposition of gold nanoparticles onto polydopamine—decorated g—C3N4 for highly efficient reduction of nitroaromatics in environmental water purification. J. Colloid Interface Sci. 2019, 534, 357–369. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, T.; Lin, M.; Liang, Y.; Fu, J.; Wang, W. Nonmetal plasmonic TiN nanoparticles significantly boost photoelectrochemical performance for hydrogen evolution of CdS nanoroad array photoanode. Renew. Energy 2021, 180, 1290–1299. [Google Scholar] [CrossRef]
- Cao, M.; Liu, X.; Wang, W.; Gao, M.; Yang, H. Bifunctional two—dimensional copper—aluminum modified filter paper composite for efficient tetracycline removal: Synergy of adsorption and reusability by degradation. Chemosphere 2022, 287, 132031. [Google Scholar] [CrossRef]
- Saravanan, R.; Mansoob Khan, M.; Gupta, V.K.; Mosquera, E.; Gracia, F.; Narayanan, V.; Stephen, A. ZnO/Ag/CdO nanocomposite for visible light—induced photocatalytic degradation of industrial textile effluents. J. Colloid Interface Sci. 2015, 452, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chankhanittha, T.; Nanan, S. Visible—light—driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-L.; Wei, X.-L.; Zhang, J.-C.; Liu, Y.; Tang, X.; Li, P.; Liu, Z.-Y. Influence of structural damage on evaluation of microscopic pore structure in marine continental transitional shale of the Southern North China Basin: A method based on the low—temperature N2 adsorption experiment. Pet. Sci. 2022, 19, 100–115. [Google Scholar] [CrossRef]
- Shi, L.; Yin, J.; Liu, Y.; Liu, H.; Zhang, H.; Tang, H. Embedding Cu3P quantum dots onto BiOCl nanosheets as a 0D/2D S—scheme heterojunction for photocatalytic antibiotic degradation. Chemosphere 2022, 309, 136607. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, S.; Zhao, W.; Liu, W. Study on the photocatalytic activity of TiN/TiO2 nanoparticle formed by ball milling. J. Nanopart. Res. 2008, 11, 931–938. [Google Scholar] [CrossRef]
- Zarei, S.; Hasheminiasari, M.; Masoudpanah, S.M.; Javadpour, J. Photocatalytic properties of ZnO/SnO2 nanocomposite films: Role of morphology. J. Mater. Res. Technol. 2022, 17, 2305–2312. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible—light photocatalytic activity of g—C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Jing, K.; Ma, W.; Ren, Y.; Xiong, J.; Guo, B.; Song, Y.; Liang, S.; Wu, L. Hierarchical Bi2MoO6 spheres in situ assembled by monolayer nanosheets toward photocatalytic selective oxidation of benzyl alcohol. Appl. Catal. B Environ. 2019, 243, 10–18. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 41. [Google Scholar] [CrossRef]
- Vivier, V.; Orazem, M.E. Impedance Analysis of Electrochemical Systems. Chem. Rev. 2022, 122, 11131–11168. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. 2D/2D g—C3N4/MnO2 Nanocomposite as a Direct Z—Scheme Photocatalyst for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 6, 965–973. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and stable Nb2O5 modified g—C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D.; et al. Enhanced Photocatalytic Degradation of Tetracycline by AgI/BiVO4 Heterojunction under Visible—Light Irradiation: Mineralization Efficiency and Mechanism. ACS Appl. Mater. Interfaces 2016, 8, 32887–32900. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P—ZnSnO3—g—C3N4 p—n—n heterojunction with multiple built—in electric fields for effectively boosting visible—light photocatalytic degradation of broad—spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Ioannidi, A.; Petala, A.; Frontistis, Z. Copper phosphide promoted BiVO4 photocatalysts for the degradation of sulfamethoxazole in aqueous media. J. Environ. Chem. Eng. 2020, 8, 104340. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.T.; Zhong, Z.; Wang, R.; Hu, X. Acetic acid—assisted fabrication of hierarchical flower—like Bi2O3 for photocatalytic degradation of sulfamethoxazole and rhodamine B under solar irradiation. J. Colloid Interface Sci. 2017, 505, 489–499. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Zhu, H. Adsorption and photocatalytic degradation of sulfamethoxazole by a novel composite hydrogel with visible light irradiation. Appl. Catal. B Environ. 2017, 217, 603–614. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g—C3N4—based materials and their application in elimination of pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
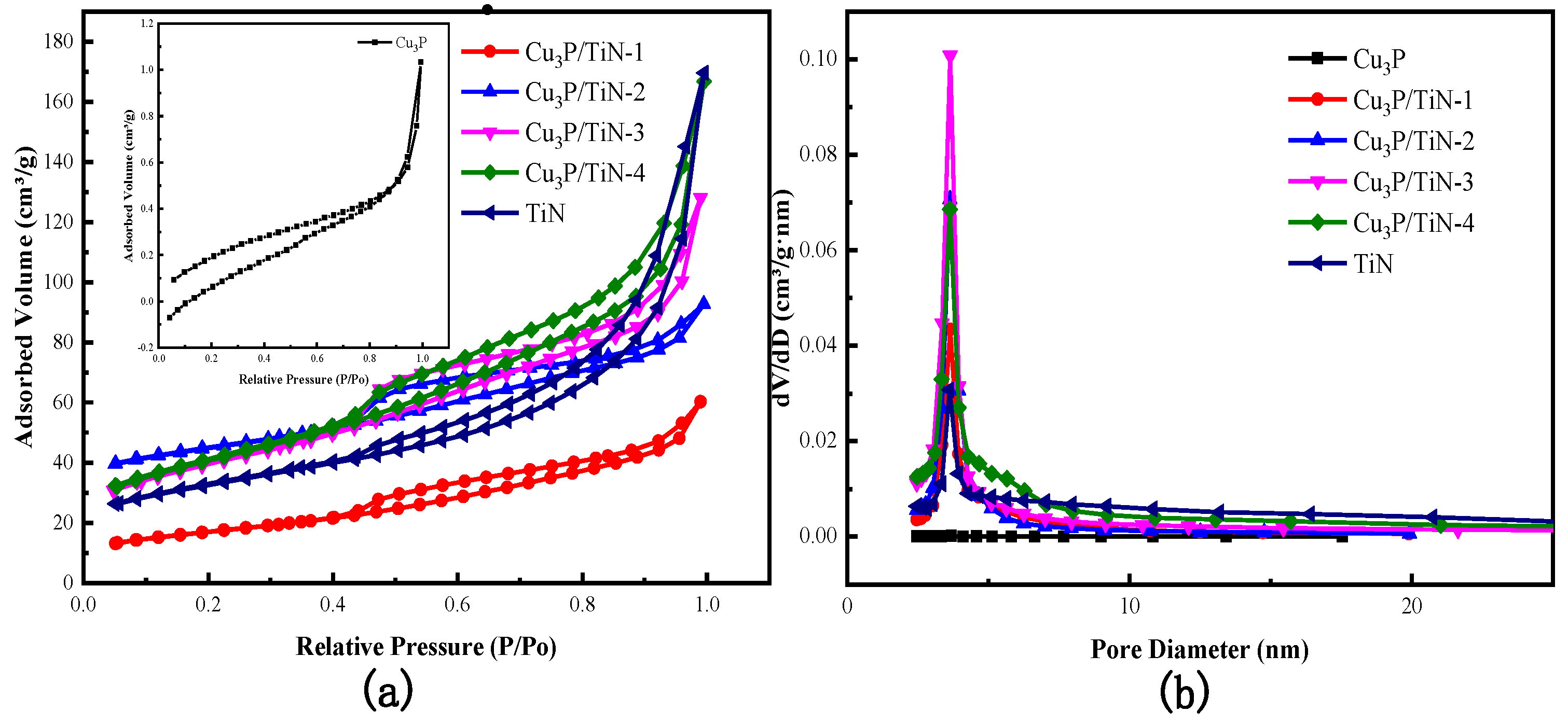

| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Cu3P | 0.8 | 0.001 | 5.4 |
| Cu3P/TiN—1 | 55 | 0.09 | 5.0 |
| Cu3P/TiN—2 | 120 | 0.16 | 4.5 |
| Cu3P/TiN—3 | 136 | 0.20 | 6.6 |
| Cu3P/TiN—4 | 142 | 0.26 | 6.8 |
| TiN | 111 | 0.26 | 10.2 |
| Catalyst | SMX Amount (mg/L) | Pollutant Concentration (g/L) | Rate Constant (min−1) | Dark (min) | Illumination (min) | Removal Rate | Reference |
|---|---|---|---|---|---|---|---|
| g—N—TiO2 | 1 | — | 0.0061 | 30 | 250 | 85% | [21] |
| Ag3PO4/Bi4Ti3O12 | 5 | — | 0.0372 | 10 | 50 | 80% | [10] |
| Cu3P/BiVO4 | 0.5 | 0.75 | 0.0217 | 10 | 120 | 77% | [45] |
| Bi2O3 | 10 | 1 | 0.009 | 30 | 200 | 52% | [46] |
| p(HEA/NMMA)—CuS | 50 | 2 | — | 30 | 210 | 75% | [47] |
| Cu3P/TiN | 50 | 0.5 | 0.0102 | 30 | 120 | 90% | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Yao, X.; Lu, S.; Zuo, Y.; Zheng, T.; Jia, L. Photocatalytically Active Semiconductor Cu3P Unites with Flocculent TiN for Efficient Removal of Sulfamethoxazole. Catalysts 2023, 13, 291. https://doi.org/10.3390/catal13020291
Shi H, Yao X, Lu S, Zuo Y, Zheng T, Jia L. Photocatalytically Active Semiconductor Cu3P Unites with Flocculent TiN for Efficient Removal of Sulfamethoxazole. Catalysts. 2023; 13(2):291. https://doi.org/10.3390/catal13020291
Chicago/Turabian StyleShi, Huancong, Xulei Yao, Shijian Lu, Yuanhui Zuo, Tao Zheng, and Liangquan Jia. 2023. "Photocatalytically Active Semiconductor Cu3P Unites with Flocculent TiN for Efficient Removal of Sulfamethoxazole" Catalysts 13, no. 2: 291. https://doi.org/10.3390/catal13020291
APA StyleShi, H., Yao, X., Lu, S., Zuo, Y., Zheng, T., & Jia, L. (2023). Photocatalytically Active Semiconductor Cu3P Unites with Flocculent TiN for Efficient Removal of Sulfamethoxazole. Catalysts, 13(2), 291. https://doi.org/10.3390/catal13020291









