Targeted Fluoxetine Delivery Using Folic Acid-Modified PLGA Nanoparticles for Selective Uptake by Glioblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells
2.3. Optimization of the Protocol for FL-Loaded PLGA NPs Preparation
2.3.1. Preparation of FL-Loaded PLGA NPs
2.3.2. Central Composite Design
2.4. Functionalization of FL-Loaded PLGA NPs with FA
2.5. Physicochemical Characterization of NPs
2.6. Determination of the FL Encapsulation Efficiency and NPs’ Loading Capacity
2.7. In Vitro Release of FL from FA-Conjugated and Non-Conjugated PLGA NPs
2.8. In Vitro Internalization Studies
2.8.1. Quantitative Analysis by Fluorescence Measurements
2.8.2. Folate Receptor Blocking Experiment
2.9. In Vitro Cytotoxicity Studies
2.9.1. Evaluation of the Antiproliferative Effect of the Developed NPs
2.9.2. Evaluation of the Ability of FL-Loaded NPs in Sensitizing GBM Cells to TMZ
2.10. Statistical Analysis
3. Results and Discussion
3.1. ANOVA Statistical Analysis of the CCD
3.1.1. Analysis of Experimental Factors Affecting NPs’ Size
3.1.2. Analysis of Experimental Factors Affecting NPs’ Zeta Potential
3.1.3. Analysis of Experimental Factors Affecting NPs’ EE
3.1.4. Analysis of Experimental Factors Affecting NPs’ LC
3.2. Optimization of FL-Loaded PLGA NPs Preparation
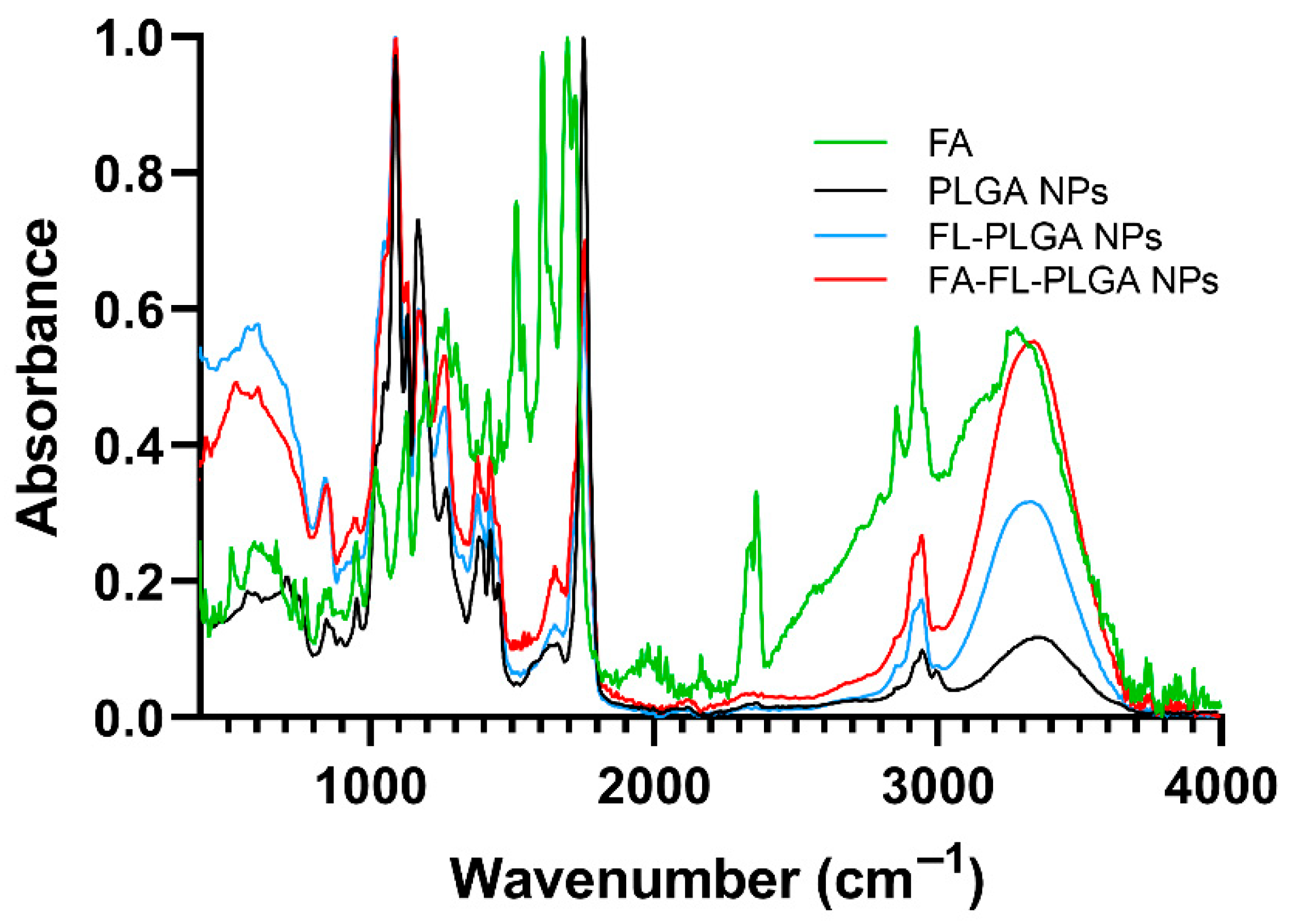
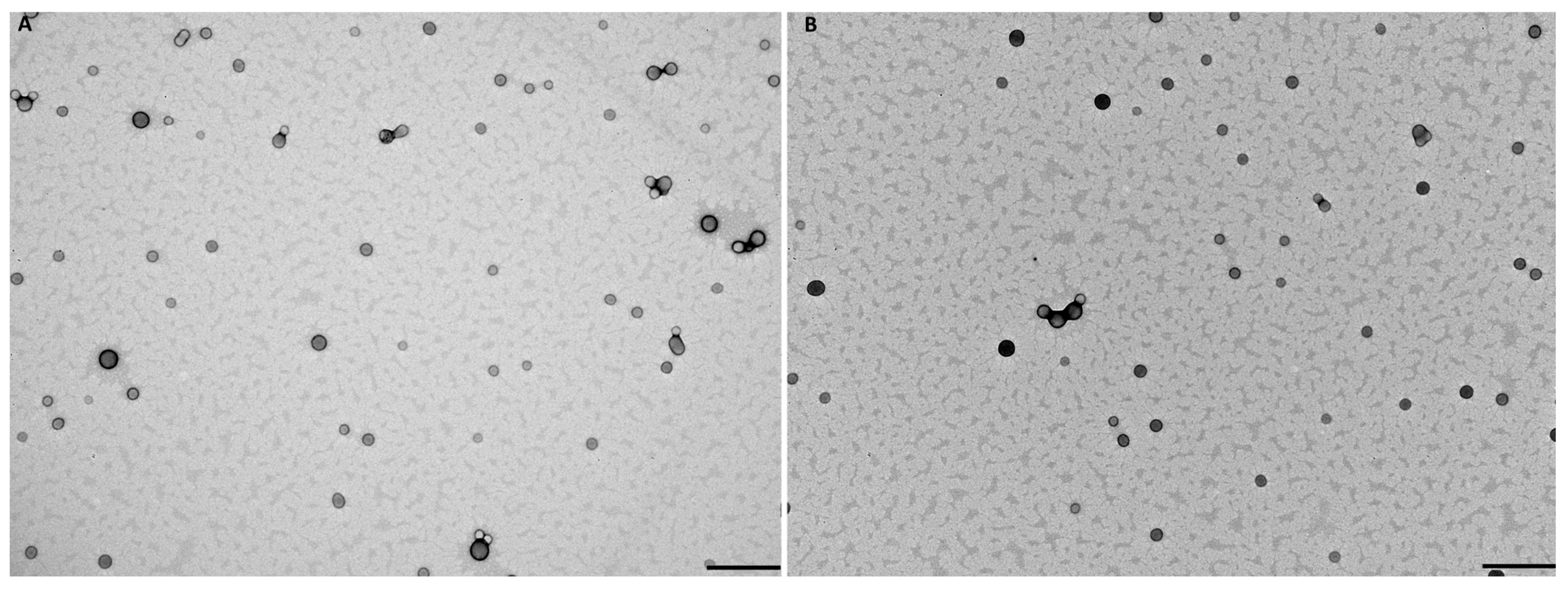
3.3. Effect of FA-Conjugation on NPs’ Properties
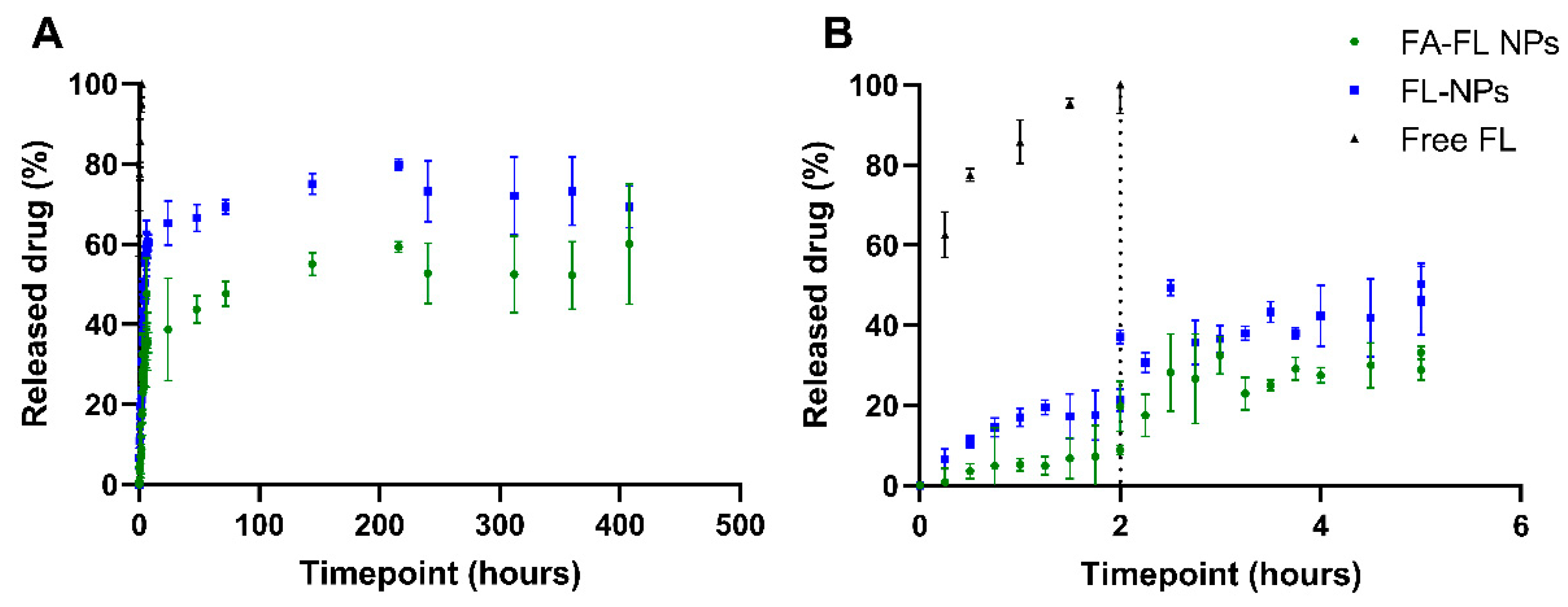
3.4. FL Release Kinetics from NPs
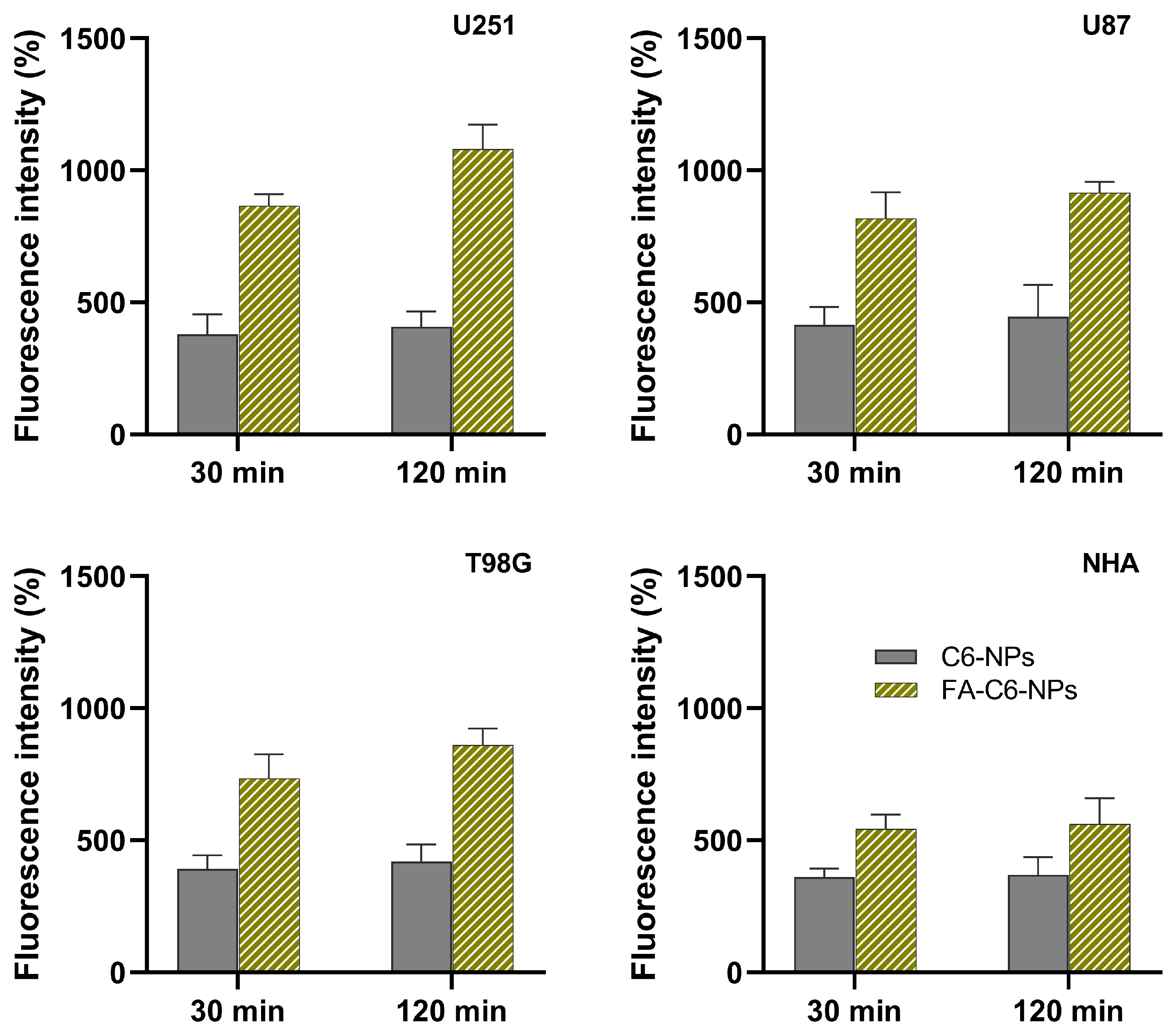
3.5. Quantitative Analysis of NP’s Cell Uptake
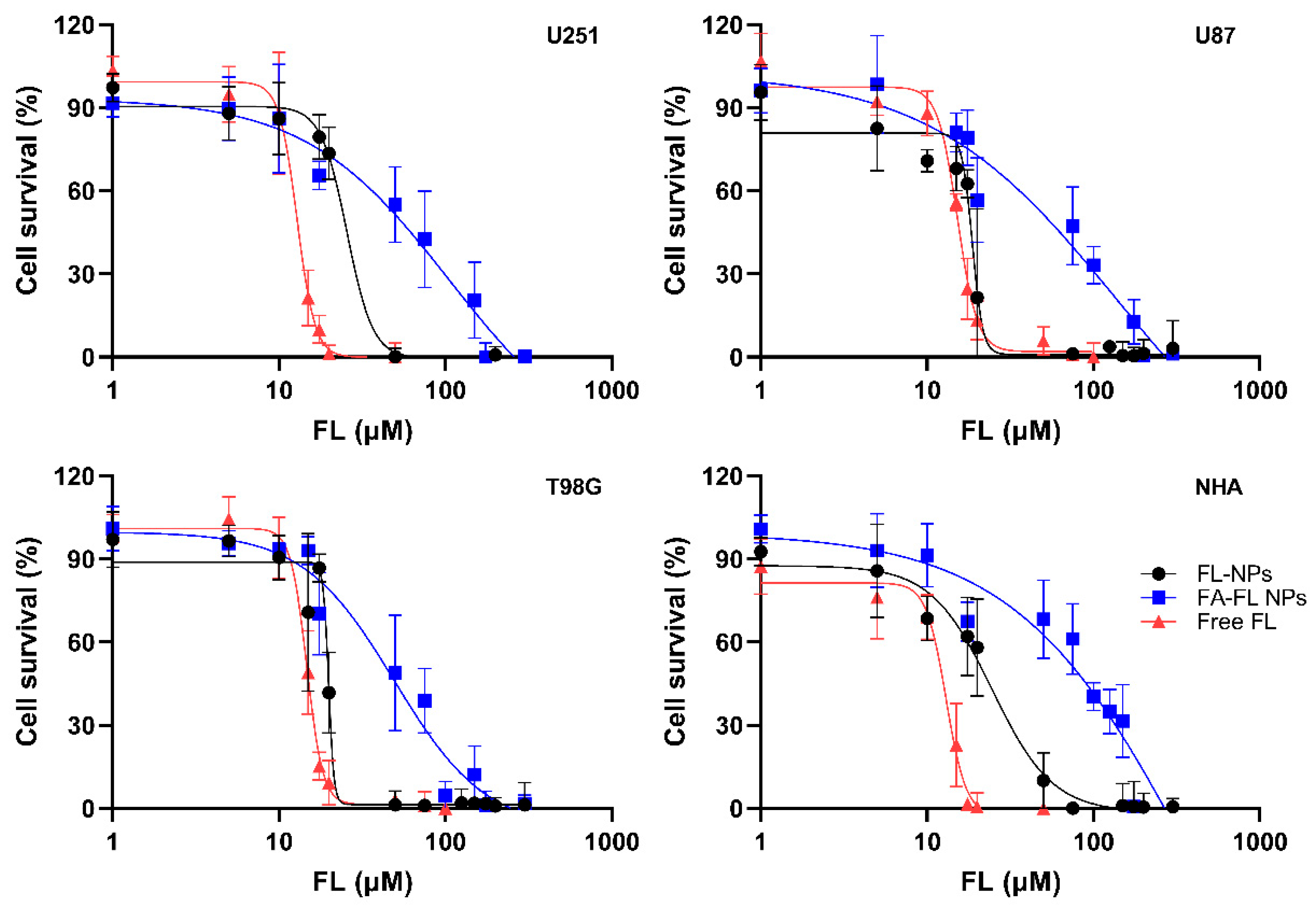
3.6. Cytotoxicity Evaluation of FL-Loaded NPs
3.7. Enhancement of TMZ Sensitivity in GBM Cells by FL-Loaded NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| CCD | Central Composite Design |
| CNS | Central Nervous System |
| C6 | Coumarin-6 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DDS | Drug Delivery Systems |
| DLS | Dynamic Light Scattering |
| DMSO | Dimethyl Sulfoxide |
| EDC | N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| EE | Encapsulation efficiency |
| FA | Folic acid |
| FaSSGF | Fasted State Simulated Gastric Fluid |
| FaSSIF | Fasted State Simulated Intestinal Fluid |
| FBS | Fetal Bovine Serum |
| FL | Fluoxetine |
| GBM | Glioblastoma |
| LC | Loading capacity |
| MGMT | O6-methylguanine DNA methyltransferase |
| NPs | Nanoparticles |
| OAT | One factor at time |
| PBS | Phosphate-buffered saline |
| PdI | Polydispersity index |
| PLGA | Poly(lactic-co-glycolic acid) |
| PVA | Polyvinyl alcohol |
| SMPD1 | Sphingomyelin phosphodiesterase 1 |
| SRB | Sulforhodamine B |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Trichloroacetic acid |
| TEM | Transmission electron microscopy |
| TMZ | Temozolomide |
References
- Zhang, P.; Zhang, Y.; Ji, N. Challenges in the Treatment of Glioblastoma by Chimeric Antigen Receptor T-Cell Immunotherapy and Possible Solutions. Front. Immunol. 2022, 13, 927132. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; Lopez-Blanch, R.; Pineda, B.; Gutierrez-Arroyo, J.L.; Loras, A.; González-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef] [PubMed]
- Ketkar, M.; Desai, S.; Rana, P.; Thorat, R.; Epari, S.; Dutt, A.; Dutt, S. Inhibition of PERK-Mediated Unfolded Protein Response Acts as a Switch for Reversal of Residual Senescence and as Senolytic Therapy in Glioblastoma. Neuro-Oncology 2024, 26, 2027–2043. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Yan, D.; Thyparambil, S.; Tian, Y.; Cecchi, F.; Rosenblum, M.; Reiner, A.S.; Panageas, K.S.; Hembrough, T.; Lin, A.L. Characterization of MGMT and EGFR Protein Expression in Glioblastoma and Association with Survival. J. Neuro-Oncol. 2020, 146, 163–170. [Google Scholar] [CrossRef]
- Szylberg, M.; Sokal, P.; Śledzińska, P.; Bebyn, M.; Krajewski, S.; Szylberg, Ł.; Szylberg, A.; Szylberg, T.; Krystkiewicz, K.; Birski, M.; et al. MGMT Promoter Methylation as a Prognostic Factor in Primary Glioblastoma: A Single-Institution Observational Study. Biomedicines 2022, 10, 2030. [Google Scholar] [CrossRef]
- Jovanović, N.; Mitrović, T.; Cvetković, V.J.; Tošić, S.; Vitorović, J.; Stamenković, S.; Nikolov, V.; Kostić, A.; Vidović, N.; Krstić, M.; et al. The Impact of MGMT Promoter Methylation and Temozolomide Treatment in Serbian Patients with Primary Glioblastoma. Medicina 2019, 55, 34. [Google Scholar] [CrossRef]
- Pham, J.; Cote, D.J.; Kang, K.; Briggs, R.G.; Gomez, D.; Prasad, A.; Daggupati, S.; Sisti, J.; Chow, F.; Attenello, F.; et al. Treatment Practices and Survival Outcomes for IDH-Wildtype Glioblastoma Patients According to MGMT Promoter Methylation Status: Insights from the U.S. National Cancer Database. J. Neuro-Oncol. 2025, 172, 655–665. [Google Scholar] [CrossRef]
- Alomari, S.; Tyler, B.; Zhang, I.; Hernandez, A.; Kraft, C.Y.; Raj, D.; Kedda, J. Drug Repurposing for Glioblastoma and Current Advances in Drug Delivery—A Comprehensive Review of the Literature. Biomolecules 2021, 11, 1870. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Ren, Y.; Zhang, X. Disulfiram: A Novel Repurposed Drug for Cancer Therapy. Cancer Chemother. Pharmacol. 2021, 87, 159–172. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Patton, C.; Stevens, G.; Tekautz, T.; Angelov, L.; Vogelbaum, M.A.; Weil, R.J.; Chao, S.; Elson, P.; Suh, J.H.; et al. Phase II Trial of Ritonavir/Lopinavir in Patients with Progressive or Recurrent High-Grade Gliomas. J. Neuro-Oncol. 2011, 102, 317–321. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Sohel, A.J.; Shutter, M.C.; Molla, M. Fluoxetine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–9. [Google Scholar] [CrossRef]
- Bi, J.; Khan, A.; Tang, J.; Armando, A.M.; Wu, S.; Zhang, W.; Gimple, R.C.; Reed, A.; Jing, H.; Koga, T.; et al. Targeting Glioblastoma Signaling and Metabolism with a Re-Purposed Brain-Penetrant Drug. Cell Rep. 2021, 37, 109957. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Mei, J.; Ji, W.; Huo, Z.; Bian, Z.; Jiao, J.; Li, X.; Sun, J.; Shao, J. MicroRNAs Involved in the EGFR Pathway in Glioblastoma. Biomed. Pharmacother. 2021, 134, 111115. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.A.; Maghe, C.; Gavard, J. Lysosomes in Glioblastoma: Pump up the Volume. Cell Cycle 2020, 19, 2094–2104. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J.; Najafi, S.H.; Shafiee, F.; Hassanzadeh, S.; Farzipour, S.; Ghasemi, A.; Asgarian-Omran, H. Fluoxetine as an Antidepressant Medicine Improves the Effects of Ionizing Radiation for the Treatment of Glioma. J. Bioenerg. Biomembr. 2020, 52, 165–174. [Google Scholar] [CrossRef]
- Hosseindoost, S.; Dehpour, A.R.; Dehghan, S.; Javadi, S.A.H.; Arjmand, B.; Fallah, A.; Hadjighassem, M. Fluoxetine Enhances the Antitumor Effect of Olfactory Ensheathing Cell-thymidine Kinase/Ganciclovir Gene Therapy in Human Glioblastoma Multiforme Cells through Upregulation of Connexin43 Levels. Drug Dev. Res. 2023, 84, 1739–1750. [Google Scholar] [CrossRef]
- Ge, Y.; Cao, Y.; Wang, Q.; Wang, Y.; Ma, W. Impact of Antidepressant Use on Survival Outcomes in Glioma Patients: A Systematic Review and Meta-Analysis. Neuro-Oncol. Adv. 2024, 6, vdae181. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Shamshiripour, P.; Rahnama, M.; Nikoobakht, M.; Hajiahmadi, F.; Moradi, A.; Ahmadvand, D. A Dynamic Study of VEGF-A SiDOX-EVs Trafficking through the in-Vitro Insert Co-Culture Blood-Brain Barrier Model by Digital Holographic Microscopy. Front. Oncol. 2024, 14, 1292083. [Google Scholar] [CrossRef]
- Miner, M.W.; Liljenbäck, H.; Virta, J.; Kärnä, S.; Viitanen, R.; Elo, P.; Gardberg, M.; Teuho, J.; Saipa, P.; Rajander, J.; et al. High Folate Receptor Expression in Gliomas Can Be Detected in Vivo Using Folate-Based Positron Emission Tomography with High Tumor-to-Brain Uptake Ratio Divulging Potential Future Targeting Possibilities. Front. Immunol. 2023, 14, 1145473. [Google Scholar] [CrossRef]
- Kamali, M.; Webster, T.J.; Amani, A.; Hadjighassem, M.R.; Malekpour, M.R.; Tirgar, F.; Khosravani, M.; Adabi, M. Effect of Folate-Targeted Erlotinib Loaded Human Serum Albumin Nanoparticles on Tumor Size and Survival Rate in a Rat Model of Glioblastoma. Life Sci. 2023, 313, 121248. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.; Mendes, M.; Silva, J.; Sereno, J.; Cova, T.; Oliveira, R.; Fortuna, A.; Castelo-Branco, M.; Falcão, A.; Sousa, J.; et al. Peptide-Lipid Nanoconstructs Act Site-Specifically towards Glioblastoma Growth Impairment. Eur. J. Pharm. Biopharm. 2020, 155, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- dos Santos, S.B.F.; Pereira, S.A.; Rodrigues, F.A.M.; da Silva, A.C.C.; de Almeida, R.R.; Sousa, A.C.C.; Fechine, L.M.U.D.; Denardin, J.C.; Araneda, F.; Sá, L.G.A.V.; et al. Antibacterial Activity of Fluoxetine-Loaded Starch Nanocapsules. Int. J. Biol. Macromol. 2020, 164, 2813–2817. [Google Scholar] [CrossRef]
- Khater, S.E.; El-khouly, A.; Abdel-Bar, H.M.; Al-mahallawi, A.M.; Ghorab, D.M. Fluoxetine Hydrochloride Loaded Lipid Polymer Hybrid Nanoparticles Showed Possible Efficiency against SARS-CoV-2 Infection. Int. J. Pharm. 2021, 607, 121023. [Google Scholar] [CrossRef]
- Fatima, F.; Aleemuddin, M.; Ahmed, M.M.; Anwer, M.K.; Aldawsari, M.F.; Soliman, G.A.; Mahdi, W.A.; Jafar, M.; Hamad, A.M.; Alshehri, S. Design and Evaluation of Solid Lipid Nanoparticles Loaded Topical Gels: Repurpose of Fluoxetine in Diabetic Wound Healing. Gels 2023, 9, 21. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Delorme, V.; Kasaeian, A.; Amiri, V.; Masoumi, M.; Sadeghinia, M.; Ebrahimzadeh, N.; Maleki, M.; Pourazar, S. An Effective Nano Drug Delivery and Combination Therapy for the Treatment of Tuberculosis. Sci. Rep. 2022, 12, 9591. [Google Scholar] [CrossRef]
- Müller, A.L.H.; De Oliveira, J.A.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Design of Experiments and Method Development. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 589–608. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Alves, B.; Andrade, S.; Lima, J.; Loureiro, J.A.; Pereira, M.C. Folic-Acid-Conjugated Poly (Lactic-Co-Glycolic Acid) Nanoparticles Loaded with Gallic Acid Induce Glioblastoma Cell Death by Reactive-Oxygen-Species-Induced Stress. Polymers 2024, 16, 2161. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Struzek, A.-M.; Scherließ, R. Quality by Design as a Tool in the Optimisation of Nanoparticle Preparation—A Case Study of PLGA Nanoparticles. Pharmaceutics 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Pattnaik, G.; Behera, A. Development and In-Vitro, in-Vivo Evaluation of Pioglitazone-Loaded Polymeric Nanoparticles Using Central Composite Design Surface Response Methodology. OpenNano 2023, 11, 100141. [Google Scholar] [CrossRef]
- Bhargav, E.; Mohammed, N.; Singh, U.N.; Ramalingam, P.; Challa, R.R.; Vallamkonda, B.; Ahmad, S.F.; DSNBK, P.; Pasala, P.K.; Rudrapal, M. A Central Composite Design-Based Targeted Quercetin Nanoliposomal Formulation: Optimization and Cytotoxic Studies on MCF-7 Breast Cancer Cell Lines. Heliyon 2024, 10, e37430. [Google Scholar] [CrossRef]
- Tedjani, M.L.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Albalawi, N. Optimizing the Antibacterial Activity of Iron Oxide Nanoparticles Using Central Composite Design. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3564–3584. [Google Scholar] [CrossRef]
- Hassan, H.; Adam, S.K.; Alias, E.; Meor Mohd Affandi, M.M.R.; Shamsuddin, A.F.; Basir, R. Central Composite Design for Formulation and Optimization of Solid Lipid Nanoparticles to Enhance Oral Bioavailability of Acyclovir. Molecules 2021, 26, 5432. [Google Scholar] [CrossRef]
- Elsayed, S.I.; Girgis, G.N.S.; El-Dahan, M.S. Formulation and Evaluation of Pravastatin Sodium-Loaded PLGA Nanoparticles: In Vitro–in Vivo Studies Assessment. Int. J. Nanomed. 2023, 18, 721–742. [Google Scholar] [CrossRef]
- Pattadar, D.K.; Zamborini, F.P. Effect of Size, Coverage, and Dispersity on the Potential-Controlled Ostwald Ripening of Metal Nanoparticles. Langmuir 2019, 35, 16416–16426. [Google Scholar] [CrossRef]
- Nasef, A.M.; Gardouh, A.R.; Ghorab, M.M. Polymeric Nanoparticles: Influence of Polymer, Surfactant and Composition of Manufacturing Vehicle on Particle Size. World J. Pharm. Sci. 2015, 3, 2308–2322. [Google Scholar]
- Kakran, M.; Sahoo, N.G.; Tan, I.L.; Li, L. Preparation of Nanoparticles of Poorly Water-Soluble Antioxidant Curcumin by Antisolvent Precipitation Methods. J. Nanopart. Res. 2012, 14, 757. [Google Scholar] [CrossRef]
- Chen, X.T.; Wang, T. Preparation and Characterization of Atrazine-Loaded Biodegradable PLGA Nanospheres. J. Integr. Agric. 2019, 18, 1035–1041. [Google Scholar] [CrossRef]
- Li, F.; Ma, H.; Shen, C.; Pan, Y.; Zhang, Y.; Liu, Y.; Xu, C.; Wu, D. From the Accelerated Production of •OH Radicals to the Crosslinking of Polyvinyl Alcohol: The Role of Free Radicals Initiated by Persulfates. Appl. Catal. B Environ. 2021, 285, 119763. [Google Scholar] [CrossRef]
- Hussain, Z.; Sahudin, S.; Article, O. Preparation, Characterisation and Colloidal Stability of Chitosan-Tripolyphosphate Nanoparticles: Optimisation of Formulation and Process Parameters. Int. J. Pharm. Pharm. Sci. 2016, 8, 297–308. [Google Scholar]
- Musumeci, T.; Ventura, C.A.; Giannone, I.; Ruozi, B.; Montenegro, L.; Pignatello, R.; Puglisi, G. PLA/PLGA Nanoparticles for Sustained Release of Docetaxel. Int. J. Pharm. 2006, 325, 172–179. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Sabri, A.H.B.; Anjani, Q.K.; Domínguez-Robles, J.; Abdul Latip, N.; Hamid, K.A. Design and Development of Levodopa Loaded Polymeric Nanoparticles for Intranasal Delivery. Pharmaceuticals 2022, 15, 370. [Google Scholar] [CrossRef]
- Heriyanto, H. A Review of Encapsulation Using Emulsion Crosslinking Method. World Chem. Eng. J. 2021, 5, 37–43. [Google Scholar] [CrossRef]
- Piacentini, E.; Bazzarelli, F.; Poerio, T.; Albisa, A.; Irusta, S.; Mendoza, G.; Sebastian, V.; Giorno, L. Encapsulation of Water-Soluble Drugs in Poly(vinyl alcohol) (PVA) Microparticles via Membrane Emulsification: Influence of Process and Formulation Parameters on Structural and Functional Properties. Mater. Today Commun. 2020, 24, 100967. [Google Scholar] [CrossRef]
- Rajamma, A.J.; Shekar, H.S.; Sateesha, S.B. Ultrasound-Induced Microencapsulation of Simvastatin for Gastric Retention and Controlled Delivery. Indian J. Pharm. Sci. 2018, 80, 647–656. [Google Scholar]
- Lee, S.Y.; Kim, S.Y.; Ku, S.H.; Park, E.J.; Jang, D.J.; Kim, S.T.; Kim, S.B. Polyhydroxyalkanoate Decelerates the Release of Paclitaxel from Poly(Lactic-Co-Glycolic Acid) Nanoparticles. Pharmaceutics 2022, 14, 1618. [Google Scholar] [CrossRef]
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The Normal and Brain Tumor Vasculature: Morphological and Functional Characteristics and Therapeutic Targeting. Front. Physiol. 2021, 12, 622615. [Google Scholar] [CrossRef]
- Calatayud, M.P.; Sanz, B.; Raffa, V.; Riggio, C.; Ibarra, M.R.; Goya, G.F. The Effect of Surface Charge of Functionalized Fe3O4 Nanoparticles on Protein Adsorption and Cell Uptake. Biomaterials 2014, 35, 6389–6399. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein Adsorption and Cellular Uptake of Cerium Oxide Nanoparticles as a Function of Zeta Potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent Effect of Polymeric Nanoparticle Zeta Potential/Surface Charge, on Their Cytotoxicity and Affinity to Cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, H.; Li, Y.; Yang, K.; Lei, P.; Gu, Y.; Sun, L.; Xu, H.; Wang, R. Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate. Foods 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Ow, V.; Lin, Q.; Wong, J.H.M.; Sim, B.; Tan, Y.L.; Leow, Y.; Goh, R.; Loh, X.J. Understanding the Interplay between PH and Charges for Theranostic Nanomaterials. Nanoscale 2025, 17, 6960–6980. [Google Scholar] [CrossRef]
- Madani, F.; Morovvati, H.; Webster, T.J.; Najaf Asaadi, S.; Rezayat, S.M.; Hadjighassem, M.; Khosravani, M.; Adabi, M. Combination Chemotherapy via Poloxamer 188 Surface-Modified PLGA Nanoparticles That Traverse the Blood–Brain–Barrier in a Glioblastoma Model. Sci. Rep. 2024, 14, 19516. [Google Scholar] [CrossRef]
- Seko, I.; Tonbul, H.; Tavukçuoğlu, E.; Şahin, A.; Akbas, S.; Yanık, H.; Öztürk, S.C.; Esendagli, G.; Khan, M.; Capan, Y. Development of Curcumin and Docetaxel Co-Loaded Actively Targeted PLGA Nanoparticles to Overcome Blood Brain Barrier. J. Drug Deliv. Sci. Technol. 2021, 66, 102867. [Google Scholar] [CrossRef]
- Riva’I, I.; Wulandari, I.O.; Sulistyarti, H.; Sabarudin, A. Ex-Situ Synthesis of Polyvinyl Alcohol(PVA)-Coated Fe3O4 Nanoparticles by Coprecipitation-Ultrasonication Method. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; Institute of Physics Publishing: Bristol, UK, 2018; Volume 299. [Google Scholar]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Hossein, H.H.S.; Jabbari, I.; Zarepour, A.; Zarrabi, A.; Ashrafizadeh, M.; Taherian, A.; Makvandi, P. Functionalization of Magnetic Nanoparticles by Folate as Potential MRI Contrast Agent for Breast Cancer Diagnostics. Molecules 2020, 25, 4053. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Abriata, J.P.; Raspantini, G.L.; Tofani, L.B.; Fumagalli, F.; de Melo, S.M.G.; da Silva Emery, F.; Swiech, K.; Marcato, P.D.; Lee, R.; et al. In Vitro Evaluation of Folate-Modified PLGA Nanoparticles Containing Paclitaxel for Ovarian Cancer Therapy. Mater. Sci. Eng. C 2019, 105, 110038. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, T.; Kaur, R.; Kaur, A. Recent Biomedical Applications and Patents on Biodegradable Polymer-PLGA. Int. J. Pharmacol. Pharm. Sci. 2014, 2, 30–42. [Google Scholar]
- Raouf, A.; Mohammed, J.; Dulimyi, E. Qualitative and Quantitative Determination of Folic in Tablets by FTIR Spectroscopy. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 773–780. [Google Scholar]
- Oraby, M.; Ahmed, A.S.; Abdel-Lateef, M.A.; Mostafa, M.A.H.; Hassan, A.I. Employ FTIR Spectroscopic Method for Determination of Certain Multiple Sclerosis Medications in Plasma and Pharmaceutical Formulations. Microchem. J. 2021, 167, 106329. [Google Scholar] [CrossRef]
- Kaplan, M.; Öztürk, K.; Öztürk, S.C.; Tavukçuoğlu, E.; Esendağlı, G.; Calis, S. Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation. Pharmaceutics 2023, 15, 175. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle Size Distribution Quantification from Transmission Electron Microscopy (TEM) of Ruthenium Tetroxide Stained Polymeric Nanoparticles. J. Colloid Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and Pharmacodynamics: A Comprehensive Analysis of the Absorption, Distribution, Metabolism, and Excretion of Psychiatric Drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kim, Y.-S.; Kim, I.-S.; Park, R.-W.; Oh, S.J.; Moon, D.H.; Kim, S.Y.; Kwon, I.C. Tumoral Accumulation of Long-Circulating, Self-Assembled Nanoparticles and Its Visualization by Gamma Scintigraphy. Macromol. Res. 2008, 16, 15–20. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Preparation, Optimization by 23 Factorial Design, Characterization and in Vitro Release Kinetics of Lorazepam Loaded PLGA Nanoparticles. Polym. Sci. Ser. A 2016, 58, 975–986. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. PH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate Receptors’ Expression in Gliomas May Possess Potential Nanoparticle-Based Drug Delivery Opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.R.; Chen, W.; Chen, M.H.; Wang, H.; Wang, X.D.; Sun, L.L.; Wang, F.Z.; Wang, D.C. Fluoxetine Synergizes with Temozolomide to Induce the CHOP-Dependent Endoplasmic Reticulum Stress-Related Apoptosis Pathway in Glioma Cells. Oncol. Rep. 2016, 36, 676–684. [Google Scholar] [CrossRef]
- Liu, K.H.; Yang, S.T.; Lin, Y.K.; Lin, J.W.; Lee, Y.H.; Wang, J.Y.; Hu, C.J.; Lin, E.Y.; Chen, S.M.; Then, C.K.; et al. Fluoxetine, an Antidepressant, Suppresses Glioblastoma by Evoking AMPAR-Mediated Calcium-Dependent Apoptosis. Oncotarget 2015, 6, 5088–5101. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Ivanov, A.; Lengert, E.; Tulyakova, K.; Shilyagina, N.; Ermakov, A. Current Principles, Challenges, and New Metrics in PH-Responsive Drug Delivery Systems for Systemic Cancer Therapy. Pharmaceutics 2023, 15, 1566. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Arif, H.; Awan, A.N.; Khan, M.M.; Batool, S.; Ahmed, S. Synthesis and Optimization of Fluoxetine-Loaded Polymeric Nanoparticles for Dual Therapeutic Applications in Cancer and Depression. DARU J. Pharm. Sci. 2025, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Perazzoli, G.; Prados, J.; Ortiz, R.; Caba, O.; Cabeza, L.; Berdasco, M.; Gónzalez, B.; Melguizo, C. Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PLoS ONE 2015, 10, e0140131. [Google Scholar] [CrossRef]
- Milani, R.; Brognara, E.; Fabbri, E.; Finotti, A.; Borgatti, M.; Lampronti, I.; Marzaro, G.; Chilin, A.; Lee, K.K.-H.; Kok, S.H.-L.; et al. Corilagin Induces High Levels of Apoptosis in the Temozolomide-Resistant T98G Glioma Cell Line. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 1307–1315. [Google Scholar] [CrossRef]
- Khazaei, M.; Pazhouhi, M.; Khazaei, S. Temozolomide and Tranilast Synergistic Antiproliferative Effect on Human Glioblastoma Multiforme Cell Line (U87MG). Med. J. Islam. Repub. Iran 2019, 33, 39. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiang, D.; Zhu, X.; Wang, C. Preliminary Study on Relationship between Temozolomide Chemotherapy-Resistant Cells and Stem Cells in Gliomas. Turk. Neurosurg. 2019, 32, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Androutsopoulos, G.; Logotheti, S.; Adonakis, G.; Maroulis, I.; Tzelepi, V. DNA Methylation in Epithelial Ovarian Cancer: Current Data and Future Perspectives. Curr. Mol. Pharmacol. 2021, 14, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Shabani, S.; Nafissi, N.; Majidzadeh, T.; Mahjoubi, F. Investigation of Promoter Methylation Patterns Association with Genes Expression Profile of ISL1, MGMT and DMNT3b in Tissue of Breast Cancer Patients. Mol. Biol. Rep. 2022, 49, 847–857. [Google Scholar] [CrossRef]
- Karpathak, S.; Garg, R.; Ahmad, M.K.; Srivastava, A.; Misra, N.; Kumar Srivastav, A. Association of DNA Repair Gene O6-Methylguanine-DNA Methyltransferase (MGMT) Expression with Aberrant Promoter Methylation in Lung Cancer Progression. Eur. Respir. J. 2023, 62, PA2208. [Google Scholar]


| Independent Variable | Units | Experimental Level | ||||
|---|---|---|---|---|---|---|
| −α 1 | −1 | 0 | +1 | +α 1 | ||
| mPLGA | mg | 3.8 | 10.0 | 25.0 | 40.0 | 46.2 |
| mFL | mg | 0.2 | 0.5 | 1.25 | 2.0 | 2.3 |
| % PVA | % (w/v) | 0.2 | 1.0 | 3 | 5 | 5.8 |
| Sonication Cycles | s | 1.9 | 5 | 12.5 | 20 | 23.1 |
| Predicted Values | Experimental Values | ||
|---|---|---|---|
| Size (nm) | Mean value | 119 | 136 ± 2 |
| Range | (66–172) | (134–138) | |
| PdI | Mean value | 0.22 | 0.22 ± 0.04 |
| Range | (0.02–0.45) | (0.18–0.26) | |
| Zeta potential (mV) | Mean value | −15.9 | −14.6 ± 1.4 |
| Range | (−18.0–[−13.8]) | (−16.1–[−13.4]) | |
| EE (%) | Mean value | 52 | 52.3 ± 2.4 |
| Range | (42.7–61.3) | (50.5–55.0) | |
| LC (%) | Mean value | 4.1 | 3.6 ± 0.2 |
| Range | (3.3–4.9) | (3.5–3.8) |
| IC50 (µM) | ||||
|---|---|---|---|---|
| U251 | U87 | T98G | NHA | |
| Free FL | 12.8 ± 2.0 | 15.2 ± 0.6 | 14.8 ± 1.8 | 12.1 ± 2.3 |
| FL-loaded PLGA NPs | 24.7 ± 3.1 | 18.1 ± 1.2 | 19.7 ± 3.0 | 21.5 ± 0.9 |
| FA-FL-loaded PLGA NPs | 52.5 ± 5.3 | 52.7 ± 4.5 | 45.0 ± 0.9 | 77.9 ± 6.2 |
| IC50 (µM) | |||
|---|---|---|---|
| U251 | U87 | T98G | |
| Free TMZ | 40.5 ± 4.8 | 189 ± 15 | 699 ± 29 |
| Free TMZ + FL-loaded PLGA NPs | 34.8 ± 5.1 | 7.1 ± 6.8 | 11.2 ± 1.5 |
| Free TMZ + FA-FL-loaded PLGA NPs | 49.6 ± 7.4 | 53.9 ± 1.7 | 31.0 ± 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalho, M.J.; Nóbrega, C.; Andrade, S.; Lima, J.; Loureiro, J.A.; Pereira, M.C. Targeted Fluoxetine Delivery Using Folic Acid-Modified PLGA Nanoparticles for Selective Uptake by Glioblastoma Cells. Pharmaceutics 2025, 17, 1116. https://doi.org/10.3390/pharmaceutics17091116
Ramalho MJ, Nóbrega C, Andrade S, Lima J, Loureiro JA, Pereira MC. Targeted Fluoxetine Delivery Using Folic Acid-Modified PLGA Nanoparticles for Selective Uptake by Glioblastoma Cells. Pharmaceutics. 2025; 17(9):1116. https://doi.org/10.3390/pharmaceutics17091116
Chicago/Turabian StyleRamalho, Maria João, Carina Nóbrega, Stéphanie Andrade, Jorge Lima, Joana Angélica Loureiro, and Maria Carmo Pereira. 2025. "Targeted Fluoxetine Delivery Using Folic Acid-Modified PLGA Nanoparticles for Selective Uptake by Glioblastoma Cells" Pharmaceutics 17, no. 9: 1116. https://doi.org/10.3390/pharmaceutics17091116
APA StyleRamalho, M. J., Nóbrega, C., Andrade, S., Lima, J., Loureiro, J. A., & Pereira, M. C. (2025). Targeted Fluoxetine Delivery Using Folic Acid-Modified PLGA Nanoparticles for Selective Uptake by Glioblastoma Cells. Pharmaceutics, 17(9), 1116. https://doi.org/10.3390/pharmaceutics17091116











