Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. MTT Measurement
2.3. Cell Counting
2.4. ATP Measurement
2.5. Detection of Intracellular Reactive Oxygen Species Level
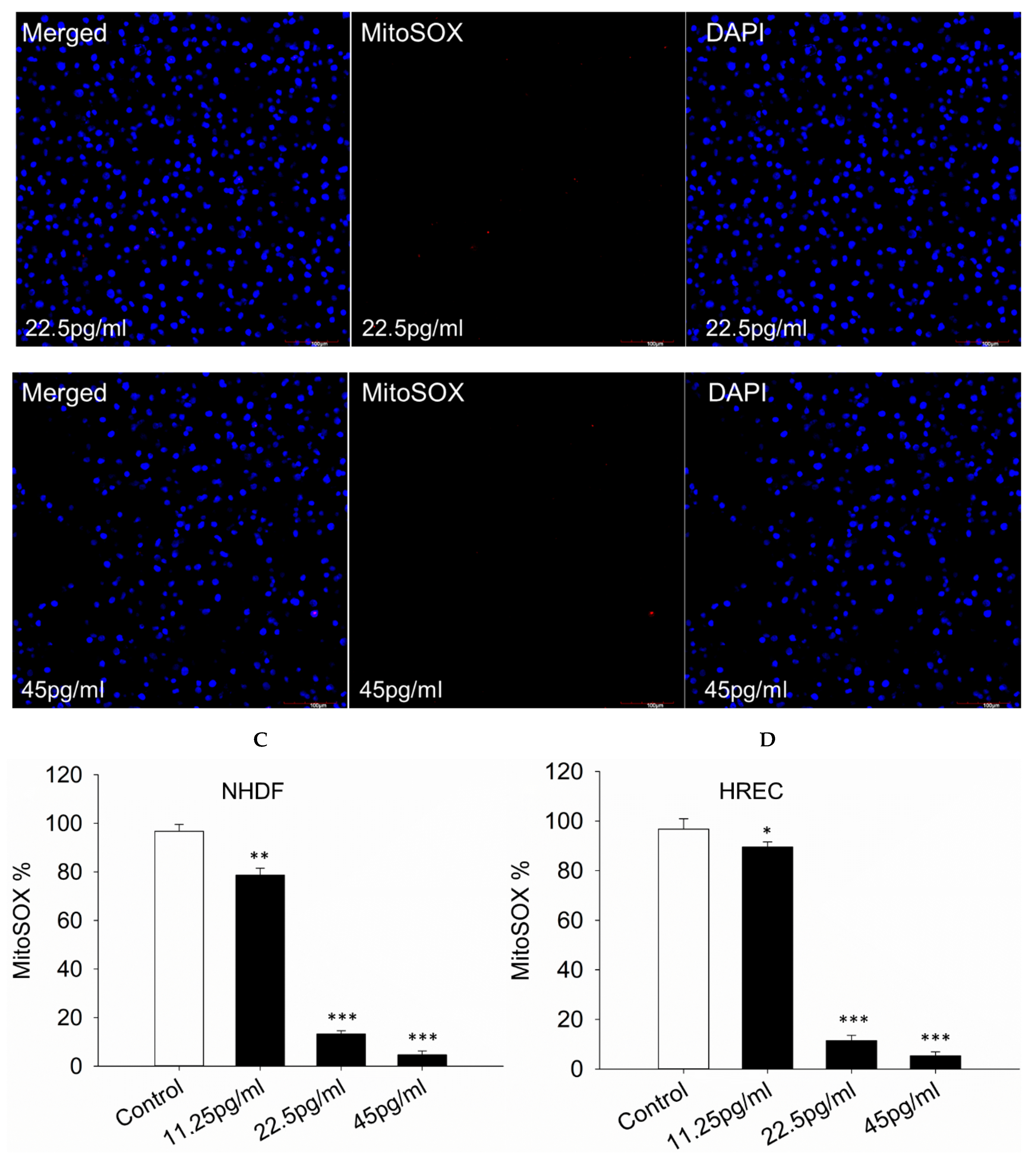
2.6. Detection of Mitochondrial Superoxide
2.7. AGE and RAGE Measurements
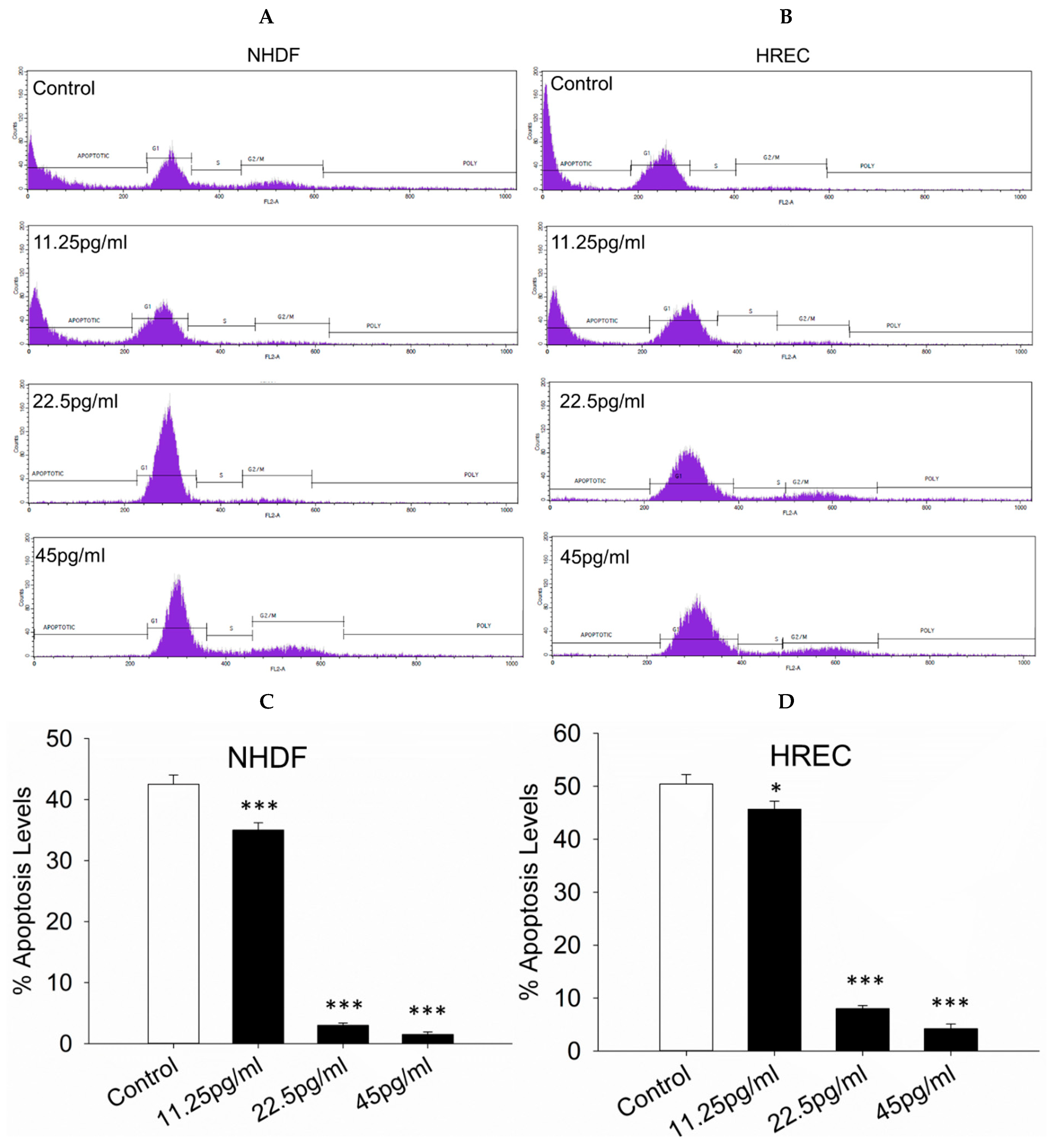
2.8. Propidium Iodide Staining Used for Detecting Apoptosis
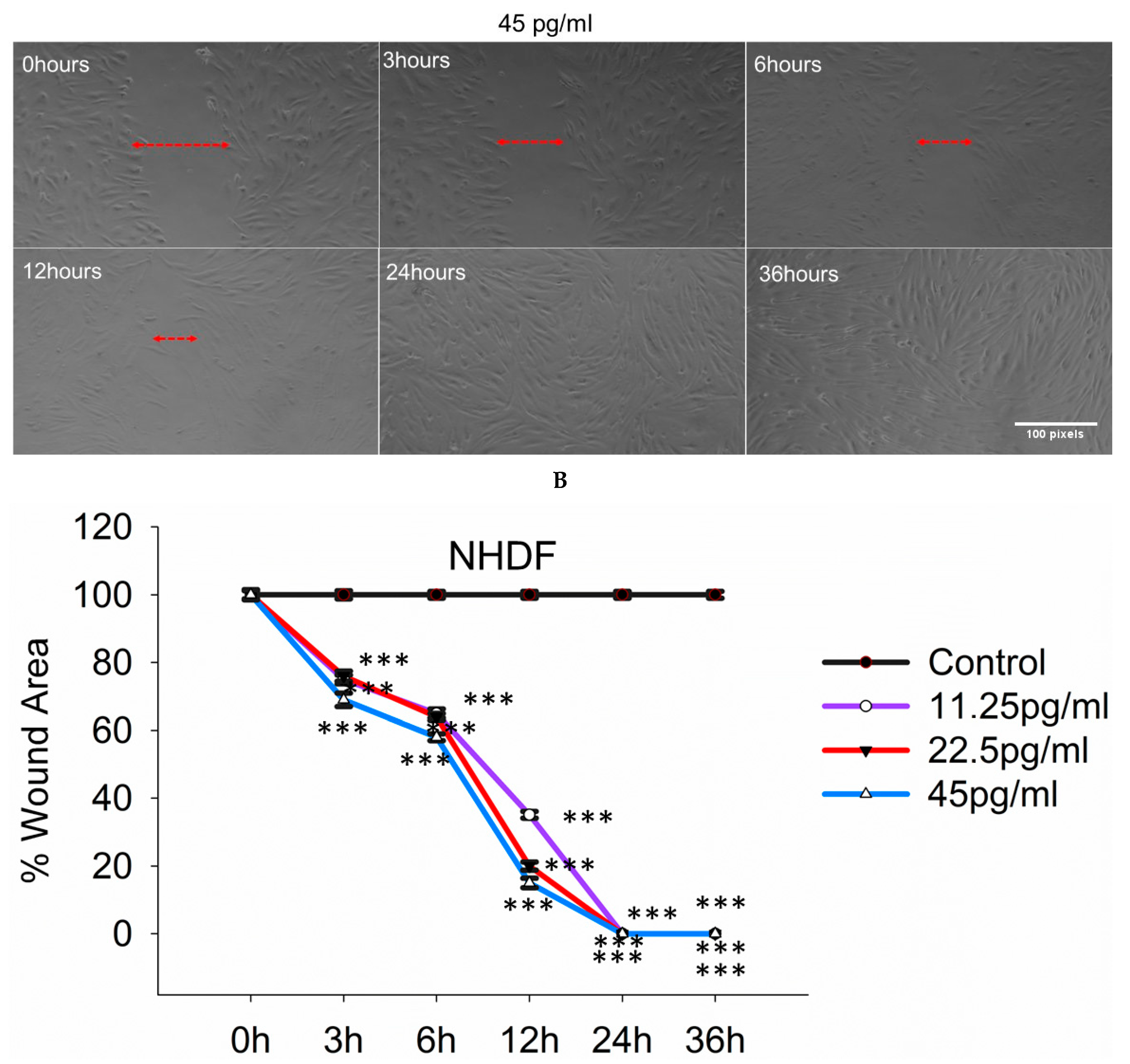
2.9. In Vitro Wound Scratch Assay
2.10. RNA Preparation and Quantitative Real-Time PCR
2.11. Graph Presentation and Statistical Analysis
3. Results
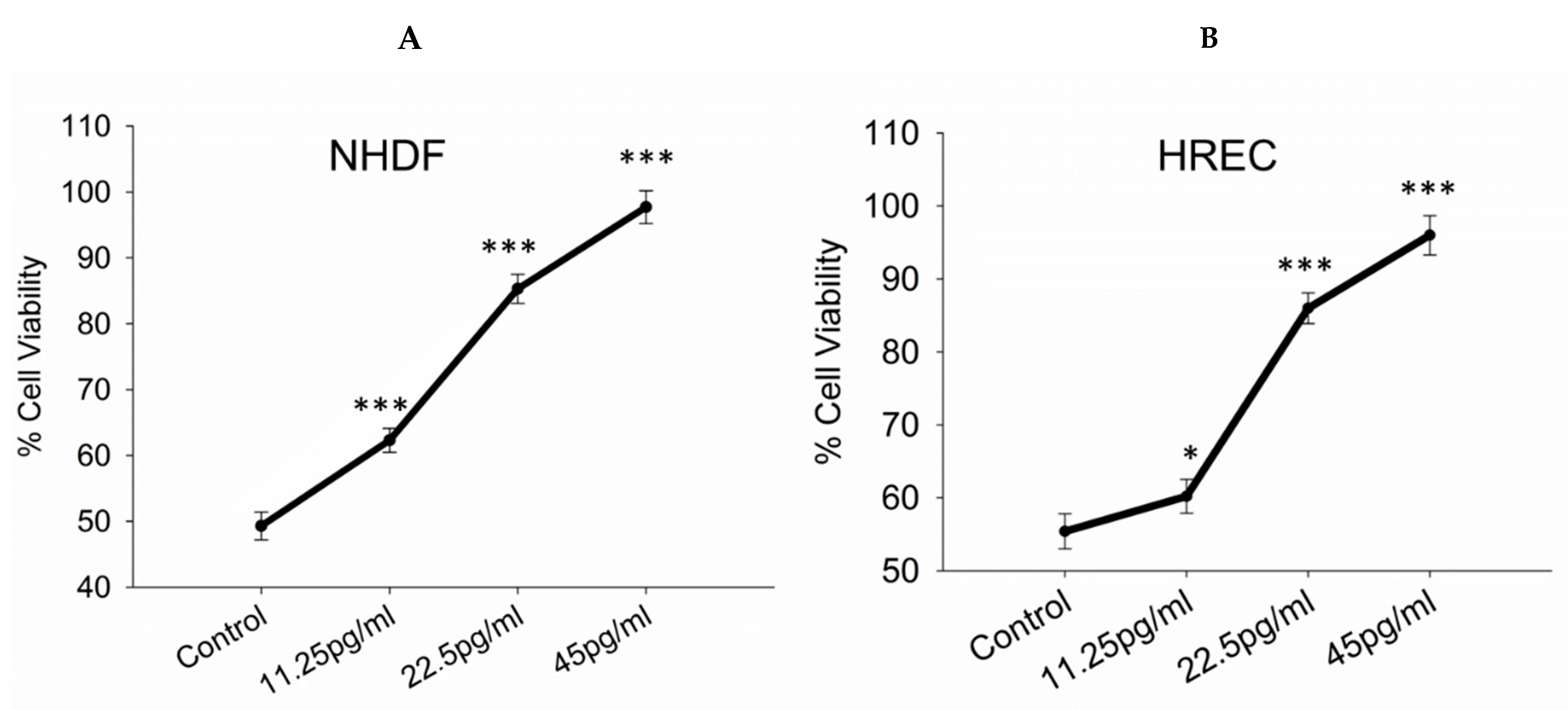
3.1. Toxicity/Viability Assessment
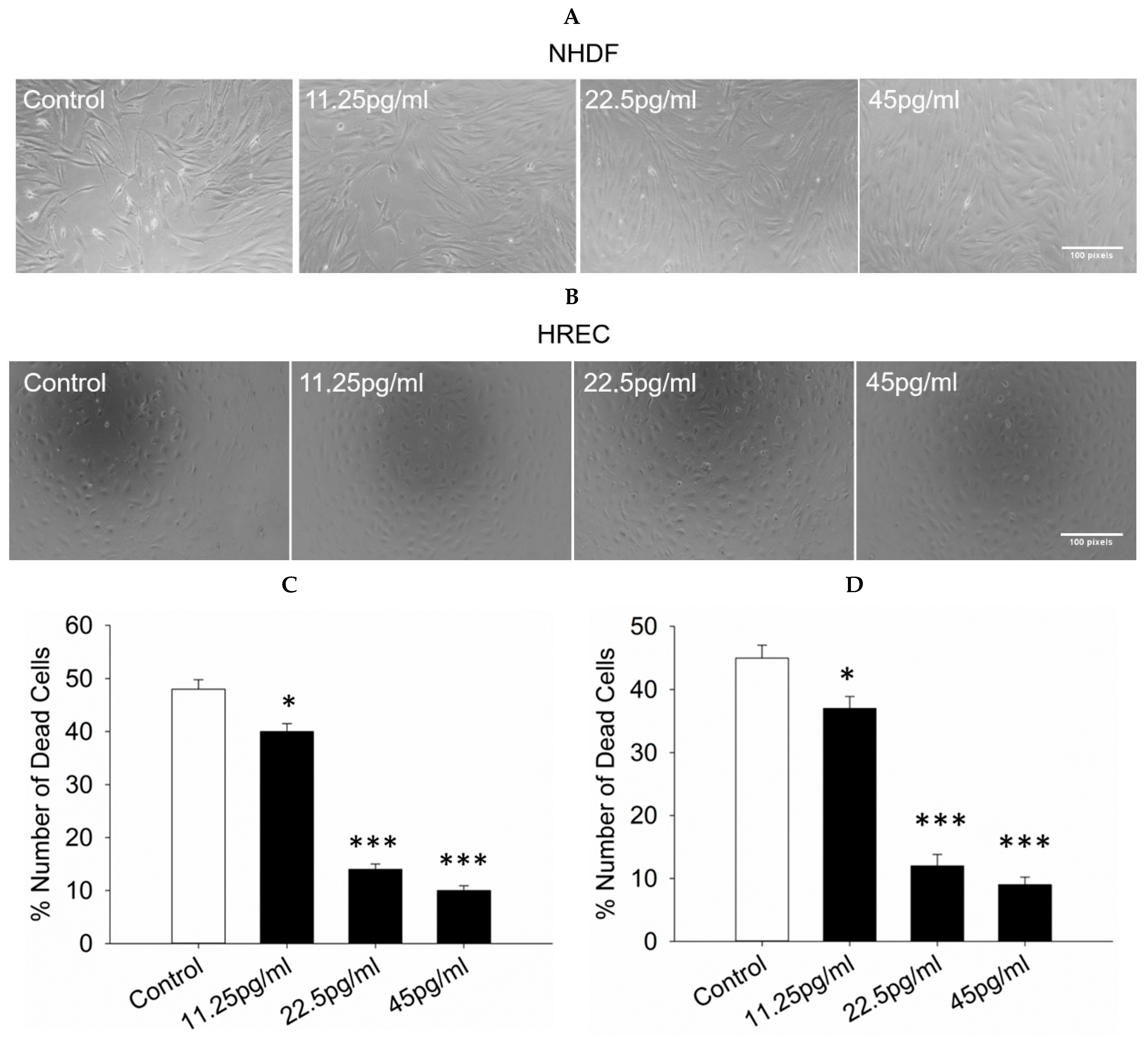
3.2. Cell Death Assessment
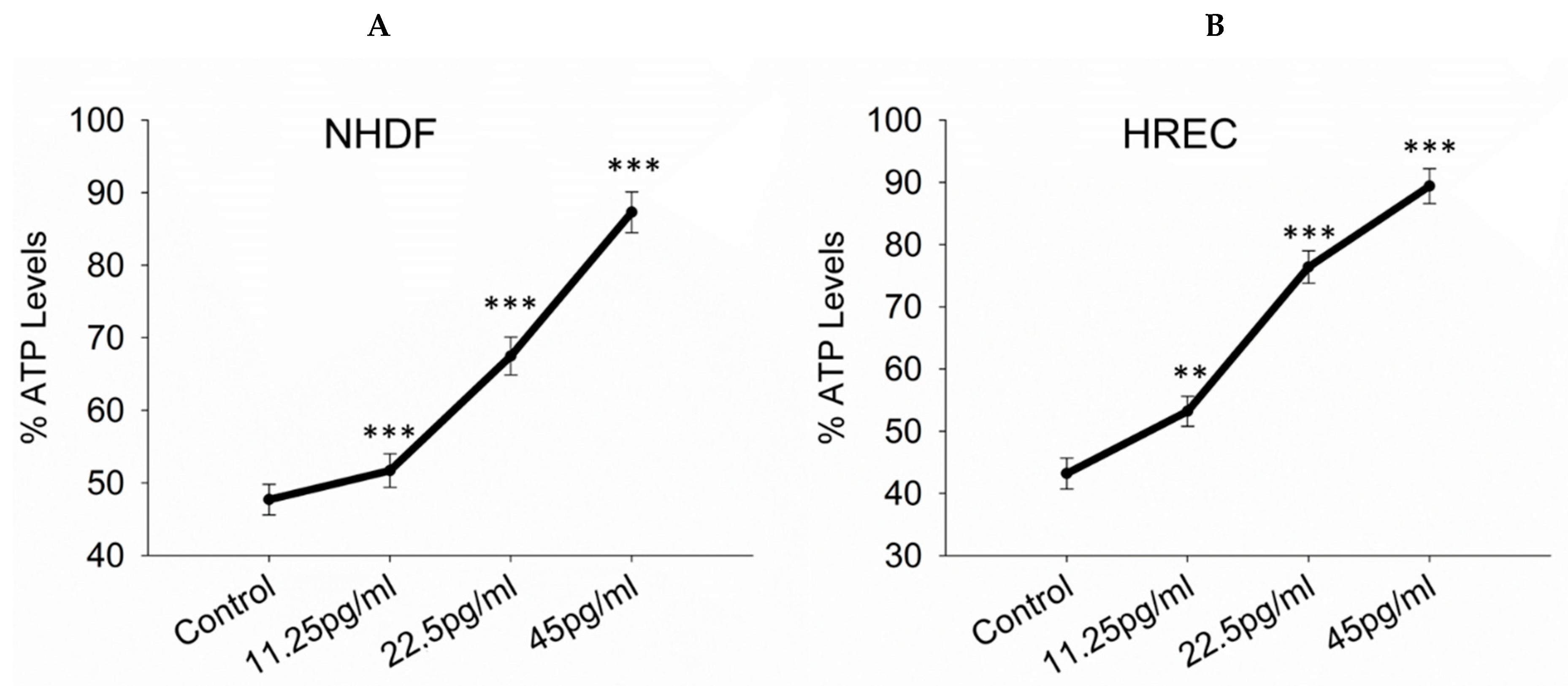
3.3. ATP Levels
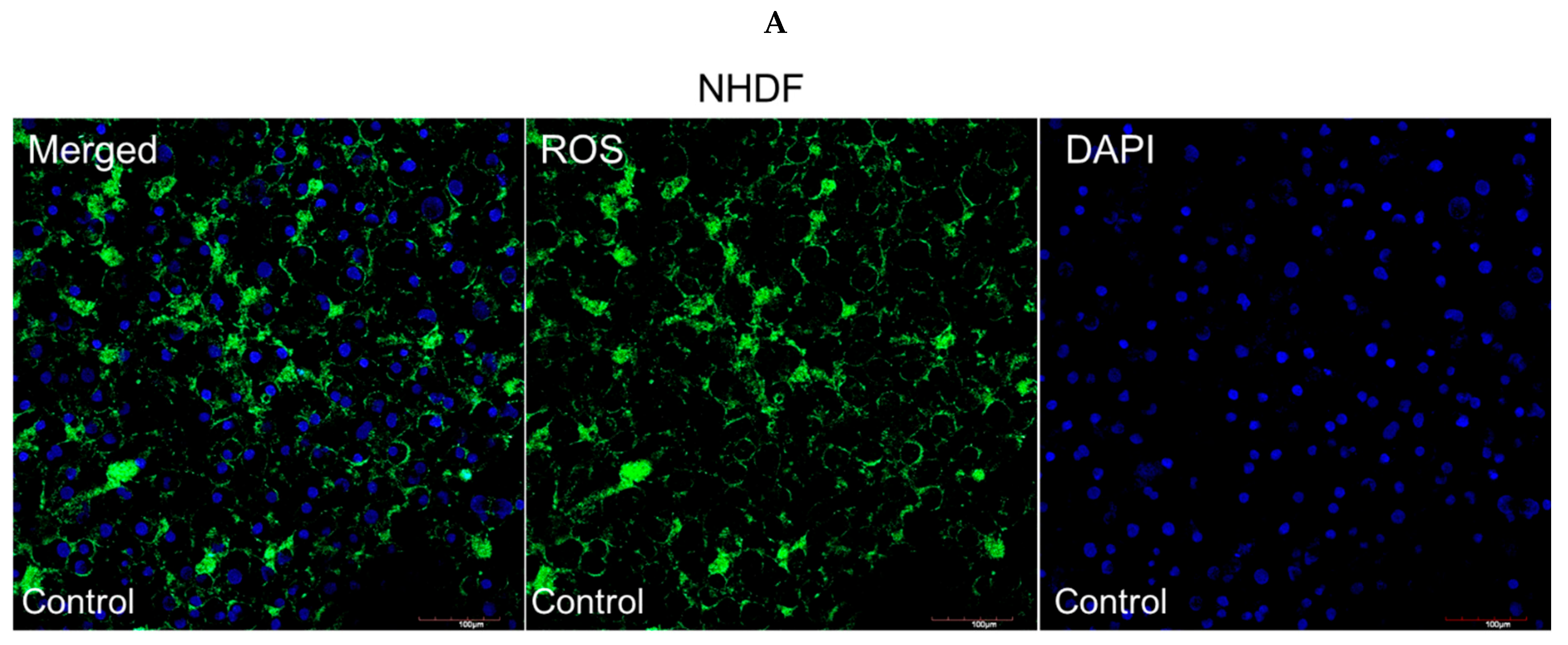
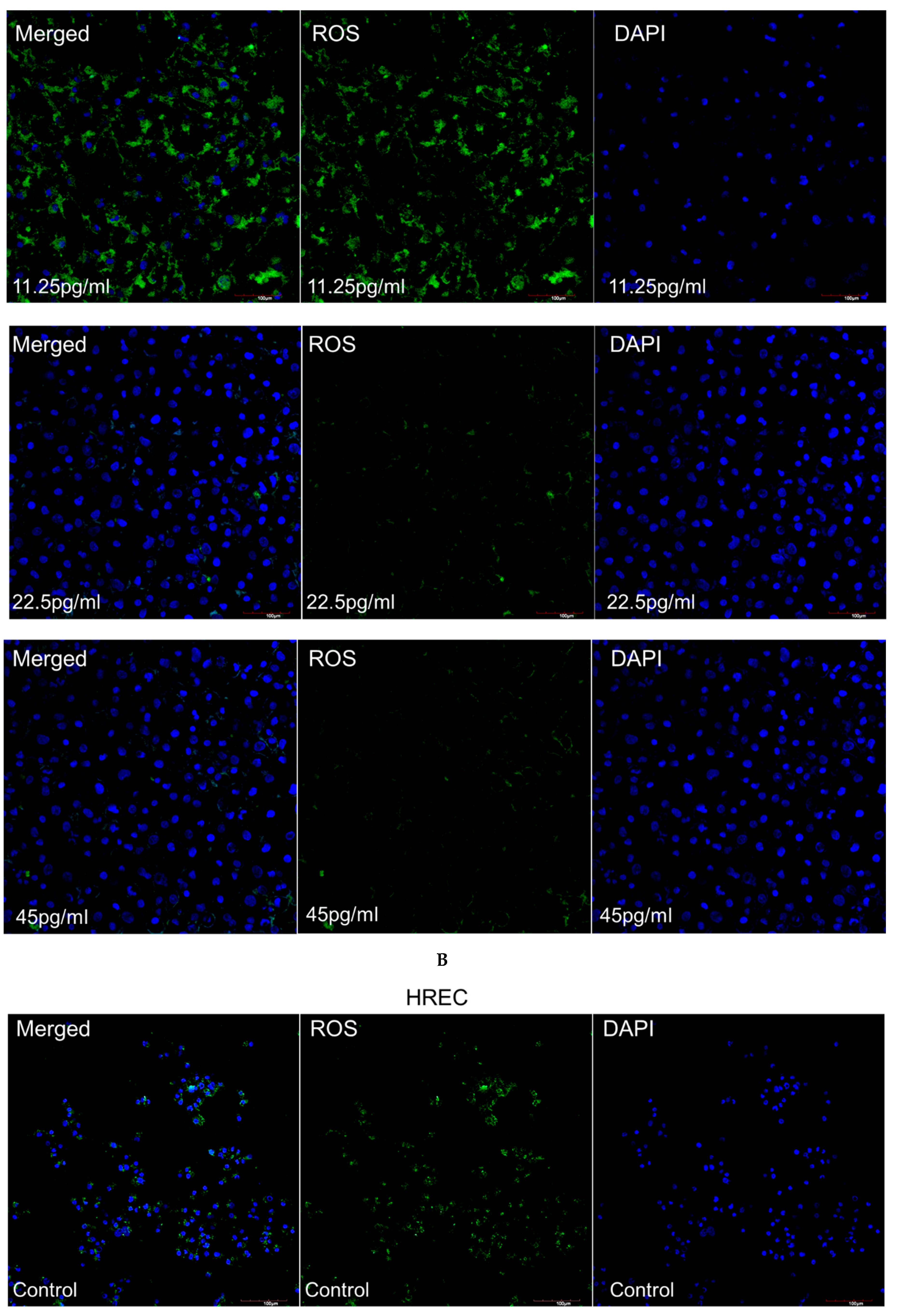
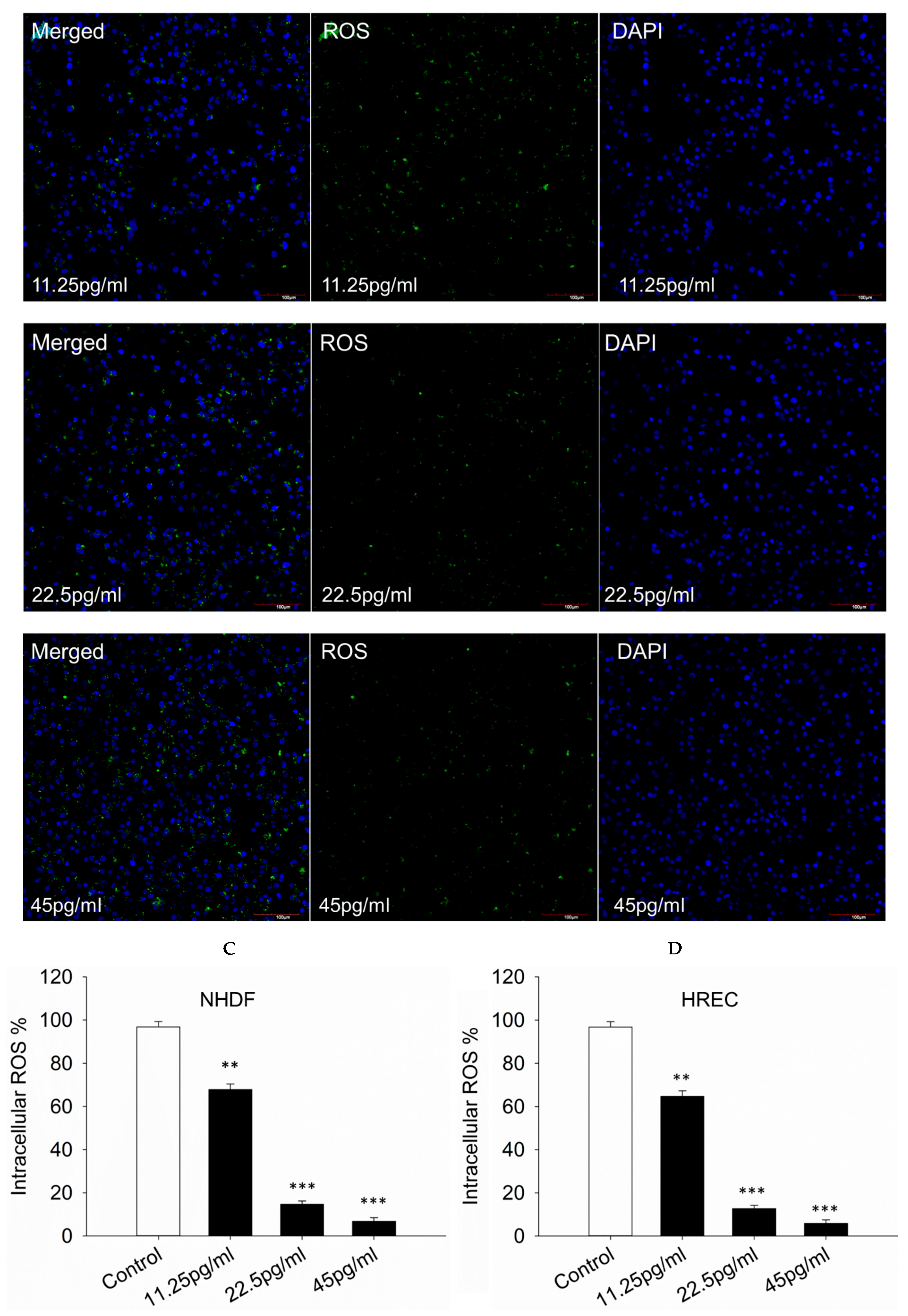
3.4. Intracellular ROS Production
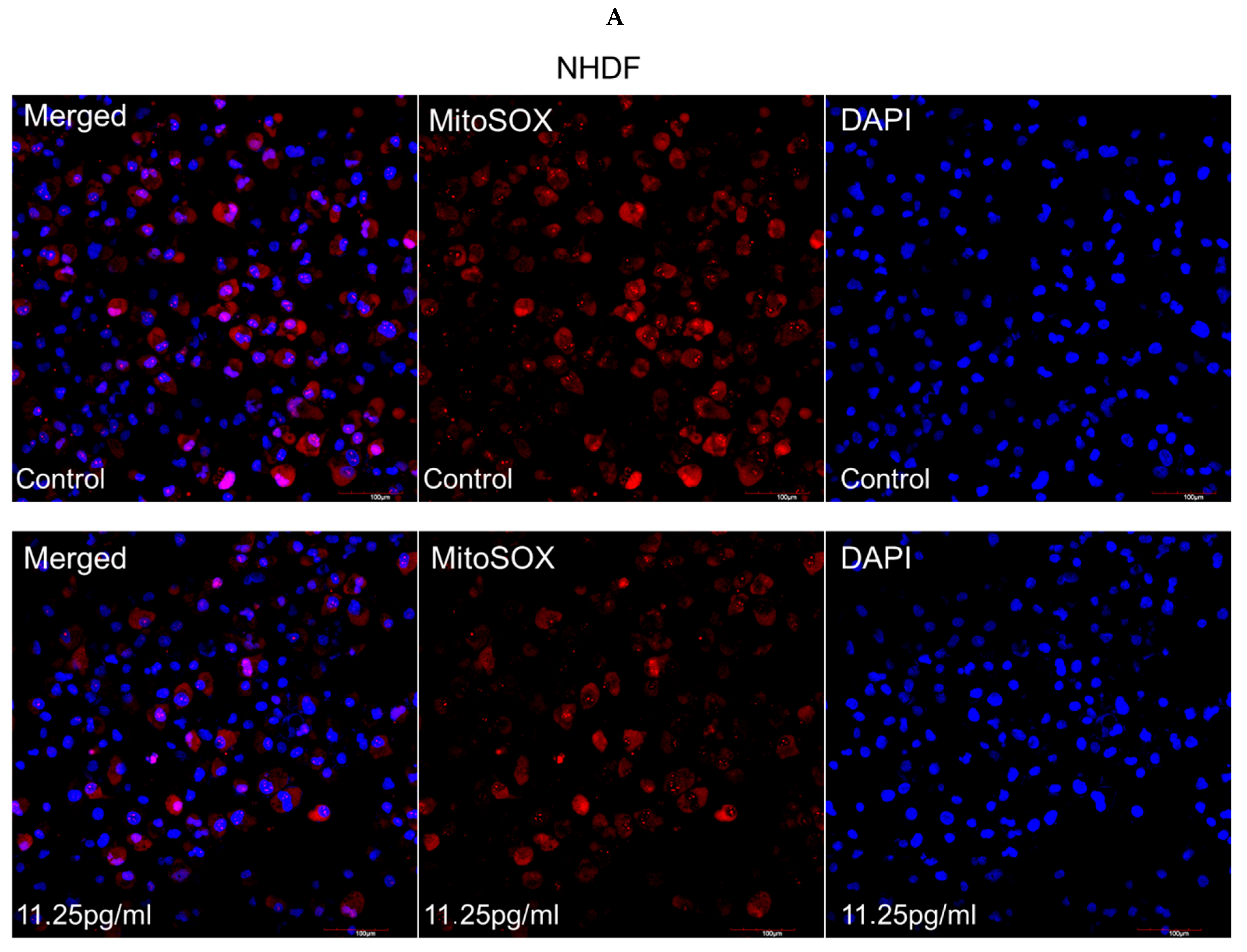
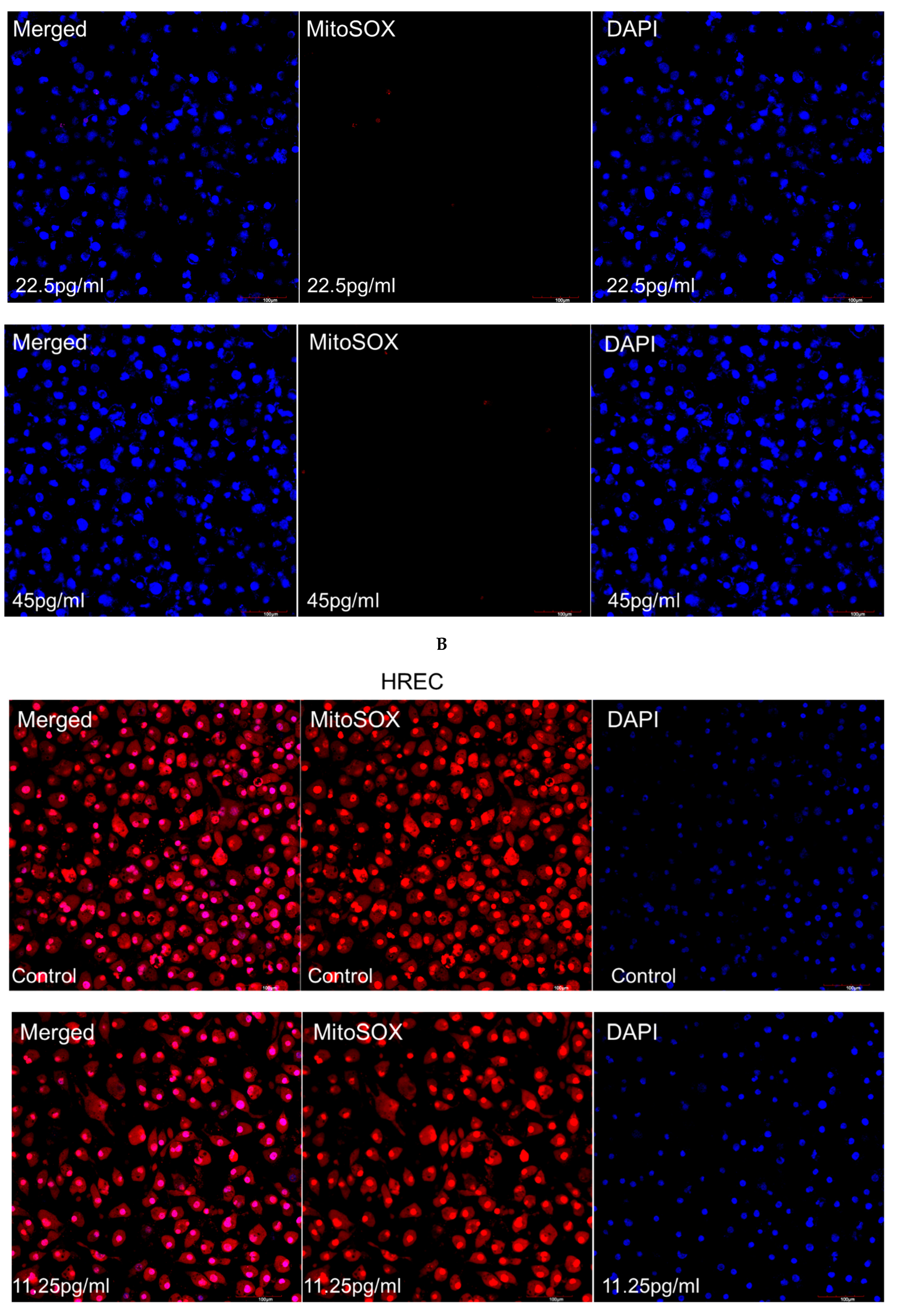
3.5. Mitochondrial Superoxide Production
3.6. AGE and RAGE Expression
3.7. Apoptosis Levels
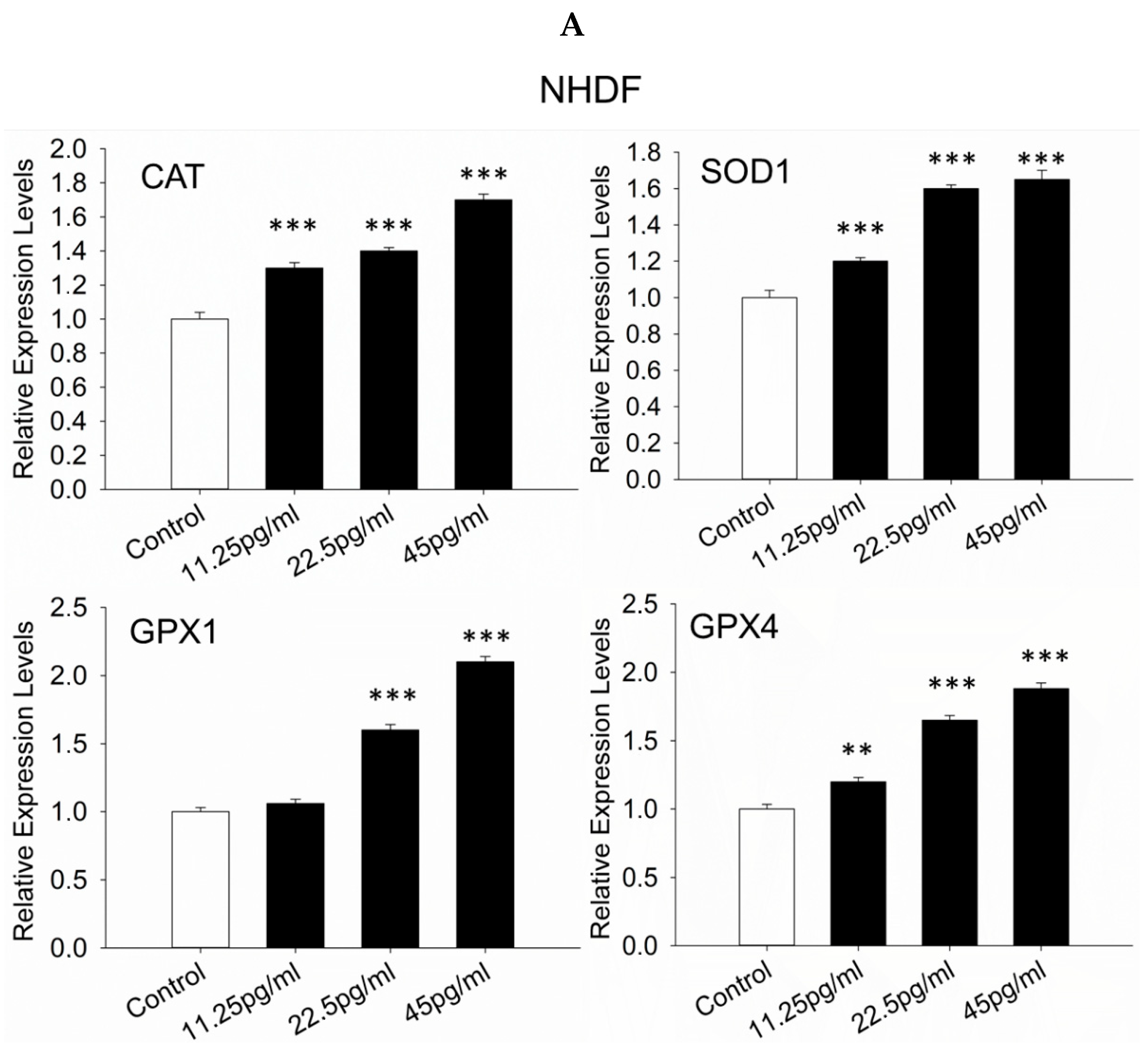
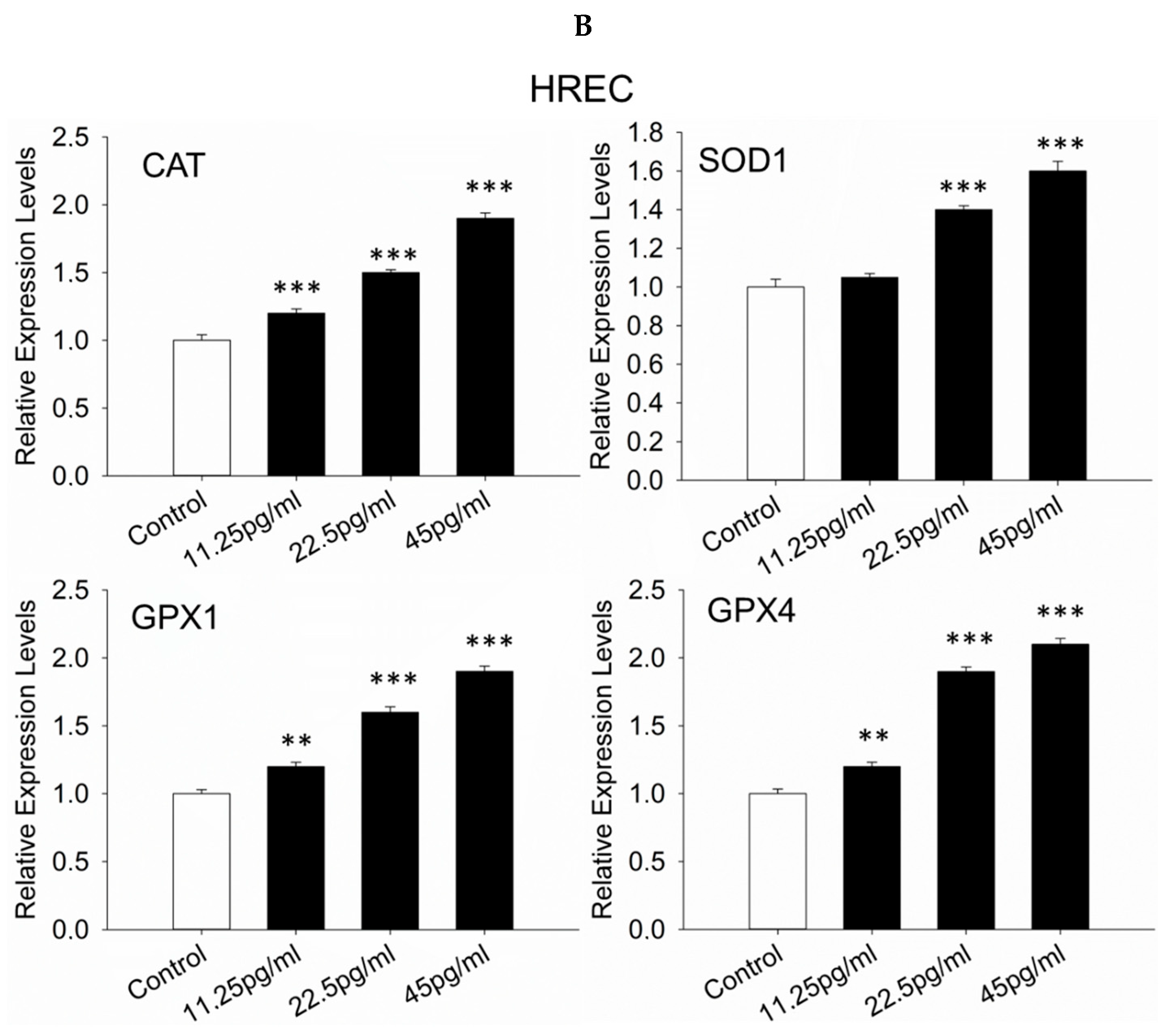
3.8. Antioxidant Response-Related Genes
3.9. Semaglutide Increased Wound Closure Capacity in NHDFs
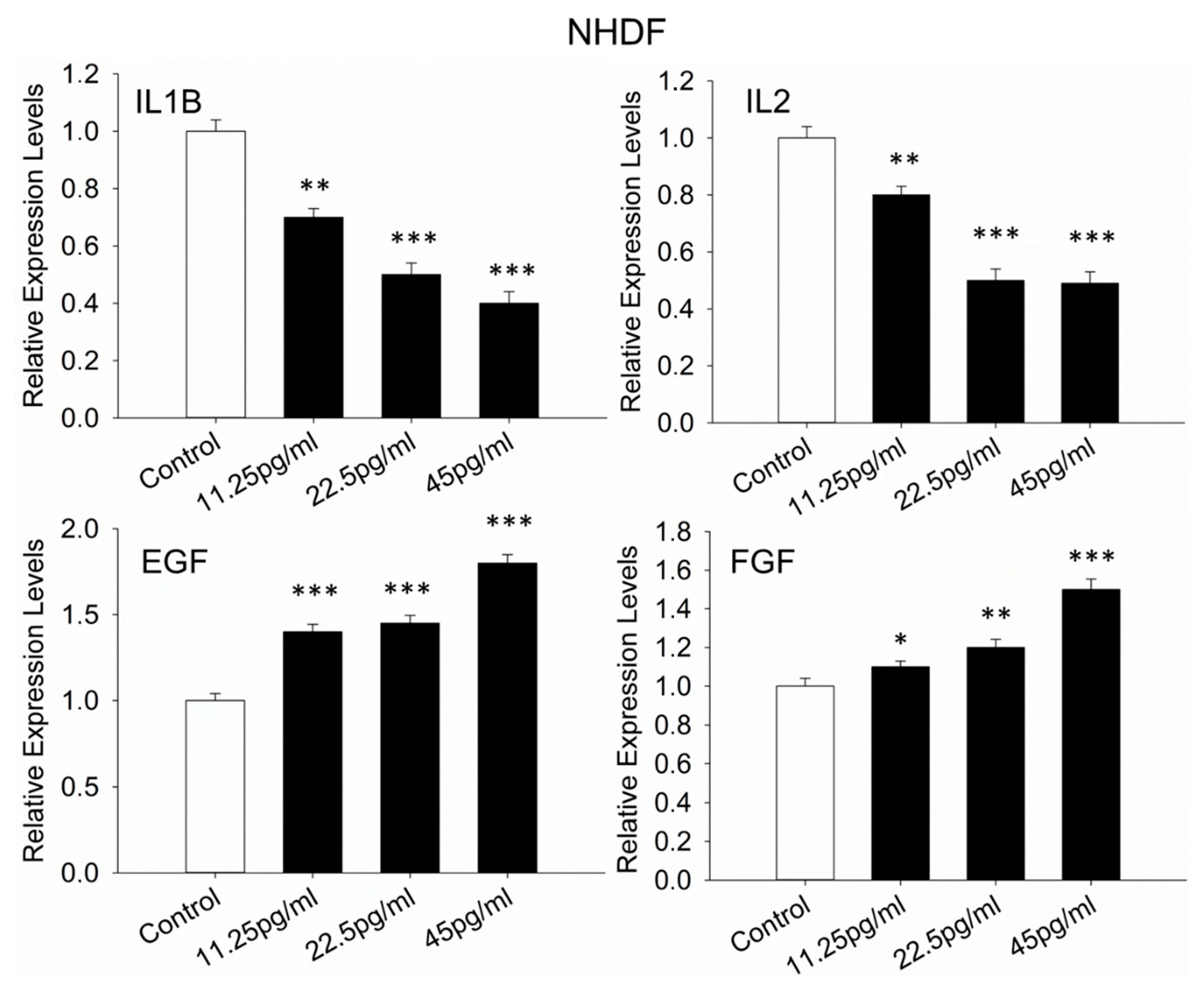
3.10. Wound-Healing-Evaluation-Related Genes in NHDFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ACTB | Actin Beta |
| AGEs | Advanced Glycation End-products |
| COL1A1 | Collagen Type I Alpha 1 Chain |
| COL3A1 | Collagen Type III Alpha 1 Chain |
| COL4A1 | Collagen Type IV Alpha 1 Chain |
| COL6A1 | Collagen Type VI Alpha 1 Chain |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DMSO | Dimethyl Sulfoxide |
| DR | Diabetic Retinopathy |
| FGF | Fibroblast Growth Factor |
| HREC | Human Retinal Endothelial Cells |
| IL1B | Interleukin 1 Beta |
| IL2 | Interleukin 2 |
| IL3 | Interleukin 3 |
| IL4 | Interleukin 4 |
| IL5 | Interleukin 5 |
| IL9 | Interleukin 9 |
| MMP3 | Matrix Metallopeptidase 3 |
| MMP9 | Matrix Metallopeptidase 9 |
| MTT | 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide |
| NHDF | Normal Human Dermal Fibroblasts |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B (Akt) |
| ROS | Reactive Oxygen Species |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| SOD | Superoxide Dismutase |
| VEGF | Vascular Endothelial Growth Factor |
References
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Al-Noshokaty, T.M.; Abdelhamid, R.; Abdelmaksoud, N.M.; Khaled, A.; Hossam, M.; Ahmed, R.; Saber, T.; Khaled, S.; Elshaer, S.S.; Abulsoud, A.I. Unlocking the multifaceted roles of GLP-1: Physiological functions and therapeutic potential. Toxicol. Rep. 2025, 14, 101895. [Google Scholar] [CrossRef]
- Goldenberg, R.M.; Gilbert, J.D.; Manjoo, P.; Pedersen, S.D.; Woo, V.C.; Lovshin, J.A. Management of type 2 diabetes, obesity, or nonalcoholic steatohepatitis with high-dose GLP-1 receptor agonists and GLP-1 receptor-based co-agonists. Obes. Rev. 2024, 25, e13663. [Google Scholar] [CrossRef]
- Brunton, S.A.; Wysham, C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: Role and clinical experience to date. Postgrad. Med. 2020, 132, 3–14. [Google Scholar] [CrossRef]
- Giannakogeorgou, A.; Roden, M. Role of lifestyle and glucagon-like peptide-1 receptor agonists for weight loss in obesity, type 2 diabetes and steatotic liver diseases. Aliment. Pharmacol. Ther. 2024, 59 (Suppl. S1), S52–S75. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Nickerson, H.D.; Dutta, S. Diabetic complications: Current challenges and opportunities. J. Cardiovasc. Transl. Res. 2012, 5, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory cytokines in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 948417. [Google Scholar] [CrossRef]
- Garibotto, G.; Carta, A.; Picciotto, D.; Viazzi, F.; Verzola, D. Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J. Nephrol. 2017, 30, 719–727. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Dawi, J.; Misakyan, Y.; Affa, S.; Kades, S.; Narasimhan, A.; Hajjar, F.; Besser, M.; Tumanyan, K.; Venketaraman, V. Oxidative Stress, Glutathione Insufficiency, and Inflammatory Pathways in Type 2 Diabetes Mellitus: Implications for Therapeutic Interventions. Biomedicines 2025, 13, 18. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Al Qassab, M.; Mneimneh, M.; Jradi, A.; Derbas, B.; Dabboussi, D.; Khoury Baini, J.; Katrib, N.; Chaarani, N.; Attieh, P.; Kanaan, A.; et al. The Expanding Role of GLP-1 Receptor Agonists: Advancing Clinical Outcomes in Metabolic and Mental Health. Curr. Issues Mol. Biol. 2025, 47, 285. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic Effects of GLP-1 and Analogs on Cell Signaling, Metabolism, and Function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 2024, 33, e15065. [Google Scholar] [CrossRef]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Kapoor, I.; Sarvepalli, S.M.; D’Alessio, D.; Grewal, D.S.; Hadziahmetovic, M. GLP-1 receptor agonists and diabetic retinopathy: A meta-analysis of randomized clinical trials. Surv. Ophthalmol. 2023, 68, 1071–1083. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kato, A.; Onsui, A.; Kanatanai, S.; Ishimura, A.; Kurita, T.; Nakajima, T. Impact of Glucagon-like Peptide-1 Receptor Agonists on Intestinal Epithelial Cell Barrier. Eur. Pharm. J. 2024, 71, 43–52. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Zhu, C.; Guo, B.; Yang, W.; He, W.; Li, X.; Wang, Y.; Li, W.; Wang, F.; et al. Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole-kindled mice. Int. J. Mol. Med. 2021, 48, 219. [Google Scholar] [CrossRef]
- Anastasiou, I.A.; Sarantis, P.; Eleftheriadou, I.; Tentolouris, K.N.; Mourouzis, I.; Karamouzis, M.V.; Pantos, K.; Tentolouris, N. Effects of Hypericin on Cultured Primary Normal Human Dermal Fibroblasts Under Increased Oxidative Stress. Int. J. Low. Extrem. Wounds 2023, 15347346231212332. [Google Scholar] [CrossRef]
- Ruan, W.; Lai, M. Actin, a reliable marker of internal control? Clin. Chim. Acta 2007, 385, 1–5. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simó, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2018, 20, 889–897. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Younessi, P.; Yoonessi, A. Advanced glycation end-products and their receptor-mediated roles: Inflammation and oxidative stress. Iran. J. Med. Sci. 2011, 36, 154–166. [Google Scholar]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Bogdanov, P.; Ramos, H.; Huerta, J.; Simó-Servat, O.; Hernández, C. Effects of the Topical Administration of Semaglutide on Retinal Neuroinflammation and Vascular Leakage in Experimental Diabetes. Biomedicines 2021, 9, 926. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory benefits of semaglutide: State of the art. J. Clin. Transl. Endocrinol. 2024, 36, 100340. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Tsioufis, K.; Katsi, V. Spotlight on the Mechanism of Action of Semaglutide. Curr. Issues Mol. Biol. 2024, 46, 14514–14541. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, N.; Gao, G.; Ma, Y.; Yang, Q.; Chen, K.; Guo, W. Semaglutide functional electrospinning nanofiber membrane rescues apoptosis and promotes diabetic wound healing. Eur. Polym. J. 2024, 220, 113448. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kang, Y.H.; Kang, M.K. Umbelliferone alleviates impaired wound healing and skin barrier dysfunction in high glucose-exposed dermal fibroblasts and diabetic skins. J. Mol. Med. 2024, 102, 1457–1470. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasiou, I.A.; Tentolouris, A.; Sarantis, P.; Katsaouni, A.; Rebelos, E.; Mourouzis, I.; Pantos, C.; Tentolouris, N. Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro. Pharmaceutics 2025, 17, 1115. https://doi.org/10.3390/pharmaceutics17091115
Anastasiou IA, Tentolouris A, Sarantis P, Katsaouni A, Rebelos E, Mourouzis I, Pantos C, Tentolouris N. Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro. Pharmaceutics. 2025; 17(9):1115. https://doi.org/10.3390/pharmaceutics17091115
Chicago/Turabian StyleAnastasiou, Ioanna A., Anastasios Tentolouris, Panagiotis Sarantis, Athanasia Katsaouni, Eleni Rebelos, Iordanis Mourouzis, Constantinos Pantos, and Nikolaos Tentolouris. 2025. "Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro" Pharmaceutics 17, no. 9: 1115. https://doi.org/10.3390/pharmaceutics17091115
APA StyleAnastasiou, I. A., Tentolouris, A., Sarantis, P., Katsaouni, A., Rebelos, E., Mourouzis, I., Pantos, C., & Tentolouris, N. (2025). Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro. Pharmaceutics, 17(9), 1115. https://doi.org/10.3390/pharmaceutics17091115







