Abstract
In this study, three new 3,7-dihydroxyflavone (1) derivatives with different sugars were designed and synthesised by click chemistry. Click chemistry requires the previously modification of building blocks with azide and alkyne groups and therefore, the 3,7-dihydroxyflavone (1) was first converted in 3,7-(prop-2-yn-yloxy)flavone (2) and acetobromo-α-D-glucose (3) was converted into 2,3,4,6-tetra-O-acetyl-β-glucopyranosyl azide (4). Subsequently, a click reaction was performed via copper-catalysed cycloaddition (CuAAC) between 2 and 4, as well as between 2 and 2-acetamido-3,4,6-tetra-O-acetyl-2-deoxy-β-D-glucopyranosyl (AG931) and, 2 and commercial 2-azidoethyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl (AG358), resulting in three distinct disubstituted flavone glycosides (5a–5c). Biological assays performed on L929 fibroblast cell lines and human glioblastoma astrocytoma U-251 cell lines indicated cytocompatibility with fibroblasts and reduced metabolic activity of GBM cells in the presence of compound 5b and 5c. To enhance therapeutic effect, improve local drug delivery, and overcome solubility issues of these high molecular weight compounds, the synthesised compounds were encapsulated in polymeric particles (polymersomes, PMs) composed of polylactic acid-polyethylene glycol (PEG-PLA) functionalized, once more by click chemistry, with 0.1 mol% transferrin mimetic (T7—HRPYIAH) peptide. The PMs were prepared by solvent displacement and exhibited stability over 100 days, encapsulation efficiency of 39–93%, and mean size diameters of 120–180 nm. The toxicity assays of the PMs on the U-251 cell line showed a significant decrease in metabolic activity, supporting the potential of this delivery system against GBM. Among the PMs tested, the flavone 5c-based PM demonstrated the highest efficacy.
1. Introduction
Glioblastoma Multiforme (GBM), a grade IV astrocytoma commonly known as Glioblastoma, is the most aggressive, invasive type and accounts for almost 80% of all Central Nervous System tumours [1,2,3,4,5,6]. GBM is considered a rare disease, and the current treatment includes surgery, radiotherapy, and/or chemotherapy [1,7]. GBM presents a significant intratumorally heterogeneity, high mitotic activity, tissue necrosis, vascular proliferation, resistance to apoptosis, and penetration into adjuvant cells [8]. This rapid growth in deep brain areas complicates local accessibility and the easiness of diffusion makes the tumour resection after surgery almost impossible [9,10]. Therefore, this cancer is associated with a poor prognosis: The median survival rate is 12 months [4,5,11,12] as patients invariably relapse [13]. After five years of post-diagnosis, the survival rate is less than 5% [14]. The worldwide incidence is less than 0.01% and is 1.6 times greater in men than women. As for the current therapeutic agents, temozolomide is the first-line chemotherapeutical drug in the use of GBM treatment [15,16,17,18,19].
The biological challenges of therapy are significant. Firstly, the ineffectiveness of antiproliferative therapies can be partly attributed to the discovery of tumour heterogeneity. This dynamic heterogeneity enables resistance to combination oncology, with therapy-resistant subclones forming as the tumour grows [20,21]. Secondly, treatment resistance and tumour recurrence in GBM may be linked to its microenvironment [22,23,24,25,26,27]. Finally, the blood–brain barrier (BBB) consists of a layer of non-fenestrated capillary endothelial cells interconnected through a network of intercellular junctions [28]. In both primary and metastatic brain tumours, the integrity of the BBB is compromised, leading to the formation of the brain–tumour barrier (BTB). A leaky BBB/BTB implies that the membrane no longer restricts drug delivery, affecting the effectiveness of treatments [29]. Emerging evidence [30,31,32,33,34] suggests that the heterogeneous breakdown of the BBB makes it challenging to achieve uniform drug concentrations inside the tumour.
Flavonoids are a large and wide family of polyphenolic metabolites derived from plants [35,36,37,38]. Flavonoids have a unique structure: a three-ring base consisting of two benzene rings linked either between a pyran heterocyclic ring or a three-carbon liker forming a fifteen-carbon skeleton (C6-C3-C6 system). Scientists have found over five thousand flavonoids from a plethora of plants [35,36,37,39]. There is evidence that many natural flavonoids possessed anticancer activity. For example, isoflavone Genistein promotes breast cancer cell arrest and induces Reactive Oxygen Species dependent apoptosis [40]. Daidzein induces apoptosis in breast cancer cells [41]. Flavanone Hesperetin triggers apoptosis in bladder carcinoma [42], oesophageal cancer [43], hepatocellular carcinoma [44], and human breast carcinoma [45]. Flavonoids such as Quercetin, Myricetin, Genistein, Delphinidin, Luteolin, Apigenin, Baicalein, Galangin, or even Diosmetin can decrease the level of proliferation and/or invasion of GBM cells [46]. Synthetic flavonoids [35,36,47] have also been reported for their antitumoral activity. From the several synthetic flavonoids reported with antitumor activity, two acetic acid flavonoids have been reported to have activity against advanced non-small cell lung cancer [48].
Several bioactive synthetic flavonoids were also reported by our group [49,50,51,52], and among them, an acetoglycosyl flavonoid was shown to have an anti-proliferative effect in human cancer cell lines, namely GBM [53].
In this study, we designed three new di-acetoglycosyl triazole-linked flavones. We describe their synthesis, structure, and anti-proliferative properties for the first time. By adding a triazole ring between the flavone and acetylated glycosides, we improve their metabolic stability compared to the more common O-glycosidic bonds [54]. To overcome their limited solubility, we encapsulated these compounds in polymersomes (PMs), using transferrin-triazole-linked PEG-PLA copolymers [55], while enhancing their potential for targeting delivery. PMs are spherical and hollow nanosystems composed of amphiphilic copolymers that can encapsulate hydrophilic and hydrophobic drugs, individually or at the same time [56]. These drug delivery systems (DDS) have more content retention [57], have superior functionalisation choices [58], higher bioavailability and biodegradability, and have the possibility of cargo release induced by stimuli, compared to others [51,54,58].
2. Results and Discussion
2.1. Synthesis and Structure Elucidation
Three new di-glycosyl flavones bearing a 1,2,3-triazole moiety and different sugar moieties were synthesised in this study.
There has been a great interest in the synthesis of 1,2,3-triazole functional groups since these moieties are more than just linkers. They have some favourable physicochemical properties and have shown importance to biological activity [59,60]. This strategy generates many biologically potential compounds [61,62,63,64] since hundreds of synthetic 1,2,3-triazole-linked flavonoid analogues have been reported to have antitumor activity [52]. For the chemical synthesis of these triazole-linked flavones, the 2022 Nobel prize “click” chemistry was chosen. Sharpless and co-workers [65] enormously impacted chemistry philosophy by discovering “click” reactions. Click chemistry is a class of nearly perfect chemical reactions that are effective in terms of atom economy, stereo-specificity, wide scope, and almost all properties that today are called the green chemistry principles. The reaction is enormously selective since it only occurs when azide and alkyl groups are present. The copper-catalysed azide–alkyne cycloaddition (CuAAC) is a variant of the classical thermal Huisgen 1,3-dipolar cycloaddition and was described by Sharpless as the ‘cream of the crop’ of click chemistry [66,67]. By using copper (CuSO4) and sodium ascorbate, the energy necessary for the activation barrier is decreased significantly, making the reaction possible to proceed at room temperature and in aqueous or organic solvents, leading to a 1,4-disubstituted triazole [68].
To achieve these flavones, the flavone 2 and the glycoside 4 were firstly synthesised (Scheme 1). The 3,7-dihydroxyflavone (1) propargylation was carried out in basic conditions, with acetone as solvent due to its polar aprotic characteristics. The usage of TBAHS was to help solubilise the negative charges of the phenoxide ion. This reaction proceeded as in previous work from our group; it was inspired by a propargylation reaction on polyphenols [69] and provided a yield of 95% of compound 2.
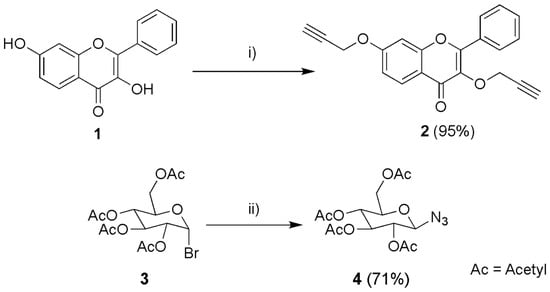
Scheme 1.
Synthesis of 3,7-(prop-2-yn-yloxy) flavone (2) and 2,3,4,6 tetra-O-Acetyl-β-D-glucopyranosyl azide (4). (i) Propargyl bromide, Tetrabutylammonium hydrogen sulphate (TBAHS), Cs2CO3, acetone, 60 °C, 8 days; (ii) NaN3, acetone:water, room temperature, 4 h.
A simple and effective glycosyl-azide synthesis was carried out by a reaction of glycosyl-halide with sodium azide in aqueous acetone as solvent. It has been shown that the combination of water-acetone as solvent is more efficient than dry DMF, caused by poor solubility of NaN3 [70]. Dissolving NaN3 in water increases the concentration of the azide ions and thus the reaction rate. After the acetone evaporated, the pure solid precipitated from the water. The reaction provided a yield of 71%.
Compounds 5a–c were synthesised by CuAAC. The reactions involved the previously formed flavone (2) together with three similar glycosyl-azide substrates: 2,3,4-tetra-O-acetyl-β-glucopyranosyl azide (4), commercial 2-acetamido-3,4,6-tetra-O-acetyl-2-deoxy-β-D-glucopyranosyl (AG931), and commercial 2-azidoethyl 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl (AG358). These reactions were performed in the presence of copper sulphate (CuSO4) and sodium ascorbate in a non-homogeneous mixture of water and THF (1:4) at room temperature (Scheme 2).
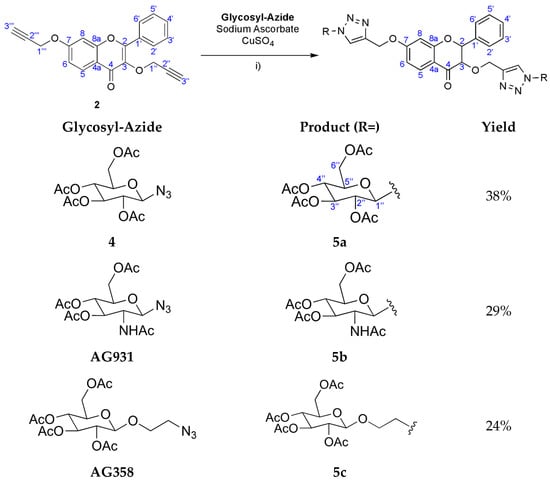
Scheme 2.
Synthesis of flavonoids 5a–5c. (i) 5a: 4, THF: water, room temperature, 24 h; 5b: AG931, THF: water, room temperature, 2 h; 5c: AG358, THF: water, room temperature, 2 h. Ac = Acetyl (COCH3).
After the THF evaporated, an impure white solid precipitated in the remaining water in the case of 5a and 5b syntheses. Therefore, by using chromatographic preparative TLC plates (5:5 CHCl3—Acetone), 5a was obtained in 35% yield and 5b in 29% yield. In the case of 5c synthesis, no solid was formed from water. This water solution was then extracted with AcOEt and the organic phase purified by crystallisation from MeOH/CH2Cl2, where 5c was obtained in 24% yield.
However, it was not the first time that chromatographic procedures together with the low yield obtained and the presence of by-products were described when using click chemistry [52,65,71].
For the flavonoid building block (2), the alkyne group could be easily identified by a strong band of the triple CC bond at 2107 cm−1, and another band at 3274 cm−1 from the alkyne group (≡C-H). By 1H NMR spectrometry, the two proton signals from the two alkynes functional groups (≡C-H) could be observed (δH-3‴ 3.68 s, δH-3″ 3.47 s). Similarly, by 13C NMR spectrometry, the four carbon signals from the two alkenes (C≡C) were also observed (δC-3‴ 79.3, δC-3″ 79.1, δC-2″ 78.7, δC-2‴ 78.3). For the glycosyl-azide building block (4), the presence of the azide could be observed by the characteristic strong band at 2119 cm−1.
The NMR spectra (see Supplementary Material) of the newly synthesised compounds 5a–5c showed characteristic signals for the flavone scaffold and the glycosyl moieties. Additionally, signals of the triazole proton (δH 8.62–7.88, s) and carbons (δC 143.1–141.6 and 125.2–123.8) were observed by NMR. The placement of the triazole ring in these compounds was confirmed by the heteronuclear multiple bond correlation (HMBC) spectra. Special nomenclature for this correlation attributions’ were used. These spectra showed a correlation between the proton signals A (-C=CH-triazole) and C (-O-CH2-triazole) with carbon B signal (-C=CH-triazole), and carbon B with the C-7 signal. The same was observed for the other triazole ring with two bridges of correlations between the proton signals D and F with carbon E signal, and proton D signal with C-3 signal (Figure 1). These correlations were observed for the three di-glycosyl flavones.
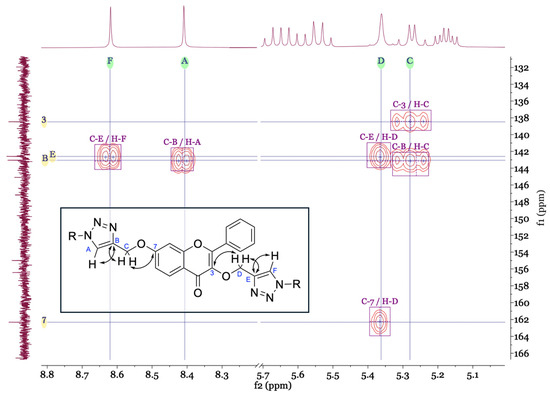
Figure 1.
Main connectivities found in HMBC for the identification of the triazole ring positions.
The molecular formula of 5a–5c was also confirmed by HRMS as C49H52N6O22 (1099.288), C49H54N8O20 (1097.319) and, C53H60N6O24 (1187.338), respectively.
Comparing the IR spectrum of 5a with 2, the spectrum of 5a has a second strong band due to carbonyl groups of the acetyl groups at 1751 cm−1 and other at 1227 cm−1 typical of C-O of the acetyl groups. Also, it is visible that the characteristic strong band of the triple CC bond (at 2107 cm−1) disappears, underscoring the reaction success. This is also visible in the IR spectrum of 5b and 5c: here, we see the new acetyl C=O bands at 1747 cm−1 and 1750 cm−1, respectively. For the acetamido 5b compound, a strong band at 1621 cm−1 was observed, characteristic of the amide C=O stretch.
2.2. Cytotoxicity of Free Compounds
Acetylated flavonoids 5a–5c were evaluated for their in vitro growth inhibitory by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay. MTT assay measures cellular metabolic activity as an indicator of cell viability and proliferation. This colorimetric assay is based on the chemical reduction in the yellow tetrazolium salt (MTT) to purple formazan crystals by metabolically active cells.
The cytotoxicity of compounds on the fibroblast L929 cell line (Figure 2a) was first evaluated according to the procedure described by the International Organization for Standardization (ISO 10993-5:2009) [72].
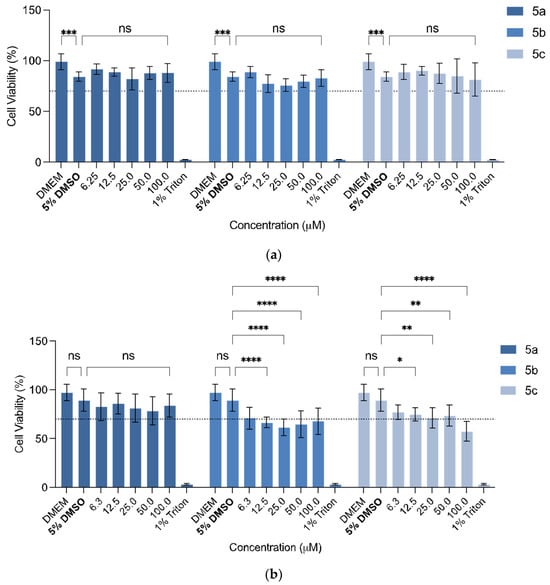
Figure 2.
Cell viability (%) effect on (a) the fibroblast L929 cell line and (b) the human glioblastoma astrocytoma U-251 cell line of free synthesised compounds. Each value represents a total of n = 3 replicates of n = 3 independent assays (a total of 9 replicates). DMEM was used as a positive control and 1% Triton X-100 as a negative control. * (One asterisk): p-value <0.05; ** (Two asterisks): p-value < 0.01; *** (Three asterisks): p-value < 0.001; **** (Four asterisks): p-value < 0.0001.
The presence of 5% DMSO had significant effect on cell viability (CV) of the L929 cell line (p = 0.0003, Figure 2). Considering the cytotoxicity of the compounds in this cell line, some significant changes in the CV percentages were observed; however, none of them decreased the CV below 70% (which is the safety threshold, according to the ISO 10993-5:2009) (Figure 2a). None of the compounds showed significant viability changes, proving that their use is safe up to 100 µM (5a: p > 0.9999; 5b: p = 0.4695; 5c: p = 0.2876).
The same compounds were also tested, with a similar procedure, against the human glioblastoma astrocytoma U-251 cell line (Figure 3b). The presence of 5% DMSO did not affect the CV of the U-251 cell line. An anticipated outcome of these studies would be the decrease in the CV vs. concentration in this cell line in contrast to the first cell line tested. Compound 5b, with the acetylated N-acetyl glucosamide, significantly decreased the CV across all concentrations (p = 0.0018, for 6.3 µM). However, the CV did not decrease below 50%, even at the highest concentration tested (100 µM). Regarding compound 5a, there was no significant differences across all concentrations, and compound 5c, the only statistically significant difference against this cell line, was at >12.5 µM (p = 0.0248). Here, once more, the CV did not decrease below 50%.
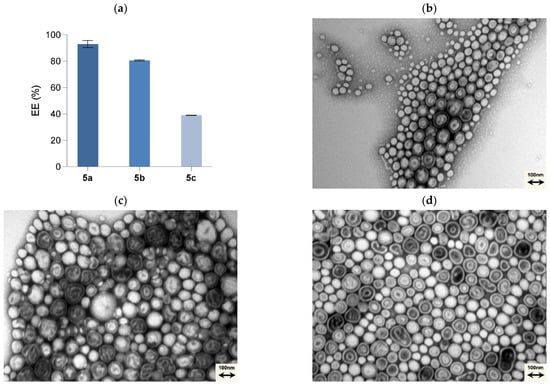
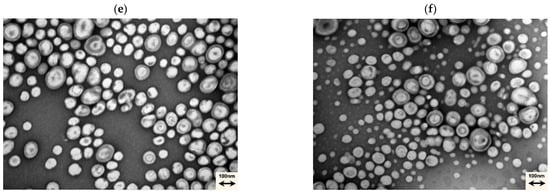
Figure 3.
Entrapment efficiency (EE%) of the compounds synthesised (a) and TEM images of the formulations 0 mol% T7-PEGPLA (control) (b), 0.1 mol% T7-PEGPLA (control) (c), 0.1 mol% T7-PEGPLA-5a (d), 0.1 mol% T7-PEGPLA-5b (e), and 0.1 mol% T7-PEGPLA-5c (f).
2.3. Formation and Characterisation of Loaded and T7-Functionalised PMs
Transferrin is a plasma protein that transports iron and binds to receptors overexpressed on the BBB due to the high iron demand for intermediary metabolism [73]. These receptors are also overexpressed in several tumour cells [74]. To access this functionality, T7 (HAIYPRH) peptide, a targeting ligand that can specially bind to transferrin receptors was employed to be part of the PMs delivery system. This peptide is a combination of seven amino acids (His-Ala-Ile-Tyr-Pro-Arg-His) and was commercially functionalised to have an alkyl end-group. T7-functionalised PEG20PLA112 (T7-PEGPLA) polymer was prepared as reported in Ref. [55].
The same solvent displacement method was employed in the assembly of these functionalised copolymers. T7-PEGPLA and PEGPLA were mixed with a molar ratio of 0,1%. The mean diameter and the PDI of the five formulations of T7-PEGPLA, mixed with PEGPLA and loaded with 5a–5c, were evaluated by DLS (Table 1). The zeta potential (ζ) was also measured.

Table 1.
The mean diameter, PDI, and zeta potential (ζ) of loaded and functionalised PMs prepared by the solvent displacement method (mean ± SD, n = 3).
In a statistical analysis of the mean diameter, the empty PMs’ formulations (0 mol% and 0.1 mol%) did not show any statistical difference (p = 0.9993), proving that the presence of the T7-PEGPLA does not alter the morphology of the particle.
Regarding the loaded PMs, the biggest increase in size was with the 0.1 mol% T7-PEGPLA-5a formulation, from a mean of 122.7 to 184.4 nm (p < 0.0001). The 0.1 mol% T7-PEGPLA-5b formulation had a mean of 161.6 nm (p = 0.0010), whereas the 0.1 mol% T7-PEGPLA-5c formulation had a mean of 153.2 nm (p = 0.0030). These differences in size can be correlated to the entrapment efficiency (Figure 3a) of each formulation, given that the empty formulations (0 mol% and 0.1 mol%) show similar mean diameters and PDIs. Compound 5a had the biggest entrapment efficacy (93 ± 2.73%), followed by 5b (80 ± 0.49%), and finally 5c (39 ± 0.12%). Higher EE% seems to increase more the mean diameter than a lower EE% formulation.
Also, by TEM analysis (Figure 3b–f), it is possible to visually affirm that no morphological change occurs.
The functionalised PMs were stored over time at 4 °C, their stability was determined by DLS and Zeta potential, and showed no significant changes for 100 days, indicating the strong stability of the prepared diblock copolymers’ PMs and the stability of the system with the entrapped compounds (Figure 4).
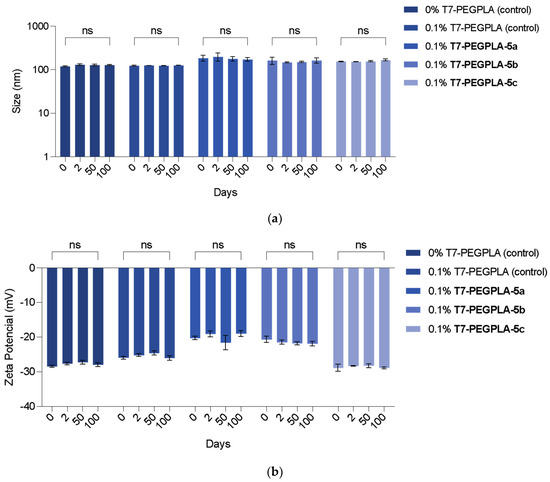
Figure 4.
Effective mean diameter (a) and zeta potential (b) of functionalised PMs formulations stored at 4 °C for 100 consecutive days (each value represents a total of n = 3 replicates). The Size (nm) y-axis is presented in a logarithmical (log10) scale.
2.4. Anti-Glioblastoma Activity of Compounds Entrapped in Functionalised PMs
The three formulations containing the entrapped flavonoids were evaluated for their in vitro growth inhibitory, also by the MTT assay (Figure 5). The usage of 5% DMSO was no longer needed given that the compounds could be administered in an aqueous media with the help of the PMs’ system. The assay was carried out taking considering the compounds’ concentration inside the PMs, given that the intrinsic toxicity of this PMs was already proven to not be significant [55]. There was a visible effect of the exposure of the U-251 cell line to the prepared PMs. The PMs’ system with compounds 5a and 5b only decreased the cell viability of the U-251 cell line below 50% at 200 µM, resulting in these systems only exhibiting significant effects at higher concentrations. For the PMs with compound 5c, with the ethyl linker, almost all concentrations tested decreased the cell viability below the 50%, with the highest concentration resulting in only 21% of GBM cell line viability. Compared with the free compounds (Figure 2b), it is possible to see that, in the same dose, this compound can achieve a higher effect on the cell viability. Therefore, this compound, by having a substitution of a 2-azidoethyl in the anomeric carbon, proved to be the most promising against the U-251 cell line of the three analogues.
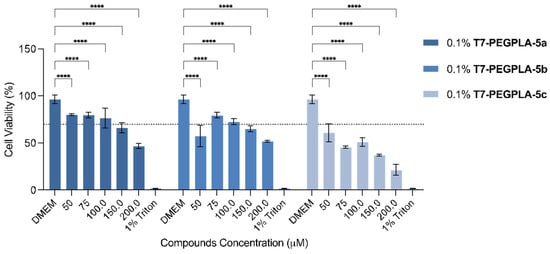
Figure 5.
Cell viability (%) effect on the human glioblastoma astrocytoma U-251 cell line of loaded PMs with synthesised flavones. Each value represents a total of n = 3 replicates of n = 3 independent assays (a total of 9 replicates). DMEM was used as the positive control and 1% Triton X-100 as a negative control. **** (Four asterisks): p-value < 0.0001.
By entrapping the flavonoids into PMs, we were able to apply higher dosages to the cells and achieve more effects compared to the free compounds, highlighting the higher bioavailability that these systems provide for the entrapped compounds.
Many published studies already involve the encapsulation of drugs into PMs. Some of the examples of these drugs already encapsulated are the antitumoral Doxorubicin [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89] and Paclitaxel [84,90,91,92], and others, such as Azobenzene [93], Hypericin [93], Avidin [94], Rhodamine B [82,95,96], Calcein [97], and Insulin [98,99].
However, none of them are in clinical trials or in therapeutics [100,101,102].
3. Materials and Methods
3.1. General Information
All solvents used were from Sigma-Aldrich Co. (St. Louis, MO, USA) and Thermo Fisher Scientific (Waltham, MA, USA). Sodium Azide (NaN3, S2002 and 71290), (+)-sodium-L-ascorbate (A7631), 3,7-dihydroxyflavone (1, 419826), propargyl alcohol (P50803), sodium cyanoborohydride (NaBH3CN, 156159), glucose (101175P), glucosamine (G4875), and silver carbonate (AgCO3, 179647) were acquired from Sigma-Aldrich Co. Acetobromo-ɑ-D-glucose (3, L04151.18), tetrabutylammonium hydroxide (TBAHS, AC168380250), caesium carbonate (Cs2CO3, 12887), and propargyl bromide (AC131482500) were acquired from Alfa Aesar (Thermo Fisher Scientific). 2-acetamido-3,4,6-tetra-O-acetyl-2-deoxy-β-D-glucopyranosyl azide (AG931) and 2-azidoethyl 2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl (AG904) were acquired from Synthose Inc. (Concord, ON, Canada). Boron trifluoride ethyl etherate (BF3.OEt2, B0527) were acquired from Tokyo Chemical Industry Co., Ltd. (TCI, Tokyo, Japan). Copper (II) sulphate (CuSO4, CL00.1127.1000) was acquired from Chem-Lab NV (Zedelgem, Belgium).
The reactions were monitored by Thin Layer Chromatography (TLC) aluminium sheets, silica gel 60 with a fluorescent indicator (ALUGRAM® Xtra SIL G UV254) from Macherey–Nagel GmbH & Co. KG. (Düren, Germany). The purifications by preparative TLC were performed with 20 × 20 cm glass plates covered with silica gel G/UV254 with a fluorescent indicator (816320.5) from Macherey–Nagel GmbH & Co. KG. High-performance liquid chromatography (HPLC) was performed on a Dionex Ultimate 3000 (Thermo Scientific, Darmstadt, Germany), with AcclaimTM 120 C18 (100 × 4.6 mm) column with a particle size of 5 μm, from Thermo Fisher Scientific (Bremen, Germany), detector 220 nm, 265 nm, and 330 nm, and 1 mL/min isocratic flow. IR spectra were obtained in KBr microplates in a Fourier transform infrared spectroscopy (FTIR) spectrometer Nicolet iS10 from Thermo Scientific with Smart OMNITransmisson accessory (Software OMNIC 8.3) (cm−1). The 1H and 13C-NMR spectra were obtained at the CEMUP (Centro de Materiais da Universidade do Porto), Porto, Portugal, on a Bruker Ascend III 400 instrument (1H: 400.14 MHz; 13C: 100.63 MHz). The 13C-NMR assignments were made by bidimensional HSQC and HMBC experiments (long-range C, H coupling constants were optimised to 7 and 1 Hz). The chemical shifts are expressed in ppm values relative to tetramethylsilane (TMS) as an internal reference, and coupling constants are reported in hertz (Hz). The assignment’s abbreviations are as follows: doublet (d), double doublet (dd), double double doublet (ddd), double double double doublet (dddd), double quartet (dq), double triplet (dt), multiplet (m), singlet (s), triplet (t), and triple doublet (td). HRMS spectra were obtained at the CEMUP (Centro de Materiais da Universidade do Porto), Porto, Portugal, on the LTQ Orbitrap XL hybrid mass spectrometer (Thermo Fischer Scientific, Bremen, Germany).
Cell reagents were purchased from Gibco (Invitrogen Corporation, Life Technologies, Cramlington, UK). Cell reagents (Dulbecco’s Modified Eagle’s Medium (DMEM), Fetal Bovine Serum (FBS), Penicillin–Streptomycin (Pen Strep), and Trypsin EDTA) were provided by VWR (Leuven, Belgium), Biowest (Nuaillé, France) and Gibco (Life Technologies, S.A. (Madrid, Spain)). The 929 Fibroblast cell line was obtained from the ‘European Collection of Authenticated Cell Cultures’ (ECACC, UK), and the U-251 Glioblastoma Astrocytoma cell line was kindly donated by Dr Salette Reis. In figures, different asterisks (*) in the same sample represent significant differences: p-value > 0.05; * (One asterisk): p-value < 0.05; ** (Two asterisks): p-value < 0.01; *** (Three asterisks): p-value < 0.001; **** (Four asterisks): p-value < 0.0001. All the statistical analyses were performed with GraphPad Prism 10 for macOS (Version 10.1.1, 21 November 2023).
3.2. Chemistry
3.2.1. Synthesis of 3,7-(Prop-2-yn-yloxy)flavone (2)
3,7-Dihydroxyflavone (1, 2.00 g, 7.87 mmol), TBAHS (26.71 g, 78.67 mmol, 5 equiv/OH), and Cs2CO3 (38.45 g, 118.00 mmol, 7.5 equiv/OH) were added in acetone (250 mL). The mixture was refluxed for 1 h at 60 °C until maximum solubilisation. A propargyl bromide solution (80% wt.% in toluene, 21.35 mL, 196.66 mmol, 12.5 equiv/OH) was added and left stirring for eight days until the starting material 1 had been consumed. The product showed a purple fluorescence (normal-phase analytical TLC, 95:5 CHCl3—MeOH, Rf = 0.80). The mixture was filtered and washed with acetone, providing a brown filtrate that, after being evaporated under reduced pressure, provided a residue oil that was purified by crystallisation with MeOH providing 3,7-(prop-2-yn-yloxy) flavone (2, 2.46 g, 7.44 mmol, 95% yield) as a white solid. Melting point: 130–132 °C; HPLC (reverse-phase, 7:3 acetonitrile—water, Rt = 5.65 min.); IR (KBr) υ max (cm−1): 3274 (≡C-H), 3173 (=C-H), 2946 (-C-H), 2107 (C≡C), 1618 (C=O), 1559 (C=C); 1H NMR (400.14 MHz, DMSO-d6) δ: 8.09 (2H, m, H-2′, H-6′), 8.02 (1H, d, J = 8.9 Hz, H-5), 7.58 (3H, m, H-3′, H-4′, H-5′), 7.36 (1H, d, J = 2.3 Hz, H-8), 7.14 (1H, dd, J = 8.9, 2.4 Hz, H-6), 5.00 (2H, d, J = 2.4 Hz, H-1‴), 4.97 (2H, d, J = 2.5 Hz, H-1″), 3.68 (1H, t, J = 2.4 Hz, H-3‴), 3.47 (1H, t, J = 2.4 Hz, H-3″); 13C NMR (100.63 MHz, DMSO-d6) δ: 173.2 (C-4), 161.7 (C-7), 156.3 (C-8a), 155.5 (C-2), 137.8 (C-3), 130.8 (C-4′), 130.4 (C-1′), 128.5 (C-2′, C-3′, C-5′, C-6′), 126.4 (C-5), 117.5 (C-4a), 115.2 (C-6), 101.9 (C-8), 79.3 (C-3‴), 79.1 (C-3″), 78.7 (C-2″), 78.3 (C-2‴), 58.7 (C-1″), 56.3 (C-1‴). HRMS (ESI+) m/z calculated for C21H14O4 [M + H+] 331.096, found 331.096.
3.2.2. Synthesis of 2,3,4,6 tetra-O-Acetyl-β-D-glucopyranosyl Azide (4)
Acetobromo-α-D-glucose (3, 500 mg, 1.22 mmol) was dissolved in acetone (5 mL). Sodium azide (98.81 mg, 1.52 mmol, 1.25 equiv) was dissolved in water (3 mL), added to the reaction mixture, and left stirring at room temperature. After 4 h, the starting material was completely consumed, and only one spot, with the same Rf as the previously synthesised 2,3,4,6 tetra-O-Acetyl-β-D-glucopyranosyl azide, was observed (normal-phase analytical TLC, 6:4 n-Hexane—AcOEt, Rf = 0.45). The mixture was concentrated until the acetone completely evaporated and a white solid precipitated from the water. The solid was filtered and washed with water to provide 2,3,4,6 tetra-O-Acetyl-β-D-glucopyranosyl azide (4, 322.6 mg, 864.14 µmol, 71% yield). Melting point: 120–124 °C; IR (KBr) υ max (cm−1): 2970 and 2910 (-C-H aliphatic), 2119 (-N3), 1755 (C=O), 1241, and 1213 (C-O); 1H NMR (300.13 MHz, CDCl3) δ: 5.22 (1H, t, J = 9.5 Hz, H-3), 5.12 (1H, t, J = 9.8 Hz, H-4), 4.95 (1H, t, J = 9.1 Hz, H-2), 4.64 (1H, d, J = 8.9 Hz, H-1), 4.27 (1H, dd, J = 12.5, 4.7 Hz, H-6a), 4.16 (1H, dd, J = 12.5, 2.3 Hz, H-6b), 3.79 (1H, ddd, J = 9.9, 4.7, 2.3 Hz, H-5), 2.10 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.03 (3H, s, COCH3), 2.01 (3H, s, COCH3); 13C NMR (75.48 MHz, CDCl3) δ: 170.6 (COCH3), 170.1 (COCH3), 169.3 (COCH3), 169.2 (COCH3), 87.9 (C-1), 74.0 (C-5), 72.6 (C-3), 70.6 (C-2), 67.8 (C-4), 61.6 (C-6), 20.6 (4 COCH3). HRMS (ESI+) m/z calculated for C14H19N3O9 [M + Na+] 396.102, found 396.094.
3.2.3. Synthesis of 3,7-((2,3,4,6-tetra-O-Acetyl-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl) methoxy) flavone (5a)
3,7-(prop-2-yn-yloxy) flavone (2, 400 mg, 1.21 mmol) and 2,3,4,6-tetra-O-Acetyl-b-D-glucopyranosyl azide (4, 949.29 mg, 2.54 mmol; 2 equiv/alkyne) were dissolved in THF (16 mL). After solubilisation, an aqueous mixture (4 mL) of sodium ascorbate (1.92 g, 9.69 mmol, 4 equiv/alkyne) and CuSO4 (773.04 mg, 4.84 mmol, 2 equiv/alkyne) was added to the solution. The mixture was protected from light with aluminium foil and left stirring at room temperature. After 24 h, the starting material 2 was completely consumed (normal-phase analytical TLC, 5:5 CHCl3–Acetone). The mixture was filtered and washed with THF. The filtrated solution was concentrated until the THF completely evaporated and a solid precipitated from water. The solid was filtered and washed with water. The solid obtained was further purified through preparative TLC (normal-phase, 5:5 CHCl3–Acetone) to provide 3,7-((2,3,4,6-tetra-O-Acetyl-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl)methoxy) flavone (Rf = 0.85) as a yellow solid (5a, 458 mg, 425.27 µmol, 35% yield). Melting point: 185–187 °C; IR (KBr) υ max (cm−1): 3468 and 3135 (=C-H triazole and Ar.), 2946 (-C-H), 1751 (C=O acetyl), 1623 (C=O flavone), 1497, and 1449 (C-C Ar.), 1227 (C-O); 1H NMR (400.14 MHz, DMSO-d6) δ: 8.62 (1H, s, -C=CH-triazole), 8.41 (1H, s, -C=CH-triazole), 8.04 (1H, d, J = 8.9 Hz, H-5), 8.02–7.93 (2H, m, H-2′, H-6′), 7.56–7.47 (3H, m, H-3′, H-4′, H-5′), 7.43 (1H, d, J = 2.4 Hz, H-8), 7.15 (1H, dd, J = 8.9 and 2.3 Hz, H-6), 6.39 (1H, d, J = 9.2 Hz, H-1″), 6.31 (1H, d, J = 9.2 Hz, H-1″), 5.72–5.59 (2H, dt, J = 18.4 and 9.3 Hz, 2 H-2″), 5.59–5.50 (2H, dd, J = 9.6 and 9.5 Hz, 2 H-3″), 5.36 (2H, t, J = 13.4 Hz, -O-CH2-triazole), 5.27 (2H, td, J = 12.3 and 5.9 Hz, -O-CH2-triazole), 5.22–5.11 (2H, td, J = 9.8 and 5.3 Hz, 2 H-4″), 4.42–4.30 (2H, dddd, J = 11.9, 10.1, 5.3 and 2.6 Hz, 2 H-5‴), 4.13 (2H, dd, J = 12.6 and 5.4 Hz, 2 H-6″a), 4.08 (2H, dd, J = 13.0 and 2.4 Hz, 2 H-6″b), 2.03 (6H, s, 2 COCH3), 2.00 (6H, d, J = 6.7 Hz, 2 COCH3), 1.96 (6H, s, 2 COCH3), 1.77–1.68 (6H, m, 2 COCH3); 13C NMR (100.63 MHz, DMSO-d6) δ: 173.3 (C-4), 169.9 (2 COCH3), 169.5 (2 COCH3), 169.3 (2 COCH3), 168.3 (2 COCH3), 162.3 (C-7), 156.4 (C-8a), 154.9 (C-2), 143.1 (-C=CH-triazole), 142.6 (-C=CH-triazole), 138.5 (C-3), 130.6 (C-4′), 130.3 (C-1′), 128.3 (C-2′, C-3′, C-5′, C-6′), 126.4 (C-5), 123.8 (2 -C=CH-triazole), 117.5 (C-4a), 115.1 (C-6), 101.6 (C-8), 83.8 (2 C-1″), 73.2 (2 C-5″), 72.1 (2 C-3″), 70.0 (2 C-2″), 67.5 (2 C-4″), 63.9 (-O-CH2-triazole), 61.7 (-O-CH2-triazole, 2 C-6″), 20.3 (6 COCH3), 19.7 (2 COCH3); HRMS (ESI+) m/z calculated for C49H52N6O22 [M + Na+] 1099.303, found 1099.288.
3.2.4. Synthesis of 3,7-((2-Acetamido-3,4,6-tetra-O-Acetyl-2-deoxy-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl)methoxy) flavone (5b)
3,7-(prop-2-yn-yloxy) flavone (2, 250 mg, 716.80 µmol) was dissolved in THF (12 mL) with 2-acetamido-3,4,6-tetra-O-Acetyl-2-deoxy-β-D-glucopyranosyl azide (AG931, 619.92 mg, 1.66 mmol, 1.10 equiv/alkyne). After solubilisation, an aqueous mixture (3 mL) of CuSO4 (438.15 mg, 3.03 mmol, 2 equiv/alkyne) and sodium ascorbate (1.20 g, 6.05 mmol, 4 equiv/alkyne) was added to the solution. The mixture was protected from light with aluminium foil and left stirring at room temperature. After 2 h, the starting material 2 was completely consumed (normal-phase analytical TLC, 5:5 CHCl3—Acetone). The mixture was filtered and washed with THF. The filtrated solution was concentrated until the THF completely evaporated and a solid precipitated from water. The solid was filtered and washed with water. The solid obtained was further purified through preparative TLC (normal-phase, 5:5 CHCl3—Acetone) to provide 3,7-((2-acetamido-3,4,6-tetra-O-Acetyl-2-deoxy-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl)methoxy) flavone (Rf = 0.28) as a white solid (5b, 232 mg, 215.81 µmol, 29% yield). Melting point: 267–270 °C; IR (KBr) υ max (cm−1): 3347 and 3077 (=C-H triazole and Ar.), 2958 (-C-H), 1747 (C=O acetyl), 1670 (C=O flavone), 1621 (C=O amide), 1540 (C=C triazole and Ar.), 1449 (C-C Ar.), 1232 (C-O); 1H NMR (400.14 MHz, DMSO-d6) δ: 8.49 (1H, s, -C=CH-triazole), 8.28 (1H, s, -C=CH-triazole), 8.08 (1H, d, J = 9.2 Hz, H-5,), 8.04 (2H, d, J = 9.0 Hz, 2 -NH-COCH3), 8.00–7.96 (2H, m, H-2′, H-6′), 7.57–7.49 (3H, m, H-3′, H-4′, H-5′), 7.43 (1H, d, J = 2.4 Hz, H-8), 7.15 (1H, dd, J = 8.9 and 2.4 Hz, H-6), 6.14 (1H, d, J = 10.0 Hz, H-1″), 6.09 (1H, d, J = 10.0 Hz, H-1″), 5.39–5.30 (4H, m, 2 H-3″), 5.25 (2H, d, J = 1.9 Hz, 2 -O-CH2-triazole), 5.10 (2H, dt, J = 9.7 and 2.9 Hz, 2 H-4″), 4.60 (2H, dq, J = 19.5 and 9.9 Hz, 2 H-2″), 4.27–4.19 (2H, dddd, J = 10.7, 10.7, 5.0 and 2.3, 2 H-5″), 4.19–4.02 (4H, m, 2 H-6″, H-6‴), 2.02–2.00 (6H, m, 2 COCH3), 1.99 (6H, s, 2 COCH3), 1.94 (6H, s, 2 COCH3), 1.54 (6H, d, J = 6.5 Hz, 2 -NH-COCH3); 13C NMR (100.63 MHz, DMSO-d6) δ: 173.3 (C-4), 169.9 (2 COCH3), 169.5 (2 COCH3), 169.3 (2 COCH3, 2 -NH-COCH3), 162.4 (C-7), 156.5 (C-8a), 155.1 (C-2), 142.7 (-C=CH-triazole), 142.1 (-C=CH-triazole), 138.6 (C-3), 130.6 (C-4′), 130.3 (C-1′), 128.3 (C-2′, C-3′, C-5′, C-6′), 126.4 (C-5), 123.7 (2 -C=CH-triazole), 117.6 (C-4a), 115.1 (C-6), 101.6 (C-8), 84.7 (2 C-1″), 73.3 (2 C-5″), 72.3 (2 C-3″), 67.9 (2 C-4″), 64.1 (2 -O-CH2-triazole), 61.7 (2 C-6″), 51.9 (2 C-2″), 22.2 (2 -NH-COCH3), 20.4 (6 COCH3); HRMS (ESI+) m/z calculated for C49H54N8O20 [M + Na+] 1097.335, found 1097.319.
3.2.5. Synthesis of 3,7-((2-Azidoethyl 2,3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl)methoxy) flavone (5c)
3,7-(prop-2-yn-yloxy) flavone (2, 200 mg, 605.44 µmol) was dissolved in THF (8 mL) with 2-azidoethyl 2,3,4,6-tri-O-acetyl-β-D-glucopyranosyl azide (AG904, 555.92 mg, 1.33 mmol, 1.10 equiv/alkyne). After solubilisation, an aqueous mixture (2 mL) of CuSO4 (386.52 mg, 2.42 mmol, 2 equiv/alkyne) and sodium ascorbate (959.38 mg, 4.84 mmol, 4 equiv/alkyne) was added to the solution. The mixture was protected from light with aluminium foil and left stirring at room temperature. After 2 h, the starting material 2 was completely consumed (normal-phase analytical TLC, 5:5 CHCl3–Acetone). The mixture was filtered and washed with THF. The filtrated solution was concentrated until the THF completely evaporated. The water solution was extracted with AcOEt. The organic layer was dried with Na2SO2, followed by crystallisation from MeOH/CH2Cl2. The crystallisation provided 3,7-((2-azidoethyl 2,3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl-1H-1,2,3-triazole-4-yl)methoxy) flavone (Rf = 0.65) as a yellow solid (5c, 170 mg, 145.91 µmol, 24% yield). Melting point: 190–193 °C; IR (KBr) υ max (cm−1): 3470 and 3134 (=C-H triazole and Ar.), 2961 (-C-H), 1750 (C=O acetyl), 1637 (C=O flavone), 1621 (C=C triazole and Ar.), 1501 and 1448 (C-C Ar.), 1227 (C-O); 1H NMR (400.14 MHz, DMSO-d6) δ: 8.16 (1H, s, -C=CH-triazole), 8.04 (1H, d, J = 8.9 Hz, H-5), 7.97 (2H, m, H-2′, H-6′), 7.88 (1H, s, -C=CH-triazole), 7.59–7.48 (3H, m, H-3’, H-4′, H-5′), 7.46 (1H, d, J = 2.4 Hz, H-8), 7.13 (1H, dd, J = 8.9 and 2.3 Hz, H-6), 5.31 (2H, s, -O-CH2-triazole), 5.22 (2H, t, J = 9.6 Hz, 2 H-2″), 5.21 (2 H, s, -O-CH2-triazole), 4.89 (2H, td, J = 9.7 and 2.9 Hz, 2 H-3″), 4.81 (2H, dd, J = 14.7 and 8.0 Hz, 2 H-1″), 4.72 (2H, td, J = 9.5 and 8.0 Hz, 2 H-4″), 4.58 (2H, m, -O-CH2-CH2-triazole), 4.46 (2H, m, -O-CH2-CH2-triazole), 4.22–3.78 (10H, m, 2 H-5″, 2 H-6″, 2 -O-CH2-CH2-triazole), 2.01 (6H, d, J = 4.1 Hz, 2 COCH3), 1.97 (6H, s, 2 COCH3), 1.91 (6H, s, 2 COCH3), 1.86 (6H, d, J = 12.8 Hz, 2 COCH3); 13C NMR (100.63 MHz, DMSO-d6) δ: 173.3 (C-4), 170.0 (2 COCH3), 169.5 (2 COCH3), 169.2 (2 COCH3), 168.9 (2 COCH3), 162.5 (C-7), 156.5 (C-8a), 155.3 (C-2), 142.2 (-C=CH-triazole), 141.6 (-C=CH-triazole), 138.5 (C-3), 130.6 (C-4′), 130.3 (C-1′), 128.3 (C-2′, C-3′, C-5′, C-6′), 126.4 (C-5), 125.2 (-C=CH-triazole), 124.9 (-C=CH-triazole), 117.5 (C-4a), 115.1 (C-6), 101.4 (C-8), 99.1 (2 C-1″), 71.8 (2 C-2″), 70.5 (2 C-4″), 68.0 (2 C-3″), 67.4 (2 -O-CH2-CH2-triazole, 2 C-5″), 64.2 (-O-CH2-triazole), 61.8 (-O-CH2-triazole), 61.6 (2 C-6″), 49.3 (-O-CH2-CH2-triazole), 49.1 (-O-CH2-CH2-triazole), 20.1 (8 COCH3); HRMS (ESI+) m/z calculated for C53H60N6O24 [M + Na+] 1187.356, found 1187.338.
3.3. Nanotechnology
3.3.1. Preparation of Empty and Loaded PMs
Self-assembled structures were prepared by a solvent displacement method with PEG45PLA106 diblock copolymer (PEGPLA), and the targeted PMs were prepared by the co-assembly of PEGPLA diblock copolymer and T7-functionalized PEG20PLA106 (T7-PEGPLA) in 0.1 mol% of T7-PEGPLA to 99.9 mol% PEGPLA ratio. Briefly, 20 mg of copolymers was solubilized in DMF (and 2 mg of each compound for loaded PMs). A total of 600 μL of the solution was then injected into 1.4 mL of MiliQ water (eq. 30% v/v) at a flow rate of 100 μL/min under magnetic stirring at 500 rpm using a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY, USA). The solution containing the self-assembled nanostructures was then dialyzed using a 3.5 kDa dialysis membrane (Cellu.Sept®, membrane filtration products Inc., Seguin, TX, USA) over-night in 1000 mL of purified water.
3.3.2. Particle Size, Zeta Potential, and Polydispersion Index
The samples were prepared by adding 250 μL of PM sample in 1000 μL of purified water and analysed using Dynamic Light Scattering (DLS) with a ZETASIZER Pro apparatus (Malvern Panalytical, Worcestershire, UK). The collected data, mean diameter, zeta potential, and polydispersion index (PDI) were obtained by the ZS XPLORER 3.3.1.5 software (Malvern Panalytical, Worcestershire, UK) and were expressed as mean ± standard deviation throughout the work.
3.3.3. Negative-Staining Transmission Electronic Microscopy (TEM)
For negative staining, 5 µL of samples were mounted on Formvar/carbon film-coated 300 mesh nickel grids (Electron Microscopy Sciences, Hatfield, PA, USA) and left standing for 2 min. The liquid in excess was removed with filter paper, and 5 µL of 2% uranyl acetate was added onto the grids and left standing for 30 s, after which the liquid in excess was removed with filter paper. The visualisation was performed on a JEOL JEM 1400 TEM at 120 kV (Tokyo, Japan), and the images were digitally recorded using a PHURONA digital camera (Munich, Germany). The TEM was performed at the HEMS core facility at i3S, University of Porto, Portugal, with the assistance of Sofia Pacheco and Rui Fernandes.
3.3.4. Stability Study
The stability investigation was conducted on the PMs. The samples were intermittently assessed for the mean diameter and PDI at determined time points post-manufacturing, while the formulations were preserved at a temperature of 4 °C.
3.3.5. Entrapment Efficiency
The obtained formulations were centrifuged (24,000× g for 25 min) (Model 5804, Eppendorf, Hauppauge, NY, USA), and the supernatant was discarded. The pellet was resuspended in 1 mL of acetonitrile, and it was filtered (0.45 μm PTFE filter, OlimPeak®, Teknokroma, Barcelona, Spain) to obtain the compounds entrapped amount sample. The obtained samples were then evaluated by HPLC (Dionex UltiMateTM 3000 from Thermo Fisher Scientific, Bremen, Germany) equipped with a quaternary pump and a quaternary Variable Wavelength Detector. Chromeleon 7.2 software was used for data acquisition.
The chromatographic conditions included a commercially available AcclaimTM 120 C18 (100 × 4.6 mm) column with a particle size of 5 μm from Thermo Fisher Scientific (Bremen, Germany). The optimised mobile phase for 5a–5c consisted of 50:50 H2O—Acetonitrile, following an isocratic flow of 1.0 mL/min for 15 min. The injection volume was 10 μL, and the detection was performed at 259 nm. An individual calibration curve for all compounds was prepared at concentrations of 100.0, 50.0, 25.0, 12.5, 6.13 and 3.1 µM in triplicate. The drug concentration in the pellet was obtained by interpolation from the calibration curve. The theoretical concentration of the compound was calculated by considering the amount of compound placed during the production of the PMs and the dilutions performed throughout the procedure, and the direct method of the encapsulation efficiency (EE%) can be calculated using Equation (1):
where the total amount is the initial concentration of the compound in the formulation (μM) and the entrapped amount is the final concentration of the compound in the pellet (μM).
3.3.6. MTT Assays
The human tumour cell lines U-251 (glioblastoma astrocytoma) and L929 (fibroblasts) were grown in DMEM, both supplemented with 10% FBS and 1% Pen-Strep. All cell lines were maintained at 37 °C in a 5% CO2 humidified atmosphere (Hera Cell, Heraeus). The L929 and U-251 cell lines were cultivated as reported by Mendes et al. [13,14]. The passages used for the L929 cell line were P18–P21 and P23–P27, and the U-251 cell line was P + 5–P + 8.
For the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the cells were seeded in 96-well plates (5.00 × 104 cells/well for L929 and 1.00 × 104 cells/well for U-251) and exposed to either different concentrations of compounds dissolved in medium with 5% DMSO or different concentrations of the PMs formulations for 24 h. The quantification of viable cells was conducted by introducing the MTT reagent and allowing for an incubation period of 2 h at a temperature of 37 °C.
To dissolve the crystals, DMSO was employed. The following controls were used: DMEM, DMEM with 5% DMSO, and DMEM with 1% Triton. The absorbance was read at 570 nm and 630 nm for background subtraction, and the results of cell viability of all wells were expressed as CV%.
3.4. Statistical Analysis
The results are shown as the mean ± standard deviation of three or five batches of the same formulation. The results of mean diameter, EE, and cell viability were statistically analysed using ANOVA (two-way). The differences between the groups for ANOVA were compared with a post hoc test (Dunnett’s test). The significance was set at p < 0.05.
In the figures, different asterisks (*) in the same sample represent significant differences: p-value > 0.05; * (One asterisk): p-value < 0.05; ** (Two asterisks): p-value < 0.01; *** (Three asterisks): p-value < 0.001; **** (Four asterisks): p-value < 0.0001.
All the statistical analyses were performed using GraphPad Prism 10 for macOS (Version 10.1.1, 21 November 2023).
4. Conclusions
In this study, three acetoglycosyl triazole-linked flavonoids were successfully synthesised using click chemistry. These flavonoids were encapsulated within functionalized T7 peptide PMs, utilising the same click chemistry strategy, for local delivery and to ensure the solubility of the synthesised flavones. The results demonstrated significant reductions in the viability of human glioblastoma U-251 cells at given concentrations, namely the PM system containing compound 5c, reducing cell viability by more than 50% across nearly all tested concentrations. All PMs showed a mean size between 120 and 180 nm, which constitutes a favourable size range for nanoparticle extravasation in vascular tissues. Also, the entrapment efficacy of this 5c-PM (39%) underlines the potential of this particles in local high dosage therapies.
Therefore, the developed “click-on-click” DDS shows promising potential for targeting not only the world’s most aggressive and deadly brain cancers but also in modern therapy. The rapid and precise synthesis enabled by click chemistry not only paves the way for the development of highly targeted therapies but also opens up opportunities to revolutionise cancer treatment. This DDS represents an exciting step toward providing more efficient and localised therapeutic options for GBM patients, a cancer notoriously resistant to conventional treatments.
We can further refine and expand the applicability of this system, offering the potential for a more effective, targeted approach to GBM therapy. By addressing these goals, this study can provide an innovative and targeted approach to GBM treatment, moving towards the possibility of transforming glioblastoma from a terminal to a more manageable health condition.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pharmaceutics17060771/s1. Figure S1: 1H NMR (A) and 13C NMR (B) spectra of 5a; Figure S2: HSQC (A) and HMBC (B) spectra of 5a; Figure S3: HMRS (A) and IR (B) spectra of 5a; Figure S4: 1H NMR (A) and 13C NMR (B) spectra of 5b; Figure S5: HSQC (A) and HMBC (B) spectra of 5b; Figure S6: HMRS (A) and IR (B) spectra of 5b; Figure S7: 1H NMR (A) and 13C NMR (B) spectra of 5c; Figure S8: HSQC (A) and HMBC (B) spectra of 5c; Figure S9: HMRS (A) and IR (B) spectra of 5c.
Author Contributions
Conceptualisation, A.A., P.C.C. and M.C.-d.-S.; methodology, N.M.S. and A.A.; investigation, N.M.S.; data curation, N.M.S., A.A., A.I.B. and A.M.; writing—original draft preparation, N.M.S.; writing—review and editing, N.M.S., A.A., A.I.B., A.M., S.R., M.C.-d.-S. and P.C.C.; supervision, M.C.-d.-S. and P.C.C.; funding, S.R., M.C.-d.-S. and P.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by national funds from FCT, Fundação para a Ciência e a Tecnologia, I.P., in the scope of the strategic fundings UIDB/04423/2020, UIDP/04423/2020 (CIIMAR—Marine Natural Products and Medicinal Chemistry group), UIDP/04378/2020, UIDB/04378/2020 (UCIBIO—Research Unit on Applied Molecular Biosciences), and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB. This work also received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the project UIDB/50006/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
The authors thank Sofia Pacheco and Rui Fernandes from the HEMS core facility at i3S, University of Porto, Portugal, for the TEM analysis assistance, and Sara Cravo for the all the technical and scientific support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pourgholi, F.; Hajivalili, M.; Farhad, J.-N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel Vehicles in Treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant Astrocytic Glioma: Genetics, Biology, and Paths to Treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Oppenheimer, D.R. Oppenheimer’s Diagnostic Neuropathology: A Practical Manual, 3rd ed.; Blackwell Scientific Publications: Oxford, UK, 2007; Volume 33. [Google Scholar]
- Vanza, J.; Jani, P.; Pandya, N.; Tandel, H. Formulation and Statistical Optimization of Intravenous Temozolomide-Loaded PEGylated Liposomes to Treat Glioblastoma Multiforme by Three-Level Factorial Design. Drug Dev. Ind. Pharm. 2018, 44, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.C.; Queiroz, V.C.J.; Cavalcante, J.E.S.; Matozinho, H.H.S.; Silva, F.H.R.; Costa, M.G.P.; Pereira I, F.Y.; Tavares, L.C.P.; Guimarães, G.M. Treatment and Prognosis of Glioblastoma Multiforme: A Literature Review. J. Neurol. Sci. 2015, 357, e183. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dung Nguyen, T.T.; Vo, T.K.; Tran, N.-M.-A.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-Based Drug Delivery for Central Nervous System Disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Alphandéry, E. Glioblastoma Treatments: An Account of Recent Industrial Developments. Front. Pharmacol. 2018, 9, 879. [Google Scholar] [CrossRef]
- Tilak, M.; Holborn, J.; New, L.A.; Lalonde, J.; Jones, N. Receptor Tyrosine Kinase Signaling and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2021, 22, 1831. [Google Scholar] [CrossRef]
- Mujokoro, B.; Adabi, M.; Sadroddiny, E.; Adabi, M.; Khosravani, M. Nano-Structures Mediated Co-Delivery of Therapeutic Agents for Glioblastoma Treatment: A Review. Mater. Sci. Eng. C 2016, 69, 1092–1102. [Google Scholar] [CrossRef]
- Soda, Y.; Myskiw, C.; Rommel, A.; Verma, I.M. Mechanisms of Neovascularization and Resistance to Anti-Angiogenic Therapies in Glioblastoma Multiforme. J. Mol. Med. 2013, 91, 439–448. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging Treatment Strategies for Glioblastoma Multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in Brain Targeting Drug Delivery System by Nasal Route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, P.; Arozarena, I. Tyrosine Kinase Inhibitors in Adult Glioblastoma: An (Un)Closed Chapter? Cancers 2021, 13, 5799. [Google Scholar] [CrossRef]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005–2009. Neuro-Oncol. 2012, 14, v1–v49. [Google Scholar] [CrossRef]
- Yang, L.-J.; Zhou, C.-F.; Lin, Z.-X. Temozolomide and Radiotherapy for Newly Diagnosed Glioblastoma Multiforme: A Systematic Review. Cancer Invest. 2014, 32, 31–36. [Google Scholar] [CrossRef]
- Rønning, P.A.; Helseth, E.; Meling, T.R.; Johannesen, T.B. A Population-Based Study on the Effect of Temozolomide in the Treatment of Glioblastoma Multiforme. Neuro-Oncol. 2012, 14, 1178–1184. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Stupp, R.; van den Bent, M.J.; Hegi, M.E. Optimal Role of Temozolomide in the Treatment of Malignant Gliomas. Curr. Neurol. Neurosci. Rep. 2005, 5, 198–206. [Google Scholar] [CrossRef]
- Ruiz-Molina, D.; Mao, X.; Alfonso-Triguero, P.; Lorenzo, J.; Bruna, J.; Yuste, V.J.; Candiota, A.P.; Novio, F. Advances in Preclinical/Clinical Glioblastoma Treatment: Can Nanoparticles Be of Help? Cancers 2022, 14, 4960. [Google Scholar] [CrossRef]
- Hutóczki, G.; Virga, J.; Birkó, Z.; Klekner, A. Novel Concepts of Glioblastoma Therapy Concerning Its Heterogeneity. Int. J. Mol. Sci. 2021, 22, 10005. [Google Scholar] [CrossRef]
- Habib, A.; Pease, M.; Kodavali, C.V.; Amankulor, N.; Zinn, P.O. A Contemporary Update on Glioblastoma: Molecular Biology, Current Management, and a Vision towards Bio-Adaptable Personalized Care. J. Neurooncol 2021, 151, 103–112. [Google Scholar] [CrossRef]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When Immune Cells Turn Bad—Tumor-Associated Microglia/Macrophages in Glioma. Int. J. Mol. Sci. 2018, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the Immune System in Glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della Bella, S.; Passoni, L.; Mavilio, D.; et al. Role of Myeloid Cells in the Immunosuppressive Microenvironment in Gliomas. Immunobiology 2020, 225, 151853. [Google Scholar] [CrossRef]
- Abels, E.R.; Maas, S.L.N.; Tai, E.; Ting, D.T.; Broekman, M.L.D.; Breakefield, X.O.; El Khoury, J. GlioM&M: Web-Based Tool for Studying Circulating and Infiltrating Monocytes and Macrophages in Glioma. Sci. Rep. 2020, 10, 9898. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The Role of Microglia and Macrophages in Glioma Maintenance and Progression. Nat. Neurosci. 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Dejana, E. Interendothelial Junctions: Structure, Signalling and Functional Roles. Curr. Opin. Cell Biol. 1997, 9, 674–682. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Shusta, E.V. Blood–Brain Barrier Modulation to Improve Glioma Drug Delivery. Pharmaceutics 2020, 12, 1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-K.; Tsai, C.-L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects and Bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research Trends in Flavonoids and Health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Agarwal, S.; Sharma, R.; Nag, T.C.; Dwivedi, A.K.; Balapure, A.K. Genistein Potentiates Centchroman Induced Antineoplasticity in Breast Cancer via PI3K/Akt Deactivation and ROS Dependent Induction of Apoptosis. Life Sci. 2019, 239, 117073. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, Q.Y.; Kang, X.M.; Wang, J.X.; Zhao, W.H. Daidzein Induces MCF-7 Breast Cancer Cell Apoptosis via the Mitochondrial Pathway. Ann. Oncol. 2010, 21, 263–268. [Google Scholar] [CrossRef]
- Pandey, P.; Sayyed, U.; Tiwari, R.K.; Siddiqui, M.H.; Pathak, N.; Bajpai, P. Hesperidin Induces ROS-Mediated Apoptosis along with Cell Cycle Arrest at G2/M Phase in Human Gall Bladder Carcinoma. Nutr. Cancer 2019, 71, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin Induces Apoptosis of Esophageal Cancer Cells via Mitochondrial Pathway Mediated by the Increased Intracellular Reactive Oxygen Species. Tumor Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, J.; Wu, D.; Wang, J.; Dong, W. Hesperetin Induces the Apoptosis of Hepatocellular Carcinoma Cells via Mitochondrial Pathway Mediated by the Increased Intracellular Reactive Oxygen Species, ATP and Calcium. Med. Oncol. 2015, 32, 101. [Google Scholar] [CrossRef]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway. J. Cell Physiol. 2015, 230, 1729–1739. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, X.; Han, X.; Yao, L.; Lan, W. Natural Flavonoids Alleviate Glioblastoma Multiforme by Regulating Long Non-Coding RNA. Biomed. Pharmacother. 2023, 161, 114477. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481. [Google Scholar] [CrossRef]
- Genoux, E.; Nicolle, E.; Boumendjel, A. Flavonoids as Anticancer Agents: Recent Progress and State of the Art? Curr. Org. Chem. 2011, 15, 2608–2615. [Google Scholar] [CrossRef]
- Fonseca, J.; Marques, S.; Silva, P.; Brandão, P.; Cidade, H.; Pinto, M.; Bousbaa, H. Prenylated Chalcone 2 Acts as an Antimitotic Agent and Enhances the Chemosensitivity of Tumor Cells to Paclitaxel. Molecules 2016, 21, 982. [Google Scholar] [CrossRef]
- Mendanha, D.; Vieira de Castro, J.; Moreira, J.; Costa, B.M.; Cidade, H.; Pinto, M.; Ferreira, H.; Neves, N.M. A New Chalcone Derivative with Promising Antiproliferative and Anti-Invasion Activities in Glioblastoma Cells. Molecules 2021, 26, 3383. [Google Scholar] [CrossRef]
- Alves, A.; Silva, A.M.; Moreira, J.; Nunes, C.; Reis, S.; Pinto, M.; Cidade, H.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. Polymersomes for Sustained Delivery of a Chalcone Derivative Targeting Glioblastoma Cells. Brain Sci. 2024, 14, 82. [Google Scholar] [CrossRef]
- Pereira, D.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Recent Advances in Bioactive Flavonoid Hybrids Linked by 1,2,3-Triazole Ring Obtained by Click Chemistry. Molecules 2021, 27, 230. [Google Scholar] [CrossRef]
- Neves, A.R.; Correia-da-Silva, M.; Silva, P.M.A.; Ribeiro, D.; Sousa, E.; Bousbaa, H.; Pinto, M. Synthesis of New Glycosylated Flavonoids with Inhibitory Activity on Cell Growth. Molecules 2018, 23, 1093. [Google Scholar] [CrossRef]
- Alves, A.; Silva, A.M.; Nunes, C.; Cravo, S.; Reis, S.; Pinto, M.; Sousa, E.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. The Synthesis and Characterization of a Delivery System Based on Polymersomes and a Xanthone with Inhibitory Activity in Glioblastoma. Life 2024, 14, 132. [Google Scholar] [CrossRef]
- Alves, A.; Pfeifer, P.; Marinho, A.; Nunes, C.; Reis, S.; Ferreira, D.; Correia-da-Silva, M.; Costa, P.; Battaglia, G.; Batalha, Í.L.; et al. Sometimes Less Is More: Avidity-Dependent Transport of Targeted Polymersomes across the Blood-Brain-Barrier. RSC Pharm. 2025, 2, 535–540. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Sajadi, S.M.; Seidi, F.; Rabiee, N.; Fatahi, Y.; Rabiee, M.; Dominic, C.D.M.; Zarrintaj, P.; Formela, K.; Saeb, M.R.; et al. Clickable Polysaccharides for Biomedical Applications: A Comprehensive Review. Prog. Polym. Sci. 2022, 133, 101590. [Google Scholar] [CrossRef]
- Photos, P.J.; Bacakova, L.; Discher, B.; Bates, F.S.; Discher, D.E. Polymer Vesicles in Vivo: Correlations with PEG Molecular Weight. J. Control. Release 2003, 90, 323–334. [Google Scholar] [CrossRef]
- Saraiva, N.M.; Alves, A.; Costa, P.C.; Correia-da-Silva, M. Click Chemistry in Polymersome Technology. Pharmaceuticals 2024, 17, 747. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New Click-Chemistry Methods for 1,2,3-Triazoles Synthesis: Recent Advances and Applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Shen, J.; Zhao, G.; Wang, P.G. The Impact of Click Chemistry in Medicinal Chemistry. Expert Opin. Drug Discov. 2012, 7, 489–501. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click Chemistry Reactions in Medicinal Chemistry: Applications of the 1,3-dipolar Cycloaddition between Azides and Alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Sheng, J.; Ma, N.; Wang, Y.; Ye, W.-C.; Zhao, B.-X. The Application of Click Chemistry in the Synthesis of Agents with Anticancer Activity. Drug Des. Dev. Ther. 2015, 9, 1585–1599. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1136. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kondengadan, S.M.; Bansal, S.; Yang, C.; Liu, D.; Fultz, Z.; Wang, B. Click Chemistry and Drug Delivery: A Bird’s-Eye View. Acta Pharm. Sin. B 2023, 13, 1990–2016. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Chen, Q.; Bian, N.; Cao, C.; Qiu, X.-L.; Qi, A.-D.; Han, B.-H. Glucosamine Hydrochloride Functionalized Tetraphenylethylene: A Novel Fluorescent Probe for Alkaline Phosphatase Based on the Aggregation-Induced Emission. Chem. Commun. 2010, 46, 4067. [Google Scholar] [CrossRef] [PubMed]
- Ibatullin, F.M.; Shabalin, K.A. A Simple and Convenient Synthesis of Glycosyl Azides. Synth. Commun. 2000, 30, 2819–2823. [Google Scholar] [CrossRef]
- Pereira, D.; Gonçalves, C.; Martins, B.T.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Cidade, H. Flavonoid Glycosides with a Triazole Moiety for Marine Antifouling Applications: Synthesis and Biological Activity Evaluation. Mar. Drugs 2020, 19, 5. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2009.
- Pardridge, W.M.; Eisenberg, J.; Yang, J. Human Blood-Brain Barrier Transferrin Receptor. Metabolism 1987, 36, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Almeida, G.M.; Santos-Silva, F.; do Carmo Pereira, M.; Coelho, M.A.N. Transferrin Surface-Modified PLGA Nanoparticles-Mediated Delivery of a Proteasome Inhibitor to Human Pancreatic Cancer Cells. J. Biomed. Mater. Res. A 2015, 103, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Haldar, M.K.; Confeld, M.I.; Langaas, C.J.; Yang, X.; Qian, S.Y.; Mallik, S. Mitochondria-Targeted Fluorescent Polymersomes for Drug Delivery to Cancer Cells. Polym. Chem. 2016, 7, 4151–4154. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Yang, H.; Bao, C.; Fan, J.; Wang, C.; Lin, Q.; Zhu, L. Light-Responsive Polymersomes with a Charge-Switch for Targeted Drug Delivery. J. Mater. Chem. B 2020, 8, 727–735. [Google Scholar] [CrossRef]
- Figueiredo, P.; Balasubramanian, V.; Shahbazi, M.-A.; Correia, A.; Wu, D.; Palivan, C.G.; Hirvonen, J.T.; Santos, H.A. Angiopep2-Functionalized Polymersomes for Targeted Doxorubicin Delivery to Glioblastoma Cells. Int. J. Pharm. 2016, 511, 794–803. [Google Scholar] [CrossRef]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Self-Targeted Polymersomal Co-Formulation of Doxorubicin, Camptothecin and FOXM1 Aptamer for Efficient Treatment of Non-Small Cell Lung Cancer. J. Control. Release 2021, 335, 369–388. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Le Meins, J.F.; Misra, A.; Voisin, P.; Bouchaud, V.; Ibarboure, E.; Schatz, C.; Lecommandoux, S. Biomimetic Doxorubicin Loaded Polymersomes from Hyaluronan-Block- Poly(γ-Benzyl Glutamate) Copolymers. Biomacromolecules 2009, 10, 2802–2808. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I Clinical Trial and Pharmacokinetic Evaluation of NK911, a Micelle-Encapsulated Doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Shahriari, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. Synthesis of Hyaluronic Acid-Based Polymersomes for Doxorubicin Delivery to Metastatic Breast Cancer. Int. J. Pharm. 2019, 572, 118835. [Google Scholar] [CrossRef]
- Pramod, P.S.; Shah, R.; Jayakannan, M. Dual Stimuli Polysaccharide Nanovesicles for Conjugated and Physically Loaded Doxorubicin Delivery in Breast Cancer Cells. Nanoscale 2015, 7, 6636–6652. [Google Scholar] [CrossRef]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I Dose Escalation and Pharmacokinetic Study of Pluronic Polymer-Bound Doxorubicin (SP1049C) in Patients with Advanced Cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable Polymersomes Loaded with Both Paclitaxel and Doxorubicin Permeate and Shrink Tumors, Inducing Apoptosis in Proportion to Accumulated Drug. J. Control. Release 2006, 116, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, L.; Qu, X.; Yang, Z. PH-Sensitive Polymeric Vesicles from Coassembly of Amphiphilic Cholate Grafted Poly(L-Lysine) and Acid-Cleavable Polymer–Drug Conjugate. Langmuir 2012, 28, 11988–11996. [Google Scholar] [CrossRef]
- Qiao, Z.-Y.; Ji, R.; Huang, X.-N.; Du, F.-S.; Zhang, R.; Liang, D.-H.; Li, Z.-C. Polymersomes from Dual Responsive Block Copolymers: Drug Encapsulation by Heating and Acid-Triggered Release. Biomacromolecules 2013, 14, 1555–1563. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; Guo, J.; Zhao, X.; Zhong, Z. Robust and Smart Polypeptide-Based Nanomedicines for Targeted Tumor Therapy. Adv. Drug Deliv. Rev. 2020, 160, 199–211. [Google Scholar] [CrossRef]
- Oliveira, H.; Pérez-Andrés, E.; Thevenot, J.; Sandre, O.; Berra, E.; Lecommandoux, S. Magnetic Field Triggered Drug Release from Polymersomes for Cancer Therapeutics. J. Control. Release 2013, 169, 165–170. [Google Scholar] [CrossRef]
- Yang, X.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Steeber, D.A.; Gong, S. Multifunctional SPIO/DOX-Loaded Wormlike Polymer Vesicles for Cancer Therapy and MR Imaging. Biomaterials 2010, 31, 9065–9073. [Google Scholar] [CrossRef]
- Pan, X.Q.; Gong, Y.C.; Li, Z.L.; Li, Y.P.; Xiong, X.Y. Folate-Conjugated Pluronic/Polylactic Acid Polymersomes for Oral Delivery of Paclitaxel. Int. J. Biol. Macromol. 2019, 139, 377–386. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Chen, Y.; Han, T.; Chen, Y.; Wang, C. A Preliminary Study on Surface Bioactivation of Polyaryletherketone by UV-Grafting with PolyNaSS: Influence on Osteogenic and Antibacterial Activities. J. Biomater. Sci. Polym. Ed. 2022, 33, 1845–1865. [Google Scholar] [CrossRef]
- Li, S.; Byrne, B.; Welsh, J.; Palmer, A.F. Self-Assembled Poly(Butadiene)-b-Poly(Ethylene Oxide) Polymersomes as Paclitaxel Carriers. Biotechnol. Prog. 2007, 23, 278–285. [Google Scholar] [CrossRef]
- Kulkarni, P.; Haldar, M.K.; Karandish, F.; Confeld, M.; Hossain, R.; Borowicz, P.; Gange, K.; Xia, L.; Sarkar, K.; Mallik, S. Tissue-Penetrating, Hypoxia-Responsive Echogenic Polymersomes For Drug Delivery To Solid Tumors. Chem. A Eur. J. 2018, 24, 12490–12494. [Google Scholar] [CrossRef] [PubMed]
- Robbins, G.P.; Saunders, R.L.; Haun, J.B.; Rawson, J.; Therien, M.J.; Hammer, D.A. Tunable Leuko-Polymersomes That Adhere Specifically to Inflammatory Markers. Langmuir 2010, 26, 14089–14096. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Yuan, J.; Cai, Z.; Xin, Y.; Kang, Y.; Yin, Y. Voltage-Responsive Vesicles Based on Orthogonal Assembly of Two Homopolymers. J. Am. Chem. Soc. 2010, 132, 9268–9270. [Google Scholar] [CrossRef] [PubMed]
- Pramod, P.S.; Takamura, K.; Chaphekar, S.; Balasubramanian, N.; Jayakannan, M. Dextran Vesicular Carriers for Dual Encapsulation of Hydrophilic and Hydrophobic Molecules and Delivery into Cells. Biomacromolecules 2012, 13, 3627–3640. [Google Scholar] [CrossRef]
- Jia, L.; Cui, D.; Bignon, J.; Di Cicco, A.; Wdzieczak-Bakala, J.; Liu, J.; Li, M.-H. Reduction-Responsive Cholesterol-Based Block Copolymer Vesicles for Drug Delivery. Biomacromolecules 2014, 15, 2206–2217. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y.J.; Kang, S.; Kim, K.T. Monosaccharide-Responsive Release of Insulin from Polymersomes of Polyboroxole Block Copolymers at Neutral PH. J. Am. Chem. Soc. 2012, 134, 4030–4033. [Google Scholar] [CrossRef]
- Tai, W.; Mo, R.; Di, J.; Subramanian, V.; Gu, X.; Buse, J.B.; Gu, Z. Bio-Inspired Synthetic Nanovesicles for Glucose-Responsive Release of Insulin. Biomacromolecules 2014, 15, 3495–3502. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K.; et al. Emerging Era of “Somes”: Polymersomes as Versatile Drug Delivery Carrier for Cancer Diagnostics and Therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef]
- Bobde, S.S. Polymersomes for Targeting to Brain Tumors. In Nanocarriers for Drug-Targeting Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 451–481. ISBN 9780323907736. [Google Scholar]
- Poma, A.; Pei, Y.; Ruiz-Perez, L.; Rizzello, L.; Battaglia, G. Polymersomes: Synthesis and Applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–43. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
