Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
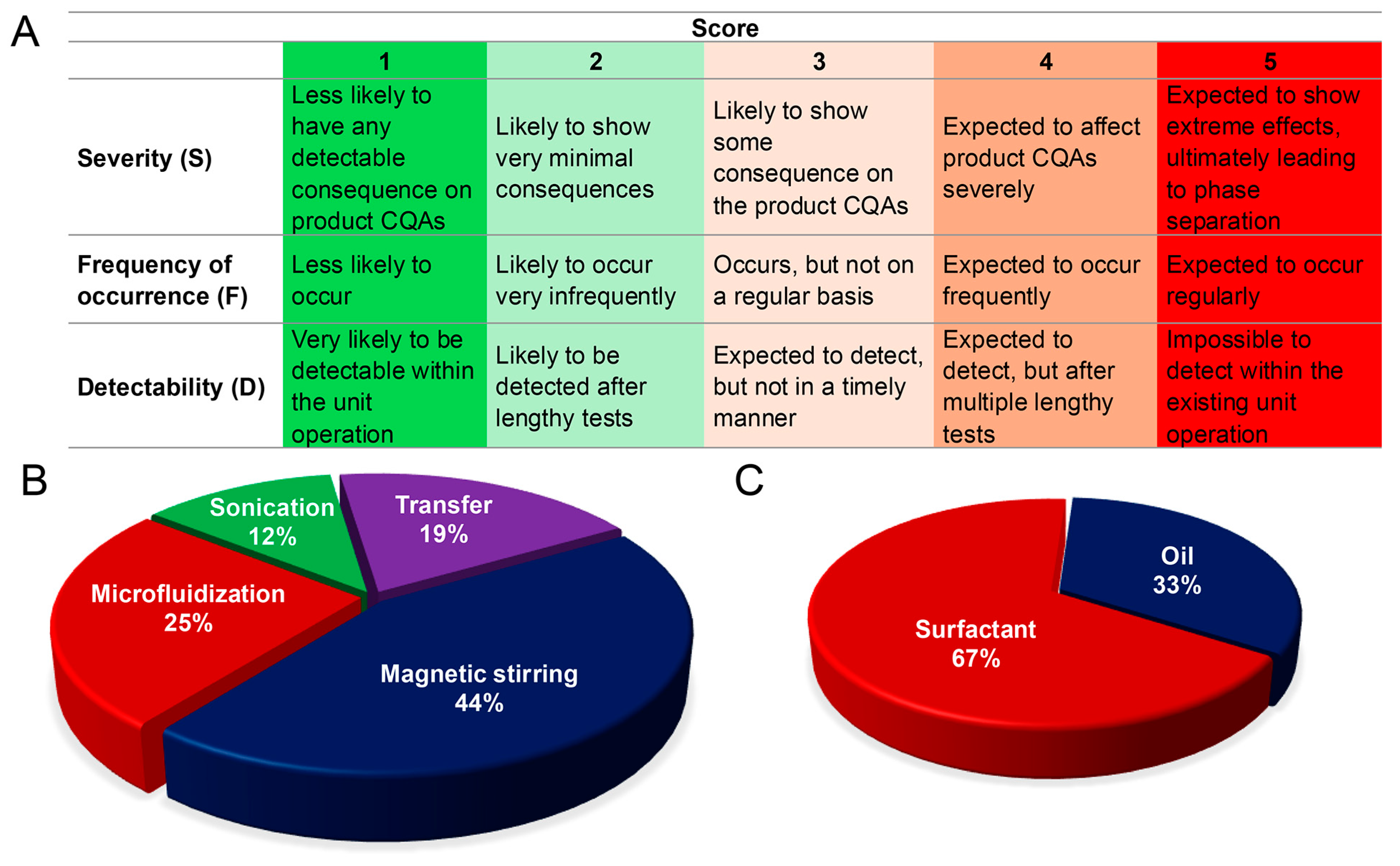
2.2.1. Risk Assessment and Experimental Design
2.2.2. Micelle and Nanoemulsion Preparation
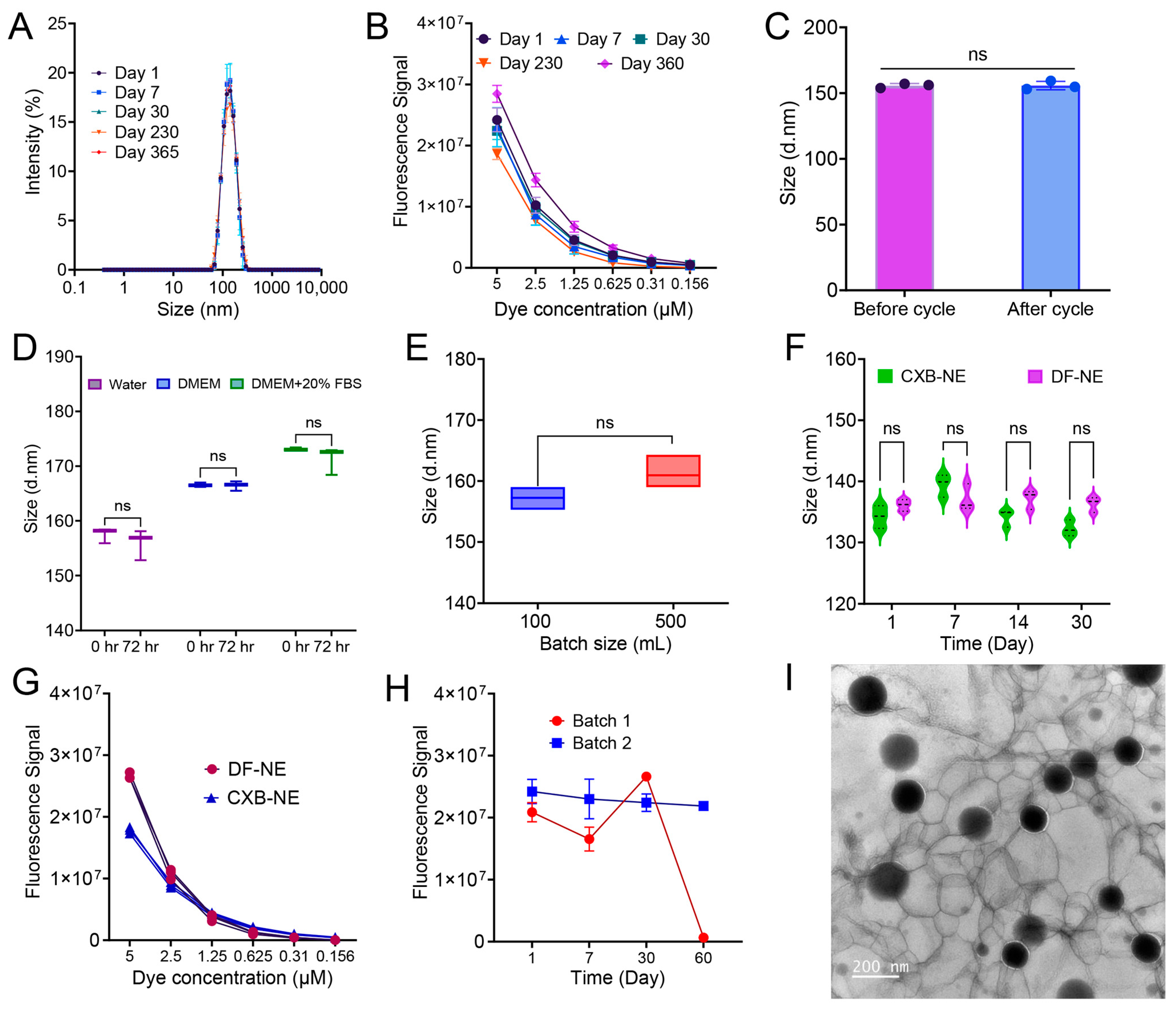
2.2.3. Colloidal, Fluorescence, and Morphological Analysis
2.2.4. Stability Study Under Stress Conditions
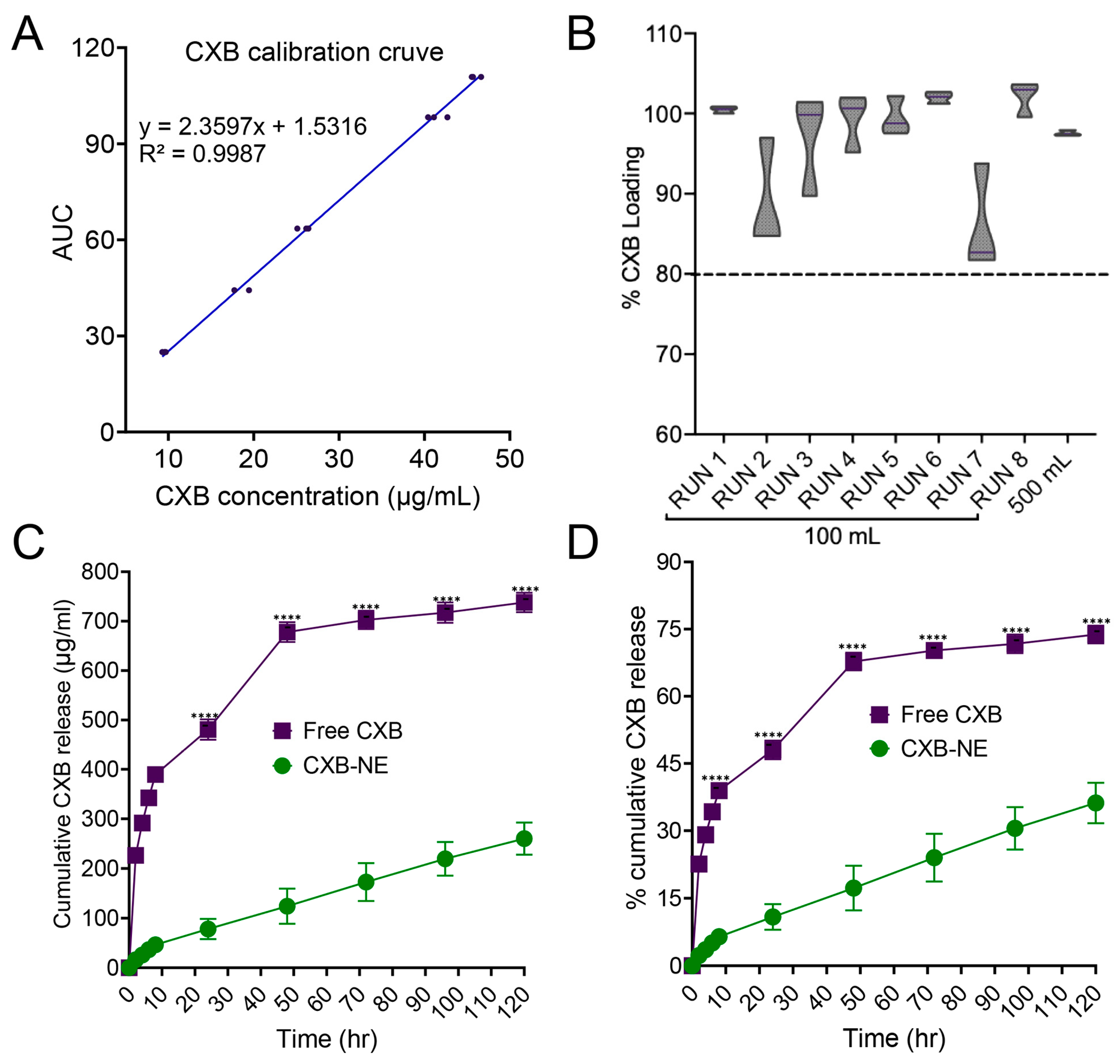
2.2.5. Celecoxib Quantification and In Vitro Release Study
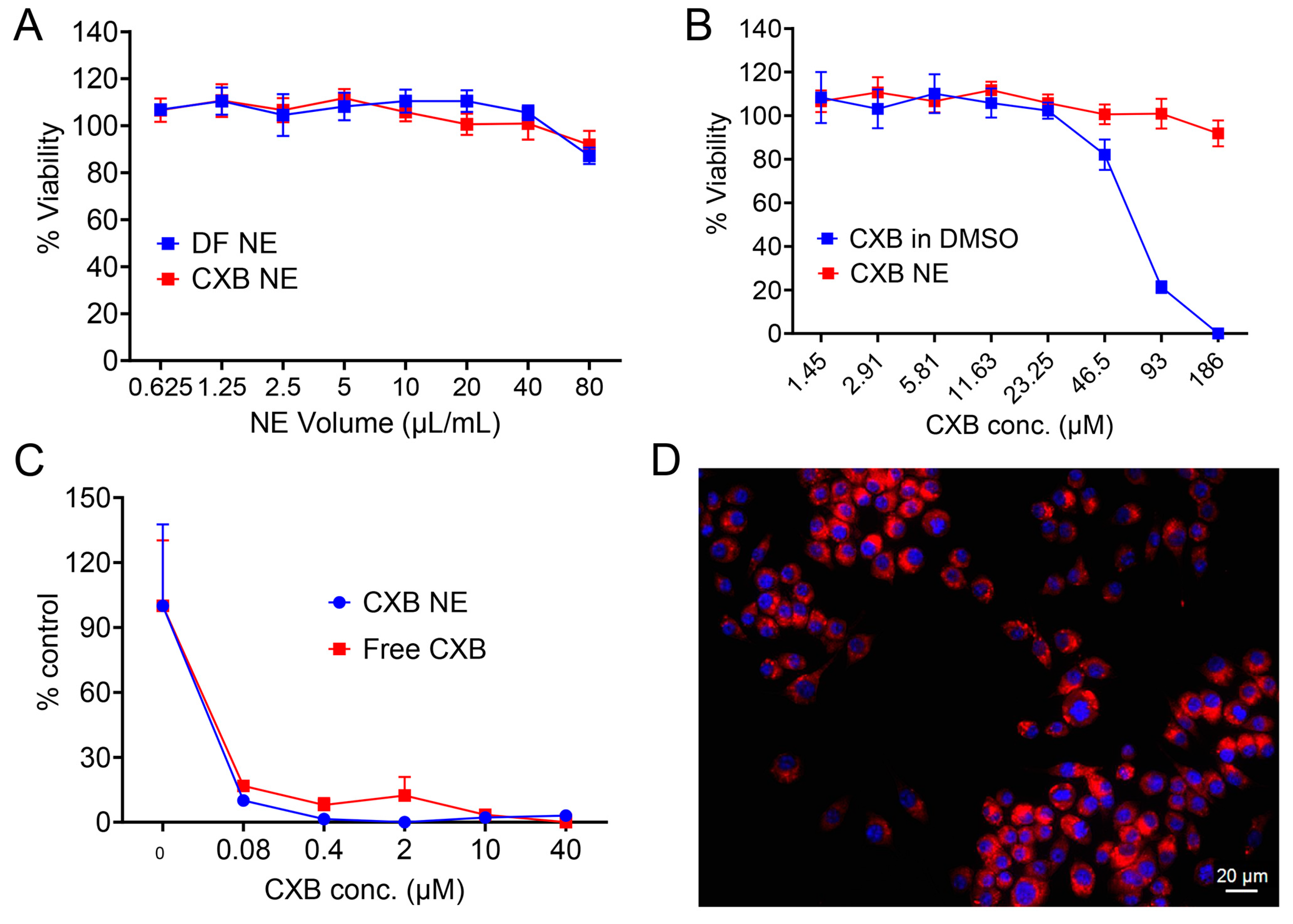
2.2.6. In Vitro Cell Viability and Cellular Uptake Study
2.2.7. In Vitro Pharmacological Response
2.2.8. Statistical Analysis
3. Results
3.1. Identification of Critical Quality Attributes (CQAs)
3.2. Manufacturing Feasibility, Formulation Compatibility, and Process Parameter Screening
3.3. Risk Assessment and High-Risk Factor Distribution
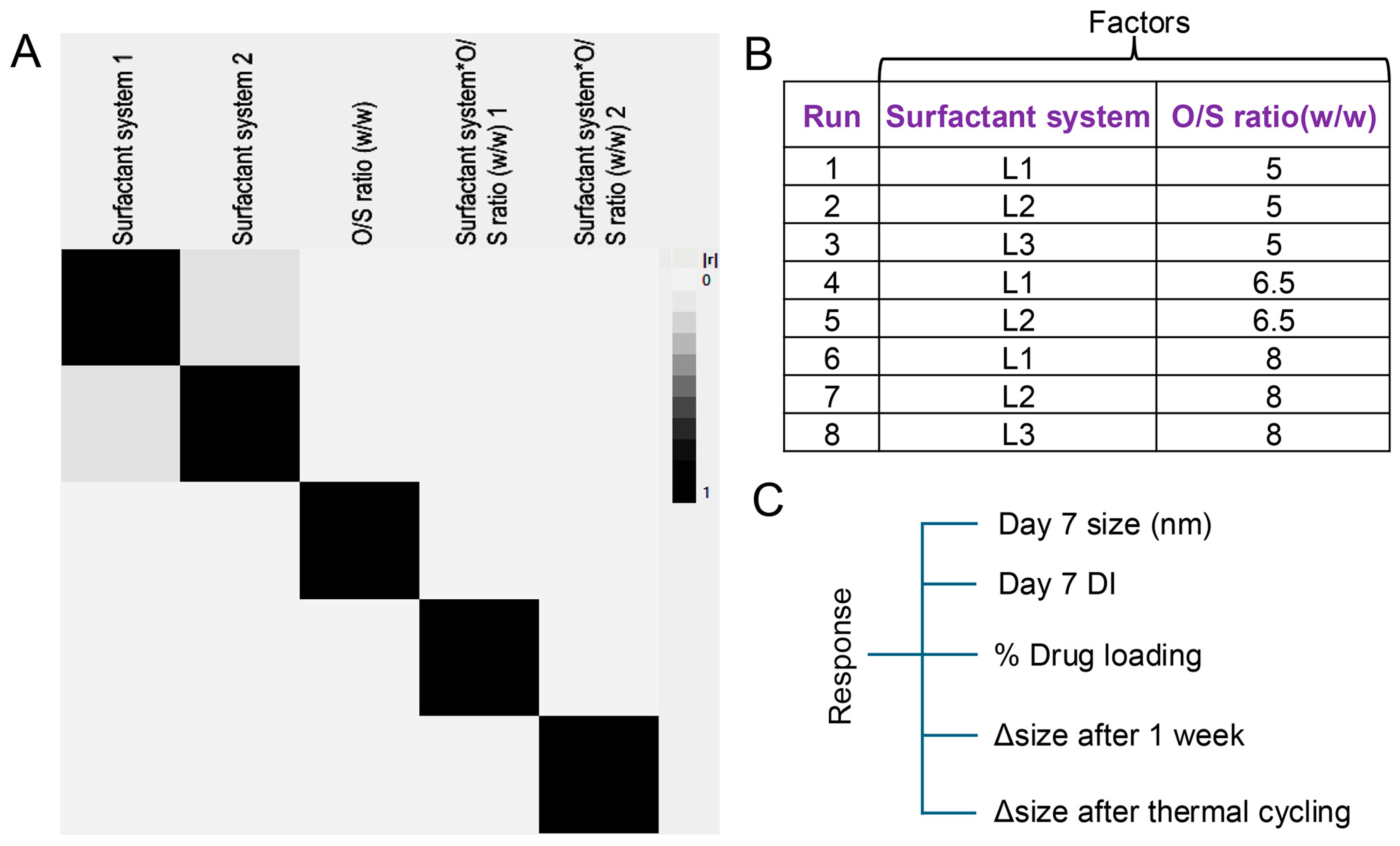
3.4. Full Factorial Design of Experiment
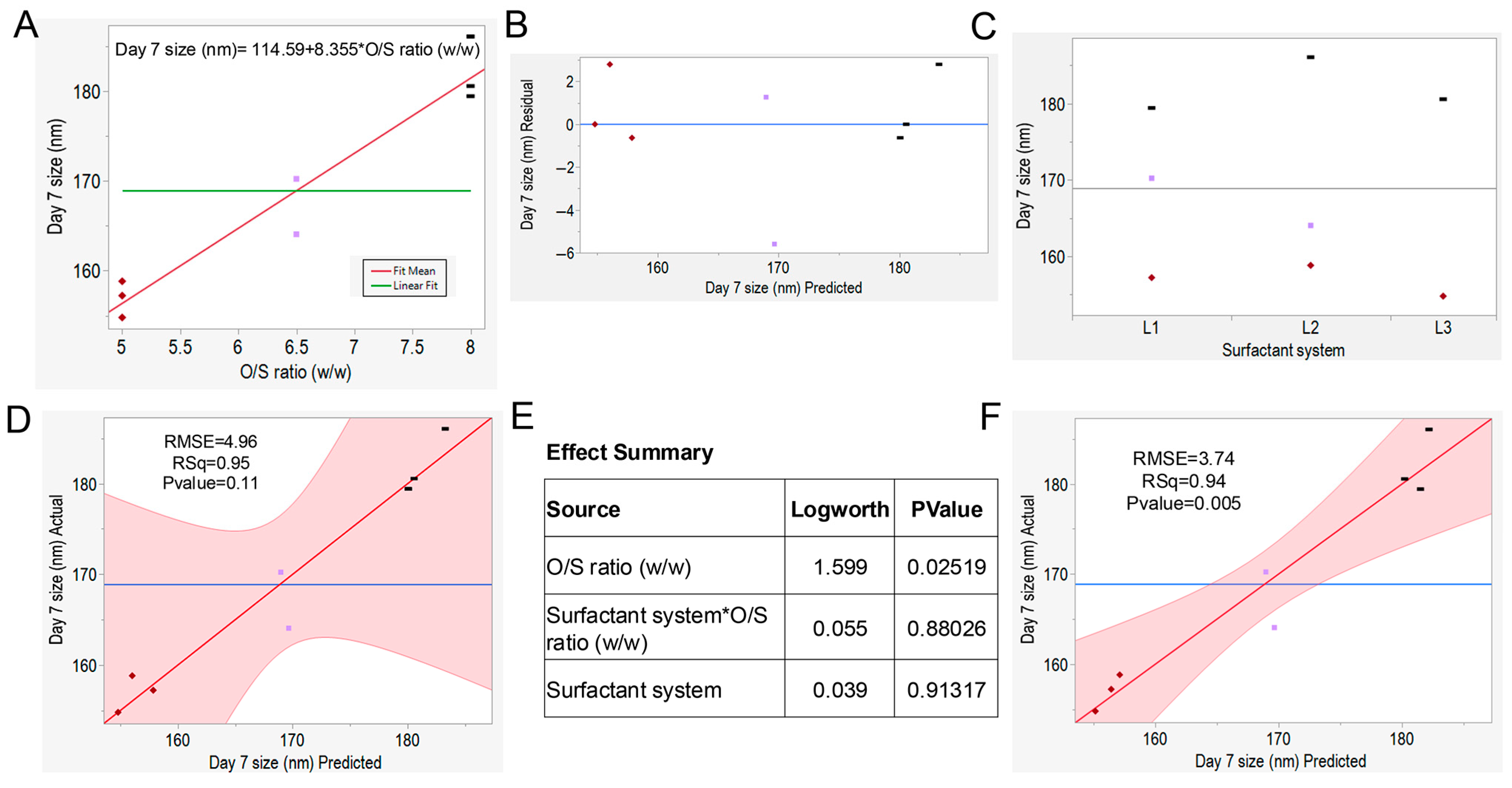
3.5. Regression Analysis and Predictive Modeling
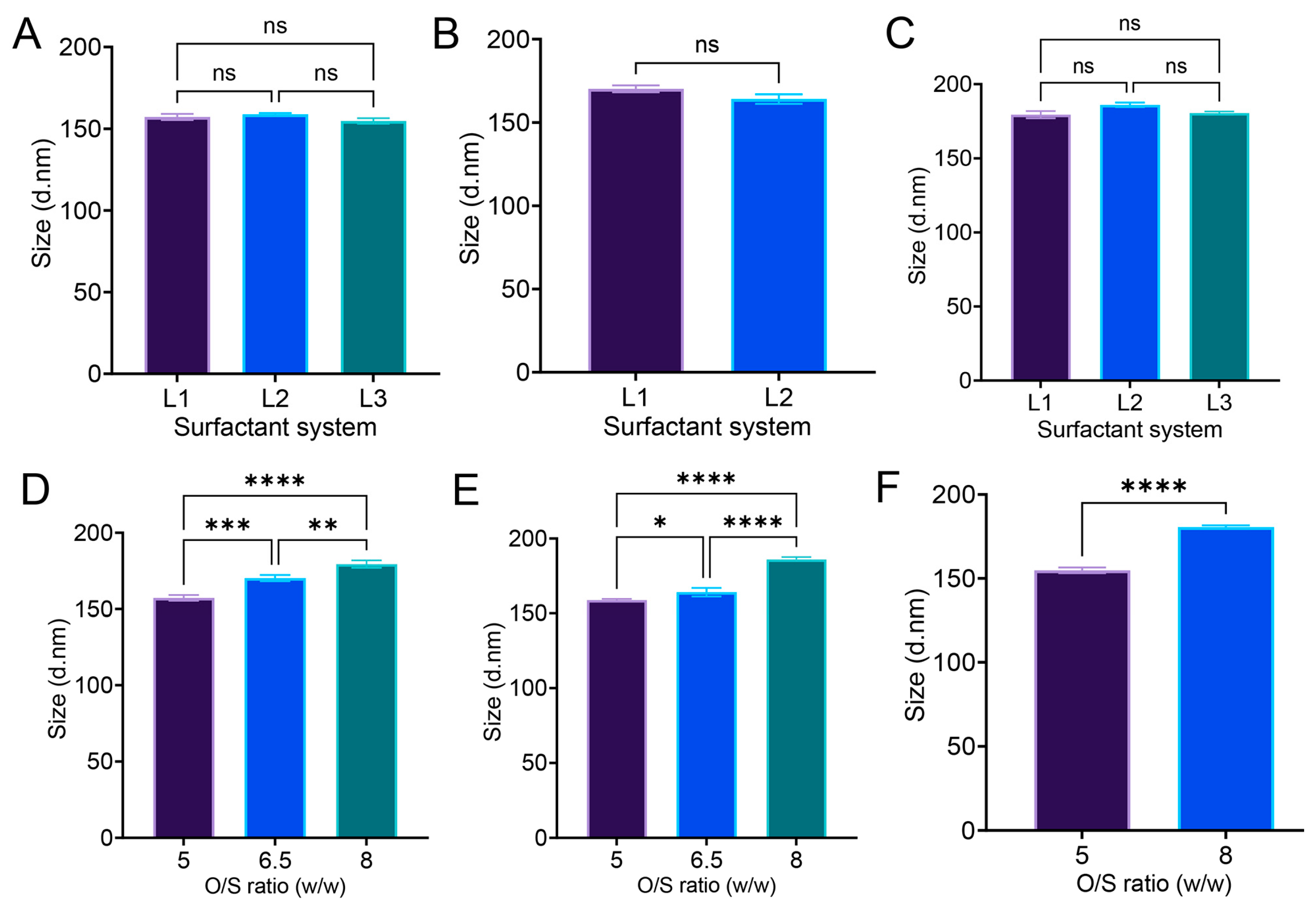
3.6. Effect of O/S Ratio (w/w) and Surfactant System on Nanoemulsion Droplet Size
3.7. Colloidal and Fluorescence Stability, Morphology, and Scale-Up
3.8. Celecoxib Quantification and In Vitro Release Study
3.9. In Vitro Cell Viability, PGE2 Release Inhibition, and Cellular Uptake Study
4. Discussion
5. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yong, R.J.; Mullins, P.M.; Bhattacharyya, N. Prevalence of chronic pain among adults in the United States. PAIN 2022, 163, e328–e332. [Google Scholar] [CrossRef] [PubMed]
- Rasu, R.S.; Vouthy, K.; Crowl, A.N.; Stegeman, A.E.; Fikru, B.; Bawa, W.A.; Knell, M.E. Cost of Pain Medication to Treat Adult Patients with Nonmalignant Chronic Pain in the United States. J. Manag. Care Pharm. 2014, 20, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.M.; Connor, E.M.; Bose, J.; Lucas, J.W.; Zelaya, C.E. Prescription Opioid Use Among Adults with Chronic Pain: United States, 2019; National Center for Health Statistics: Hyattsville, MD, USA, 2021.
- Hazam, H.; Prades, L.; Cailleau, C.; Mougin, J.; Feng, J.; Benhamou, D.; Gobeaux, F.; Hamdi, L.; Couvreur, P.; Sitbon, P.; et al. A nanomedicine approach for the treatment of long-lasting pain. J. Control. Release 2024, 373, 688–698. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Janjic, J.M.; Bai, M. Design and development of theranostic nanomedicines. In Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications; Wiley: Hoboken, NJ, USA, 2014; pp. 429–465. [Google Scholar] [CrossRef]
- Herneisey, M.; Liu, L.; Lambert, E.; Schmitz, N.; Loftus, S.; Janjic, J.M. Development of Theranostic Perfluorocarbon Nanoemulsions as a Model Non-Opioid Pain Nanomedicine Using a Quality by Design (QbD) Approach. AAPS PharmSciTech 2019, 20, 65. [Google Scholar] [CrossRef]
- Holm, P.; Allesø, M.; Bryder, M.C.; Holm, R. Q8 (R2) Pharmaceutical Development. In ICH Quality Guidelines: An Implementation Guide; Wiley: Hoboken, NJ, USA, 2017; pp. 535–577. [Google Scholar] [CrossRef]
- Javed, M.N.; Alam, M.S.; Waziri, A.; Pottoo, F.H.; Yadav, A.K.; Hasnain, M.S.; Almalki, F.A. QbD applications for the development of nanopharmaceutical products. In Pharmaceutical Quality by Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–253. [Google Scholar]
- Herneisey, M.; Lambert, E.; Kachel, A.; Shychuck, E.; Drennen, J.K.; Janjic, J.M. Quality by Design Approach Using Multiple Linear and Logistic Regression Modeling Enables Microemulsion Scale Up. Molecules 2019, 24, 2066. [Google Scholar] [CrossRef]
- Izat, N.; Yerlikaya, F.; Capan, Y. A Glance on the History of Pharmaceutical Quality by Design; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Vichare, R.; Crelli, C.; Liu, L.; McCallin, R.; Cowan, A.; Stratimirovic, S.; Herneisey, M.; Pollock, J.A.; Janjic, J.M. Folate-conjugated near-infrared fluorescent perfluorocarbon nanoemulsions as theranostics for activated macrophage COX-2 inhibition. Sci. Rep. 2023, 13, 15229. [Google Scholar] [CrossRef]
- Janjic, J.; Liu, L.; Komatsu, T.; Herneisey, M.; Loftus, S.; Lambert, E.; Zor, F.; Gorantla, V. (136) Dual Targeted Theranostic Nanomedicines for Local and Systemic Non-Opioid Analgesia in Trauma. J. Pain 2019, 20, S10. [Google Scholar] [CrossRef]
- Jou, I.-M.; Lin, C.-F.; Tsai, K.-J.; Wei, S.-J. Macrophage-Mediated Inflammatory Disorders. Mediat. Inflamm. 2013, 2013, 316482. [Google Scholar] [CrossRef]
- Ganguly, R.; Kumar, S.; Soumya, M.; Khare, A.; Bhainsa, K.C.; Aswal, V.K.; Kohlbrecher, J. Structural and therapeutic properties of salicylic acid-solubilized Pluronic solutions and hydrogels. Soft Matter 2024, 20, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Wu, D. Nutrition and Age-Associated Inflammation: Implications for Disease Prevention. J. Parenter. Enter. Nutr. 2008, 32, 626–629. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Zhang, Y.; Ruan, Y.; Chen, Q.; Gong, W.; Yu, J.; Xia, W.; He, J.C.-J.; Huang, S.; et al. COX-2/mPGES-1/PGE2 cascade activation mediates uric acid-induced mesangial cell proliferation. Oncotarget 2016, 8, 10185–10198. [Google Scholar] [CrossRef]
- Clemett, D.; Goa, K.L. Celecoxib: A review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef]
- Rahme, E.; Bernatsky, S. NSAIDs and risk of lower gastrointestinal bleeding. Lancet 2010, 376, 146–148. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Shakeel, F.; Shafiq, S.; Haq, N.; Alanazi, F.K.; A Alsarra, I. Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: An overview. Expert Opin. Drug Deliv. 2012, 9, 953–974. [Google Scholar] [CrossRef]
- Tang, S.Y.; Sivakumar, M.; Ng, A.M.-H.; Shridharan, P. Anti-inflammatory and analgesic activity of novel oral aspirin-loaded nanoemulsion and nano multiple emulsion formulations generated using ultrasound cavitation. Int. J. Pharm. 2012, 430, 299–306. [Google Scholar] [CrossRef]
- Ghiasi, Z.; Esmaeli, F.; Aghajani, M.; Ghazi-Khansari, M.; Faramarzi, M.A.; Amani, A. Enhancing analgesic and anti-inflammatory effects of capsaicin when loaded into olive oil nanoemulsion: An in vivo study. Int. J. Pharm. 2019, 559, 341–347. [Google Scholar] [CrossRef] [PubMed]
- A Gaber, D.; Alsubaiyel, A.M.; Alabdulrahim, A.K.; Alharbi, H.Z.; Aldubaikhy, R.M.; Alharbi, R.S.; Albishr, W.K.; Mohamed, H.A. Nano-Emulsion Based Gel for Topical Delivery of an Anti-Inflammatory Drug: In vitro and in vivo Evaluation. Drug Des. Dev. Ther. 2023, ume 17, 1435–1451. [Google Scholar] [CrossRef]
- Sakeena, M.H.F.; Yam, M.F.; Elrashid, S.M.; Munavvar, A.S.; Azmin, M.N. Anti-inflammatory and Analgesic Effects of Ketoprofen in Palm Oil Esters Nanoemulsion. J. Oleo Sci. 2010, 59, 667–671. [Google Scholar] [CrossRef]
- Janjic, J.M.; Vasudeva, K.; Saleem, M.; Stevens, A.; Liu, L.; Patel, S.; Pollock, J.A. Low-dose NSAIDs reduce pain via macrophage targeted nanoemulsion delivery to neuroinflammation of the sciatic nerve in rat. J. Neuroimmunol. 2018, 318, 72–79. [Google Scholar] [CrossRef]
- Vasudeva, K.; Andersen, K.; Zeyzus-Johns, B.; Hitchens, T.K.; Patel, S.K.; Balducci, A.; Janjic, J.M.; Pollock, J.A.; Gao, J.-X. Imaging Neuroinflammation In Vivo in a Neuropathic Pain Rat Model with Near-Infrared Fluorescence and 19F Magnetic Resonance. PLoS ONE 2014, 9, e90589. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflügers Arch. Eur. J. Physiol. 2020, 473, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2014, 92, 1–15. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Paroutoglou, E.; Fojan, P.; Gurevich, L.; Afshari, A. Thermal Properties of Novel Phase-Change Materials Based on Tamanu and Coconut Oil Encapsulated in Electrospun Fiber Matrices. Sustainability 2022, 14, 7432. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S. Studies on the Effect of Oil and Surfactant on the Formation of Alginate-Based O/W Lidocaine Nanocarriers Using Nanoemulsion Template. Pharmaceutics 2020, 12, 1223. [Google Scholar] [CrossRef]
- Alotaibi, H.F.; Khafagy, E.-S.; Abu Lila, A.S.; Alotaibe, H.F.; Elbehairi, S.E.; Alanazi, A.S.; Alfaifi, M.Y.; Alamoudi, J.A.; Alamrani, S.S.; Mokhtar, F.A. Anticancer potentials of metformin loaded coconut oil nanoemulsion on MCF-7, HepG2 and HCT-116 cell lines. Artif. Cells Nanomed. Biotechnol. 2023, 51, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A. Shadab Coconut oil-based resveratrol nanoemulsion: Optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem. 2021, 357, 129721. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kim, J.-K.; Lim, J.-S.; Lim, S.-J. Enhancement of indocyanine green stability and cellular uptake by incorporating cationic lipid into indocyanine green-loaded nanoemulsions. Colloids Surf. B Biointerfaces 2015, 136, 305–313. [Google Scholar] [CrossRef]
- Alotaibi, H.; Hatahet, T.; Al-Jamal, W.T. Understanding the formulation parameters for engineering indocyanine green J-aggregate lipid nanocapsules and solid lipid nanoparticles as promising photothermal agents. Eur. J. Pharm. Sci. 2025, 207, 107034. [Google Scholar] [CrossRef]
- Van Zyl, A.J.; de Wet-Roos, D.; Sanderson, R.D.; Klumperman, B. The role of surfactant in controlling particle size and stability in the miniemulsion polymerization of polymeric nanocapsules. Eur. Polym. J. 2004, 40, 2717–2725. [Google Scholar] [CrossRef]
- Debraj, D.; Carpenter, J.; Vatti, A.K. Understanding the Effect of the Oil-to-Surfactant Ratio on Eugenol Oil-in-Water Nanoemulsions Using Experimental and Molecular Dynamics Investigations. Ind. Eng. Chem. Res. 2023, 62, 16766–16776. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB value on oil-in-water emulsions: Droplet size, rheological behavior, zeta-potential, and creaming index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Seyed-Hosseini, S.; Safaei, N.; Asgharpour, M. Reprioritization of failures in a system failure mode and effects analysis by decision making trial and evaluation laboratory technique. Reliab. Eng. Syst. Saf. 2006, 91, 872–881. [Google Scholar] [CrossRef]
- Vichare, R.; Kulahci, Y.; McCallin, R.; Zor, F.; Selek, F.N.; Liu, L.; Crelli, C.; Troidle, A.; Herneisey, M.; Nichols, J.M.; et al. Theranostic nanoemulsions suppress macrophage-mediated acute inflammation in rats. J. Nanobiotechnol. 2025, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Janjic, J.; McCallin, R.; Liu, L.; Crelli, C.; Das, A.; Troidle, A. In vitro Quality Assessments of Perfluorocarbon Nanoemulsions for Near-infrared Fluorescence Imaging of Inflammation in Preclinical Models. Bio-Protocol 2023, 13, e4842. [Google Scholar] [CrossRef]
- Das, A.C.; Nichols, J.M.; Crelli, C.V.; Liu, L.; Vichare, R.; Pham, H.V.; Gaffney, C.M.; Cherry, F.R.; Grace, P.M.; Shepherd, A.J.; et al. Injectable, reversibly thermoresponsive captopril-laden hydrogel for the local treatment of sensory loss in diabetic neuropathy. Sci. Rep. 2024, 14, 18978. [Google Scholar] [CrossRef]
- Seedher, N.; Bhatia, S. Solubility enhancement of cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003, 4, 36–44. [Google Scholar] [CrossRef]
- Nichols, J.M.; Crelli, C.V.; Liu, L.; Pham, H.V.; Janjic, J.M.; Shepherd, A.J. Tracking macrophages in diabetic neuropathy with two-color nanoemulsions for near-infrared fluorescent imaging and microscopy. J. Neuroinflammation 2021, 18, 299. [Google Scholar] [CrossRef]
- Vichare, R.; Crelli, C.; Liu, L.; Das, A.C.; McCallin, R.; Zor, F.; Kulahci, Y.; Gorantla, V.S.; Janjic, J.M. A Reversibly Thermoresponsive, Theranostic Nanoemulgel for Tacrolimus Delivery to Activated Macrophages: Formulation and In Vitro Validation. Pharmaceutics 2023, 15, 2372. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Martins, J.; Santos, J.; da Conceição, M. Comparative study of physico-chemical properties of coconut oil (Cocos nucifera L.) obtained by industrial and artisanal processes. Biotechnol. Ind. J. 2020, 16, 210. [Google Scholar]
- Bertani, A.; Di Paola, G.; Russo, E.; Tuzzolino, F. How to describe bivariate data. J. Thorac. Dis. 2018, 10, 1133–1137. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Ermer, J. ICH Q2 (R2): Validation of analytical procedures. In Method Validation in Pharmaceutical Analysis: A Guide to Best Practice; Wiley: Hoboken, NJ, USA, 2025; pp. 351–372. [Google Scholar]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Peterchristoper, G.V. Release kinetics–concepts and applications. Int. J. Pharm. Res. Technol. (IJPRT) 2018, 8, 12–20. [Google Scholar]
- Mohamadi, P.S.; Hivechi, A.; Bahrami, H.; Hemmatinegad, N.; Milan, P.B. Antibacterial and biological properties of coconut oil loaded poly(ε-caprolactone)/gelatin electrospun membranes. J. Ind. Text. 2021, 51, 906S–930S. [Google Scholar] [CrossRef]
- Chandran, J.; Nayana, N.; Roshini, N.; Nisha, P. Oxidative stability, thermal stability and acceptability of coconut oil flavored with essential oils from black pepper and ginger. J. Food Sci. Technol. 2016, 54, 144–152. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P. Coconut oil as base oil for industrial lubricants—evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly (lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J. Photochem. Photobiol. B Biol. 2004, 74, 29–38. [Google Scholar] [CrossRef]
- Li, P.; Huang, H.; Fang, Y.; Wang, Y.; No, D.S.; Bhatnagar, R.S.; Abbaspourrad, A. Interfacial engineering of clear emulsions: Surfactant hydrophobicity and the hidden role of chain structure. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676. [Google Scholar] [CrossRef]
- Pourshamohammad, S.; Amrani, M.; Sarabi-Aghdam, V.; Abedi, A.-S.; Mahmoudzadeh, M. Investigating the surfactant-to-oil ratio, type of ripening inhibitor, and modified starch on the stability of Carum copticum essential oil nanoemulsions: Application in yoghurt drink. J. Agric. Food Res. 2024, 16, 101207. [Google Scholar] [CrossRef]
- Liu, L.; Karagoz, H.; Herneisey, M.; Zor, F.; Komatsu, T.; Loftus, S.; Janjic, B.M.; Gorantla, V.S.; Janjic, J.M. Sex Differences Revealed in a Mouse CFA Inflammation Model with Macrophage Targeted Nanotheranostics. Theranostics 2020, 10, 1694–1707. [Google Scholar] [CrossRef]
- Kovalchuk, V.; Loglio, G.; Bykov, A.; Ferrari, M.; Krägel, J.; Liggieri, L.; Miller, R.; Milyaeva, O.; Noskov, B.; Ravera, F.; et al. Effect of Temperature on the Dynamic Properties of Mixed Surfactant Adsorbed Layers at the Water/Hexane Interface under Low-Gravity Conditions. Colloids Interfaces 2020, 4, 27. [Google Scholar] [CrossRef]
- Oimoen, S. Classical Designs: Full Factorial Designs; STAT Center of Excellence: Dayton, OH, USA, 2019; pp. 1–25. [Google Scholar]
- Pourhoseingholi, M.A.; Baghestani, A.R.; Vahedi, M. How to control confounding effects by statistical analysis. Gastroenterol. Hepatol. Bed Bench 2012, 5, 79–83. [Google Scholar] [PubMed]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Influence of surfactant type and thermal cycling on formation and stability of flavor oil emulsions fabricated by spontaneous emulsification. Food Res. Int. 2016, 89, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bagia, C.; Janjic, J.M. The First Scale-Up Production of Theranostic Nanoemulsions. BioResearch Open Access 2015, 4, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Peshkovsky, A.S.; Bystryak, S. Continuous-flow production of a pharmaceutical nanoemulsion by high-amplitude ultrasound: Process scale-up. Chem. Eng. Process.-Process. Intensif. 2014, 82, 132–136. [Google Scholar] [CrossRef]
- Francke, N.M.; Bunjes, H. Influence of drug loading on the physical stability of phospholipid-stabilised colloidal lipid emulsions. Int. J. Pharm. X 2020, 2, 100060. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Beaino, W.; Anderson, C.J.; Janjic, J.M. Theranostic nanoemulsions for macrophage COX-2 inhibition in a murine inflammation model. Clin. Immunol. 2015, 160, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]

| QTPPs | Target | Justification |
|---|---|---|
| Dosage form | O/W nanoemulsion | Enhancement of drug bioavailability |
| Dose | 0.24 mg of CXB/kg body weight, single dose | Already proven pain relief for 4 days |
| Route of administration | Intravenous | Better efficacy in targeting macrophages and escaping first-pass metabolism |
| Encapsulation efficiency | >80% | Optimum drug encapsulation for therapeutic efficacy |
| Morphology | Spherical | For uniform release and high encapsulation efficiency |
| Droplet size | 100–160 nm | Standard quality of nanoemulsion for stability |
| Polydispersity index | <0.20 | Standard quality of nanoemulsion for stability |
| Stability | Stable under normal and stress conditions | Measure of normal and accelerated study |
| CQAs | Target | Justification |
|---|---|---|
| Size at day 7 | 100–160 nm | By day 7, nanoemulsion droplets will equilibrate to a stable size |
| PDI at day 7 | <0.2 | By day 7, nanoemulsion droplets will equilibrate, and DI will be stable |
| Δ size after 1 week | <10% | Gives an idea of the storage stability of nanoemulsion |
| Δ PDI after 1 week | <0.1 | Gives an idea of the storage stability of nanoemulsion |
| Encapsulation efficiency | >80% | Evaluate the efficiency of the nanoemulsion to encapsulate the drug |
| Δ size after thermal cycling | <10% | Evaluate the shelf-life and storage conditions of nanoemulsions in harsh environments |
| Δ size after serum stability | <20 nm | Tested in medium and serum to assess the stability as the nanoemulsion is intended to be used intravenously, where it will encounter body fluid with electrolytes and protein. |
| Run | Droplet Size (nm) | PDI | Δ Size After 1 Week | Encapsulation Efficiency | Δ Size After Thermal Cycling |
|---|---|---|---|---|---|
| 1 | 157.23 ± 1.52 | 0.08 ± 0.01 | 1.02 ± 0.01% | 100.47 ± 0.36% | −0.06 ± 0.01% |
| 2 | 158.83 ± 0.59 | 0.10 ± 0.02 | 1.81 ± 0.01% | 88.84 ± 5.75% | 15.65 ± 0.01% |
| 3 | 154.80 ± 1.40 | 0.11 ± 0.01 | 0.69 ± 0.01% | 97.00 ± 5.19% | −1.65 ± 0.01% |
| 4 | 170.23 ± 1.68 | 0.09 ± 0.02 | 0.18 ± 0.02% | 99.25 ± 2.94% | 9.52 ± 0.01% |
| 5 | 164.06 ± 2.36 | 0.08 ± 0.01 | −1.04 ± 0.01% | 99.50 ± 1.96% | 3.94 ± 0.01% |
| 6 | 179.43 ± 1.98 | 0.08 ± 0.02 | −0.09% ± 0.02% | 102.00 ± 0.60% | 9.97% ± 0.02% |
| 7 | 186.07 ± 1.29 | 0.07 ± 0.02 | 0.61 ± 0.01% | 86.05 ± 5.46% | 17.48 ± 0.01% |
| 8 | 180.56 ± 0.87 | 0.06 ± 0.03 | −0.69 ± 0.01% | 102.05 ± 1.79% | 18.25 ± 0.02% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.C.; Reddy, G.A.; Newaj, S.M.; Patel, S.; Vichare, R.; Liu, L.; Janjic, J.M. Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions. Pharmaceutics 2025, 17, 1010. https://doi.org/10.3390/pharmaceutics17081010
Das AC, Reddy GA, Newaj SM, Patel S, Vichare R, Liu L, Janjic JM. Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions. Pharmaceutics. 2025; 17(8):1010. https://doi.org/10.3390/pharmaceutics17081010
Chicago/Turabian StyleDas, Amit Chandra, Gayathri Aparnasai Reddy, Shekh Md. Newaj, Smith Patel, Riddhi Vichare, Lu Liu, and Jelena M. Janjic. 2025. "Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions" Pharmaceutics 17, no. 8: 1010. https://doi.org/10.3390/pharmaceutics17081010
APA StyleDas, A. C., Reddy, G. A., Newaj, S. M., Patel, S., Vichare, R., Liu, L., & Janjic, J. M. (2025). Design of Experiments Leads to Scalable Analgesic Near-Infrared Fluorescent Coconut Nanoemulsions. Pharmaceutics, 17(8), 1010. https://doi.org/10.3390/pharmaceutics17081010







