Biomedical Applications of Functionalized Composites Based on Metal–Organic Frameworks in Bone Diseases
Abstract
1. Introduction
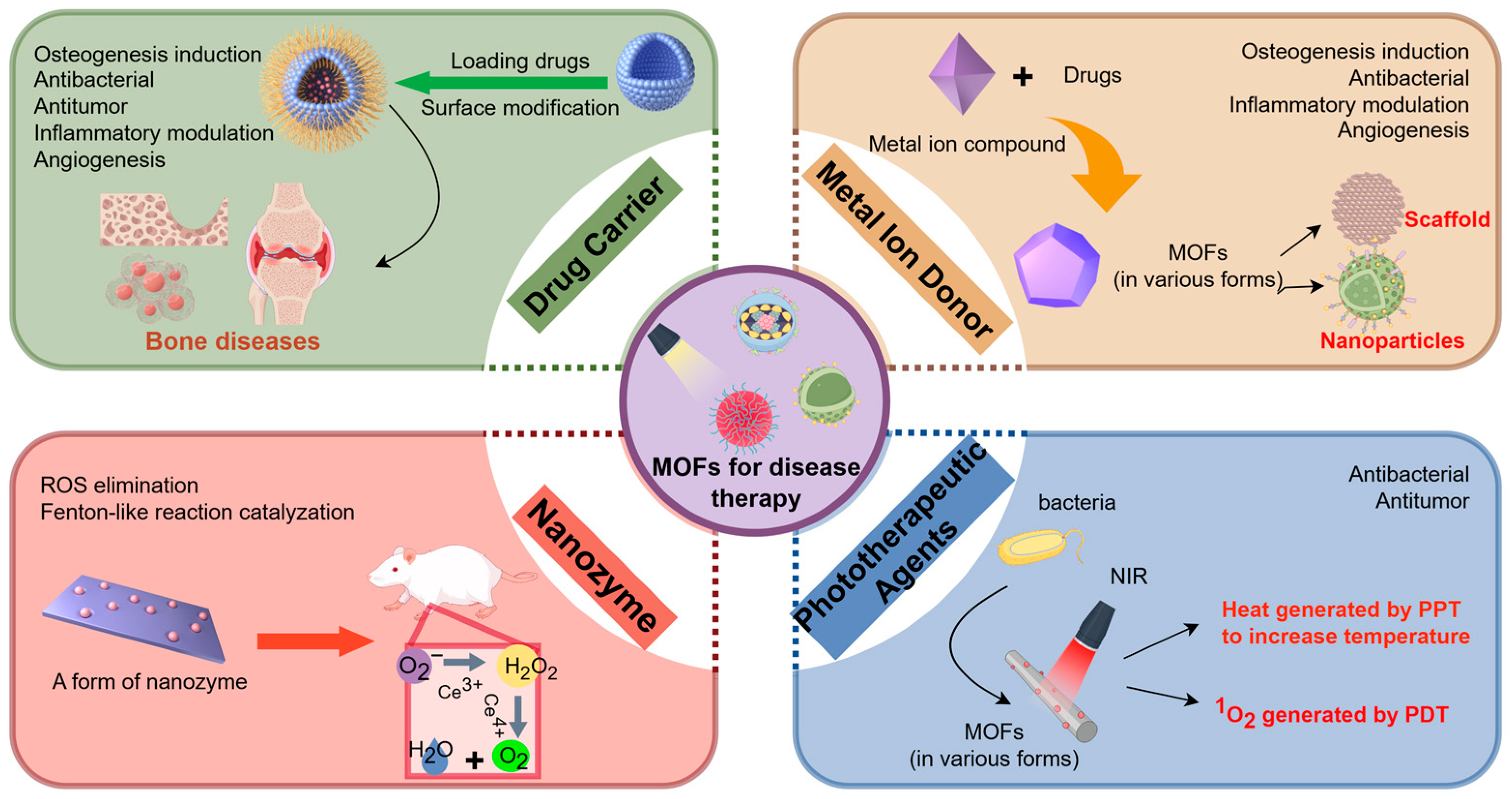
2. Classification of MOF Applications in Biomedicine
2.1. Drug Carriers
2.2. Metal Ion Donors
2.3. Nanozymes
2.4. Phototherapeutic Agents
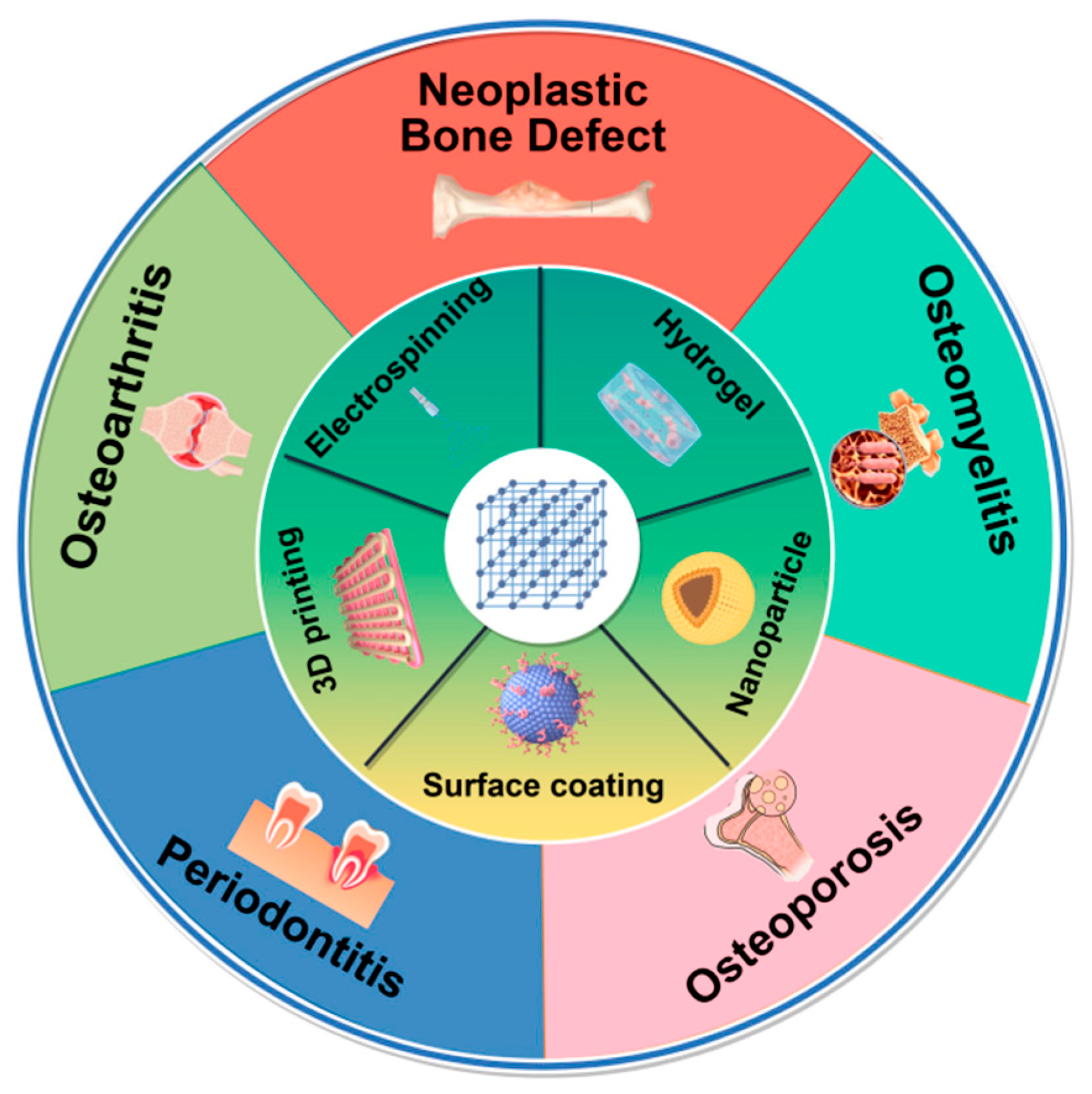
3. Synthesis Technology for MOF Composites in Bone Disease
3.1. Surface Coating Materials
3.2. Electrospinning Materials
3.3. Hydrogel Materials
3.4. Three-Dimensional (3D) Printing Materials
3.5. Nanoparticle (NP) Materials
4. Applications of MOFs in Bone Disease Therapy
4.1. MOFs in Osteoarthritis (OA)
| MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs and Loading Efficiency | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|
| MIL-100(Fe)@HA@PCA | Nanoparticle | Drug carrier | Hyaluronic acid (~21.6%); protocatechuic acid (~19.4%) | Fe3+ | Promoting chondrocyte proliferation; relieving inflammation | / | Promoting cartilage regeneration: inhibit MMP-13 expression | [62] |
| Qu@ZIF-8 | Nanoparticle | Drug carrier | Quercetin (~23%) | Zn2+ | Promoting cartilage anabolism; anti-inflammatory; inhibiting IL-1β-induced apoptosis | Inhibiting PI3K/Akt signaling pathway | Maintaining cartilage structure integrity and glycosaminoglycan synthesis | [102] |
| MIL-101-NH2 | Nanoparticle | Drug carrier | Curcumin (~25.9%); siHIF-2α; hyaluronic acid | Fe3+ | Promoting cartilage anabolism; inhibiting inflammatory factors | / | Promoting cartilage anabolism; inhibiting inflammatory factors | [103] |
| ZIF8@CRIg-CD59@HA@ZA | Nanoparticle | Drug carrier | CRIg-CD59 (>90%); Zoledronic acid (~73.6%) | Zn2+ | Inhibiting bone resorption; repairing VSIg4+ macrophage barrier | / | Reducing synovial hyperplasia; protection of articular bone | [32] |
| KZIF@HA | Nanoparticle | Drug carrier | Kartogenin; hyaluronic acid | Zn2+ | Promoting cartilage anabolism; promoting M1-to-M2 macrophage polarization | Inhibiting JNK and ERK pathways in chondrocytes | Cartilage protection; inhibiting joint inflammation | [105] |
| Bai@FA-UIO-66-NH2 | Nanoparticle | Drug carrier | Baicalin | Zr4+ | Reducting ROS; promoting M1-to-M2 macrophage polarization | Modulation of immune homeostasis | Cartilage protection; reducing bone hyperplasia; recover subchondral bone structure | [106] |
| ZIF-8-PDA-HA | Nanoparticle | Drug carrier | Diclofenac sodium (~99%) | Zn2+ | Promoting cell proliferation; accelerated lubrication | / | / | [107] |
| MIL-101(Cr)@PEG-g-PNIPAm | Hydrogel | Drug carrier | Diclofenac sodium (~29.2%) | Cr3+ | Promoting cartilage anabolism; anti-inflammatory; accelerating lubrication | / | / | [108] |
| MIL-101(Cr)@P(NIPAm-gPEGMax) | Hydrogel | Drug carrier | Diclofenac sodium (~23.8%) | Cr3+ | Anti-inflammatory; accelerating lubrication | / | / | [109] |
| Mn3O4/UIO-TPP | Nanoparticle | Nanozyme | / | Mn3O4; Zr4+ | reducting mitochondrial ROS; inhibiting oxidative and inflammatory | / | Cartilage protection; inhibiting IL-6 and MMP-13 expression | [111] |
| Cu MOF | Nanoparticle | Nanozyme | / | Cu2+ | Reducting ROS; promoting M1-to-M2 macrophage polarization | / | Inhibiting ECM degradation; improving hypoxia; inhibiting synovitis | [112] |
| miR/IrO2@ZIF-8 | Nanoparticle | Nanozyme | AntagomiR-181a | Zn2+ | Reducting ROS; relieving inflammation; promoting cartilage anabolism; | / | Inhibiting ECM degradation; reducing bone hyperplasia; recover subchondral bone structure; inhibiting MMP-13 and ADAMTS-5 expression | [110] |
4.2. MOFs in Neoplastic Bone Defects
4.2.1. Breast Cancer-Induced Osteolysis
4.2.2. Osteosarcoma (OS)
| Neoplastic Bone Defects | MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Breast Cancer | BT-isUiO-66 | Nanoparticle | Drug carrier | Zoledronic acid (~3.14 wt%) | Zr4+ | Reducting ROS; inhibiting bone resorption; promoting M1 macrophage polarization | Activating TLR9 signaling transduction | Inhibiting tumor; protection of tibial tissue structure | [115] |
| 5-Fu/ICG@ZIF-90-PEG-ZOL | Nanoparticle | Drug carrier | 5-fluorouracil; zoledronic acid (~23.13%) | Zn2+ | Inducing apoptosis of MCF-7 cells under NIR | Inhibiting DNA replication | Bone-targeted; inhibiting tumor | [116] | |
| ICG@Cu2-xSe-ZIF-8 | Nanoparticle | Photosensitizer | / | Cu2-xSe | Promoting tumor cell apoptosis; inhibiting bone resorption | Inhibiting p65 and NFATc1 pathways | Inhibiting tumor; inhibiting cancer cells-induced osteolysis | [117] | |
| Osteosarcoma | HA@MOF/D-Arg | Nanoparticle | Drug carrier | D-arginine | Fe3+ | Promoting tumor cell apoptosis | Promoting DNA damage | Enhancing tumor ablation; preventing lung metastasis; alleviating tissue hypoxia | [120] |
| PDA-cloaked Fe-MOF | Nanoparticle | Drug carrier | D-arginine; tirapazamine | Fe3+ | Promoting tumor cell apoptosis | Producing ROS | Enhancing tumor ablation; preventing lung metastasis | [121] | |
| CUR-BMS1166@ZIF-8@PEG-FA | Nanoparticle | Drug carrier | Curcumin; BMS1166 | Zn2+ | Inducing immunogenic cell death | Activating autophagy; Inhibiting PD-1/PD-L1 signaling | Inhibiting tumor growth | [122] | |
| NH2-MIL-125(Ti) | Scaffold | Drug carrier | Doxorubicin | Ti4+ | Promoting tumor cell apoptosis; promoting osteogenesis | / | Inhibiting tumor growth; promoting bone repair | [123] | |
| DOX@UiO-66-NH2 | Nanoparticle | Drug carrier | Doxorubicin | Zr4+ | Promoting tumor cell apoptosis; promoting osteogenesis | Promoting osteoblast differentiation via activating PI3K-Akt and MAPK signaling pathways | Inhibiting tumor growth | [33] | |
| PCL@Cu-HHTP | Scaffold | Photosensitizer | / | Cu2+ | Promoting tumor cell apoptosis; promoting osteogenesis | / | Inhibiting tumor growth | [69] | |
| Cu-TCPP-TCP | Scaffold | Photosensitizer | / | Cu2+ | Promoting tumor cell apoptosis; promoting osteogenesis; promoting vascularization | / | Inhibiting tumor growth; promoting bone repair | [124] | |
| Co-TCPP/CPC | Scaffold | Photosensitizer | / | Co2+ | Promoting tumor cell apoptosis; promoting osteogenesis; promoting vascularization | / | Inhibiting tumor growth; promoting bone repair | [70] | |
| PDA-MOF-E-M | Nanoparticle | Photosensitizer | Erastin | Fe3+ | Promoting tumor cell apoptosis; inhibiting osteoclastic differentiation | / | Inhibiting tumor growth; inhibiting osteolysis | [125] | |
| V-RZCD | Nanoparticle | Drug carrier | Doxorubicin; zoledronic acid | Ca2+ | Promoting tumor cell apoptosis | / | Inhibiting tumor growth; inhibiting osteolysis | [126] |
4.3. MOFs in Osteoporosis
4.4. MOFs in Periodontitis
4.5. MOFs in Osteomyelitis
| MOF Composites | Form of the Composites | Primary Role of MOFs | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|
| HNTM-Pt@Au | Nanoparticle | Sound sensitizer | Pt, Au | Antibacterial; Promoting osteogenesis and mineralization; promoting M1-to-M2 macrophage polarization | Inhibiting iNOS and promoting TGF-β in macrophages | Inhibiting MRSA-infected osteomyelitis; preventing bone destruction | [148] |
| HNTM-MoS2 | Nanosheet | Sound sensitizer | MoS2 | Antibacterial; promoting M1-to-M2 macrophage polarization | / | Eliminating bone infection; inhibiting inflammation; inhibiting bone loss | [149] |
| HN-Ti3C2 | Nanosheet | Sound sensitizer | Ti3C2 | Antibacterial; Promoting osteogenesis and mineralization | Activating calcium, MAPK, and Wnt signaling pathways | Eliminating bone infection; inhibiting bone loss | [146] |
| CeTCPP-Au | Nanoparticle | Sound sensitizer | Au | Antibacterial | / | Eliminating bone infection; relieving inflammation | [150] |
| CNT-CuHHTP | Nanotube | Microwave dynamic therapy | Cu2+ | Antibacterial | / | Eliminating bone infection; relieving inflammation | [151] |
5. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.J.; You, X.L.; Zhang, L.L.; Zhang, C.Q.; Zou, W.G. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Yin, S.H.; Lin, M.Z.; Chen, X.L.; Pan, Y.; Peng, Y.Q.; Sun, J.B.; Kumar, A.; Liu, J.Q. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243S–6249S. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A.R. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Wang, W.H.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Binaeian, E.; Nabipour, H.; Ahmadi, S.; Rohani, S. The green synthesis and applications of biological metal-organic frameworks for targeted drug delivery and tumor treatments. J. Mater. Chem. B 2023, 11, 11426–11459. [Google Scholar] [CrossRef] [PubMed]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal-Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Afshariazar, F.; Morsali, A. Mixed-valence metal-organic frameworks: Concepts, opportunities, and prospects. Chem. Soc. Rev. 2025, 54, 1318–1383. [Google Scholar] [CrossRef]
- Lin, Z.J.; Lü, J.; Hong, M.C.; Cao, R. Metal-organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef]
- Qian, Q.H.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Bo, C.; Guo, S. Luminescent Ln-MOFs for Chemical Sensing Application on Biomolecules. ACS Sens. 2024, 9, 4402–4424. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent Progress in the Development of MOF-Based Photocatalysts for the Photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef]
- Roohollahi, H.; Zeinalzadeh, H.; Kazemian, H. Recent Advances in Adsorption and Separation of Methane and Carbon Dioxide Greenhouse Gases Using Metal-Organic Framework-Based Composites. Ind. Eng. Chem. Res. 2022, 61, 10555–10586. [Google Scholar] [CrossRef]
- Saboorizadeh, B.; Zare-Dorabei, R.; Safavi, M.; Safarifard, V. Applications of Metal-Organic Frameworks (MOFs) in Drug Delivery, Biosensing, and Therapy: A Comprehensive Review. Langmuir 2024, 40, 22477–22503. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, C.; Guo, J.; Zhang, X.; Wu, L.; Zhu, L.; Du, B. Research Progress of Metal-Organic Frameworks as Drug Delivery Systems. ACS Appl. Mater. Interfaces 2024, 16, 43156–43170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Su, Z. Design of metal-organic framework composites in anti-cancer therapies. Nanoscale 2021, 13, 12102–12118. [Google Scholar] [CrossRef]
- Deng, L.E.; Guo, M.L.; Deng, Y.J.; Pan, Y.; Wang, X.X.; Maduraiveeran, G.; Liu, J.Q.; Lu, C.Y. MOF-Based Platform for Kidney Diseases: Advances, Challenges, and Prospects. Pharmaceutics 2024, 16, 793. [Google Scholar] [CrossRef]
- Fatah, S.A.; Omer, K.M. Aptamer-Modified MOFs (Aptamer@MOF) for Efficient Detection of Bacterial Pathogens: A Review. ACS Appl. Mater. Interfaces 2025, 17, 11578–11594. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically derived metal organic frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef]
- Lan, G.X.; Ni, K.Y.; Lin, W.B. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Cui, J.; Wang, S.; Han, Y.; Shao, H.; Wang, C.; Hu, Y.; Li, X.; Zhou, Q.; et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023, 9, eabo7868. [Google Scholar] [CrossRef]
- Li, Q.; Wang, R.J.; Xue, J.F.; Wang, R.Y.; Zhang, S.; Kang, H.; Wang, Y.; Zhu, H.D.; Lv, C.Z. ZIF-8-Modified Black Phosphorus Nanosheets Incorporated into Injectable Dual-Component Hydrogels for Enhanced Photothermal Antibacterial and Osteogenic Activities. ACS Appl. Mater. Interfaces 2024, 16, 32058–32077. [Google Scholar] [CrossRef]
- Lao, A.; Wu, J.; Li, D.; Shen, A.; Li, Y.; Zhuang, Y.; Lin, K.; Wu, J.; Liu, J. Functionalized Metal-Organic Framework-Modified Hydrogel That Breaks the Vicious Cycle of Inflammation and ROS for Repairing of Diabetic Bone Defects. Small 2023, 19, e2206919. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yu, H.; You, T.; Kong, X.; Wei, X.; Zheng, Z.; Zheng, L.; Feng, Z.; Huang, B.; Zhang, X.; et al. A Dual-Targeted Metal-Organic Framework Based Nanoplatform for the Treatment of Rheumatoid Arthritis by Restoring the Macrophage Niche. ACS Nano 2023, 17, 13917–13937. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Zeng, Y.X.; Pan, Z.X.; Feng, Z.Z.; Bao, Y.; Ye, Z.Y.; Li, Y.S.; Tang, J.Z.; Liu, X.J.; He, Y. Amino-Functionalized Zirconium-Based Metal-Organic Frameworks as Bifunctional Nanomaterials to Treat Bone Tumors and Promote Osteogenesis. ACS Appl. Mater. Interfaces 2023, 15, 53217–53227. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile Nanoscale Metal-Organic Frameworks (nMOFs): An Emerging 3D Nanoplatform for Drug Delivery and Therapeutic Applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef]
- Wan, Z.; Chung, C.H.Y.; Lau, C.M.L.; Chung, J.T.; Chau, Y.; Fan, Z.; Zhang, S.; Yao, S. Metal-Organic Frameworks-Based Microrockets for Controlled and Sustained Drug Release. Nano Lett. 2025, 25, 5989–5996. [Google Scholar] [CrossRef]
- Tohidi, S.; Aghaie-Khafri, M. Chitosan-Coated MIL-100(Fe) as an Anticancer Drug Carrier: Theoretical and Experimental Investigation. ACS Med. Chem. Lett. 2023, 14, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Chen, S.P.; Zhao, H.; Yang, Y.; Chen, X.Q.; Sun, J.; Fan, H.S.; Zhang, X.D. PPy@MIL-100 Nanoparticles as a pH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217. [Google Scholar] [CrossRef]
- Wan, M.; Song, S.; Feng, W.; Shen, H.; Luo, Y.; Wu, W.; Shen, J. Metal–Organic Framework (UiO-66)-Based Temperature-Responsive Pesticide Delivery System for Controlled Release and Enhanced Insecticidal Performance against Spodoptera frugiperda. ACS Appl. Bio Mater. 2022, 5, 4020–4027. [Google Scholar] [CrossRef]
- Long, Y.L.; Cheng, X.; Tang, Q.M.; Chen, L.L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem. B 2021, 9, 8365–8377. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-edged effects and mechanisms of Zn(2+) microenvironments on osteogenic activity of BMSCs: Osteogenic differentiation or apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu Monometallic and Ag/Cu Bimetallic Nanoparticle-Graphene Composites with Enhanced Antibacterial Performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Chen, X.R.; Sun, Y.; Yang, P.X.; Gu, X.S.; Dai, X. Application of metal-organic frameworks-based functional composite scaffolds in tissue engineering. Regen. Biomater. 2024, 11, rbae009. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.J.; Wang, Y.T.; Zhao, Y.J.; Luo, Y.; Wang, Y.A. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100056. [Google Scholar] [CrossRef]
- Lian, C.; Liu, J.; Wei, W.; Wu, X.; Goto, T.; Li, H.; Tu, R.; Dai, H. Mg-gallate metal-organic framework-based sprayable hydrogel for continuously regulating oxidative stress microenvironment and promoting neurovascular network reconstruction in diabetic wounds. Bioact. Mater. 2024, 38, 181–194. [Google Scholar] [CrossRef]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, e2001369. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, C.; Meng, L.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26–41. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Tang, H.; Niu, X.; Yang, H.; Bai, Y.; Gu, X.; Zheng, Y. Fabrication of a Nanoscale Magnesium/Copper Metal–Organic Framework on Zn-Based Guided Bone Generation Membranes for Enhancing Osteogenesis, Angiogenesis, and Bacteriostasis Properties. ACS Appl. Mater. Interfaces 2024, 16, 5648–5665. [Google Scholar] [CrossRef]
- Shu, C.; Qin, C.; Chen, L.; Wang, Y.; Shi, Z.; Yu, J.; Huang, J.; Zhao, C.; Huan, Z.; Wu, C.; et al. Metal-Organic Framework Functionalized Bioceramic Scaffolds with Antioxidative Activity for Enhanced Osteochondral Regeneration. Adv. Sci. 2023, 10, 2206875. [Google Scholar] [CrossRef]
- Wang, B.; Xie, X.; Jiang, W.; Zhan, Y.; Zhang, Y.; Guo, Y.; Wang, Z.; Guo, N.; Guo, K.; Sun, J. Osteoinductive micro-nano guided bone regeneration membrane for in situ bone defect repair. Stem Cell Res. Ther. 2024, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Zulpya, M.; Zhang, X.Y.; Xu, S.A.; Sun, J.; Dong, B.A. Recent Advances of Metal-Organic Frameworks-based Nanozymes for Bio-applications. Chem. Res. Chin. Univ. 2022, 38, 1324–1343. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, B.; Lang, L.M.; Liu, G.X.; Xia, X.H. Bioinspired Fabrication of Two-Dimensional Metal-Organic Framework-Based Nanozyme for Sensitive Colorimetric Detection of Glutathione. ACS Appl. Nano Mater. 2022, 5, 18761–18769. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, R.; Hui, Y.; Chen, D.; Zhao, C.-X. Boosting Enzyme Activity in Enzyme Metal-Organic Framework Composites. Chem Bio Eng. 2024, 1, 99–112. [Google Scholar] [CrossRef]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-Modified Metal–Organic Frameworks with Multienzymes Activity as Biomimetic Catalysts and Electrocatalytic Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef]
- Niu, X.H.; Li, X.; Lyu, Z.Y.; Pan, J.M.; Ding, S.C.; Ruan, X.F.; Zhu, W.L.; Du, D.; Lin, Y.H. Metal-organic framework based nanozymes: Promising materials for biochemical analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vitro and in vivo. J. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, L.; Liu, J.; Ma, F.; Zhang, J.; Wang, Y.; Tang, B.; Cao, S. Peek@ZIF-8(CEL) as a Novel Bone Implant for Large Defect Repair and Enhanced Bone Healing via a Long-Term Stable Bioactive Releaser. ACS Appl. Mater. Interfaces 2024, 16, 44127–44138. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Lv, S.; Xu, D.; Li, S.; Hou, W.; Wang, C.; Yu, D. Synergistic effect of hierarchical topographic structure on 3D-printed Titanium scaffold for enhanced coupling of osteogenesis and angiogenesis. Mater. Today Bio 2023, 23, 100866. [Google Scholar] [CrossRef]
- Pan, H.; Miao, X.; Deng, J.; Pan, C.; Cheng, X.; Wang, X. Bimetallic Metal-Organic Framework for Mitigating Aseptic Osteolysis. ACS Appl. Mater. Interfaces 2023, 15, 4935–4946. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Qin, Z.; Chen, H.; Lan, Q.; Wang, Z.; Lan, N.; Yang, Y.; Zheng, L.; Zhao, J.; Kai, D. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 2020, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The recent progress on metal-organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, Y.; Yan, T.-H.; Li, J.; Li, Y.; Drake, H.F.; Zhong, H.; Jin, Y.; Zhao, R.; Zhou, H.-C. Metal-Organic Framework-Based Nanoheater with Photo-Triggered Cascade Effects for On-Demand Suppression of Cellular Thermoresistance and Synergistic Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2200004. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem.-Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Y.; Shen, J.; Wang, Q. Modified nanoscale metal organic framework-based nanoplatforms in photodynamic therapy and further applications. Photodiagnosis Photodyn. Ther. 2020, 32, 102026. [Google Scholar] [CrossRef]
- Chien, W.C.; Cheng, P.H.; Cheng, X.J.; Chuang, C.C.; Huang, Y.T.; Anilkumar, T.S.; Liu, C.H.; Lu, Y.J.; Wu, K.C.W. MCP-1-Functionalized, Core-Shell Gold Nanorod@Iron-Based Metal-Organic Framework (MCP-1/GNR@MIL-100(Fe)) for Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 52092–52105. [Google Scholar] [CrossRef]
- Huang, B.; Li, G.; Cao, L.; Wu, S.; Zhang, Y.; Li, Z.; Zhou, F.; Xu, K.; Wang, G.; Su, J. Nanoengineered 3D-printing scaffolds prepared by metal-coordination self-assembly for hyperthermia-catalytic osteosarcoma therapy and bone regeneration. J. Colloid Interface Sci. 2024, 672, 724–735. [Google Scholar] [CrossRef]
- Qu, Y.; Zhuang, H.; Zhang, M.; Wang, Y.; Zhai, D.; Ma, B.; Wang, X.; Qin, C.; Huan, Z.; Wu, C. Bone cements for therapy and regeneration for minimally invasive treatment of neoplastic bone defects. J. Mater. Chem. B 2021, 9, 4355–4364. [Google Scholar] [CrossRef]
- Jin, L.G.; Liu, H.P.; Wang, C.F.; Mao, C.Y.; Wu, S.L.; Zhang, Y.; Li, Z.Y.; Zhu, S.L.; Jiang, H.; Cui, Z.D.; et al. Interface/Dipole Polarized Antibiotics-Loaded Fe3O4/PB Nanoparticles for Non-Invasive Therapy of Osteomyelitis Under Medical Microwave Irradiation. Adv. Mater. 2024, 36, e2410917. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, Y.; Hu, W.; Li, R.; Wang, J.; Han, M.; Li, Z. Evaluation of the Antibacterial, Anti-Inflammatory, And Bone-Promoting Capacity of UiO-66 Loaded with Thymol or Carvacrol. ACS Appl. Mater. Interfaces 2024, 16, 36017–36029. [Google Scholar] [CrossRef]
- Li, J.; Song, S.; Meng, J.; Tan, L.; Liu, X.; Zheng, Y.; Li, Z.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; et al. 2D MOF Periodontitis Photodynamic Ion Therapy. J. Am. Chem. Soc. 2021, 143, 15427–15439. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Liang, N.; Dong, M.; Feng, Z.; Meng, L.; Sun, C.; Wang, A.; Yu, X.; Wang, W.; Xie, J.; et al. Stem Cell Membrane-Encapsulated Zeolitic Imidazolate Framework-8: A Targeted Nano-Platform for Osteogenic Differentiation. Small 2022, 18, e2202485. [Google Scholar] [CrossRef]
- Li, Y.X.; Xu, C.C.; Mao, J.; Mao, L.X.; Li, W.Q.; Liu, Z.Y.; Shin, A.; Wu, J.Q.; Hou, L.L.; Li, D.J.; et al. ZIF-8-based Nanoparticles for Inflammation Treatment and Oxidative Stress Reduction in Periodontitis. ACS Appl. Mater. Interfaces 2024, 16, 36077–36094. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lazaro-Martinez, J.M.; Rodriguez-Castellon, E.; Bandosz, T.J. Carbon Quantum Dot Surface-Chemistry-Dependent Ag Release Governs the High Antibacterial Activity of Ag-Metal-Organic Framework Composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fang, J.; Wang, Y.; Sun, J.; Sun, Y.; Sun, X.; Qi, M.; Li, W.; Li, C.; Zhou, Y.; et al. Antibacterial Zeolite Imidazole Frameworks with Manganese Doping for Immunomodulation to Accelerate Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2101515. [Google Scholar] [CrossRef]
- Zhang, W.S.; Wang, B.J.; Xiang, G.L.; Jiang, T.Z.; Zhao, X. Photodynamic Alginate Zn-MOF Thermosensitive Hydrogel for Accelerated Healing of Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 22830–22842. [Google Scholar] [CrossRef]
- Huang, S.; Ye, Y.; Jiang, C.; Wang, R.; Hu, W.; Raza, S.; Jie, O.; Pan, Y.; Liu, J. Current and promising applications of MOFs loaded with PTAs on photothermal therapy. React. Funct. Polym. 2023, 193, 105743. [Google Scholar] [CrossRef]
- Tong, P.H.; Yang, J.J.; Zhou, Y.F.; Tang, Y.F.; Tang, M.T.; Zang, Y.; Pan, Y.F.; Dong, L.W.; Tan, Y.X.; Nam, K.T.; et al. Metal-organic frameworks (MOFs) for phototherapy and synergistic phototherapy of cancer. Coord. Chem. Rev. 2025, 526, 216381. [Google Scholar] [CrossRef]
- Zhou, B.X.; Jiang, X.L.; Zhou, X.X.; Tan, W.Y.; Luo, H.; Lei, S.R.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Fardjahromi, M.A.; Ejeian, F.; Razmjou, A.; Vesey, G.; Mukhopadhyay, S.C.; Derakhshan, A.; Warkiani, M.E. Enhancing osteoregenerative potential of biphasic calcium phosphates by using bioinspired ZIF8 coating. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 123, 111972. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, Y.; Wang, W.; Han, M.; Wan, X.; Li, Q.; Chen, X.; Cao, J.; Zhang, Y.; Li, J.; et al. Ultrasound-Activated Probiotics Vesicles Coating for Titanium Implant Infections Through Bacterial Cuproptosis-Like Death and Immunoregulation. Adv. Mater. 2024, 36, e2405953. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Zhang, Z.; Wang, Y.; Ye, Y.; Yinwang, E.; Liu, A.; Zhou, X.; Xu, J.; Zhou, C.; Sun, H.; et al. Iodine Immobilized Metal-Organic Framework for NIR-Triggered Antibacterial Therapy on Orthopedic Implants. Small 2021, 17, 2102315. [Google Scholar] [CrossRef]
- He, M.M.; Huang, Y.; Xu, H.; Feng, G.J.; Liu, L.M.; Li, Y.B.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, J.; Chan, Y.K.; Bai, D.; Shu, R.; Shi, X.; Li, Y.; Li, L.; Yang, X.; Yang, W. Heterostructured Metal-Organic Frameworks/Polydopamine Coating Endows Polyetheretherketone Implants with Multimodal Osteogenicity and Photoswitchable Disinfection. Adv. Healthc. Mater. 2022, 11, e2200641. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, X.; Yang, H.; Li, L.; He, C.; Cheng, C.; Haag, R. ZnO/Nanocarbons-Modified Fibrous Scaffolds for Stem Cell-Based Osteogenic Differentiation. Small 2020, 16, e2003010. [Google Scholar] [CrossRef]
- Toprak, O.; Topuz, B.; Abou Monsef, Y.; Oto, C.; Orhan, K.; Karakecili, A. BMP-6 carrying metal organic framework-embedded in bioresorbable electrospun fibers for enhanced bone regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 111738. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Zhu, Y.; Han, Y.; Shen, J.; Bao, B.; Gao, T.; Lin, J.; Huang, T.; Xu, J.; et al. Tunable and Controlled Release of Cobalt Ions from Metal-Organic Framework Hydrogel Nanocomposites Enhances Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 59051–59066. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, R.-D.; Su, S.-L.; Hao, Y.-L.; Zhou, F. Green synthesis of metal-organic framework loaded dexamethasone on wood aerogels for enhanced cranial bone regeneration. J. Mater. Chem. B 2023, 11, 9496–9508. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Hu, H.; Xu, Z.; Liu, J.; Chen, J.; Chen, B.; Shi, L.; Luo, H.; Chen, G.; et al. Engineered Metallic Ion-Based Hydrogel for Tendon-Bone Reconstruction. ACS Appl. Mater. Interfaces 2024, 16, 6837–6848. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Zhang, X.; Xu, Z.; Hu, H.; Liu, J.; Ren, P.; Kong, X.; Chen, J.; Yang, K.; et al. Dual-Targeted Metal Ion Network Hydrogel Scaffold for Promoting the Integrated Repair of Tendon-Bone Interfaces. ACS Appl. Mater. Interfaces 2024, 16, 5582–5597. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.J.; Johnson, B.N.; Jia, X.F. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Zhong, L.N.; Chen, J.Y.; Ma, Z.Y.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.Y.; Pei, X.B.; Wang, J.; Wan, Q.B. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Hu, M.G.; Dong, M.R.; Yang, Z.H.; Zhan, X.; Chang, X.Y.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Ren, N.; Feng, Z.; Sun, Z.; Dong, M.; Wang, W.; Liu, F.; Sun, C.; Zhou, W.; Xing, Z.; et al. Biomimetic Metal-Organic Frameworks as Targeted Vehicles to Enhance Osteogenesis. Adv. Healthc. Mater. 2022, 11, 2102821. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Hu, Y.; Zhang, H.; Wang, S.C.; Xu, K.; Su, J.C. Single-cell RNA sequencing in osteoarthritis. Cell Prolif. 2023, 56, e13517. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chang, M.N.; Shi, D.; Yun, C.X.; Li, J.; Guo, H.T.; Lin, X. Therapeutic targets in aging-related osteoarthritis: A focus on the extracellular matrix homeostasis. Life Sci. 2025, 368, 123487. [Google Scholar] [CrossRef]
- Li, J.D.; Zhang, H.; Han, Y.F.; Hu, Y.; Geng, Z.; Su, J.C. Targeted and responsive biomaterials in osteoarthritis. Theranostics 2023, 13, 931–954. [Google Scholar] [CrossRef]
- Chen, H.M.; Qin, Z.N.; Zhao, J.M.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.B.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhou, J.J.; Chen, R.H.; Shi, R.H.; Xia, G.L.; Zhou, S.; Liu, Z.B.; Zhang, N.Q.; Wang, H.B.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Tian, Z.; Sun, L.; Kong, W.; Yan, R.; Yu, Z.; Tian, Q.-W.; Liu, C. Quercetin-Loaded Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles Attenuate Osteoarthritis by Activating Autophagy via the Pi3k/Akt Signaling. ACS Appl. Mater. Interfaces 2024, 16, 40444–40454. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Hou, Y.-K.; Chen, M.-W.; Yu, X.-Z.; Chen, S.-Y.; Yue, Y.-R.; Guo, X.-T.; Chen, J.-X.; Zhou, Q. A pH-responsive metal-organic framework for the co-delivery of HIF-2α siRNA and curcumin for enhanced therapy of osteoarthritis. J. Nanobiotechnol. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, W.Y. Macrophage membrane-camouflaged biomimetic nanovesicles for targeted treatment of arthritis. Ageing Res. Rev. 2024, 95, 102241. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, S.-L.; Zhao, X.; Sun, D.; Yang, Y.; Chen, M.; Zhu, C.; Jiang, B.; Gu, Q.; Liu, H.; et al. Self-Reinforced MOF-Based Nanogel Alleviates Osteoarthritis by Long-Acting Drug Release. Adv. Mater. 2024, 36, e2401094. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Y.; Ruan, Z.; Zhang, S.; Feng, X.; Lu, C.; Zhao, J.; Yin, F.; Cao, C.; Zheng, L. Baicalin nanodelivery system based on functionalized metal-organic framework for targeted therapy of osteoarthritis by modulating macrophage polarization. J. Nanobiotechnol. 2024, 22, 221. [Google Scholar] [CrossRef]
- Gong, P.; Wang, M.; Wang, J.; Li, J.; Wang, B.; Bai, X.; Liu, J.; Liu, Z.; Wang, D.; Liu, W. A biomimetic lubricating nanosystem for synergistic therapy of osteoarthritis. J. Colloid Interface Sci. 2024, 672, 589–599. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Lin, X.; He, Z.; Zhang, H.; Ji, L.; Gong, P.; Zhou, F.; Liu, W. Dual-functional MOFs-based hybrid microgel advances aqueous lubrication and anti-inflammation. J. Colloid Interface Sci. 2023, 644, 200–210. [Google Scholar] [CrossRef]
- Tian, L.; Han, S.; Wu, W.; Li, Z.; He, Z.; Liu, C.; Xue, H.; Zhou, F.; Liu, W.; Liu, J. Dose-effect relationship of copolymer on enhancing aqueous lubrication of a hybrid osteoarthritis drug delivery nanocarrier. J. Colloid Interface Sci. 2025, 679, 788–797. [Google Scholar] [CrossRef]
- Wu, S.; Nan, F.; Zhang, K.; Hao, W.; Shi, D.; Li, Y.; Deng, W.; Jarhen, N.; Li, K.; Xiao, Y.; et al. A novel metal-organic framework encapsulated iridium oxide nanozyme enhanced antisense oligonucleotide combo for osteoarthritis synergistic therapy. Aggregate 2024, 5, e635. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, J.; Yao, Y.; Huang, L.; Zheng, L.; Zhao, J. Mitochondrial-targeting Mn3O4/UIO-TPP nanozyme scavenge ROS to restore mitochondrial function for osteoarthritis therapy. Regen. Biomater. 2023, 10, rbad078. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Sun, W.; Lin, J.; Fan, C.; Wang, C.; Zhang, Z.; Wang, Y.; Tang, Y.; Lin, Y.; Zhou, D. Using Cu-Based Metal-Organic Framework as a Comprehensive and Powerful Antioxidant Nanozyme for Efficient Osteoarthritis Treatment. Adv. Sci. 2024, 11, e2307798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, B.; Tan, Y.; Su, J.; Wang, Y.; Xu, J.; Tao, L.; Zhou, H.; Liu, L.; Li, X. Sinomenine inhibits osteolysis in breast cancer by reducing IL-8/CXCR1 and c-Fos/NFATc1 signaling. Pharmacol. Res. 2019, 142, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Liang, Y.J.; Lian, C.; Peng, F.L.; Xiao, Y.S.; He, Y.F.; Ma, C.X.; Wang, Y.; Zhang, P.Y.; Deng, Y.H.; et al. CST6 protein and peptides inhibit breast cancer bone metastasis by suppressing CTSB activity and osteoclastogenesis. Theranostics 2021, 11, 9821–9832. [Google Scholar] [CrossRef]
- Pang, Y.C.; Fu, Y.; Li, C.; Wu, Z.X.; Cao, W.C.; Hu, X.; Sun, X.C.; He, W.X.; Cao, X.K.; Ling, D.S.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett. 2020, 20, 829–840. [Google Scholar] [CrossRef]
- Ting, G.; Zhang, W.; Fei, G.; Zhu, L.; Ping, S.; Li, W.; Lin, G.; Wan, D.; Tao, Y.; Kai, Y. A bone-targeting drug delivery vehicle of a metal-organic framework conjugate with zoledronate combined with photothermal therapy for tumor inhibition in cancer bone metastasis. Biomater. Sci. 2022, 10, 1831–1843. [Google Scholar] [CrossRef]
- Zou, B.; Xiong, Z.; He, L.; Chen, T. Reversing breast cancer bone metastasis by metal organic framework-capped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials 2022, 285, 121549. [Google Scholar] [CrossRef]
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA-A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Zeng, W.; Wan, R.; Zheng, Y.H.; Singh, S.R.; Wei, Y.Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011, 313, 129–136. [Google Scholar] [CrossRef]
- Du, C.; Zhou, M.; Jia, F.; Ruan, L.; Lu, H.; Zhang, J.; Zhu, B.; Liu, X.; Chen, J.; Chai, Z.; et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials 2021, 269, 120642. [Google Scholar] [CrossRef]
- Wang, Y.; Williams, G.R.; Zheng, Y.; Guo, H.; Chen, S.; Ren, R.; Wang, T.; Xia, J.; Zhu, L.-M. Polydopamine-cloaked Fe-based metal organic frameworks enable synergistic multidimensional treatment of osteosarcoma. J. Colloid Interface Sci. 2023, 651, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-X.; Zhang, T.-W.; Zhou, L.; Ding, W.; Liang, H.-F.; Hu, Z.-C.; Chen, Q.; Dong, J.; Xue, F.-F.; Yin, X.-F.; et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials 2022, 282, 121407. [Google Scholar] [CrossRef]
- Zeng, Y.; Yuan, J.; Ran, Z.; Zhan, X.; Li, X.; Ye, H.; Dong, J.; Cao, G.; Pan, Z.; Bao, Y.; et al. Chitosan/NH2-MIL-125 (Ti) scaffold loaded with doxorubicin for postoperative bone tumor clearance and osteogenesis: An in vitro study. Int. J. Biol. Macromol. 2024, 263, 130368. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Ma, B.; Li, B.; Huan, Z.; Ma, N.; Zhu, H.; Chang, J.; Xiao, Y.; Wu, C. 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 2020, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-j.; Chen, Q.-l.; Zhang, S.-f.; Song, K.; Han, Z.-t.; Liu, W.-b.; Zhang, X.-s.; Dong, S.h.; Hu, W.-h.; Han, Z.c.; et al. A multifunctional biomimetic nanoplatform for image-guideded photothermal-ferroptotic synergistic osteosarcoma therapy. Bioact. Mater. 2024, 36, 157–167. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Feng, W.; Feng, H.; Ding, Z.; Zhao, Q.; Li, X.; Tang, N.; Zhang, P.; Li, J.; et al. A Targeted Erythrocyte Membrane-Encapsulated Drug-Delivery System with Anti-osteosarcoma and Anti-osteolytic Effects. ACS Appl. Mater. Interfaces 2021, 13, 27920–27933. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, itc17–itc32. [Google Scholar] [CrossRef]
- Gao, Y.G.; Chen, N.; Fu, Z.D.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Ha, M.; Hughton, B.; Oliynyk, A.O.; Iyer, A.K.; Bernard, G.M.; Lambkin, G.; Lawrence, M.C.; Katz, M.J.; Mar, A.; et al. Alkaline Earth Metal-Organic Frameworks with Tailorable Ion Release: A Path for Supporting Biomineralization. ACS Appl. Mater. Interfaces 2019, 11, 32739–32745. [Google Scholar] [CrossRef]
- Vassaki, M.; Papathanasiou, K.E.; Hadjicharalambous, C.; Chandrinou, D.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Self-sacrificial MOFs for ultra-long controlled release of bisphosphonate anti-osteoporotic drugs. Chem. Commun. 2020, 56, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luo, C.Z.; Xie, D.H.; Hu, J.J.; Chen, J.X.; Huang, N.H.; Wang, H.; Zhang, S.Q.; Zhang, Q. Convenient synthesis of zwitterionic calcium(II)-carboxylate metal organic frameworks with efficient activities for the treatment of osteoporosis. Int. J. Pharm. 2021, 608, 121083. [Google Scholar] [CrossRef]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-osteoblastogenesis MSC Reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Lin, W.; Hu, S.; Li, K.; Shi, Y.; Pan, C.; Xu, Z.; Li, D.; Wang, H.; Li, B.; Chen, H. Breaking Osteoclast-Acid Vicious Cycle to Rescue Osteoporosis via an Acid Responsive Organic Framework-Based Neutralizing and Gene Editing Platform. Small 2024, 20, e2307595. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2000 2017, 75, 45–51. [Google Scholar] [CrossRef]
- Van Holm, W.; Carvalho, R.; Delanghe, L.; Eilers, T.; Zayed, N.; Mermans, F.; Bernaerts, K.; Boon, N.; Claes, I.; Lebeer, S.; et al. Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms. npj Biofilms Microbiomes 2023, 9, 3. [Google Scholar] [CrossRef]
- Ejeian, F.; Razmjou, A.; Nasr-Esfahani, M.H.; Mohammad, M.; Karamali, F.; Warkiani, M.E.; Asadnia, M.; Chen, V. ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 10029–10043. [Google Scholar] [CrossRef]
- Li, N.; Xie, L.; Wu, Y.; Wu, Y.; Liu, Y.; Gao, Y.; Yang, J.; Zhang, X.; Jiang, L. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment. Mater. Today Bio 2022, 16, 100360. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Xue, H.; Yu, R.; Jin, X.; Wu, X.; Huang, H. Reprogramming mitochondrial metabolism of macrophages by miRNA-released microporous coatings to prevent peri-implantitis. J. Nanobiotechnol. 2023, 21, 485. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, Y.; Ji, C.; Zhu, H.; Lao, A.; Zhao, R.; Hu, Y.; Zhou, Y.; Zhou, J.; Lin, K.; et al. A five-in-one novel MOF-modified injectable hydrogel with thermo-sensitive and adhesive properties for promoting alveolar bone repair in periodontitis: Antibacterial, hemostasis, immune reprogramming, pro-osteo-/angiogenesis and recruitment. Bioact. Mater. 2024, 41, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.; Yao, Y.; Tang, C.; Wang, Y.; Yuan, Q.; Peng, L. Bifunctional mesoporous HMUiO-66-NH2 nanoparticles for bone remodeling and ROS scavenging in periodontitis therapy. Biomaterials 2025, 314, 122872. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, Y.; Ho, C.; Li, Z.; Chiu, W.; Li, A.; Dai, Y.; Li, W.; Zhang, X. Dynamic hydrogel-metal-organic framework system promotes bone regeneration in periodontitis through controlled drug delivery. J. Nanobiotechnol. 2024, 22, 287. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Wang, H.; Mu, N.; He, Y.; Zhang, X.; Lei, J.; Yang, C.; Ma, L.; Gao, Y. Ultrasound-controlled MXene-based Schottky heterojunction improves anti-infection and osteogenesis properties. Theranostics 2023, 13, 1669–1683. [Google Scholar] [CrossRef]
- Song, Y.C.; Li, H.M.; Yuan, Y.; Zhang, D.; Wang, Z.; Qi, B.W.; Jiang, P.; Yu, A.X. Synergistic photothermal-sonodynamic therapy for antibacterial and immune reprogramming in chronic osteomyelitis. J. Control. Release 2025, 381, 113612. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, L.; Li, Z.; Liu, X.; Zheng, Y.; Feng, X.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. Single-Atom Catalysis for Efficient Sonodynamic Therapy of Methicillin-Resistant Staphylococcus aureus-Infected Osteomyelitis. ACS Nano 2021, 15, 10628–10639. [Google Scholar] [CrossRef]
- Feng, X.; Ma, L.; Lei, J.; Ouyang, Q.; Zeng, Y.; Luo, Y.; Zhang, X.; Song, Y.; Li, G.; Tan, L.; et al. Piezo-Augmented Sonosensitizer with Strong Ultrasound-Propelling Ability for Efficient Treatment of Osteomyelitis. ACS Nano 2022, 16, 2546–2557. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Liu, X.M.; Gao, S.; Cui, Z.D.; Wu, S.L.; Liang, Y.Q.; Li, Z.Y.; Zheng, Y.F.; Zhu, S.L.; Jiang, H.; et al. Engineering Dynamic Defect of CeIII/CeIV-Based Metal-Organic Framework through Ultrasound-Triggered Au Electron Trapper for Sonodynamic Therapy of Osteomyelitis. Small 2023, 19, e2207687. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Wu, S.L.; Zheng, Y.F.; Wang, C.F.; Li, Z.Y.; Zhang, Y.; Zhu, S.L.; Jiang, H.; Cui, Z.D.; Liu, X.M. Enhancing Microwave Dynamic Effects via Surface States of Ultrasmall 2D MOF Triggered by Interface Confinement for Antibiotics-Free Therapy. Adv. Sci. 2023, 10, e2300084. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, F.; Ebrahem, N.E.; Abdelhamid, H.N. A comparative study of the toxic effect of ZIF-8 and ZIF-L on the colonization and decomposition of shaded outdoor mice carrions by arthropods. Sci. Rep. 2022, 12, 14240. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.Q.; Zheng, X.H.; Li, Z.S.; Xie, Z.G. Nanoscale Fluorescent Metal-Organic Framework@Microporous Organic Polymer Composites for Enhanced Intracellular Uptake and Bioimaging. Chem.-A Eur. J. 2017, 23, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, K.Y.A.; Chen, K.F.; Jiang, X.Y.; Lin, C.H. In vitro renal toxicity evaluation of copper-based metaleorganic framework HKUST-1 on human embryonic kidney cells. Environ. Pollut. 2021, 273, 116528. [Google Scholar] [CrossRef]
| Classification | Main Functionalization Methods | Main Strengths | Main Weaknesses | Impact on Osteogenesis | Impact on Antibacterial Activity | Impact on Biocompatibility |
|---|---|---|---|---|---|---|
| Drug carriers | In situ formation; post synthesis | Diverse design; high loading capacity; adjustable size | Unknown toxicity; low solubility; unknown homogeneity of structure | Promotes osteogenic differentiation; sustained release enhances bone repair [30,31] | Limited direct antibacterial effect unless combined with antibiotics [72,73] | High biocompatibility due to natural drug carriers; risk of inflammation if uncontrolled release [72,73] |
| Metal ion donors | In situ formation | Biologically active metal ions as coordination sites | Uncontrollable release kinetics and dose | Zn2+ upregulates RUNX2/ALP; Mg2+ enhances angiogenesis; supports mineralization [74,75] | Zn2+ disrupts bacterial membranes; Cu2+ induces ROS for pathogen elimination [76] | Cytotoxicity at high ion concentrations; requires controlled release kinetics [48] |
| Nanozymes | In situ formation; post synthesis | Low cost; high stability; excellent tolerance compared with nature enzyme | Unknown metabolic toxicity; long-term biocompatibility; immune responses | Scavenges ROS to reduce oxidative stress; promotes bone formation [61,62] | Mimics enzyme activity (e.g., catalase) to degrade bacterial biofilms [77] | May trigger immune responses if not surface-modified; Requires precise activity regulation [53] |
| Phototherapeutic agents | In situ formation; post synthesis | Small side effect on target tissue | Unknown biocompatibility; light transmission; targeted aggregation of MOFs, etc. | Photothermal stimulation upregulates osteogenic genes (e.g., COL1, OCN) [69,70] | ROS generation under light eliminates pathogens [78] | Depends on external stimuli (light/US), limiting deep-tissue applications; potential thermal damage if misapplied [79,80] |
| MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs and Loading Efficiency | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|
| [Sr(H2O)3(H2PXBP)]; [SrCa(H2O)3(H2PXBP)] | Scaffold | Released ions | / | Ca2+, Sr2+ | Cell growth | / | / | [130] |
| Self-sacrificial MOFs | Nanoparticle | Drug carrier | Etidronate, pamidronate, alendronate, neridronate) | Mg2+, Ca2+ | Cell growth | / | / | [131] |
| {[Ca(Cdcbp)]·2H2O}n MOF | Compound | Released ion | / | Ca2+ | Promoting osteogenesis | / | Promoting mineralization | [132] |
| AHT-Ce/Sr MOF | Implant | Released ion | / | Ce3+, Sr2+ | Reducting ROS; enhancing osteoblast differentiation; improving mitochondrial division and autophagy | Activating AMPK signaling | Promoting bone formation | [134] |
| ZIF8-NaHCO3@Cas9 | Nanoparticle | Drug carrier | RANKL-CRISPR/Cas9 plasmids (~80%) | Zn2+ | Reducting ROS; inhibiting bone resorption; promoting osteogenesis; delaying cell senescence | / | Promoting mineralization; delaying senescence | [135] |
| MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|
| Mino@ZIF-8 | Nanoparticle | Drug carrier | Minocycline hydrochloride (~7.9%) | Zn2+ | Relieving inflammation; protecting mitochondrial function | Enhancing AKT/GSK3β/NRF2 pathway | Reducing alveolar bone resorption; improving bone density | [75] |
| PP/PDA/ZIF-8 | Membrane | Mechanical properties | / | Zn2+ | Promoting osteogenesis | / | / | [138] |
| DZIF@PGel | Hydrogel | Drug carrier | Dexamethasone (~19.2%) | Zn2+ | Antibacterial; relieving inflammation; inhibiting bone resorption; promoting osteogenesis | / | Promoting mineralization | [139] |
| L-MOF-agomir | Implant | Drug carrier | miR-27a (93–98%) | Zn2+ | Antibacterial; promoting M1-to-M2 macrophage polarization; relieving inflammation; promoting osteogenesis | Metabolic shift from glycolysis to OXPHOS | Inducing macrophage to M2 polarization; relieving peri-implantitis bone resorption | [140] |
| SFD/CS/ZIF-8@QCT | Hydrogel | Drug carrier | Quercetin (~13.51%) | Zn2+ | Antibacterial; relieving inflammation; promoting M1-to-M2 macrophage polarization; promoting osteogenesis | Activating PI3K-Akt signaling pathway | Repairing alveolar bone defects | [141] |
| Car@UiO-66; Thy@UiO-66 | Nanoparticle | Drug carrier | Carvacrol (~79.60%); Thymol (~79.65%) | Zr4+ | Antibacterial; relieving inflammation; promoting M1-to-M2 macrophage polarization; promoting vascularization; promoting osteogenesis | / | Facilitating bone defect healing | [72] |
| HMUiO-66-NH2 | Nanoparticle | Nanozyme | / | Zr4+ | Reducting ROS; inhibiting bone resorption; promoting osteogenesis | Promoting Wnt and TGF-β signaling pathways | Facilitating bone defect healing | [142] |
| CuTCPP-Fe2O3 | Ointment | Photosensitizer | / | Fe3+, Cu2+ | Antibacterial | / | Reducing alveolar bone loss; reducing tissue inflammation; promoting angiogenesis; alleviating periodontitis | [73] |
| CSBDX@MOF | Hydrogel | Drug carrier | Gallic acid | Mg2+ | Antibacterial; Relieving inflammation; promoting M1-to-M2 macrophage polarization; inhibiting bone resorption; promoting osteogenesis | / | Reducing alveolar bone loss; enhancing collagen deposition | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, C.; Yuan, Z.; El Haddaoui-Drissi, R.; Ni, R.; Xiao, Y.; Qi, Z.; Shang, J.; Lin, X. Biomedical Applications of Functionalized Composites Based on Metal–Organic Frameworks in Bone Diseases. Pharmaceutics 2025, 17, 757. https://doi.org/10.3390/pharmaceutics17060757
Yun C, Yuan Z, El Haddaoui-Drissi R, Ni R, Xiao Y, Qi Z, Shang J, Lin X. Biomedical Applications of Functionalized Composites Based on Metal–Organic Frameworks in Bone Diseases. Pharmaceutics. 2025; 17(6):757. https://doi.org/10.3390/pharmaceutics17060757
Chicago/Turabian StyleYun, Chenxi, Zhe Yuan, Rim El Haddaoui-Drissi, Ruitong Ni, Yunyun Xiao, Zhenhui Qi, Jie Shang, and Xiao Lin. 2025. "Biomedical Applications of Functionalized Composites Based on Metal–Organic Frameworks in Bone Diseases" Pharmaceutics 17, no. 6: 757. https://doi.org/10.3390/pharmaceutics17060757
APA StyleYun, C., Yuan, Z., El Haddaoui-Drissi, R., Ni, R., Xiao, Y., Qi, Z., Shang, J., & Lin, X. (2025). Biomedical Applications of Functionalized Composites Based on Metal–Organic Frameworks in Bone Diseases. Pharmaceutics, 17(6), 757. https://doi.org/10.3390/pharmaceutics17060757







