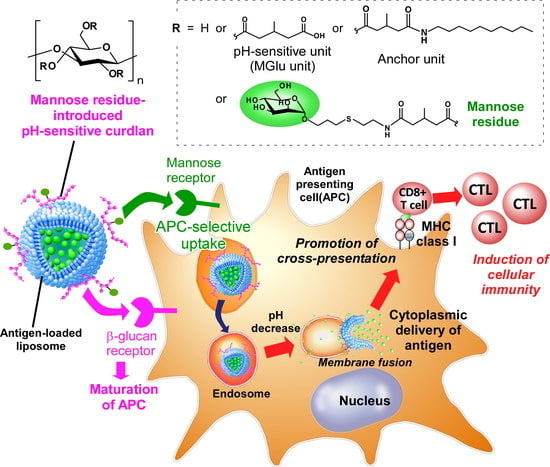
Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mannose-Modified Curdlan Derivatives
2.3. Preparation of Curdlan Derivative-Modified Liposomes
2.4. Characterization of Curdlan Derivative-Modified Liposomes
2.5. Interaction of Curdlan Derivative-Modified Liposomes with Lectin
2.6. Cellular Association of Liposomes
2.7. Confocal Laser Scanning Microscpy
2.8. Evaluation of Antigen Presentation
2.9. Mice
2.10. Interaction of Liposomes with Splenocytes
2.11. Treatment of Tumor-Bearing Mice
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Mannose Residue-Introduced Carboxylated Cudlans
3.2. Characterization of Cudlan Derivative-Modified Liposomes
3.3. Interaction of Liposomes with Lectin
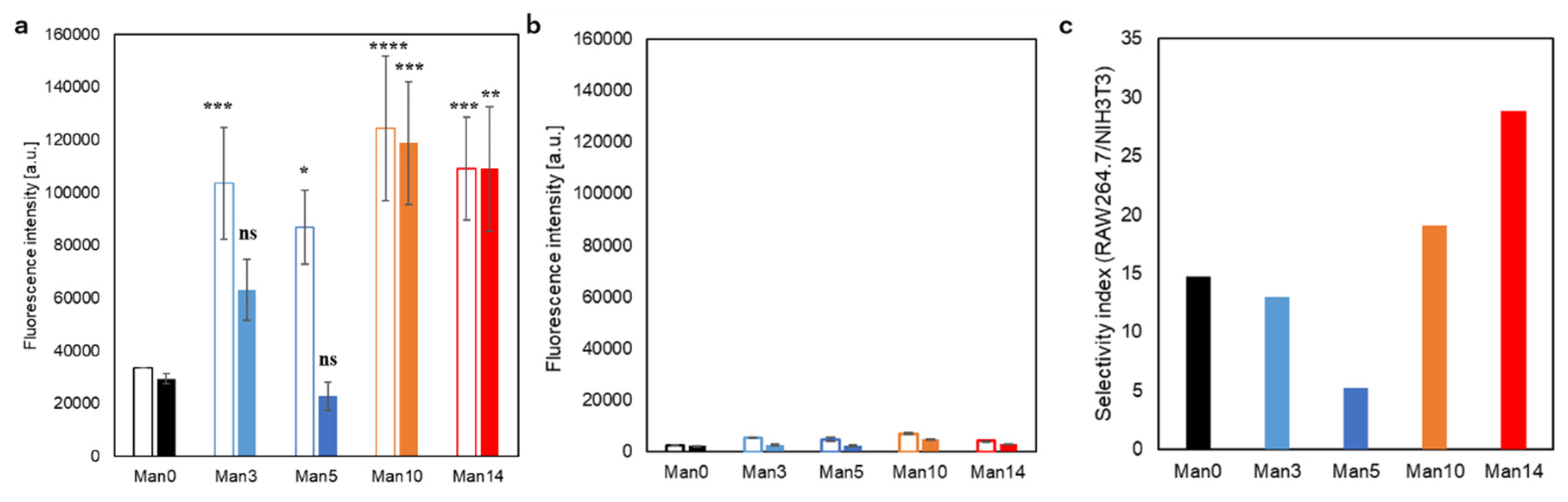
3.4. Cellular Association of Curdlan Derivative-Modified Liposomes
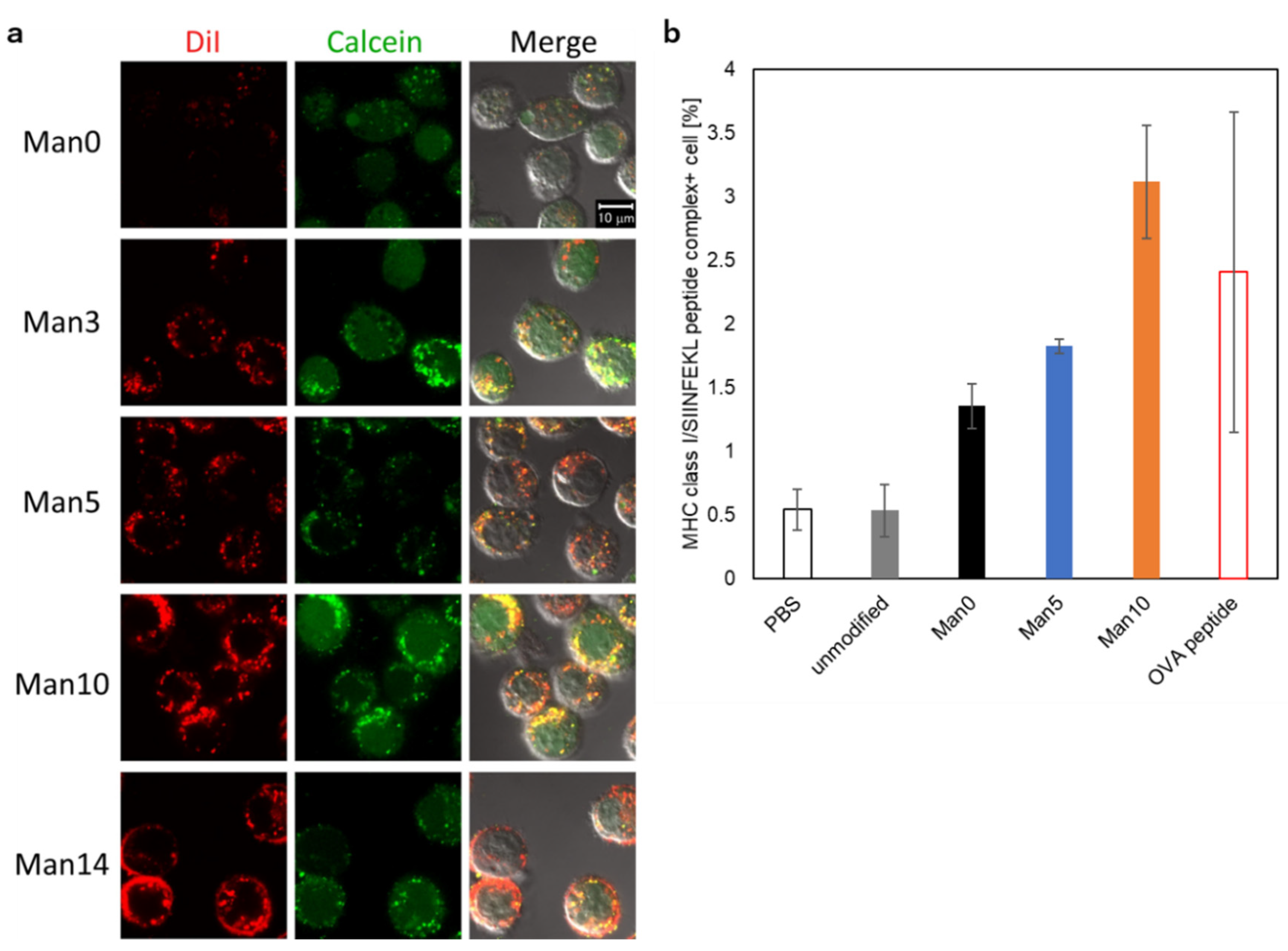
3.5. Cytoplasmic Delivery of Antigen and Evaluation of Antigen Presentation
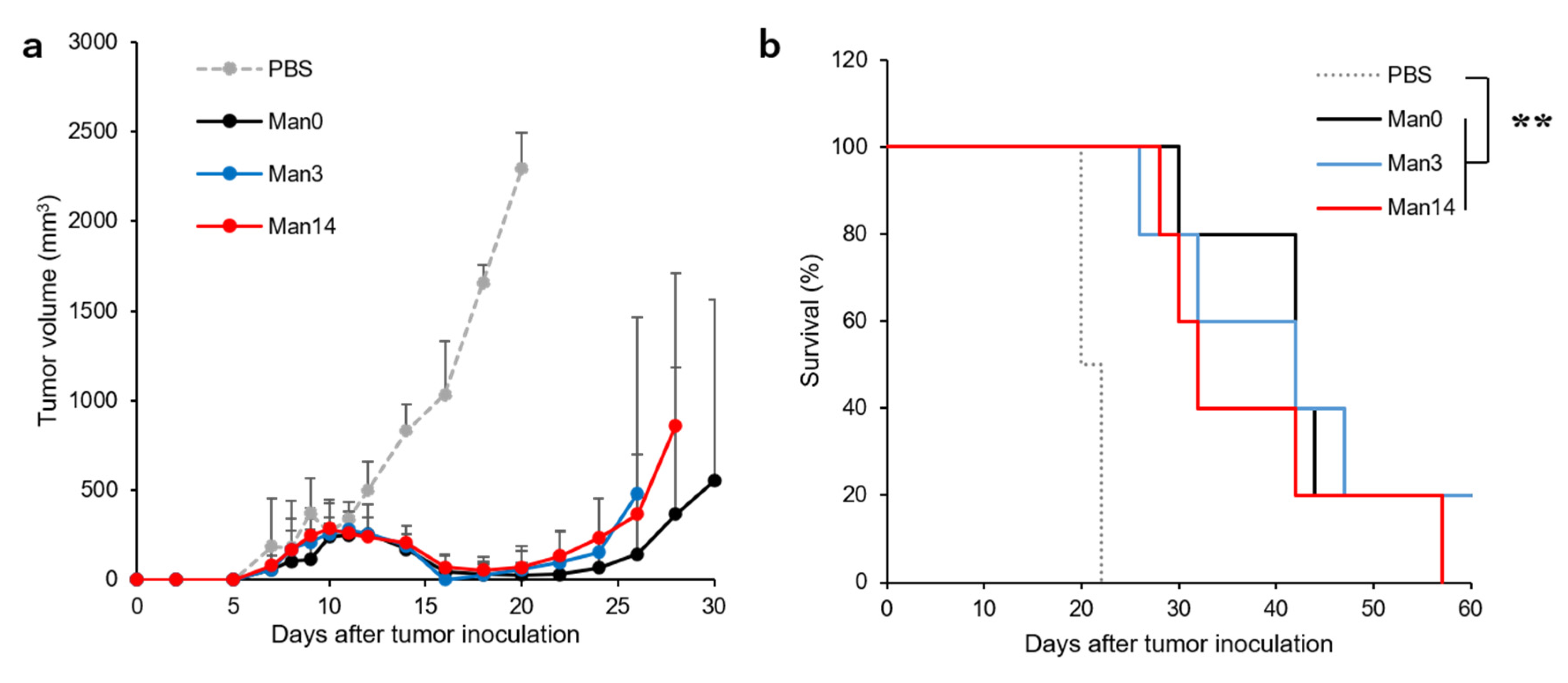
3.6. Treatment of Tumor-Bearing Mice by Curdlan Derivative-Modified Liposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 27, 568–571. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2013, 2, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Linhares, A.D.S.; Battin, C.; Jutz, S.; Leitner, J.; Hafner, C.; Tobias, J.; Wiedermann, U.; Kundi, M.; Zlabinger, G.J.; Grabmeier-Pfistershammer, K.; et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci. Rep. 2019, 9, 11472. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.K.; Wolchok, J.D.; Allison, J.P. Anti-CTLA-4 antibody therapy: Immune monitoring during clinical development of a novel immunotherapy. Semin. Oncol. 2010, 37, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Nair, V.S.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, Y.; Gao, P.; Yao, Z. Targeting CD47: The achievements and concerns of current studies on cancer immunotherapy. J. Thorac. Dis. 2017, 9, E168–E174. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Reeves, E.; James, E. Antigen processing and immune regulation in the response to tumours. Immunology 2017, 150, 16–24. [Google Scholar] [CrossRef]
- Villadangos, J.A.; Schnorrer, P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 2007, 7, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; Burgdorf, S. Current concepts of antigen cross-presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Gros, M.; Amigorena, S. Regulation of antigen export to the cytosol during cross-presentation. Front. Immunol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Ho, N.I.; Raaijmakers, T.K.; Adema, G.J. Adjuvants enhancing cross-presentation by dendritic cells: The key to more effective vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef]
- Kim, C.G.; Kye, Y.C.; Yun, C.H. The role of nanovaccine in cross-presentation of antigen-presenting cells for the activation of CD8+ T cell responses. Pharmaceutics 2019, 11, 612. [Google Scholar] [CrossRef]
- Song, C.; Noh, Y.W.; Lim, Y.T. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int. J. Nanomed. 2016, 11, 3753–3764. [Google Scholar]
- Jiang, D.; Mu, W.; Pang, X.; Liu, Y.; Zhang, N.; Song, Y.; Garg, S. Cascade cytosol delivery of dual-sensitive micelle-tailored vaccine for enhancing cancer immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 37797–37811. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, J.; Chu, C.C. Enhanced MHC-I antigen presentation from the delivery of ovalbumin by light-facilitated biodegradable poly(ester amide)s nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Ariizumi, R.; Takakura, Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol. Pharm. 2017, 14, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Hayashi, A.; Kunisawa, J.; Tsutsumi, Y.; Tanaka, K.; Yashiro-Ohtani, Y.; Nakanishi, M.; Fujiwara, H.; Hamaoka, T.; Mayumi, T. Fusogenic liposomes efficiently deliver exogenous antigen through the cytoplasm into the MHC class I processing pathway. Eur. J. Immunol. 2000, 30, 1740–1747. [Google Scholar] [CrossRef]
- Flanary, S.; Hoffman, A.S.; Stayton, P.S. Antigen delivery with poly(propylacrylic acid) conjugation enhances MHC-1 presentation and T-cell activation. Bioconjug. Chem. 2009, 20, 241–248. [Google Scholar] [CrossRef]
- Yuba, E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J. Mater. Chem. B 2020, 8, 1093–1107. [Google Scholar] [CrossRef]
- Yuba, E.; Harada, A.; Sakanishi, Y.; Watarai, S.; Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 2013, 34, 3042–3052. [Google Scholar] [CrossRef]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef]
- Sayem, M.A.; Tomita, Y.; Yuno, A.; Hirayama, M.; Irie, A.; Tsukamoto, H.; Senju, S.; Yuba, E.; Yoshikawa, T.; Kono, K.; et al. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T-cells; prolonged overall survival in cancer patients with Th cell response. OncoImmunology 2016, 5, e1062209. [Google Scholar] [CrossRef]
- Yuba, E.; Yamaguchi, A.; Yoshizaki, Y.; Harada, A.; Kono, K. Bioactive polysaccharide-based pH-sensitive polymers for cytoplasmic delivery of antigen and activation of antigen-specific immunity. Biomaterials 2017, 120, 32–45. [Google Scholar] [CrossRef]
- Azad, A.K.; Rajaram, M.V.; Schlesinger, L.S. Exploitation of the macrophage mannose receptor (CD206) in infectious disease diagnostics and therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef]
- Wagener, K.; Bros, M.; Krumb, M.; Langhanki, J.; Pektor, S.; Worm, M.; Schinnerer, M.; Montermann, E.; Miederer, M.; Frey, H.; et al. Targeting of immune cells with trimannosylated liposomes. Adv. Ther. 2020, 3, 1900185. [Google Scholar] [CrossRef]
- Kojima, N.; Ishii, M.; Kawauchi, Y.; Takagi, H. Oligomannose-coated liposome as a novel adjuvant for the induction of cellular immune responses to control disease status. Biomed. Res. Int. 2013, 2013, 562924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Huang, P.; Wang, Y.; Wang, W.; Li, C.; et al. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, W.; Wu, X.; Zhang, Y.; Chen, X.; Liu, G.; Chen, G.; Jiang, M. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small 2015, 11, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Horst, A.K.; Klemm, P.; Lindhorst, T.K. A kit for the investigation of live Escherichia coli cell adhesion to glycosylated surfaces. Chem. Commun. 2010, 46, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Orr, G.A.; Rando, R.R.; Bangerter, F.W. Synthetic glycolipids and the lectin-mediated aggregation of liposomes. J. Biol. Chem. 1979, 254, 4721–4725. [Google Scholar]
- Penberthy, K.K.; Ravichandran, K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016, 269, 44–59. [Google Scholar] [CrossRef]
- Platt, N.; Gordon, S. Scavenger receptors: Diverse activities and promiscuous binding of polyanionic ligands. Chem. Biol. 1998, 5, R193–R203. [Google Scholar] [CrossRef]
- Matsui, H.; Tamura, A.; Osawa, M.; Tonegawa, A.; Arisaka, Y.; Matsumura, M.; Miura, H.; Yui, N. Scavenger receptor A-mediated targeting of carboxylated polyrotaxanes to macrophages and the impacts of supramolecular structure. Macromol. Biosci. 2018, 18, e1800059. [Google Scholar] [CrossRef]
- Hamann, S.; Kiilgaard, J.F.; Litman, T.; Alvarez-Leefmans, F.J.; Winther, B.R.; Zeuthen, T. Measurement of cell volume changes by fluorescence self-quenching. J. Fluor. 2002, 12, 139–145. [Google Scholar] [CrossRef]



| Liposome | Size (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| Man0 | 144 ± 14 | 0.20 ± 0.03 | −20.9 ± 4.0 |
| Man3 | 174 ± 12 | 0.25 ± 0.01 | −40.3 ± 0.5 |
| Man5 | 157 ± 1 | 0.21 ± 0.02 | −30.3 ± 4.0 |
| Man10 | 158 ± 24 | 0.25 ± 0.01 | −30.9 ± 0.8 |
| Man14 | 181 ± 18 | 0.26 ± 0.01 | −33.5 ± 5.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuba, E.; Fukaya, Y.; Yanagihara, S.; Kasho, N.; Harada, A. Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery. Pharmaceutics 2020, 12, 754. https://doi.org/10.3390/pharmaceutics12080754
Yuba E, Fukaya Y, Yanagihara S, Kasho N, Harada A. Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery. Pharmaceutics. 2020; 12(8):754. https://doi.org/10.3390/pharmaceutics12080754
Chicago/Turabian StyleYuba, Eiji, Yoshiki Fukaya, Shin Yanagihara, Nozomi Kasho, and Atsushi Harada. 2020. "Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery" Pharmaceutics 12, no. 8: 754. https://doi.org/10.3390/pharmaceutics12080754
APA StyleYuba, E., Fukaya, Y., Yanagihara, S., Kasho, N., & Harada, A. (2020). Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery. Pharmaceutics, 12(8), 754. https://doi.org/10.3390/pharmaceutics12080754