Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms
Abstract
1. Introduction
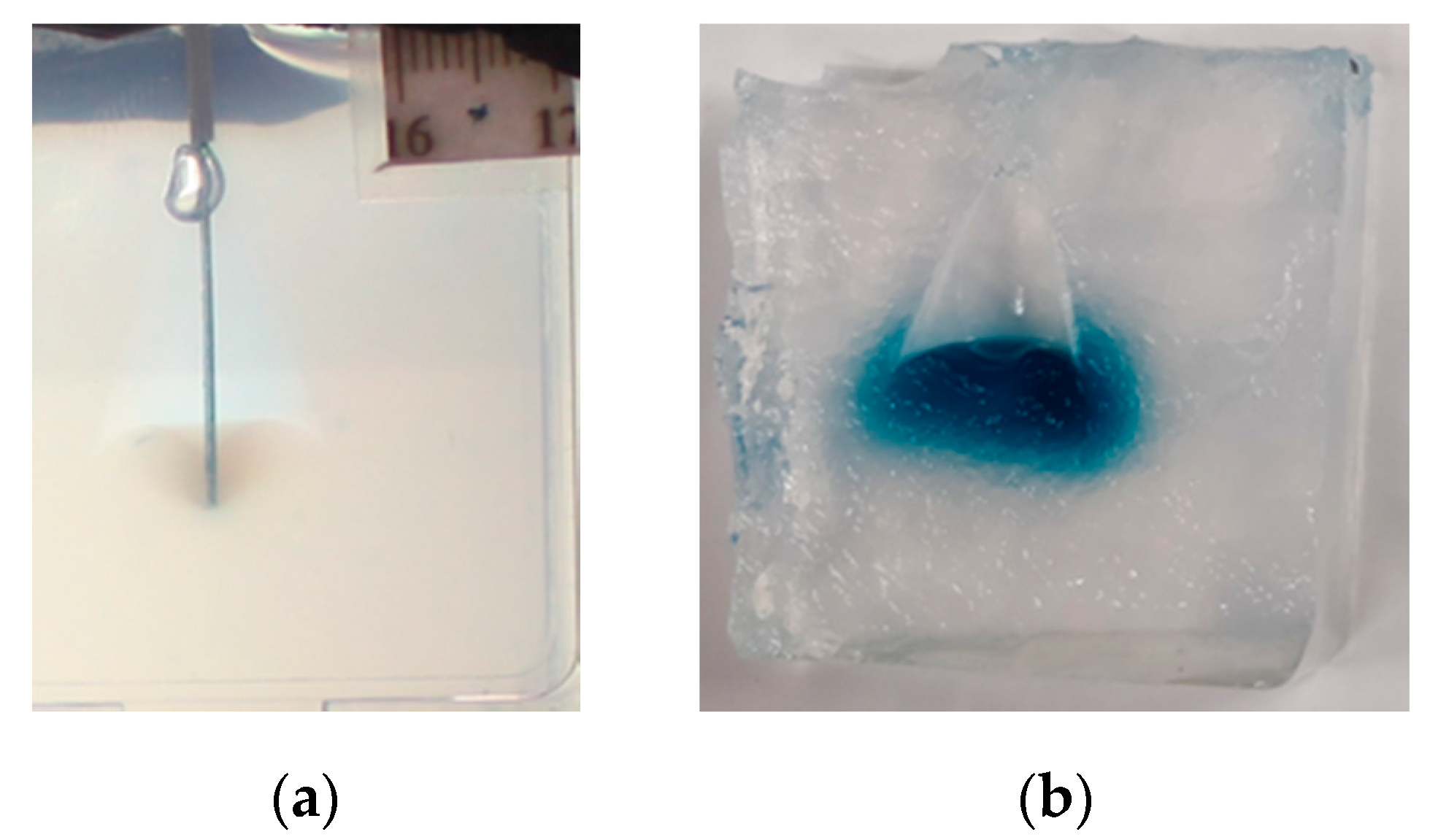
2. Materials and Methods
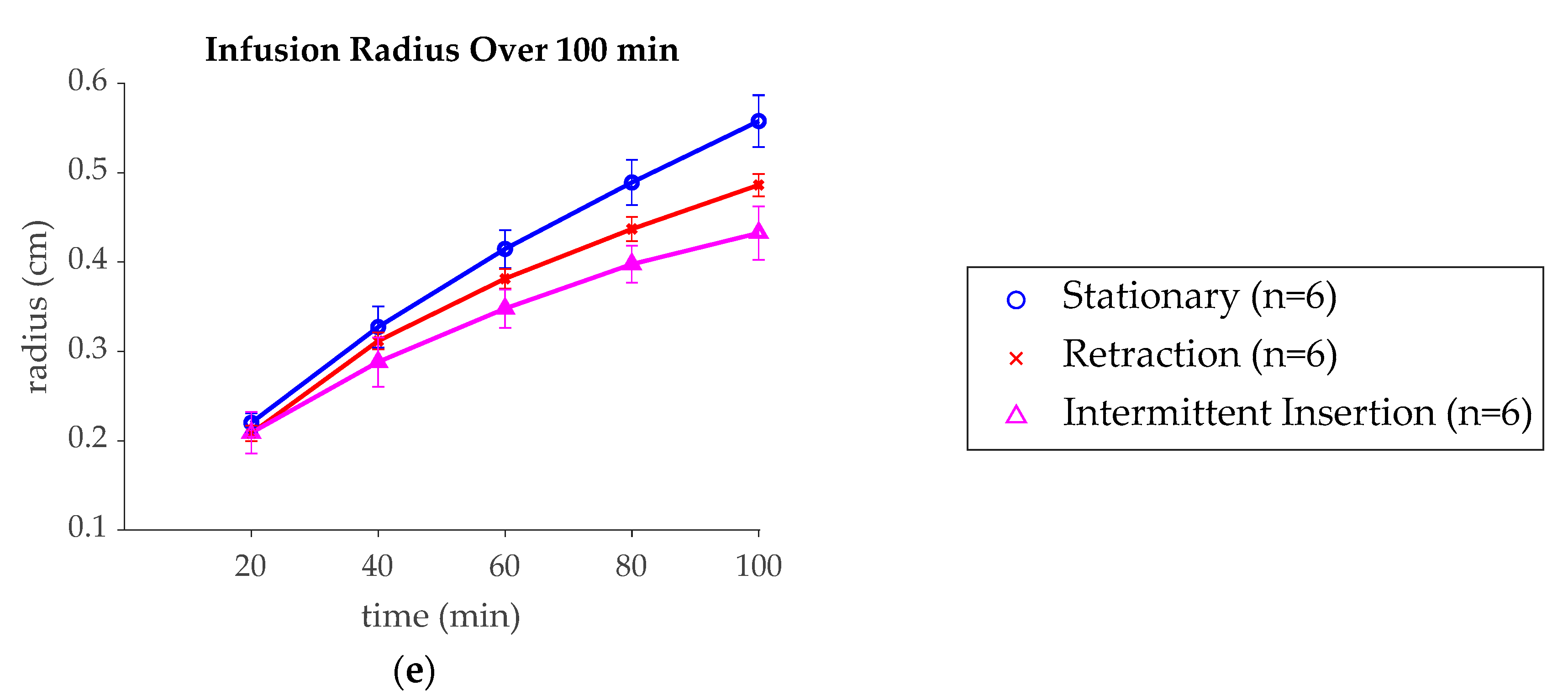
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeAngelis, L.M. Brain Tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesary, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.S. Malignant astrocytomas: Surgical aspects. Semin. Oncol. 1994, 21, 172–185. [Google Scholar] [PubMed]
- Paulsson, A.K.; Holmes, J.A.; Peiffer, A.M.; Miller, L.D.; Liu, W.; Xu, J.; Hinson, W.H.; Lesser, G.J.; Laxton, A.W.; Tatter, S.B.; et al. Comparison of clinical outcomes and genomic characteristics of single focus and multifocal glioblastoma. J. Neurooncol. 2014, 119, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.J. Central Nervous System Overview. In Clinical Radiation Oncology; Gunderson, L.L., Tepper, J.E., Eds.; Churchill-Livingstone: Philadelphia, PA, USA, 2000; pp. 314–354. [Google Scholar]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wallner, K.E.; Galicich, J.H.; Krol, G.; Arbit, E.; Malkin, M.G. Patterns of failure following treatment of glioblastoma mulitform and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 1405–1409. [Google Scholar] [CrossRef]
- Hochberg, F.H.; Pruitt, A. Assumptions in the radiotherapy of glioblastoma. Neurology 1980, 30, 907–911. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Morrison, P.F.; Laske, D.W.; Bobo, H.; Oldfield, E.H.; Dedrick, R.L. High-flow microinfusion: Tissue penetration and pharmacodynamics. Am. J. Physiol. 1994, 266, R292–R305. [Google Scholar] [CrossRef]
- Vandergrift, W.A.; Patel, S.J.; Nicholas, J.S.; Varma, A.K. Convection-enhanced delivery of immunotoxins and radioisotopes for treatment of malignant gliomas. Neurosurg. Focus. 2006, 20, E13. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: A report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Shapiro, W.R.; Laske, D.W.; Jensen, R.L.; Asher, A.L.; Wessels, B.W.; Carpenter, S.P.; Shan, J.S. Safety and Feasibility of Convection-enhanced Delivery of Cotara for the Treatment of Malignant Glioma: Initial Experience in 51 Patients. Neurosurgery 2005, 56, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Akabani, G.; Archer, G.E.; Bigner, D.D.; Berger, M.S.; Friedman, A.H.; Friedman, H.S.; Herndon Ii, J.E.; Kunwar, S.; Marcus, S.; et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-α and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J. Neurooncol. 2003, 65, 27–35. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef]
- Sampson, J.H.; Archer, G.; Pedain, C.; Wembacher-Schroder, E.; Westphal, M.; Kunwar, S.; Vogelbaum, M.A.; Coan, A.; Herndon, J.E.; Raghavan, R.; et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J. Neurosurg. 2010, 113, 301–309. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendesen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Slevin, J.T.; Gerhardt, G.A.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Improvement of bilaterial motor functions in patients with Parkinson disease through the uniteral intraputaminal infsion of glial cell line-derived neurotrophic factor. J. Neurosug. 2005, 102, 216–222. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Ai, Y.; Fischer, B.; Zhang, A.M.; Grondin, R.C.; Zhang, Z.; Gerhardt, G.A.; Gash, D.M. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006, 202, 497–505. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lonser, R.R.; Morrison, P.F.; Governale, L.S.; Oldfield, E.H. Variables affecting convection-enhanced delivery to the striatum: A systematic examination of rate of infusion, cannula size, infusate concentration, and tissue–cannula sealing time. J. Neurosurg. 1999, 90, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.F.; Chen, M.Y.; Chadwick, R.S.; Lonser, R.R.; Oldfield, E.H. Focal delivery during direct infusion to brain: Role of flow rate, catheter diameter, and tissue mechanics. Am. J. Physiol. 1999, 277, R1218–R1219. [Google Scholar] [CrossRef] [PubMed]
- Krauze, M.T.; Saito, R.; Noble, C.; Tamas, M.; Bringas, J.; Park, J.W.; Berger, M.S.; Bankiewicz, K. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J. Neurosurg. 2005, 103, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.C.; Hagel, E.; Willenberg, B.J.; Dai, W.; Casanova, F.; Batich, C.D.; Sarntinoranont, M. Polymer-coated cannulas for the reduction of backflow during intraparenchymal infusions. J. Mater. Sci. Mater. Med. 2012, 23, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Forsayeth, J.; Bankiewicz, K.S. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J. Neurosci. Methods 2010, 187, 46–51. [Google Scholar] [CrossRef]
- Gill, T.; Barua, N.U.; Woolley, M.; Bienemann, A.S.; Johnson, D.E.; Sullivan, S.O.; Murray, G.; Fennelly, C.; Lewis, O.; Irving, C.; et al. In vitro and in vivo testing of a novel recessed-step catheter for reflux-free convection-enhanced drug delivery to the brain. J. Neurosci. Methods 2013, 219, 1–9. [Google Scholar] [CrossRef]
- Rosenbluth, K.H.; Luz, M.; Mohr, E.; Mittermeyer, S.; Bringas, J.; Bankiewicz, K.S. Design of an in-dwelling cannula for convection-enhanced delivery. J. Neurosci. Methods 2011, 196, 118–123. [Google Scholar] [CrossRef]
- Elenes, E.Y.; Rylander, C.G. Maximizing Local Access to Therapeutic Deliveries in Glioblastoma. Part II: Arborizing Catheter for Convection-Enhanced Delivery in Tissue Phantoms. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M.; Peereboom, D.M.; Ahluwalia, M.S.; Gao, S. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: Results of pilot trial 1. J. Neurosurg. 2018, 1–10. [Google Scholar] [CrossRef]
- Breeze, R.E.; Wells, T.H.; Freed, C.R. Implantation of fetal tissue for the management of Parkinson’s disease: A technical note. Neurosurgery 1995, 36, 1044–1048. [Google Scholar] [CrossRef]
- Marks, W.J., Jr.; Ostrem, J.L.; Verhagen, L.; Starr, P.A.; Larson, P.S.; Bakay, R.A.; Taylor, R.; Cahn-Weiner, D.A.; Stoessl, A.J.; Olanow, C.W. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2–neurturin) to patients with idiopathic Parkinson’s disease: An open-label, phase I trial. Lancet Neurol. 2008, 7, 400–408. [Google Scholar] [CrossRef]
- Marks, W.J., Jr.; Bartus, R.T.; Siffert, J.; Davis, C.S.; Lozano, A.; Boulis, N.; Vitek, J.; Stacy, M.; Turner, D.; Verhagen, L. Gene delivery of AAV2-neurturin for Parkinson’s disease: A double-blind, randomised, controlled trial. Lancet Neurol. 2010, 9, 1164–1172. [Google Scholar] [CrossRef]
- Bartus, R.T.; Herzog, C.D.; Chu, Y.; Wilson, A.; Brown, L.; Siffert, J.; Johnson, E.M., Jr.; Olanow, C.W.; Mufson, E.J.; Kordower, J.H. Bioactivity of AAV2-neurturin gene therapy (CERE-120): Differences between Parkinson’s disease and nonhuman primate brains. Mov. Disord. 2011, 26, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sillay, K.; Hinchman, A.; Kumbier, L.; Schomberg, D.; Ross, C.; Kubota, K.; Brady, M.; Brodsky, E.; Miranpuri, G.; Raghavan, R. Strategies for the Delivery of Multiple Collinear Infusion Clouds in Convection-Enhanced Delivery in the Treatment of Parkinson’s Disease. Stereotact. Funct. Neurosurg. 2013, 91, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bankiewicz, K.S.; Sudhakar, V.; Samaranch, L.; San Sebastian, W.; Bringas, J.; Forsayeth, J. AAV viral vector delivery to the brain by shape-conforming MR-guided infusions. J. Control. Release 2016, 240, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, V.; Naidoo, J.; Samaranch, L.; Bringas, J.R.; Lonser, R.R.; Fiandaca, M.S.; Bankiewicz, K.S. Infuse-as-you-go convective delivery to enhance coverage of elongated brain targets. J. Neurosurg. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Lewis, O.; Woolley, M.; Johnson, D.E.; Fletcher, J.; Fenech, J.; Pietrzyk, M.W.; Barua, N.U.; Bienemann, A.S.; Singleton, W.; Evans, S.L. Maximising coverage of brain structures using controlled reflux, convection-enhanced delivery and the recessed step catheter. J. Neurosci. Methods 2018, 308, 337–345. [Google Scholar] [CrossRef]
- Hood, R.L.; Ecker, T.; Andriani, R.; Robertson, J.; Rossmeisl, J.; Rylander, C.G. Augmenting convection-enhanced delivery through simultaneous co-delivery of fluids and laser energy with a fiberoptic microneedle device. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 20 March 2013; p. 85760G. [Google Scholar]
- Hood, R.L.; Andriani, R.T.; Ecker, T.E.; Robertson, J.L.; Rylander, C.G. Characterizing Thermal Augmentation of Convection-Enhanced Drug Delivery with the Fiberoptic Microneedle Device. Engineering 2015, 1, 344–350. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.J.; Gillies, G.T.; Broaddus, W.C.; Prabhu, S.S.; Fillmore, H.; Mitchell, R.M.; Corwin, F.D.; Fatouros, P.P. A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg. 2004, 101, 314–322. [Google Scholar] [CrossRef]
- Casanova, F.; Carney, P.R.; Sarntinoranont, M. Influence of needle insertion speed on backflow for convection-enhanced delivery. J. Biomech. Eng. 2012, 134, 041006. [Google Scholar] [CrossRef]
- Vogelbaum, M.A. Convection enhanced delivery for treating brain tumors and selected neurological disorders: Symposium review. J. Neurooncol. 2007, 83, 97–109. [Google Scholar] [CrossRef]
- Sabliov, C.M.; Boldor, D.; Keener, K.M.; Farkas, B.E. Image Processing Method to Determine Surface Area and Volume of Axi-Symmetric Agricultural Products. Int. J. Food Prop. 2002, 5, 641–653. [Google Scholar] [CrossRef]
- Casanova, F.; Carney, P.R.; Sarntinoranont, M. In vivo evaluation of needle force and friction stress during insertion at varying insertion speed into the brain. J. Neurosci. Methods 2014, 237, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Carney, P.R.; Sarntinoranont, M. Effect of needle insertion speed on tissue injury, stress, and backflow distribution for convection-enhanced delivery in the rat brain. PLoS ONE 2014, 9, e94919. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.L.; Andriani, R.T., Jr.; Emch, S.; Robertson, J.L.; Rylander, C.G.; Rossmeisl, J.H., Jr. Fiberoptic microneedle device facilitates volumetric infusate dispersion during convection-enhanced delivery in the brain. Lasers. Surg. Med. 2013, 45, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Sillay, K.; Schomberg, D.; Hinchman, A.; Kumbier, L.; Ross, C.; Kubota, K.; Brodsky, E.; Miranpuri, G. Benchmarking the ERG valve tip and MRI Interventions Smart Flow neurocatheter convection-enhanced delivery system’s performance in a gel model of the brain: Employing infusion protocols proposed for gene therapy for Parkinson’s disease. J. Neural. Eng. 2012, 9, 026009. [Google Scholar] [CrossRef]
- Raghavan, R.; Mikaelian, S.; Brady, M.; Chen, Z.-J. Fluid infusions from catheters into elastic tissue: I. Azimuthally symmetric backflow in homogeneous media. Phys. Med. Biol. 2009, 55, 281. [Google Scholar] [CrossRef]
- Chen, Z.J.; Broaddus, W.C.; Viswanathan, R.R.; Raghavan, R.; Gillies, G.T. Intraparenchymal Drug Delivery via Positive-Pressure Infusion: Experimental and Modeling Studies of Poroelasticity in Brain Phantom Gels. IEEE Trans. Biomed. Eng. 2002, 49, 85–96. [Google Scholar] [CrossRef]
- Gillies, G.; Allison, S.; Tissue, B. Positive pressure infusion of fluorescent nanoparticles as a probe of the structure of brain phantom gelatins. Nanotechnology 2002, 13, 484. [Google Scholar] [CrossRef]
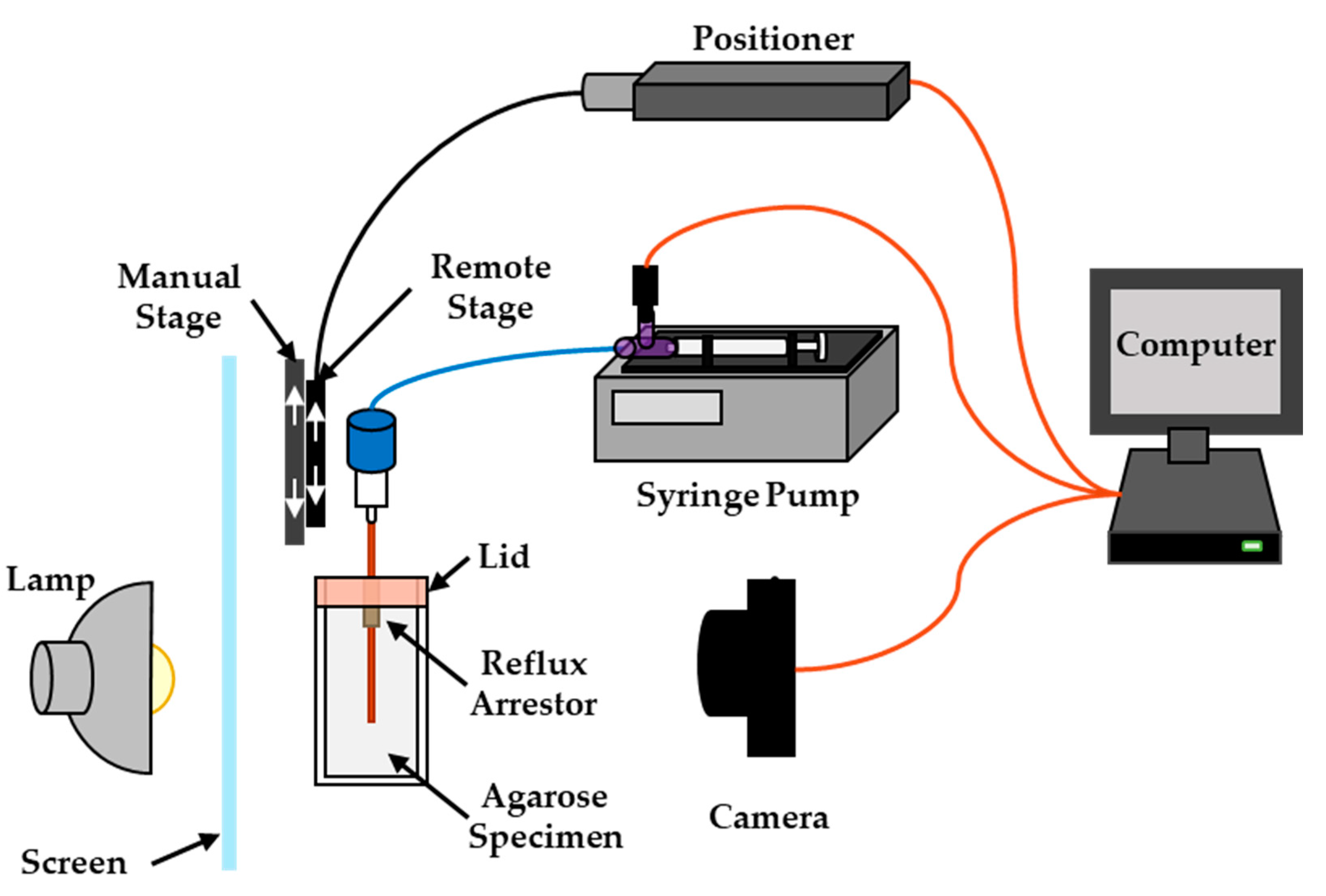
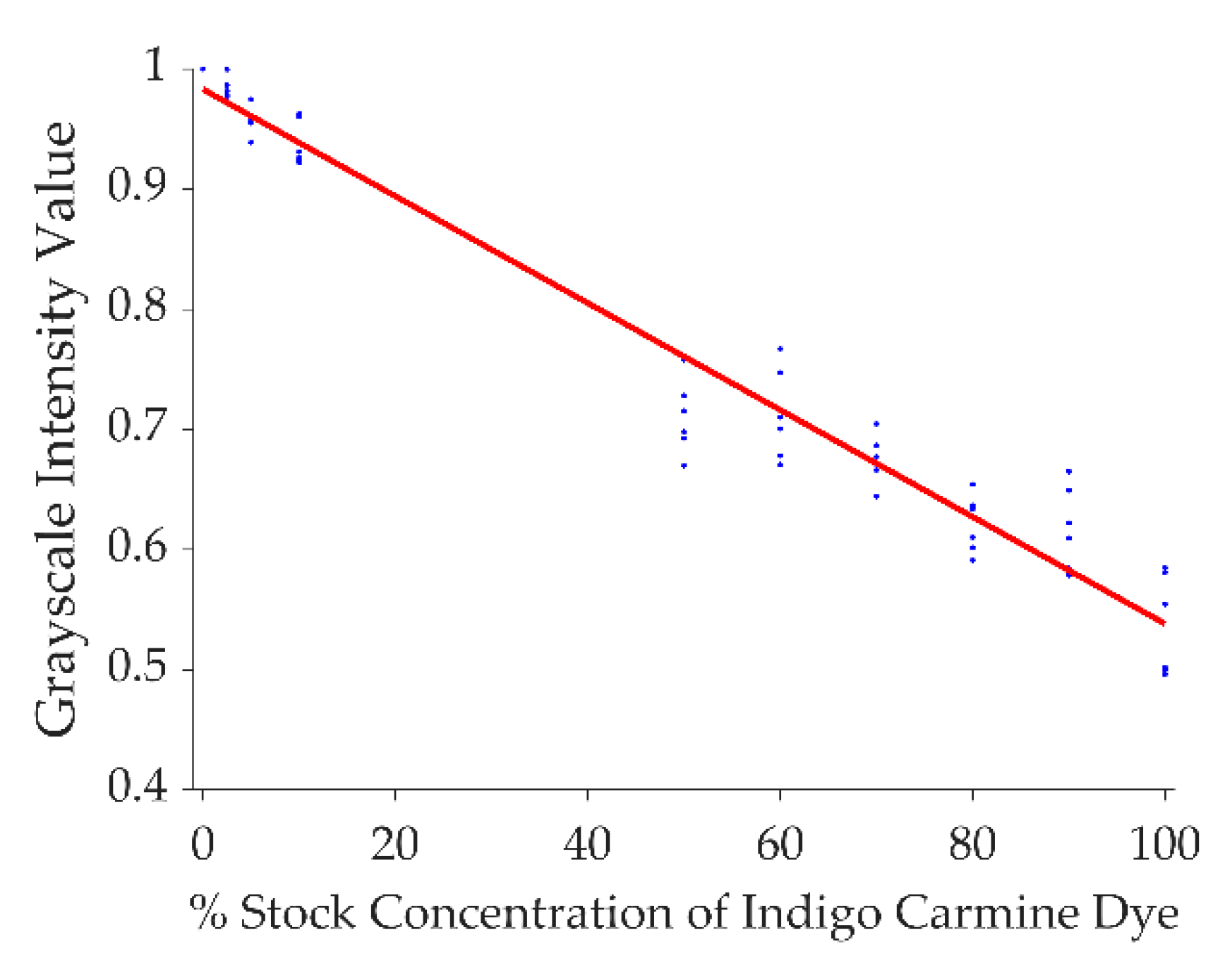
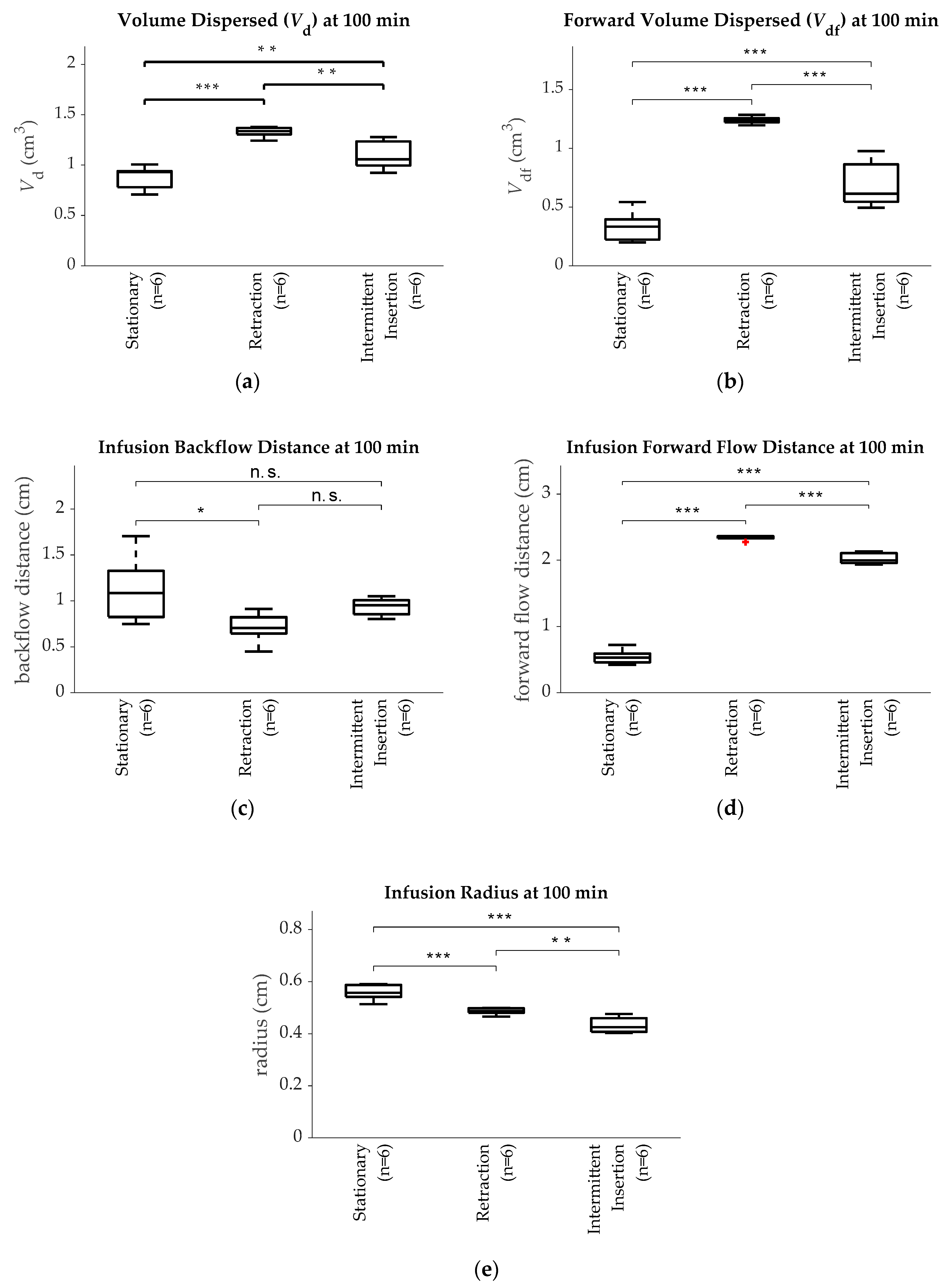
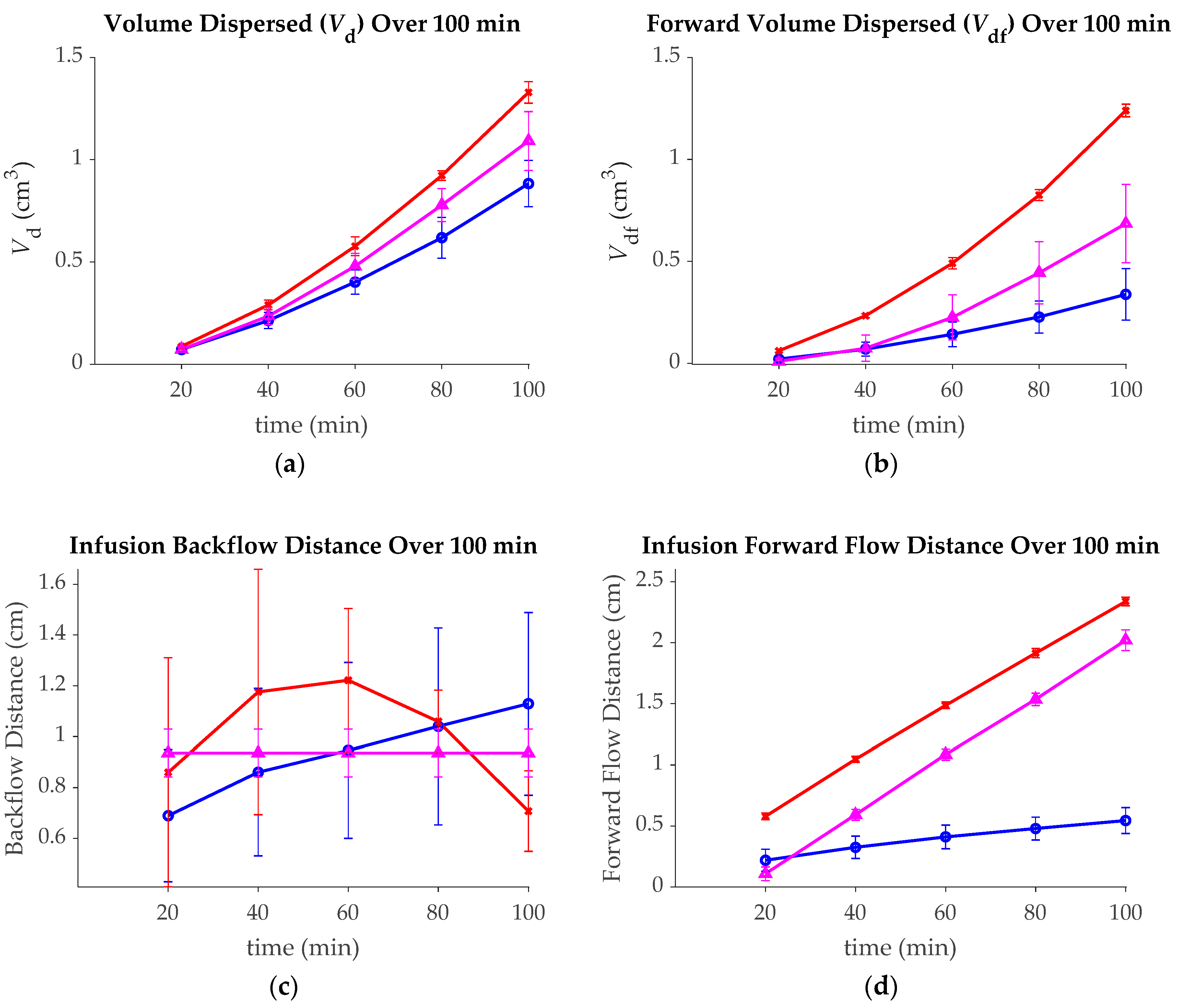
| Group | Initial Deployment Distance | Movement Direction | Movement Speed | Movement Distance | Number of Replicates |
|---|---|---|---|---|---|
| Stationary (Control) | 30 mm | None | 0 mm/min | 0 mm | 7 |
| Continuously Retracting | 30 mm | Backward | 0.25 mm/min | 25 mm | 8 |
| Continuous Insertion | 6 mm | Forward | 0.25 mm/min | 25 mm | 14 |
| Intermittent Insertion | 6 mm | Forward | 600 mm/min | 25 mm (5 mm every 16 min) | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, J.N.; McRoberts, G.R.; Rylander, C.G. Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms. Pharmaceutics 2020, 12, 753. https://doi.org/10.3390/pharmaceutics12080753
Mehta JN, McRoberts GR, Rylander CG. Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms. Pharmaceutics. 2020; 12(8):753. https://doi.org/10.3390/pharmaceutics12080753
Chicago/Turabian StyleMehta, Jason N., Gabrielle R. McRoberts, and Christopher G. Rylander. 2020. "Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms" Pharmaceutics 12, no. 8: 753. https://doi.org/10.3390/pharmaceutics12080753
APA StyleMehta, J. N., McRoberts, G. R., & Rylander, C. G. (2020). Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms. Pharmaceutics, 12(8), 753. https://doi.org/10.3390/pharmaceutics12080753




