1. Introduction
Encephalomyocarditis virus (EMCV) is an important veterinary pathogen with a wide host range. EMCV is a single-stranded positive-sense RNA picornavirus, and its genome encodes a large polyprotein, which is cleaved to produce thirteen structural and non-structural proteins. Notably, EMCV infection causes severe myocarditis and/or encephalitis in pigs with a mortality rate close to 100% in pre-weaning piglets [
1]. EMCV has the capacity to spread endemically in pig farms, contributing to a significant burden on the global swine industry. Importantly, EMCV carries a strong zoonotic potential to transmit to humans via a fecal–oral transmission route [
2]. Since its discovery in the late, we have yet to see an approved direct-acting antiviral therapy for EMCV infections in veterinary practice.
RNA interference (RNAi) technology utilizing small interfering RNAs (siRNAs) is an emerging new class of direct-acting antivirals. siRNAs are 21–25-base-pair, double-stranded RNA molecules that trigger RNA interference (RNAi), a highly conserved, sequence-specific post-transcriptional gene-silencing pathway in host cells. Unlike traditional approaches that often disrupt host–virus interactions, RNAi leverages the cell’s endogenous machinery to degrade specific viral RNA sequences in a virally infected cell, minimizing off-target effects. This mechanism has demonstrated success in combating RNA viruses, including human immunodeficiency virus (HIV), hepatitis C, SARS-CoV-2, and influenza, where siRNA-based therapies have shown the ability to suppress viral replication by silencing conserved genomic regions [
3]. RNAi presents unique opportunities to target essential viral components from replication enzymes to structural proteins, halting infection at multiple stages of its life cycle.
Multiple EMCV targets that are amenable to RNAi silencing have been tested prophylactically in vivo but utilize either viral or plasmid DNA (pDNA)-mediated RNAi delivery systems [
4,
5,
6]. This prophylaxis approach may be an ideal approach to protect animals from mortality due to viral encephalitis. Although viral vectors are highly efficient delivery vectors, they carry immunogenicity risks and could result in chromosomal integration events in the host [
7]. Despite promising in vivo prophylaxis using viral or plasmid DNA (pDNA)-mediated RNAi delivery systems, safety concerns persist regarding immunogenicity and insertional mutagenesis risks associated with viral vectors. Non-viral delivery systems, such as lipid nanoparticles (LNPs), are being explored to mitigate these issues, though their efficacy for EMCV has not yet been developed. Additionally, combinatorial approaches integrating RNAi with interferon-based therapies show potential.
Here, we want to fine-tune anti-EMCV RNAi delivery by using siRNAs encapsulated in liposomal delivery systems. In our study, we demonstrate that an anti-EMCV siRNA-based therapeutic packaged in liposomes results in the attenuation of multi-organ viremia and rescues mice from a lethal EMCV infection.
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
All animal experiments were performed in compliance with relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki. All animal care and procedures have been performed according to protocols reviewed and approved by Northwest Minzu University animal ethics committee (Animal Ethics Approval # xbmu-sm-202471/202485).
2.2. Cells, Viruses, and Animals
HEK-293 cells and BHK-21 cells were cultured in DMEM supplemented with 10% new bovine serum (NBS) in 5% CO2 environment at 37 °C. The EMCV GS01 strain was used in this study. Female BALB/c mice (6–8 weeks old, ~20 g) (Lanzhou Veterinary Research Institute, Lanzhou, China) were used to assess in vivo antiviral siRNA efficacy, with each experimental group consisting of n = 6–8 mice.
2.3. siRNAs and Nucleic Acids
SiRNAs (
Table 1) were designed against conserved regions of the EMCV genome, and all siRNAs are synthesized by RIBOBIO Co. (Guangzhou, China). SiRNAs were not chemically modified. Poly I:C was obtained from Invivogen (San Diego, CA, USA).
2.4. Antiviral siRNA In Vitro Screening and Immunostimulation
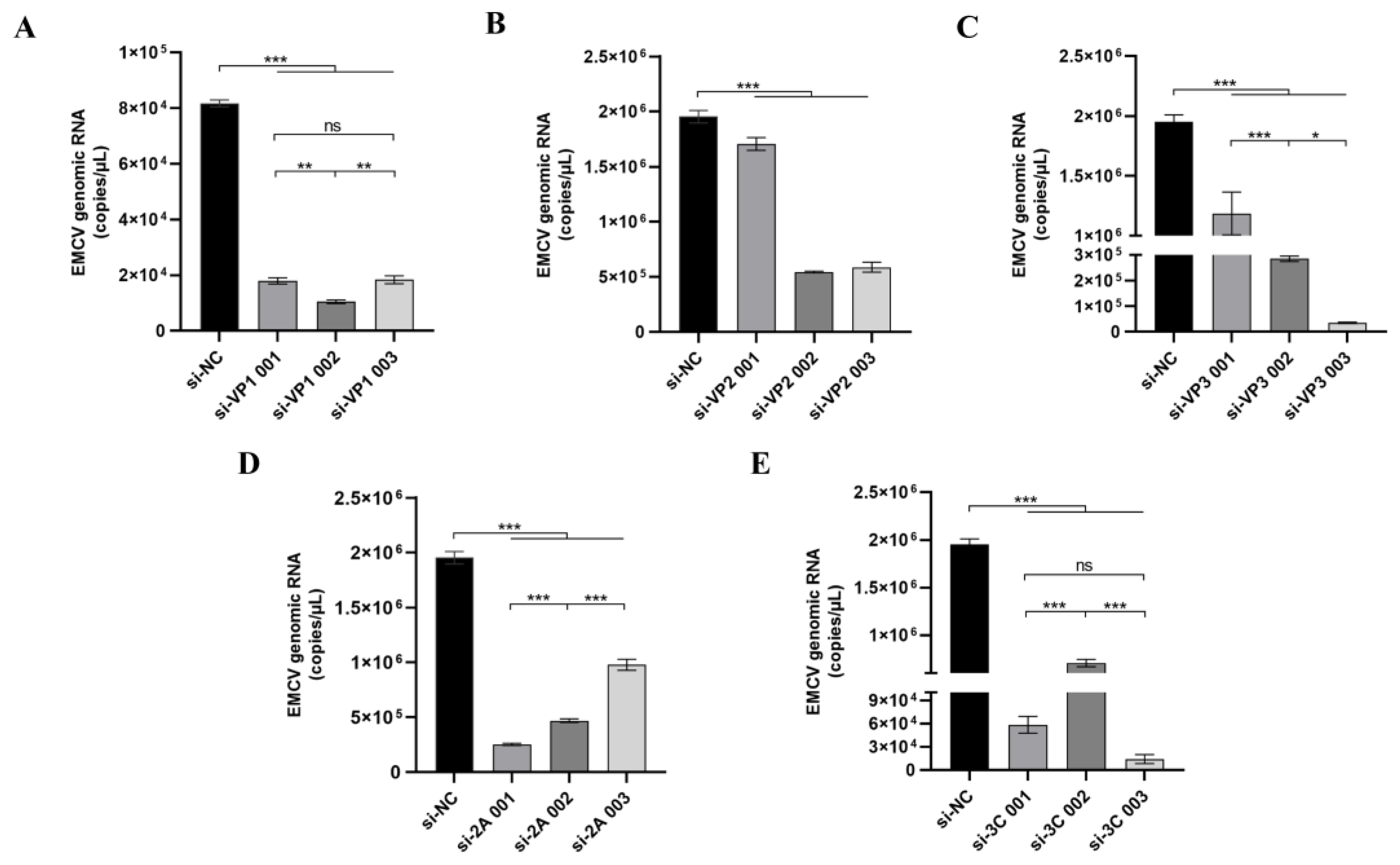
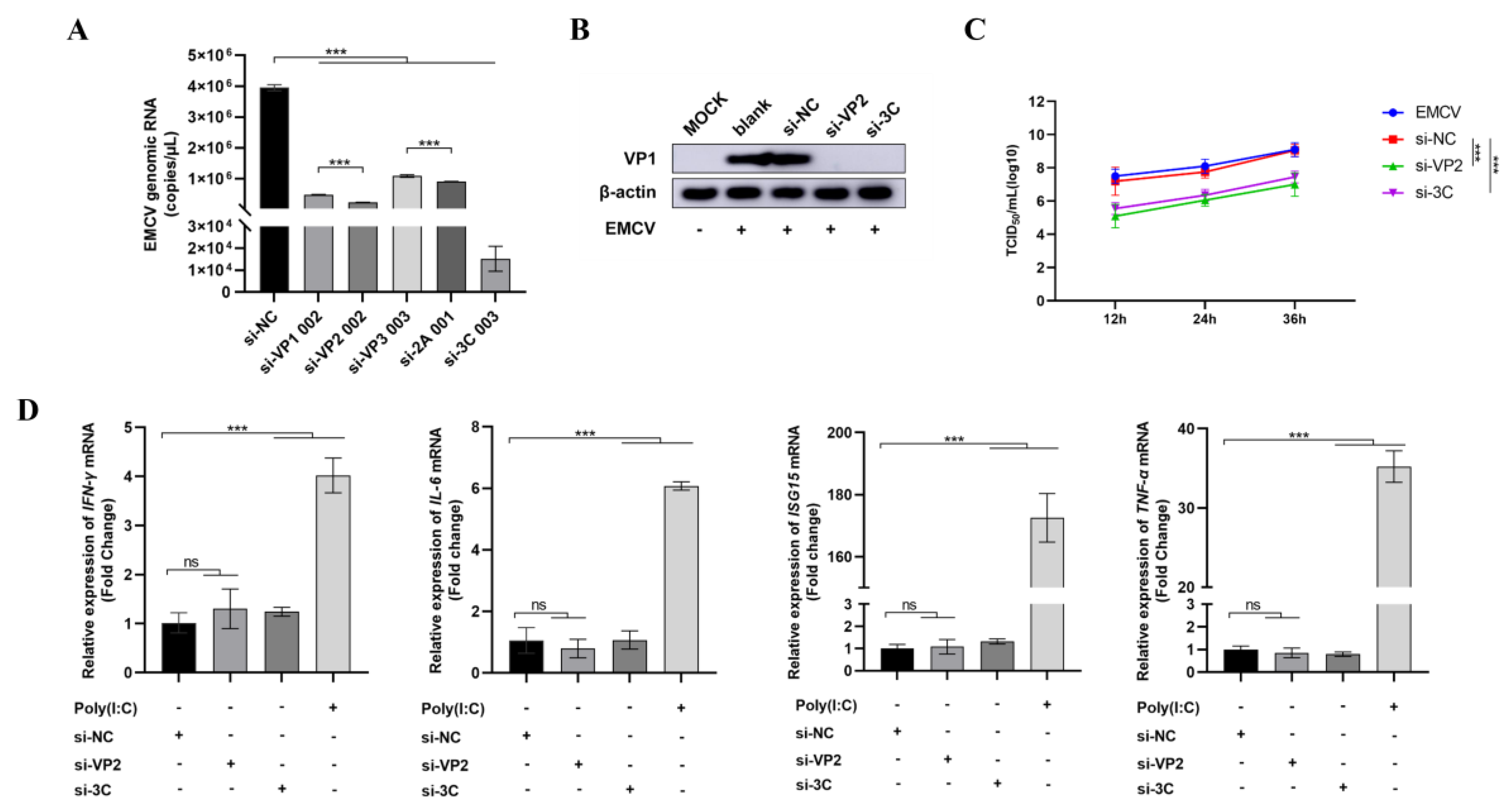
HEK-293 cells seeded overnight were treated with siRNA complexed in Lipofectamine 2000 transfection reagent (1:1.25 ratio, Invitrogen, Carlsbad, CA, USA) in Opti-MEM media (Invitrogen) before infecting with EMCV at a dose of 100 TCID50 24 h. Scrambled siRNA, si-NC, was used as a negative control. After 16 h of transfection, the transfected HEK-293 cells were infected with the EMCV GS01 strain virus with 100 TCID50/well for 24 h. To check the immunostimulatory activity of siRNAs, HEK293 cells were either transfected with siRNA or poly I:C (positive control) for 16 h before extracting RNA to assess inflammatory cytokine mRNA expression by qPCR (IFNγ, IL-6, ISG15, and TNFα).
2.5. Immunoblotting
Cells harvested after 24 h of EMCV infection were lysed in radio immunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China) and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before proteins were transferred onto a polyvinylidene Fluoride Membrane (PVDF) membrane (Millipore, Belington, MA, USA). The blots were either probed with a mouse monoclonal antibody against EMCV VP1 protein (Gene Create, Wuhan, China) or a rabbit monoclonal antibody to β-actin (Abcam, Cambridge, UK) before visualizing blots using an ECL reagent (Bio-Rad, Hercules, CA, USA).
2.6. Quantitative PCR (qPCR)
Cells were harvested after 24 h of EMCV infection, and total RNA was extracted using Trizol reagent. RNA samples were then converted into cDNA by reverse transcription before measuring IFN-γ, IL-6, ISG15, and TNF-α mRNA expression using a SYBR Green assay (Accurate Biology, Wuhan, China). For measuring viral genomic load, cell supernatant was subjected to three rounds of freeze–thawing before extracting viral RNA in Trizol reagent. For mouse organs, the brain, heart, spleen, and kidney of mice in each group were collected, and RNA was extracted by Trizol reagent post-homogenization before measuring EMCV viral load by qPCR using EMCV-specific primers as previously carried out.
2.7. 50% Tissue Culture Infective Dose (TCID50)
TCID50 assays were used to measure infectious virus titres. Infected cells were subjected to three rounds of freeze–thaw cycles, and the sample clarified by centrifugation before the supernatant was collected. Supernatants were serially diluted 10-fold before exposing a monolayer of BHK-21 cells in 96-well plates. EMCV titres were calculated by the Reed and Muench method.
2.8. Polyethyleneimine (PEI)-siRNA Preparation
PEI (Merck #408719, Darmstadt, Germany) powder was prepared as a 1 mg/mL solution in sterile water. A final concentration of 1 mg/kg/dose (for a ~20 g mouse) of PEI-siRNA was made for every dose (PEI solution and siRNA were mixed with sterile water) and left for 20 min before performing retro-orbital intravenous injection in mice.
2.9. 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1′racglycerol) (POPG) Liposome-siRNA Preparation
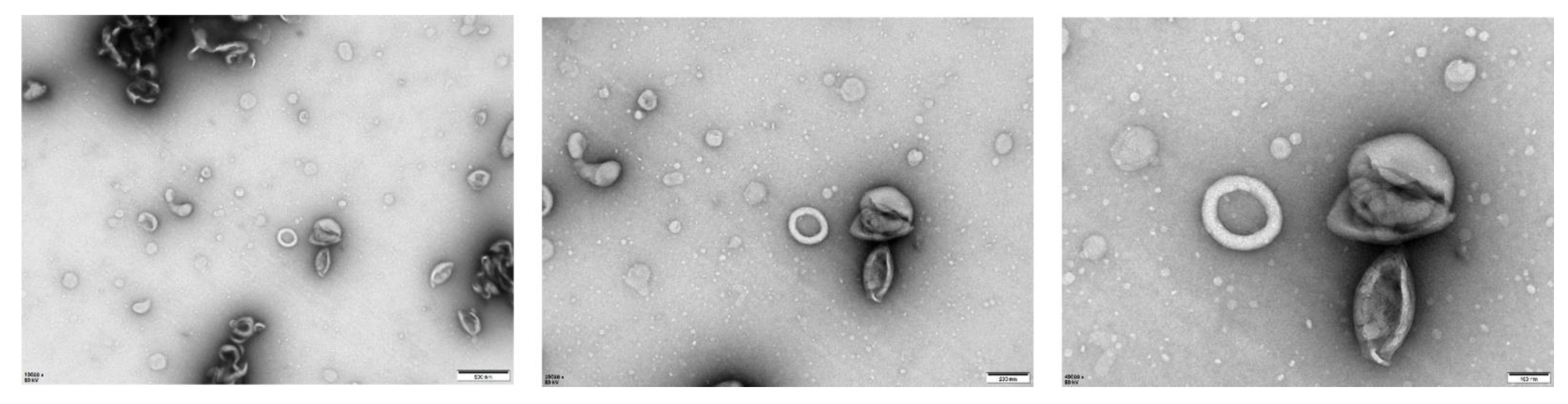
1-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1′racglycerol) (POPG) liposomes were made with 16:0–18:1 POPG (Avanti #840457P, Alabaster, AL, USA). The POPG powder was dissolved in chloroform and gently swirled until the solution formed a thin film at the bottom of the flask. An appropriate amount of siRNA was then added before the flask was shaken in a water bath at 40 °C until the thin film at the bottom of the flask was dissolved into a milky white solution (i.e., siRNA complexed in liposomes). This solution was then aspirated and extruded using a gas-tight syringe (Avanti #610017, Alabaster, AL, USA) repeatedly (up to 40 times) before collecting and storing at 4 °C. The physical characteristics of the liposomes were verified by transmission electron microscopy (TEM).
2.10. Transmission Electron Microscopy (TEM) Analysis of POPG Liposomes
An amount of 20 uL of resuspended siRNA-encapsulated POPG liposome samples was added dropwise to 200-mesh grids and incubated at room temperature for 10 min. The grids were then negatively stained with 2% phosphotungstic acid for 3 min, and the remaining liquid was removed using a filter paper before observing liposomes with a JEM1400 transmission electron microscope (Biomisp, Wuhan, China).
2.11. In Vivo Experiments
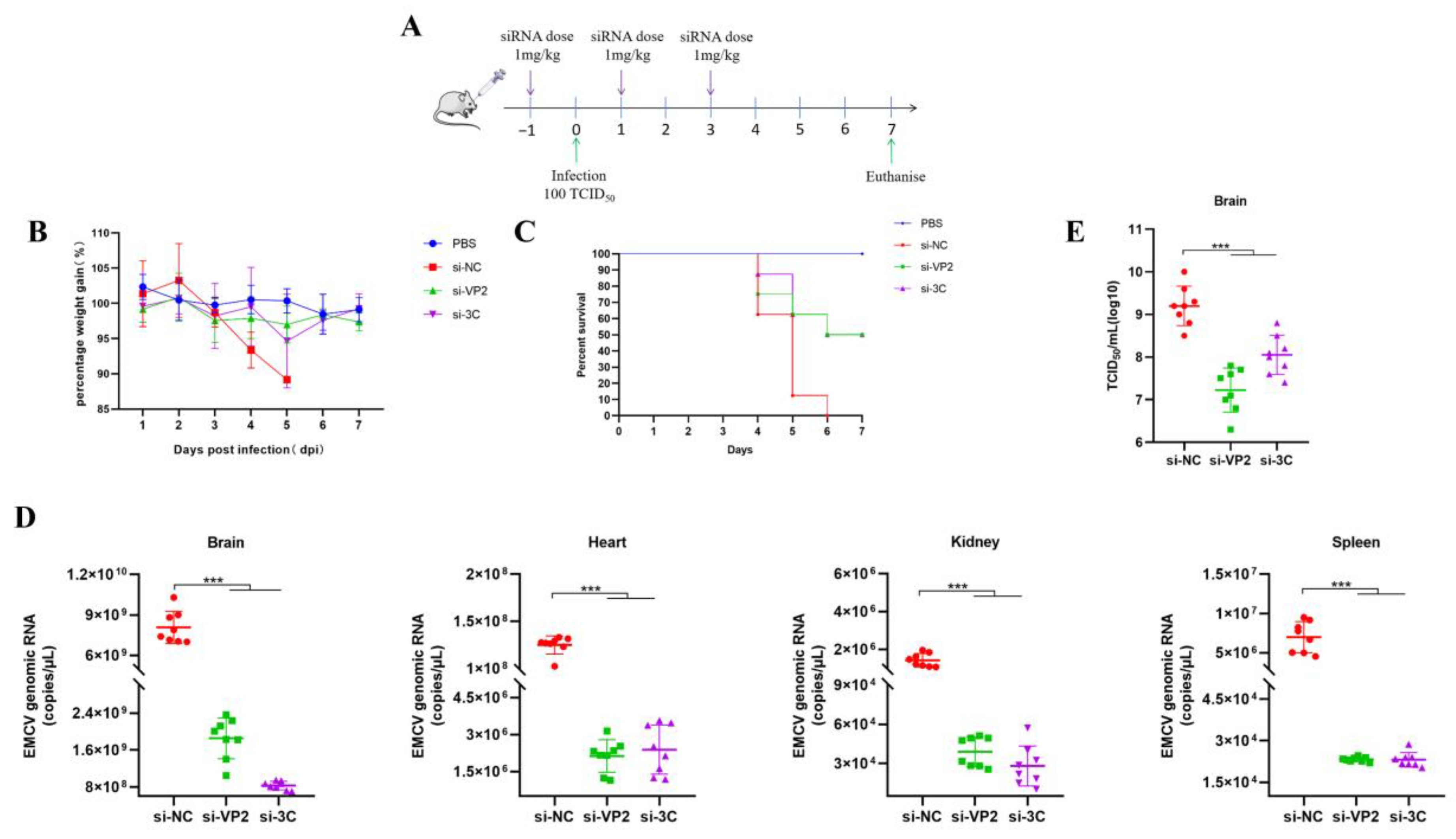
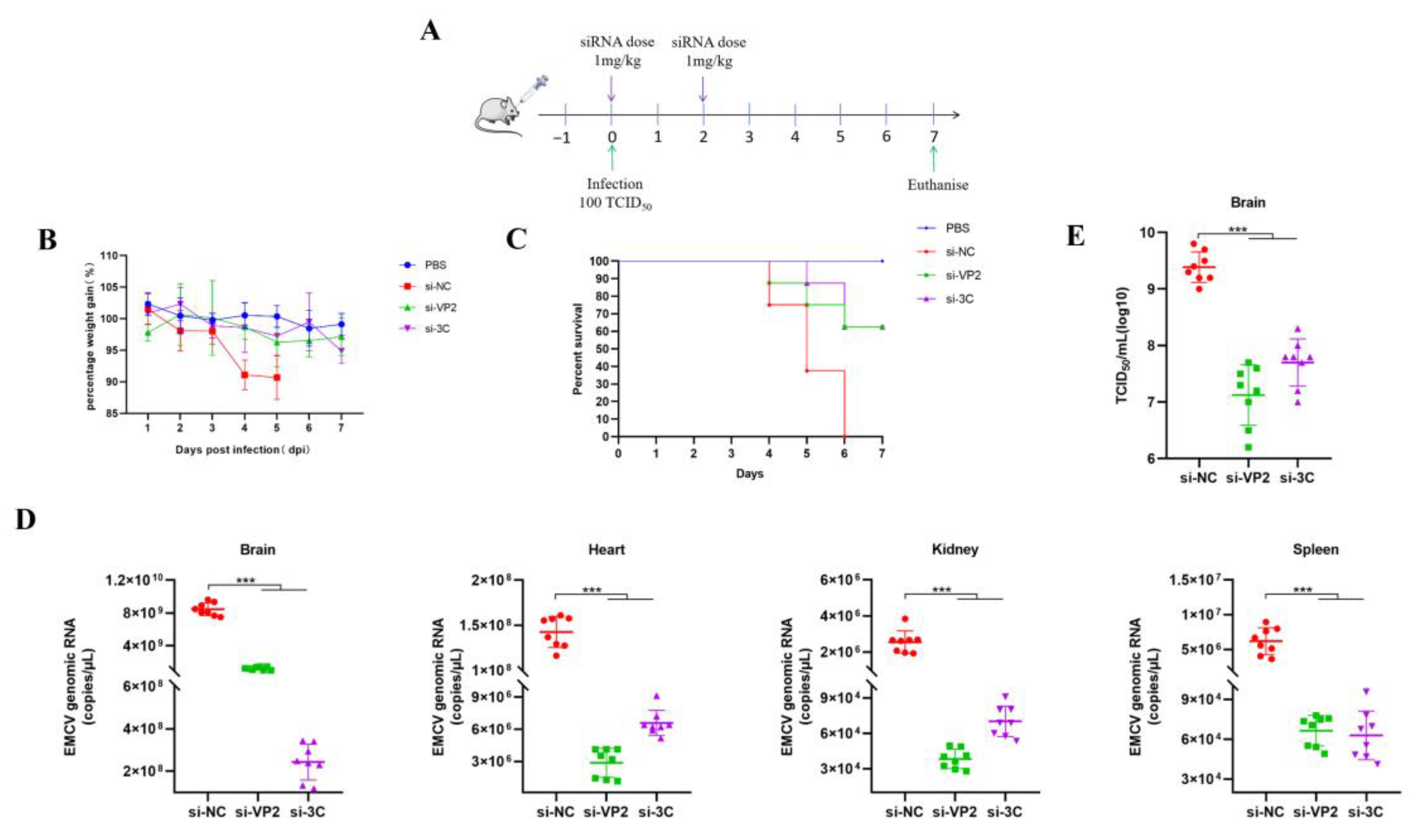
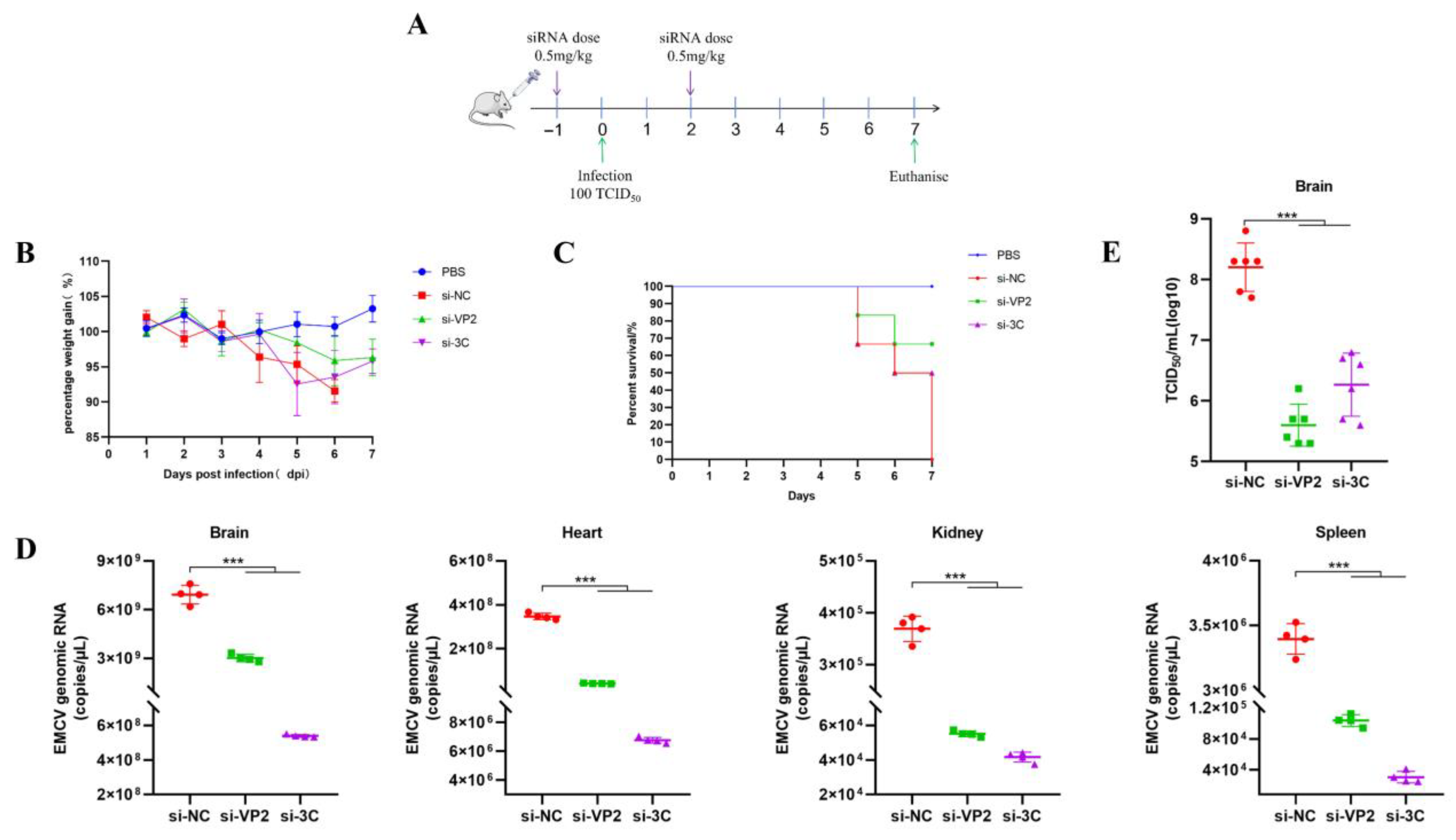
Female BALB/c mice (~20 g, 6–8-week-old) were infected by intramuscular injection with either PBS (uninfected) or 100 TCID50/500 μL of EMCV. PEI or POPG liposome complexed siRNAs retro-orbitally (IV) (100 μL total volume, 1 mg/kg dose) administered while under isoflurane anesthesia. Mice were monitored daily for weighing and clinical scoring.
2.12. Statistical Analysis
All statistical analyses were performed on GraphPad Prism 8. The data was analyzed by either Student’s t-test or one-way ANOVA. For all experiments, data were representative of three independent experiments with n = 3 technical replicates (shown as either mean ± standard deviation (SD) or mean ± standard error of mean (SEM)).
4. Discussion
EMCV is a zoonotic RNA virus with a broad host range spanning multiple mammalian species, including swine, mice, and potentially humans, thereby posing substantial risks to agricultural productivity and public health security [
13]. Swine, as the primary natural host, are particularly vulnerable to EMCV infection, exhibiting clinical manifestations such as sudden death, severe pathological damage to vital organs, and reproductive disorders (e.g., abortion and stillbirth in sows), leading to significant economic losses in the farming industry [
13]. Additionally, the detection of EMCV-specific antibodies in humans highlights its potential zoonotic transmission risk, underscoring its relevance to human biosecurity [
14].
Here, we report an important finding constituting significant progress in the development of direct-acting RNA-based antivirals against an important zoonotic virus by targeting highly conserved regions in its genome. Our findings lay a good foundation for the clinical application of siRNAs as antiviral agents against veterinary viruses such as EMCV, especially in the absence of effective vaccines and antivirals. Our study presents a novel RNA interference (RNAi)-based therapeutic strategy targeting EMCV, with findings that hold important implications for advancing antiviral development. A key strength of our work lies in the rational design of siRNAs targeting highly conserved regions of the EMCV genome. Through analysis of 449 EMCV sequences from the NCBI Virus database, we identified that the VP4 region exhibits the highest mutation frequency, prompting us to focus on more conserved regions such as VP2 and 3C for siRNA design. This targeted approach addresses a critical challenge in antiviral development, which is viral genetic variability. In our study, si-VP2 and si-3C significantly reduced EMCV genomic load and viral protein expression, while also suppressing viral replication over time. Notably, these siRNAs showed no immunostimulatory activity, eliminating the possibility that their antiviral effects stem from off-target immune activation and enhancing their safety profile.
The in vivo validation of our siRNA-based strategy further reinforces its potential. Using both prophylactic and therapeutic regimens with PEI-complexed siRNAs, we observed marked reductions in multi-organ viremia (brain, heart, spleen, and kidney) in lethally infected mice. Concomitantly, treated mice showed near-restoration of body weight to uninfected levels and significantly improved survival rates. These results align with the known tropism of EMCV, which infects multiple organs beyond the brain [
9], and confirm that our siRNAs effectively target viral replication across key infected tissues. However, it is essential to acknowledge the limitations of the mouse model in contextualizing clinical relevance for natural hosts like swine. A primary consideration is the differences in EMCV tropism between mice and swine. In swine, EMCV primarily causes severe myocarditis and encephalitis in pre-weaning piglets with near-100% mortality [
1], whereas in our mouse model, the infection manifests as multi-organ viremia with lethality driven by systemic viral replication. Additionally, physiological differences, such as immune system maturation and organ structure, may influence the efficacy and pharmacokinetics of siRNA delivery. For example, the retro-orbital injection route used in mice may not be translatable to large-scale swine administration, and the dose (1 mg/kg) may require adjustment based on swine physiology. Future studies should therefore include swine models to validate efficacy and optimize delivery routes, ensuring that the findings can be translated to veterinary practice.
To address the safety concerns associated with PEI [
8], we further evaluated an alternative delivery system using POPG liposomes. TEM analysis confirmed that siRNA-encapsulated POPG liposomes form large unilamellar vesicles with diameters of 180–200 nm, a size range conducive to cellular uptake. Our in vivo experiments using a two-dose prophylactic regimen (0.5 mg/kg per dose) demonstrated that POPG liposome-delivered siRNAs effectively reduced multi-organ viremia, rescued mice from weight loss, and achieved 50–60% survival rates. This is comparable to the efficacy of PEI-siRNA complexes. This finding is significant as anionic liposomes offer a safer alternative to cationic delivery systems, with lower toxicity and better biocompatibility [
12], making them more suitable for potential clinical applications.
When placed in the context of current EMCV treatment efforts, our study fills a critical gap. Antiviral drugs targeting EMCV’s replication machinery, such as the non-nucleoside inhibitor GPC-N114, are limited by the rapid emergence of viral resistance due to mutations in the RNA-dependent RNA polymerase (RdRp) [
15]. In contrast, our RNAi-based strategy targets highly conserved viral regions (VP2 and 3C), reducing the likelihood of resistance development. VP2, a structural protein, plays a key role in evading the host’s innate immune response by antagonizing the IFN-β pathway. Specifically, VP2 interacts with melanoma differentiation-associated gene 5 (MDA5), mitochondrial Antiviral Signaling Protein (MAVS), and TANK-binding kinase 1 (TBK1) through its C-terminal domain, leading to the degradation of RIG-I-like receptors (RLRs) via both proteasomal and lysosomal pathways [
16]. Furthermore, VP2 inhibits signal transducer and activator of transcription 1 (STAT1) phosphorylation, a key step in the JAK-STAT signaling cascade, thereby suppressing the expression of downstream interferon-stimulated genes (ISGs), while 3C, a non-structural protease, is indispensable for viral polyprotein processing and particle assembly [
1]. Its proteolytic activity, which mediates the cleavage of viral polyproteins into functional subunits, makes it an attractive target for small-molecule inhibitors. For instance, curcumol has been shown to specifically bind to the C159 active site of 3C protease, inhibiting its ability to cleave host proteins like TANK and thereby blocking viral replication [
17]. By targeting these two essential proteins, our siRNAs disrupt multiple stages of the viral life cycle, enhancing their antiviral potency.
Compared to previous RNAi studies targeting EMCV, our work offers distinct advantages. Early studies used siRNAs targeting the BJC3 strain but only validated efficacy in vitro [
18], while others employed viral vectors (recombinant adenoviruses) or plasmid DNA (pDNA) to deliver short hairpin RNAs (shRNAs) [
4,
5,
6]. However, viral vectors carry risks of immunogenicity and chromosomal integration [
8], and pDNA-based systems may have variable transfection efficiency. Our use of synthetic siRNAs complexed with either PEI or POPG liposomes avoids these issues, providing a safer and more scalable approach. Furthermore, the conservation of our target regions (VP2 and 3C) across EMCV isolates addresses the genetic variability observed in genes like VP1 and 2A [
19,
20], ensuring broader applicability of our siRNAs.
Despite these promising results, several avenues for future research remain. First, optimizing siRNA delivery systems to improve target organ specificity and stability is crucial. Lipid nanoparticles (LNPs), which have already been used in approved RNAi drugs like Patisiran [
16], offer enhanced stability against nuclease degradation and targeted tissue delivery [
21]. Testing LNPs for EMCV siRNA delivery could further improve efficacy and reduce off-target effects. Second, multiplexing siRNAs targeting multiple conserved viral regions could enhance antiviral potency and mitigate the risk of resistance. Finally, validating our strategy in swine models is essential to confirm its clinical relevance and guide the development of veterinary formulations.
In conclusion, our study demonstrates that liposome-delivered siRNAs targeting conserved EMCV VP2 and 3C regions effectively attenuate multi-organ viremia and improve survival in a lethal mouse model. This work provides a safe and scalable RNAi-based therapeutic approach for EMCV infection, addressing critical limitations of existing vaccination and drug strategies. While the mouse model has inherent limitations in translating to swine, our findings lay a solid foundation for further development and validation in natural hosts, with the potential to reduce the economic burden of EMCV on the global swine industry and mitigate zoonotic risks. With the advent of RNA therapeutics in the post-pandemic era, the use of RNAi technology could become the next generation of RNA medicines for veterinary practice. The use of RNAi can be applied as an emerging treatment modality against other important viruses that impact the livestock industry, including equine influenza, bovine viral diarrhea virus, and foot and mouth disease virus.