Insights into Persistent SARS-CoV-2 Reservoirs in Chronic Long COVID
Abstract
1. Introduction
2. Long COVID Pathophysiology
3. Persistent SARS-CoV-2 Virus Reservoirs in Patients with LC
4. Persistent Reservoirs of Viral RNA (vRNA) in Patients with LC
5. Residual SARS-CoV-2 Antigens in Patients with LC
5.1. Residual Spike Protein Is Associated with LC Symptoms
5.2. Residual Nucleoprotein and Other Viral Antigens in Patients with LC
6. Animal Models of LC to Study Persistent Reservoirs of Virus and Viral RNA (vRNA)
7. The Path Toward Therapeutics to Target and Clear the Virus and vRNA Reservoirs, and Cure LC
7.1. Antiviral Therapies for LC
7.2. Immune Therapies to Eliminate or Reduce Persistent Virus and vRNA Reservoirs in LC
8. Conclusions
- A potential causative factor of LC, in a large subset of patients, is that reservoirs of virus and/or viral RNA (vRNA) or fragments may persist and replicate in multiple sites of the body, which may drive chronic inflammation and provide continuous viral antigenic stimuli to exhausted CD4+ and CD8+ T cells [31,33,34,35,36]. However, other hypotheses regarding the causative factors of LC include metabolic disturbances, immune dysbiosis, micro-clotting, autonomic dysfunction [38,43,45,46,47], and the reactivation of other non-SARS-CoV-2 viruses, such as HSV-1, HSV-2, EBV, CMV, and HHV-6, which may be a driver of LC [48,49].
- While a growing body of literature has shown that persistent virus and vRNA reservoirs within cells from various body tissues correlate with some of the LC symptoms, it remains to be confirmed whether the various symptomatology of LC and pro-inflammatory signatures are a direct consequence of persistent viral antigens.
- Although viral persistence may be linked to inflammation and immunological overactivation in patients with LC, the underlying mechanism of such stimulation remains to be fully elucidated. Nevertheless, SARS-CoV-2-derived vRNA and protein antigens (i.e., Spike protein and Nucleoprotein) appeared to be released in various organs (e.g., gut, brain, heart, and reproductive organs) and in the circulation, possibly inducing inflammation and T cell exhaustion that persists months after the acute COVID-19 infection [23,35,38,39,40,41,42]. This suggests at least one immune evasion mechanism by which the virus may establish its reservoir in LC patients.
9. Future Directions
- Knowledge about chronic LC and its lingering health effects, months and years following acute infection, is still in its embryonic stage. Currently, there are more questions than answers regarding the underlying mechanisms by which the virus and vRNA persistence may lead to the symptomology of LC, as well as how to reverse this outcome.
- Future research should aim to develop reliable animal models that more accurately replicate virus reservoirs and the symptoms of LC in humans. As with most diseases, no single animal model can fully replicate LC as it occurs in humans; however, studies conducted on different species may yield biomarkers and help develop drugs and immunotherapies for LC.
- The integration of multi-omics approaches, including genomics, proteomics, and metabolomics, can provide a more comprehensive understanding of symptomologies of LC. Enhanced efforts to model chronic symptoms, combined with the implementation of artificial intelligence, deep learning, organoids, and organ-on-chip models, will further advance the field, enabling more precise and effective therapeutic strategies for LC.
- While growing evidence suggests that persistent virus and viral vRNA detected in patients with LC may produce consistent antigenic stimulation [23,35,38,39,40,41,42], it remains to be determined whether persistent virus and vRNA reservoirs consistently express residual viral antigens in multiple organs and circulation (e.g., Spike protein and Nucleoprotein), and whether this is directly responsible for the chronic inflammation, as well as T cell dysfunction/exhaustion associated with LC symptoms. This will require large LC patient and control groups, as well as reliable animal models of persistent virus and vRNA reservoirs associated with LC-like symptoms, as seen in humans [120].
- The mechanism by which residual Spike protein, S1 subunit, and other SARS-CoV-2 antigens may persist in the plasma and other organs of some patients remains to be explored. While persistent Spike protein has been detected in some patients with LC, the finding should be regarded for now as an association, rather than a cause-and-effect relationship [117]. Whether Spike or any residual SARS-CoV-2 antigen contributes to chronic inflammation and T cell exhaustion that led to LC symptoms requires investigation in large LC patient and control groups, as well as in reliable animal models of LC using multiple pathophysiological and neuro-immunological approaches [120].
- There remains an urgent need to develop drugs or immunotherapeutic strategies that clear persistent virus and vRNA reservoirs. This will likely contribute to curbing the symptoms that target twelve major organ systems, causing dyspnea, vascular damage, cognitive impairments (“brain fog”), physical and mental fatigue, anxiety, and depression in at least a subset of patients with LC. This significant gap in our knowledge will likely require the development of a tissue-targeted immunotherapeutic strategy that increases the frequency and function of antiviral CD4+ and CD8+ TRM cells within affected tissues, thereby clearing persistent virus reservoirs and alleviating symptoms of LC.
- We are currently investigating the mechanisms by which SARS-CoV-2 causes immune dysfunction and contributes to the progression of LC disease. Information gained from these studies will be crucial to the development of novel immune therapies for treating LC. In a ‘humanized” mouse model of LC, we are examining the PD-1, TIM-3, PSGL-1, and/or LAG-3 blockade approach as a potential target for purging the virus reservoirs (Figure 5, Figure 6 and Figure 7). One goal is to utilize this knowledge to design strategies for enhancing the efficacy of immune therapy in patients with LC.
- Our ultimate and long-term goal is to identify protective T cell antigens and epitopes that are preferentially recognized by CD4+ and CD8+ T cells from patients who have resolved acute COVID-19 and never developed LC (recovered asymptomatic patients). These protective T cell antigens and epitopes will then be used to design a T cell immunotherapeutic strategy, such as the recently described Prime/Pull/Keep immunotherapy recently developed for other viral pathogens [289,290], to boost strong and long-lasting tissue-resident SARS-CoV-2-specific CD4+ and CD8+ TRM cells, that will then clear or reduce the persistent virus and vRNA reservoirs, and reverse chronic inflammatory and severe symptoms of LC.
- To treat LC patients with T cell immunotherapy, one would first need to select the subset of LC patients who exhibit persistent virus and vRNA reservoirs detected, either directly using ultrasensitive assays to trace virus, or vRNA, or residual viral proteins from, blood, stool, and gut/rectum biopsies or indirectly through virus-specific B and T cell responses, in patients with LC [105,119,125,291,292,293,294,295]. SARS-CoV-2 protein fragments (such as Spike, nucleoprotein, and other viral proteins) are found in the blood of many patients with LC using highly sensitive tests like Simoa (Single Molecule Array) [117,292,293]. Virus vRNA and proteins can also be detected in biopsies of the gut, rectum, tonsils, and tongue [105,111,119,125,291,292,294,295]. Biomarker-guided trials have emerged as a cornerstone of future research efforts and may be a promising approach for personalized medicine in LC [218]. In the future, a combination of biomarkers—blood-borne viral proteins and persistent viral vRNA in stool—is being investigated as a potential diagnostic test to identify LC patients with viral reservoirs [117,296,297]. However, many of these methods are still under clinical development, and no single test has been universally confirmed. Nevertheless, early results are promising for differentiating patients with LC who have underlying viral persistence from those with other causes.
- Treating LC presents a unique set of challenges, including the heterogeneity of symptoms and lack of specific biomarkers and diagnostic tests [29,218]. This variability not only complicates patient selection but also makes it difficult to establish uniform treatment protocols [218]. This heterogeneity may necessitate a more nuanced approach to trial design, incorporating stratified analyses and subgroup-specific interventions to address the diverse patients with LC.
- Since LC is present in various pathophysiology and clinical presentations, patients with LC may respond differently to treatment. While a large subset of patients with LC appear to express persistent reservoirs of virus, vRNA, and/or residual viral proteins, the general utility of T cell-based immunotherapy relies on the proportion of LC patients for whom these reservoirs are the etiology of the disease. However, a T cell immunotherapy that targets T cell antigens selected as being preferentially recognized by the immune system in patients who recovered by clearing acute infections and never progressed to LC (i.e., recovered, or “asymptomatic” patients) may prevent progression to LC. Hence, this strategy may also be effective as a post-exposure prophylaxis treatment for preventing LC.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sala, M.A.; Koralnik, I.J. Five years later: No short answers for Long COVID. Geroscience 2025. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Committee on Examining the Working Definition for Long COVID; Goldowitz, I.; Worku, T.; Brown, L.; Fineberg, H.V. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; National Academics Press: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Gourishankar, A. Geographic disparities and emerging hotspot trends of long COVID in the United States. Am. J. Med. Sci. 2025, 369, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, S.S.; Sadr, A.V.; Shahjouei, S.; Vemuri, A.; Abedi, V.; Zand, R. Prevalence and Determinants of Long-Term Post-COVID Conditions in the United States: 2022 Behavioral Risk Factor Surveillance System. Am. J. Med. 2025, 138, 513–523.e10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. A nationwide study of risk factors for long COVID and its economic and mental health consequences in the United States. Commun. Med. 2025, 5, 104. [Google Scholar] [CrossRef]
- Liu-Galvin, R.; Orlando, F.A.; Khan, T.; Wozniak, G.D.; Mainous, A.G., 3rd. Long COVID and Days of Work Missed Due to Illness or Injury by Adults in the United States, 2022. J. Am. Board Fam. Med. 2025, 38, 551–555. [Google Scholar] [CrossRef]
- Hejazian, S.S.; Sadr, A.V.; Shahjouei, S.; Vemuri, A.; Shouhao, Z.; Abedi, V.; Zand, R. Prevalence and determinant of long-term Post-COVID conditions among stroke survivors in the United States. J. Stroke Cerebrovasc. Dis. 2024, 33, 108007. [Google Scholar] [CrossRef]
- Hung, C.T.; Hung, Y.C.; Suk, C.W. Prevalence and characteristics in long COVID among adults with asthma in the United States. J. Asthma 2024, 61, 736–744. [Google Scholar] [CrossRef]
- Ford, N.D.; Slaughter, D.; Edwards, D.; Dalton, A.; Perrine, C.; Vahratian, A.; Saydah, S. Long COVID and Significant Activity Limitation Among Adults, by Age—United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 866–870. [Google Scholar] [CrossRef]
- Vahratian, A.; Adjaye-Gbewonyo, D.; Lin, J.S.; Saydah, S. Long COVID in Children: United States, 2022. NCHS Data Brief 2023, 479, 1–6. [Google Scholar]
- Adjaye-Gbewonyo, D.; Vahratian, A.; Perrine, C.G.; Bertolli, J. Long COVID in Adults: United States, 2022. NCHS Data Brief 2023, 480, 1–8. [Google Scholar]
- Zang, C.; Guth, D.; Bruno, A.M.; Xu, Z.; Li, H.; Ammar, N.; Chew, R.; Guthe, N.; Hadley, E.; Kaushal, R.; et al. Long COVID after SARS-CoV-2 during pregnancy in the United States. Nat. Commun. 2025, 16, 3005. [Google Scholar] [CrossRef]
- Blanchflower, D.G.; Bryson, A. Long COVID in the United States. PLoS ONE 2023, 18, e0292672. [Google Scholar] [CrossRef]
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 941009. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.D.; Bartsch, Y.C.; Davis, J.P.; Boribong, B.P.; Loiselle, M.; Kang, J.; Kane, A.S.; Edlow, A.G.; Fasano, A.; Alter, G.; et al. Long-term humoral signatures following acute pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children. Pediatr. Res. 2023, 94, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Johnson, J.N.; Spagnoli, J.; Amin, N.; McCoy, M.; Swaminathan, N.; Yohannan, T.; Philip, R. Long-Term Cardiovascular Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Using an Institution Based Algorithm. Pediatr. Cardiol. 2023, 44, 367–380. [Google Scholar] [CrossRef]
- Constantin, T.; Pek, T.; Horvath, Z.; Garan, D.; Szabo, A.J. Multisystem inflammatory syndrome in children (MIS-C): Implications for long COVID. Inflammopharmacology 2023, 31, 2221–2236. [Google Scholar] [CrossRef]
- Gupte, A.; Sriram, S.; Gunasekaran, V.; Chaudhari, K.; Kamat, D. The Triad of COVID-19 in Children: Acute COVID-19, Multisystem Inflammatory Syndrome, and Long COVID-Part I. Pediatr. Ann. 2024, 53, e473–e477. [Google Scholar] [CrossRef]
- Ptak, K.; Olszewska, M.; Szymonska, I.; Olchawa-Czech, A.; Mol, N.; Rudek-Budzynska, A.; Kukla, K.; Cisowska, M.; Sabat, O.; Grzyb, A.; et al. Should we be afraid of long-term cardiac consequences in children with multisystem inflammatory syndrome? Experience from subsequent waves of COVID-19. Eur. J. Pediatr. 2024, 183, 2683–2692. [Google Scholar] [CrossRef]
- Gupte, A.; Sriram, S.; Gunasekaran, V.; Chaudhari, K.; Kamat, D. The Triad of COVID-19 in Children: Acute COVID-19, Multisystem Inflammatory Syndrome, and Long COVID-Part II. Pediatr. Ann. 2025, 54, e40–e44. [Google Scholar] [CrossRef]
- Singla, R.; Sankar, J.; Tayal, A.; Bhadani, H.; Bagri, N.K.; Kabra, S.; Lodha, R. Long-Term Outcomes of Survivors of COVID-19 with Moderate to Severe Infection and Children with Multisystem Inflammatory Syndrome or MIS-C. Indian J. Pediatr. 2025, 92, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B.; Aleman, S.; Bach, K.; Boribong, B.P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. 2023, 24, 1616–1627. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Hou, Y.; Gu, T.; Ni, Z.; Shi, X.; Ranney, M.L.; Mukherjee, B. Global Prevalence of Long COVID, its Subtypes and Risk factors: An Updated Systematic Review and Meta-Analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Gross, R.S.; Thaweethai, T.; Salisbury, A.L.; Kleinman, L.C.; Mohandas, S.; Rhee, K.E.; Snowden, J.N.; Tantisira, K.G.; Warburton, D.; Wood, J.C.; et al. Characterizing Long COVID Symptoms During Early Childhood. JAMA Pediatr. 2025, 179, 781–792. [Google Scholar] [CrossRef]
- Gross, R.S.; Carmilani, M.; Stockwell, M.S. Long COVID in Young Children, School-Aged Children, and Teens. JAMA Pediatr. 2025, 179, 809. [Google Scholar] [CrossRef]
- Ford, N.D.; Vahratian, A.; Pratt, C.Q.; Yousaf, A.R.; Gregory, C.O.; Saydah, S. Long COVID Prevalence and Associated Activity Limitation in US Children. JAMA Pediatr. 2025, 179, 471–473. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Park, S.O.; Nanda, N. Long COVID: A Systematic Review of Preventive Strategies. Infect. Dis. Rep. 2025, 17, 56. [Google Scholar] [CrossRef]
- Proal, A.D.; Aleman, S.; Bomsel, M.; Brodin, P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; Deeks, S.G.; et al. Targeting the SARS-CoV-2 reservoir in long COVID. Lancet Infect. Dis. 2025, 25, e294–e306. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Piazza, M.; Boner, A.L.; Bellanti, J.A. Long COVID: A proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022, 43, 187–193. [Google Scholar] [CrossRef]
- Roe, K. A role for T-cell exhaustion in Long COVID-19 and severe outcomes for several categories of COVID-19 patients. J. Neurosci. Res. 2021, 99, 2367–2376. [Google Scholar] [CrossRef]
- Eaton-Fitch, N.; Rudd, P.; Er, T.; Hool, L.; Herrero, L.; Marshall-Gradisnik, S. Immune exhaustion in ME/CFS and long COVID. JCI Insight 2024, 9, e183810. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Jacka, B.; Ballouz, S.; Jackson, K.J.L.; Wilson, D.B.; Manandhar, B.; Klemm, V.; Tan, H.X.; Wheatley, A.; Aggarwal, A.; et al. Improvement of immune dysregulation in individuals with long COVID at 24-months following SARS-CoV-2 infection. Nat. Commun. 2024, 15, 3315. [Google Scholar] [CrossRef]
- Lupi, L.; Vitiello, A.; Parolin, C.; Calistri, A.; Garzino-Demo, A. The Potential Role of Viral Persistence in the Post-Acute Sequelae of SARS-CoV-2 Infection (PASC). Pathogens 2024, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Cavarelli, M. Ghosts of the virus: Unmasking the persistent threat of SARS-CoV-2 in Long COVID. Virologie 2025, 29, 57–68. [Google Scholar]
- Coulon, P.G.; Prakash, S.; Dhanushkodi, N.R.; Srivastava, R.; Zayou, L.; Tifrea, D.F.; Edwards, R.A.; Figueroa, C.J.; Schubl, S.D.; Hsieh, L.; et al. High frequencies of alpha common cold coronavirus/SARS-CoV-2 cross-reactive functional CD4(+) and CD8(+) memory T cells are associated with protection from symptomatic and fatal SARS-CoV-2 infections in unvaccinated COVID-19 patients. Front. Immunol. 2024, 15, 1343716. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Fajardo-Rosas, V.; Yu, E.D.; Galvez, R.I.; Abawi, A.; Escarrega, E.A.; Martinez-Perez, A.; Johansson, E.; Goodwin, B.; Frazier, A.; et al. Evolution of SARS-CoV-2 T cell responses as a function of multiple COVID-19 boosters. Cell Rep. 2025, 44, 115907. [Google Scholar] [CrossRef]
- Ahsan, F.; Rahmawati, N.Y.; Dachlan, E.G.; Alditia, F.N.; Santoso, B. Memory T cell reactivity to a broad range of conserved SARS-CoV-2-derived ORF1ab epitopes in first wave COVID-19 convalescents. Vaccine 2025, 62, 127571. [Google Scholar] [CrossRef] [PubMed]
- Asaba, C.N.; Bitazar, R.; Labonte, P.; Bukong, T.N. Bronchoalveolar lavage single-cell transcriptomics reveals immune dysregulations driving COVID-19 severity. PLoS ONE 2025, 20, e0309880. [Google Scholar] [CrossRef]
- Long, Q.; Song, S.; Xue, J.; Yu, W.; Zheng, Y.; Li, J.; Wu, J.; Hu, X.; Jiang, M.; Ye, H.; et al. The CD38(+)HLA-DR(+) T cells with activation and exhaustion characteristics as predictors of severity and mortality in COVID-19 patients. Front. Immunol. 2025, 16, 1577803. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- McMillan, P.; Turner, A.J.; Uhal, B.D. Mechanisms of Gut-Related Viral Persistence in Long COVID. Viruses 2024, 16, 1266. [Google Scholar] [CrossRef]
- Prakash, S.; Ulmer, B.J.; BenMohamed, L. Long COVID-19: A Comprehensive Review of Pathophysiology, Organ-Specific Manifestations, Animal Models, and Therapeutic Advances. bioRxiv 2025. [Google Scholar]
- Livieratos, A.; Gogos, C.; Akinosoglou, K. Beyond Antivirals: Alternative Therapies for Long COVID. Viruses 2024, 16, 1795. [Google Scholar] [CrossRef]
- Lindeboom, R.G.H.; Worlock, K.B.; Dratva, L.M.; Yoshida, M.; Scobie, D.; Wagstaffe, H.R.; Richardson, L.; Wilbrey-Clark, A.; Barnes, J.L.; Kretschmer, L.; et al. Human SARS-CoV-2 challenge uncovers local and systemic response dynamics. Nature 2024, 631, 189–198. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Zhong, W. Emerging small-molecule antiviral agents in long COVID prevention. Front. Pharmacol. 2024, 15, 1457672. [Google Scholar] [CrossRef]
- Kanwal, A.; Zhang, Z. Exploring common pathogenic association between Epstein Barr virus infection and long-COVID by integrating RNA-Seq and molecular dynamics simulations. Front. Immunol. 2024, 15, 1435170. [Google Scholar] [CrossRef]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, eabf8396. [Google Scholar] [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.O. Postacute Sequelae of COVID (PASC or Long COVID): An Evidenced-Based Approach. Open Forum Infect. Dis. 2024, 11, ofae462. [Google Scholar] [CrossRef]
- Sitbon, A.; Hauw-Berlemont, C.; Mebarki, M.; Heming, N.; Mayaux, J.; Diehl, J.L.; Demoule, A.; Annane, D.; Marois, C.; Demeret, S.; et al. Treatment of COVID-19-associated ARDS with umbilical cord-derived mesenchymal stromal cells in the STROMA-CoV-2 multicenter randomized double-blind trial: Long-term safety, respiratory function, and quality of life. Stem Cell Res. Ther. 2024, 15, 109. [Google Scholar] [CrossRef]
- Wagenlechner, C.; Wendt, R.; Reichardt, B.; Mildner, M.; Mascherbauer, J.; Aigner, C.; Auer, J.; Ankersmit, H.J.; Graf, A.C. Short and long-term outcomes of children and adolescents hospitalized with COVID-19 or influenza: Results of the AUTCOV study. Sci. Rep. 2025, 15, 22692. [Google Scholar] [CrossRef] [PubMed]
- Gusmao, A.C.S.; Scalea, A.C.R.; Uehara, S. Symptoms of long COVID in children and adolescents: A scoping review. Rev. Esc. Enferm. USP 2025, 59, e20240435. [Google Scholar] [CrossRef]
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.L.; Idzikowski, E.; et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 2021, 54, 797–814 e6. [Google Scholar] [CrossRef]
- Strahm, C.; Kahlert, C.R.; Gusewell, S.; Vuichard-Gysin, D.; Stocker, R.; Kuster, S.P.; Kohler, P. Evolution of symptoms compatible with post-acute sequelae of SARS-CoV-2 (PASC) after Wild-type and/or Omicron BA.1 infection: A prospective healthcare worker cohort. J. Infect. 2024, 88, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Hoshijima, H.; Mihara, T.; Seki, H.; Hyuga, S.; Kuratani, N.; Shiga, T. Incidence of long-term post-acute sequelae of SARS-CoV-2 infection related to pain and other symptoms: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0250909. [Google Scholar] [CrossRef] [PubMed]
- Hope, A.A.; Evering, T.H. Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Infect. Dis. Clin. N. Am. 2022, 36, 379–395. [Google Scholar] [CrossRef]
- Patel, S.K.; Torous, J. Exploring the Neuropsychiatric Sequalae of Perceived COVID-19 Exposure in College Students: A Pilot Digital Phenotyping Study. Front. Psychiatry 2021, 12, 788926. [Google Scholar] [CrossRef]
- Sacks-Zimmerman, A.; Bergquist, T.F.; Farr, E.M.; Cornwell, M.A.; Kanellopoulos, D. Rehabilitation of Neuropsychiatric Symptoms in Patients with Long-COVID: Position Statement. Arch. Phys. Med. Rehabil. 2022, 104, 350–354. [Google Scholar] [CrossRef]
- Hugon, J.; Msika, E.F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2022, 269, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Michelutti, M.; Furlanis, G.; Buoite Stella, A.; Bellavita, G.; Frezza, N.; Torresin, G.; Ajcevic, M.; Manganotti, P. Sex-dependent characteristics of Neuro-Long-COVID: Data from a dedicated neurology ambulatory service. J. Neurol. Sci. 2022, 441, 120355. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, A.; Schaenman, J.; Jayavelu, N.D.; Milliren, C.E.; Calfee, C.S.; Cairns, C.B.; Kraft, M.; Baden, L.R.; Shaw, A.C.; Krammer, F.; et al. Phenotypes of disease severity in a cohort of hospitalized COVID-19 patients: Results from the IMPACC study. EBioMedicine 2022, 83, 104208. [Google Scholar] [CrossRef]
- Bungenberg, J.; Humkamp, K.; Hohenfeld, C.; Rust, M.I.; Ermis, U.; Dreher, M.; Hartmann, N.K.; Marx, G.; Binkofski, F.; Finke, C.; et al. Long COVID-19: Objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022, 9, 141–154. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Milan, A.; Salles, P.; Pelayo, C.; Uribe-San-Martin, R. Acute to Chronic Electro-Clinical Manifestations of Neuro-COVID and the Long-Haul Consequences in People With Epilepsy: A Review. Cureus 2022, 14, e26020. [Google Scholar] [CrossRef]
- Beghi, E.; Helbok, R.; Ozturk, S.; Karadas, O.; Lisnic, V.; Grosu, O.; Kovacs, T.; Dobronyi, L.; Bereczki, D.; Cotelli, M.S.; et al. Short- and long-term outcome and predictors in an international cohort of patients with neuro-COVID-19. Eur. J. Neurol. 2022, 29, 1663–1684. [Google Scholar] [CrossRef]
- Pinzon, R.T.; Wijaya, V.O.; Jody, A.A.; Nunsio, P.N.; Buana, R.B. Persistent neurological manifestations in long COVID-19 syndrome: A systematic review and meta-analysis. J. Infect. Public Health 2022, 15, 856–869. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Rako, Z.A.; Ziegler, S.; Richter, M.J.; GS, A.T.; Roller, F.; Grimminger, F.; Vadasz, I.; Seeger, W.; Herold, S.; et al. Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir. Res. 2022, 23, 263. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Benito Ballesteros, A.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Cheke, L.G. COVCOG 1: Factors Predicting Physical, Neurological and Cognitive Symptoms in Long COVID in a Community Sample. A First Publication From the COVID and Cognition Study. Front. Aging Neurosci. 2022, 14, 804922. [Google Scholar] [CrossRef]
- Stincarelli, M.A.; Abbate, I.; Matusali, G.; Tanturli, M.; Camici, M.; Arvia, R.; Lazzari, E.; Cimini, E.; Vergori, A.; Maggi, F.; et al. Reduced Presence of SARS-CoV-2 microRNA-like Small RNA in the Serum of Patients with Post-Acute Sequelae SARS-CoV-2 Infection. Microorganisms 2025, 13, 126. [Google Scholar] [CrossRef]
- Niemczak, C.E.; Ford, J.C.; Roth, R.M.; Leigh, S.M.; Parsonnet, J.; Martin, C.; Soule, S.O.; Haron, T.M.; Buckey, J.C., Jr.; Wylie, G.R. Neuroimaging markers of cognitive fatigue in individuals with post-acute sequelae of SARS-CoV-2 infection. Brain Cogn. 2025, 183, 106254. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Beydoun, H.A.; Von Ah, D.; Shadyab, A.H.; Wong, S.C.; Freiberg, M.; Ikramuddin, F.; Nguyen, P.K.; Gradidge, P.J.; Qi, L.; et al. Pre-pandemic leukocyte count is associated with severity of post-acute sequelae of SARS-CoV-2 infection among older women in the Women’s Health Initiative. Menopause 2025, 32, 197–206. [Google Scholar] [CrossRef]
- Babalola, T.K.; Clouston, S.A.P.; Sekendiz, Z.; Chowdhury, D.; Soriolo, N.; Kawuki, J.; Meliker, J.; Carr, M.; Valenti, B.R.; Fontana, A.; et al. SARS-COV-2 re-infection and incidence of post-acute sequelae of COVID-19 (PASC) among essential workers in New York: A retrospective cohort study. Lancet Reg. Health Am. 2025, 42, 100984. [Google Scholar] [CrossRef]
- Maart, S.; Hofmeyr, R.A.; Muller, J.J.; Tserere, L.B. A cross-sectional study on the long-term impact of COVID-19: Symptoms, disability and daily functioning. Health SA 2025, 30, 2880. [Google Scholar] [CrossRef]
- do Amaral, C.; da Luz Goulart, C.; da Silva, B.M.; Valente, J.; Rezende, A.G.; Fernandes, E.; Cubas-Vega, N.; Borba, M.G.S.; Sampaio, V.; Monteiro, W.; et al. Low handgrip strength is associated with worse functional outcomes in long COVID. Sci. Rep. 2024, 14, 2049. [Google Scholar] [CrossRef]
- Gheorghita, R.; Soldanescu, I.; Lobiuc, A.; Caliman Sturdza, O.A.; Filip, R.; Constantinescu-Bercu, A.; Dimian, M.; Mangul, S.; Covasa, M. The knowns and unknowns of long COVID-19: From mechanisms to therapeutical approaches. Front. Immunol. 2024, 15, 1344086. [Google Scholar] [CrossRef]
- Pires, L.; Marreiros, A.; Saraiva, C.; Reis, C.; Neves, D.; Guerreiro, C.; Tome, J.B.; Luz, M.I.; Pereira, M.I.; Barroso, A.S.; et al. Association of acute COVID-19 severity and long COVID fatigue and quality of life: Prospective cohort multicenter observational study. Medicine 2025, 104, e42891. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, V.; Anandh, U.; Bell, S.; Kronbichler, A.; Soler, M.J.; Bruchfeld, A. Long COVID and the kidney. Nat. Rev. Nephrol. 2025. [Google Scholar] [CrossRef]
- Reiss, A.B.; Greene, C.; Dayaramani, C.; Rauchman, S.H.; Stecker, M.M.; De Leon, J.; Pinkhasov, A. Long COVID, the Brain, Nerves, and Cognitive Function. Neurol. Int. 2023, 15, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K., 3rd; Levine, B.D.; Baptiste, D.; Bhave, N.; Desai, S.; Dineen, E.; Durstenfeld, M.; Edward, J.; Huang, M.; Jacobsen, R.; et al. Exercise Intolerance and Response to Training in Patients With Postacute Sequelae of SARS-CoV2 (Long COVID): A Scientific Statement From the American Heart Association. Circulation 2025, 152, e50–e62. [Google Scholar] [CrossRef] [PubMed]
- Khakshooy, A.; Chiappelli, F. Post-acute CoVid-19 syndrome (PACS) linked cardiovascular symptoms. Bioinformation 2024, 20, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Soril, L.J.J.; Damant, R.W.; Lam, G.Y.; Smith, M.P.; Weatherald, J.; Bourbeau, J.; Hernandez, P.; Stickland, M.K. The effectiveness of pulmonary rehabilitation for Post-COVID symptoms: A rapid review of the literature. Respir. Med. 2022, 195, 106782. [Google Scholar] [CrossRef]
- Sorets, T.R.; Finley, J.A.; LaFrance, W.C., Jr.; Patten, R.V.; Mordecai, K.; Jimenez, M.; Suchy, S.; Cahan, J.; Koralnik, I.J.; Cherney, L.R.; et al. Beyond mood screening: A pilot study of emotional, cognitive, and somatic concerns in patients with Long COVID. Front. Psychol. 2025, 16, 1517299. [Google Scholar] [CrossRef]
- Pietzner, M.; Denaxas, S.; Yasmeen, S.; Ulmer, M.A.; Nakanishi, T.; Arnold, M.; Kastenmuller, G.; Hemingway, H.; Langenberg, C. Complex patterns of multimorbidity associated with severe COVID-19 and long COVID. Commun. Med. 2024, 4, 94. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Takahashi, T.; Wood, J.; Lu, P.; Tabachnikova, A.; Gehlhausen, J.R.; Greene, K.; Bhattacharjee, B.; Monteiro, V.S.; Lucas, C.; et al. Sex differences in symptomatology and immune profiles of Long COVID. medRxiv 2024. [Google Scholar] [CrossRef]
- Schafer, A.; Leist, S.R.; Powers, J.M.; Baric, R.S. Animal models of Long Covid: A hit-and-run disease. Sci. Transl. Med. 2024, 16, eado2104. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Ganesh, R.; Yadav, S.; Hurt, R.T.; Mueller, M.R.; Aakre, C.A.; Gilman, E.A.; Grach, S.L.; Overgaard, J.; Snyder, M.R.; Collins, N.M.; et al. Pro Inflammatory Cytokines Profiles of Patients With Long COVID Differ Between Variant Epochs. J. Prim. Care Community Health 2024, 15, 21501319241254751. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Petsini, F.; Detopoulou, M.; Theoharides, T.C.; Demopoulos, C.A. Is there an interplay between the SARS-CoV-2 spike protein and Platelet-Activating factor? BioFactors 2022, 48, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D. Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID. Cells 2023, 12, 688. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Recombinant SARS-CoV-2 Spike Protein and Its Receptor Binding Domain Stimulate Release of Different Pro-Inflammatory Mediators via Activation of Distinct Receptors on Human Microglia Cells. Mol. Neurobiol. 2023, 60, 6704–6714. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Recombinant SARS-CoV-2 Spike Protein Stimulates Secretion of Chymase, Tryptase, and IL-1beta from Human Mast Cells, Augmented by IL-33. Int. J. Mol. Sci. 2023, 24, 9487. [Google Scholar] [CrossRef]
- Kempuraj, D.; Tsilioni, I.; Aenlle, K.K.; Klimas, N.G.; Theoharides, T.C. Long COVID elevated MMP-9 and release from microglia by SARS-CoV-2 Spike protein. Transl. Neurosci. 2024, 15, 20220352. [Google Scholar] [CrossRef]
- Mandel, H.; Yoo, Y.J.; Allen, A.J.; Abedian, S.; Verzani, Z.; Karlson, E.W.; Kleinman, L.C.; Mudumbi, P.C.; Oliveira, C.R.; Muszynski, J.A.; et al. Long COVID Incidence Proportion in Adults and Children Between 2020 and 2024: An Electronic Health Record-Based Study From the RECOVER Initiative. Clin. Infect. Dis. 2025, 80, 1247–1261. [Google Scholar] [CrossRef]
- Esposito, S.; Puntoni, M.; Deolmi, M.; Ramundo, G.; Maglietta, G.; Poeta, M.; Zampogna, S.; Colomba, C.; Suppiej, A.; Cardinale, F.; et al. Long COVID in pediatric age: An observational, prospective, longitudinal, multicenter study in Italy. Front. Immunol. 2025, 16, 1466201. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Mai, H.; Ebert, G.; Kapoor, S.; Puelles, V.G.; Czogalla, J.; Hu, S.; Su, J.; Prtvar, D.; Singh, I.; et al. Persistence of spike protein at the skull-meninges-brain axis may contribute to the neurological sequelae of COVID-19. Cell Host Microbe 2024, 32, 2112–2130.e10. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ryder, D.; Flavell, R.R.; Wang, Y.; Levi, J.; LaFranchi, B.H.; Deveau, T.M.; Buck, A.M.; Munter, S.E.; Asare, K.A.; et al. Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadk3295. [Google Scholar] [CrossRef]
- Rendeiro, A.F.; Ravichandran, H.; Kim, J.; Borczuk, A.C.; Elemento, O.; Schwartz, R.E. Persistent alveolar type 2 dysfunction and lung structural derangement in post-acute COVID-19. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Sariol, A.; Perlman, S. Lung inflammation drives Long Covid. Science 2025, 387, 1039–1040. [Google Scholar] [CrossRef]
- Goh, D.; Lim, J.C.T.; Fernaindez, S.B.; Joseph, C.R.; Edwards, S.G.; Neo, Z.W.; Lee, J.N.; Caballero, S.G.; Lau, M.C.; Yeong, J.P.S. Case report: Persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID. Front. Immunol. 2022, 13, 939989. [Google Scholar]
- Visvabharathy, L.; Orban, Z.S.; Koralnik, I.J. Case report: Treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence. Front. Med. 2022, 9, 1003103. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rossler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Wick, N.; Wick, G.; Rossler, A.; Kimpel, J.; Adolph, T.E.; et al. Clearance of Gut Mucosal SARS-CoV-2 Antigens and Postacute COVID-19 After 2 Years in Patients With Inflammatory Bowel Disease. Gastroenterology 2024, 167, 604–607.e8. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.; Martins, R.B.; Miura, C.S.; Souza, M.V.O.; Cassiano, M.H.A.; Rodrigues, T.S.; Veras, F.P.; Sousa, J.F.; Gomes, R.; Almeida, G.M.; et al. Tonsils are major sites of persistence of SARS-CoV-2 in children. Microbiol. Spectr. 2023, 11, e0134723. [Google Scholar] [CrossRef]
- Schwartz, J.; Capistrano, K.; Hussein, H.; Hafedi, A.; Shukla, D.; Naqvi, A. Oral SARS-CoV-2 Infection and Risk for Long Covid. Rev. Med. Virol. 2025, 35, e70029. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. The Oral Cavity Potentially Serving as a Reservoir for SARS-CoV-2 but Not Necessarily Facilitating the Spread of COVID-19 in Dental Practice. Eur. J. Dent. 2023, 17, 310–318. [Google Scholar] [CrossRef]
- Yao, Q.; Doyle, M.E.; Liu, Q.R.; Appleton, A.; O’Connell, J.F.; Weng, N.P.; Egan, J.M. Long-Term Dysfunction of Taste Papillae in SARS-CoV-2. NEJM Evid. 2023, 2, EVIDoa2300046. [Google Scholar] [CrossRef] [PubMed]
- Hallak, J.; Caldini, E.G.; Teixeira, T.A.; Correa, M.C.M.; Duarte-Neto, A.N.; Zambrano, F.; Taubert, A.; Hermosilla, C.; Drevet, J.R.; Dolhnikoff, M.; et al. Transmission electron microscopy reveals the presence of SARS-CoV-2 in human spermatozoa associated with an ETosis-like response. Andrology 2024, 12, 1799–1807. [Google Scholar]
- Dai, P.; Qiao, F.; Chen, Y.; Chan, D.Y.L.; Yim, H.C.H.; Fok, K.L.; Chen, H. SARS-CoV-2 and male infertility: From short- to long-term impacts. J. Endocrinol. Investig. 2023, 46, 1491–1507. [Google Scholar] [CrossRef]
- Lu, S.; Peluso, M.J.; Glidden, D.V.; Davidson, M.C.; Lugtu, K.; Pineda-Ramirez, J.; Tassetto, M.; Garcia-Knight, M.; Zhang, A.; Goldberg, S.A.; et al. Early biological markers of post-acute sequelae of SARS-CoV-2 infection. Nat. Commun. 2024, 15, 7466. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef]
- Peluso, M.J.; Swank, Z.N.; Goldberg, S.A.; Lu, S.; Dalhuisen, T.; Borberg, E.; Senussi, Y.; Luna, M.A.; Chang Song, C.; Clark, A.; et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect. Dis. 2024, 24, e345–e347. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Media Statement from CDC Director Rochelle P. Walensky, MD, MPH, on Signing the Advisory Committee on Immunization Practices’ Recommendation to Use Janssen’s COVID-19 Vaccine in People 18 and Older [Press Release]. 2021. Available online: https://archive.cdc.gov/www_cdc_gov/media/releases/2021/s0228-JJ-vaccine.html (accessed on 23 September 2025).
- Cruz, T.; Albacar, N.; Ruiz, E.; Lledo, G.M.; Perea, L.; Puebla, A.; Torvisco, A.; Mendoza, N.; Marrades, P.; Sellares, J.; et al. Persistence of dysfunctional immune response 12 months after SARS-CoV-2 infection and their relationship with pulmonary sequelae and long COVID. Respir. Res. 2025, 26, 152. [Google Scholar] [CrossRef]
- Heydemann, L.; Ciurkiewicz, M.; Stork, T.; Zdora, I.; Hulskotter, K.; Gregor, K.M.; Michaely, L.M.; Reineking, W.; Schreiner, T.; Beythien, G.; et al. Respiratory long COVID in aged hamsters features impaired lung function post-exercise with bronchiolization and fibrosis. Nat. Commun. 2025, 16, 2080. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229. [Google Scholar] [CrossRef]
- Roden, A.C.; Boland, J.M.; Johnson, T.F.; Aubry, M.C.; Lo, Y.C.; Butt, Y.M.; Maleszewski, J.J.; Larsen, B.T.; Tazelaar, H.D.; Khoor, A.; et al. Late Complications of COVID-19. Arch. Pathol. Lab. Med. 2022, 146, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Yonker, L.M.; Kane, A.S.; Swank, Z.; Papadakis, L.; Kenyon, V.; Han, S.; Lima, R.; Guthrie, L.B.; Alvarez-Carcamo, B.; Lahoud-Rahme, M.; et al. Viral spike antigen clearance and augmented recovery in children with post-COVID multisystem inflammatory syndrome treated with larazotide. Sci. Transl. Med. 2025, 17, eadu4284. [Google Scholar] [CrossRef]
- Xu, Q.; Milanez-Almeida, P.; Martins, A.J.; Radtke, A.J.; Hoehn, K.B.; Oguz, C.; Chen, J.; Liu, C.; Tang, J.; Grubbs, G.; et al. Adaptive immune responses to SARS-CoV-2 persist in the pharyngeal lymphoid tissue of children. Nat. Immunol. 2023, 24, 186–199. [Google Scholar] [CrossRef]
- Tan, H.X.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; Dixon, B.J.; Lau, J.S.Y.; Flanagan, K.L.; van de Sandt, C.E.; Kedzierska, K.; McMahon, J.H.; et al. Cutting Edge: SARS-CoV-2 Infection Induces Robust Germinal Center Activity in the Human Tonsil. J. Immunol. 2022, 208, 2267–2271. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; He, L.; Xu, X.; Piao, M.; Wang, B.; Liu, T.; Cao, H. Gut microbiota in post-acute COVID-19 syndrome: Not the end of the story. Front. Microbiol. 2024, 15, 1500890. [Google Scholar] [CrossRef]
- Dziadzko, M.; Belhassen, M.; Van Ganse, E.; Marant-Micallef, C.; Martinez, V.; Aubrun, F. Are Healthcare Resource Utilization Patterns for Pain Management Specific to Post-Acute COVID-19 Syndrome? A Study of Survivors from the First French Pandemic Wave. J. Clin. Med. 2024, 13, 7680. [Google Scholar] [CrossRef]
- Malioukis, A.; Snead, R.S.; Marczika, J.; Ambalavanan, R. Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence. Biomedicines 2024, 12, 2831. [Google Scholar] [CrossRef]
- Mundra, P.; Kailani, Z.; Yaghoobi, M.; Matthews, P.; Tobis, M.; Sadeghian, S.; Albashir, S. Liver injury in post-acute COVID-19 syndrome: A systematic review and meta-analysis of early observational studies. Can. Liver J. 2024, 7, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, A.; Crisan-Vida, M.; Tudoran, C.; Stoicu-Tivadar, L. ML-Based Framework to Predict the Severity of the Symptomatology in Patients with Post-Acute COVID-19 Syndrome. Stud. Health Technol. Inform. 2024, 321, 99–103. [Google Scholar] [PubMed]
- Ovechkin, A.; Moshonkina, T.; Shamantseva, N.; Lyakhovetskii, V.; Suthar, A.; Tharu, N.; Ng, A.; Gerasimenko, Y. Spinal Neuromodulation for Respiratory Rehabilitation in Patients with Post-Acute COVID-19 Syndrome. Life 2024, 14, 1518. [Google Scholar] [CrossRef] [PubMed]
- Platschek, B.; Boege, F. The Post-Acute COVID-19-Vaccination Syndrome in the Light of Pharmacovigilance. Vaccines 2024, 12, 1378. [Google Scholar] [CrossRef]
- Salvador-Ruiz, A.J.; Moral-Munoz, J.A.; Salazar, A.; Lucena-Anton, D.; De Sola, H.; Failde, I.; Duenas, M. Enhancing exercise intervention for patients with post-acute COVID-19 syndrome using mobile health technology: The COVIDReApp randomised controlled trial protocol. Digit. Health 2024, 10, 20552076241247936. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, N.K.; Yadav, R.; Paul, S.; Gupta, S.; Sankalp; Dixit, P. Acute gastrointestinal and post-acute COVID-19 gastrointestinal syndrome assessment on the Gastrointestinal Symptom Rating Scale scoring system: A questionnaire-based survey. J. Family Med. Prim. Care 2024, 13, 5787–5798. [Google Scholar] [CrossRef]
- Brandao, M.L.; Hermsdorff, H.H.M.; Leal, A.C.G.; Bressan, J.; Pimenta, A.M. Vaccination and food consumption: Association with Post-Acute COVID-19 Syndrome in Brazilian adults (CUME Study). Front. Nutr. 2025, 12, 1549747. [Google Scholar] [CrossRef]
- Dai, J.; He, F.; Chen, Q.; Li, Q.; Zhao, L.; Du, Y. Animal models of post-acute COVID-19 syndrome: A call for longitudinal animal studies. Front. Immunol. 2025, 16, 1521029. [Google Scholar] [CrossRef]
- Fallah, A.; Sedighian, H.; Kachuei, R.; Fooladi, A.A.I. Human microbiome in post-acute COVID-19 syndrome (PACS). Curr. Res. Microb. Sci. 2025, 8, 100324. [Google Scholar] [CrossRef]
- Hazumi, M.; Kataoka, M.; Narita, Z.; Usuda, K.; Okazaki, E.; Nishi, D. Psychological distress after COVID-19 recovery and subsequent prolonged post-acute COVID-19 syndrome: A longitudinal study with one-year follow-up in Japan. J. Psychosom. Res. 2025, 196, 112323. [Google Scholar] [CrossRef]
- Huang, L.W.; Li, H.M.; He, B.; Wang, X.B.; Zhang, Q.Z.; Peng, W.X. Prevalence of cardiovascular symptoms in post-acute COVID-19 syndrome: A meta-analysis. BMC Med. 2025, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Son, Y.; Park, J.; Kim, S.; Jo, H.; Lee, H.; Yon, D.K. Post-Acute Sequelae of COVID-19 on Irritable Bowel Syndrome in Individuals With Mental Illness in South Korea: A Population-Based Cohort Study. J. Med. Virol. 2025, 97, e70345. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.H.J.; Gu, J.T.; Kung, J.T.Y.; Liang, S.S.; Gonzalez, P.C.; Toh, F.M.; Sin, E.; Fong, K.N.K. User experiences of patients with post-acute COVID-19 syndrome receiving occupational therapy telerehabilitation. Front. Hum. Neurosci. 2025, 19, 1551631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Ding, W.; Liu, Y.; Li, W.; Guan, S.; Liu, X.; Wang, G.; Liu, Q.; Jiang, C.; et al. Food and medicine homology: A potential nutritional intervention strategy for post-acute COVID-19 syndrome. Front. Pharmacol. 2025, 16, 1588037. [Google Scholar] [CrossRef]
- Patel, V.; Korsun, M.; Cervia, J. Protective effects of booster dose of SARS-COV-2 vaccination against post-acute COVID-19 syndrome: A systematic review. J. Investig. Med. 2025. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Gopel, S.; Merle, U.; Steinacker, J.M.; Deibert, P.; Friedmann-Bette, B.; Niess, A.; Muller, B.; Schilling, C.; et al. Persistent symptoms and clinical findings in adults with post-acute sequelae of COVID-19/post-COVID-19 syndrome in the second year after acute infection: A population-based, nested case-control study. PLoS Med. 2025, 22, e1004511. [Google Scholar] [CrossRef]
- Rajai Firouzabadi, S.; Mohammadi, I.; Alinejadfard, M.; Shafiee, A. E-cigarettes are not associated with post-acute COVID-19 syndrome among US adults. Sci. Rep. 2025, 15, 2870. [Google Scholar] [CrossRef]
- Sandoval, A.; Li, M.; Jason, L.A. Two neurocognitive domains identified for patients with myalgic encephalomyelitis/chronic fatigue syndrome and post-acute sequelae of COVID-19. Front. Neurol. 2025, 16, 1612548. [Google Scholar] [CrossRef]
- Saunders, D.; Arnold, T.B.; Lavender, J.M.; Bi, D.; Alcover, K.; Hellwig, L.D.; Leazer, S.T.; Mohammed, R.; Markos, B.; Perera, K.; et al. Comparative cohort study of post-acute COVID-19 infection with a nested, randomized controlled trial of ivabradine for those with postural orthostatic tachycardia syndrome (the COVIVA study). Front. Neurol. 2025, 16, 1550636. [Google Scholar] [CrossRef]
- Sugihara, J.; Iwamura, C.; Tateishi, T.; Hosoya, T.; Shimada, S.; Hirahara, K.; Yasuda, S.; Miyazaki, Y. Prolonged high Myl9 levels are associated with the pathogenesis and respiratory symptom of post-acute COVID-19 syndrome: A 6-month follow-up study. Clinics 2025, 80, 100584. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Chaudhari, D.; Ryan, E.P.; Rossi, N.F.; Koka, O.; Baxter, B.; Tipton, M.; Dutt, T.S.; Tobi, Y.; McVicker, B.; et al. Immune Signatures in Post-Acute Sequelae of COVID-19 (PASC) and Myalgia/Chronic Fatigue Syndrome (ME/CFS): Insights from the Fecal Microbiome and Serum Cytokine Profiles. Biomolecules 2025, 15, 928. [Google Scholar] [CrossRef]
- Wu, J.S.; Xu, C.Y.; Mo, S.M.; Wu, X.M.; Du, Z.B.; Che, L.; Zhang, Y.L.; Yang, K.L.; Li, T.D.; Ge, S.X.; et al. Palmitoylated COX-2(Cys555) reprogrammed mitochondrial metabolism in pyroptotic inflammatory injury in patients with post-acute COVID-19 syndrome. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Zhang, Y.; Bharathi, V.; Dokoshi, T.; de Anda, J.; Ursery, L.T.; Kulkarni, N.N.; Nakamura, Y.; Chen, J.; Luo, E.W.C.; Wang, L.; et al. Viral afterlife: SARS-CoV-2 as a reservoir of immunomimetic peptides that reassemble into proinflammatory supramolecular complexes. Proc. Natl. Acad. Sci. USA 2024, 121, e2300644120. [Google Scholar] [CrossRef]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28568. [Google Scholar] [CrossRef]
- Patterson, B.K.; Yogendra, R.; Francisco, E.B.; Guevara-Coto, J.; Long, E.; Pise, A.; Osgood, E.; Bream, J.; Kreimer, M.; Jeffers, D.; et al. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Hum. Vaccines Immunother. 2025, 21, 2494934. [Google Scholar] [CrossRef]
- de Melo, B.P.; da Silva, J.A.M.; Rodrigues, M.A.; Palmeira, J.D.F.; Amato, A.A.; Arganaraz, G.A.; Arganaraz, E.R. SARS-CoV-2 Spike Protein and Long COVID-Part 2: Understanding the Impact of Spike Protein and Cellular Receptor Interactions on the Pathophysiology of Long COVID Syndrome. Viruses 2025, 17, 619. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.P.; da Silva, J.A.M.; Rodrigues, M.A.; Palmeira, J.D.F.; Saldanha-Araujo, F.; Arganaraz, G.A.; Arganaraz, E.R. SARS-CoV-2 Spike Protein and Long COVID-Part 1: Impact of Spike Protein in Pathophysiological Mechanisms of Long COVID Syndrome. Viruses 2025, 17, 617. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2021, 12, 746021. [Google Scholar] [CrossRef] [PubMed]
- Chansaenroj, J.; Yorsaeng, R.; Puenpa, J.; Wanlapakorn, N.; Chirathaworn, C.; Sudhinaraset, N.; Sripramote, M.; Chalongviriyalert, P.; Jirajariyavej, S.; Kiatpanabhikul, P.; et al. Long-term persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein-specific and neutralizing antibodies in recovered COVID-19 patients. PLoS ONE 2022, 17, e0267102. [Google Scholar] [CrossRef]
- Talotta, R. Impaired VEGF-A-Mediated Neurovascular Crosstalk Induced by SARS-CoV-2 Spike Protein: A Potential Hypothesis Explaining Long COVID-19 Symptoms and COVID-19 Vaccine Side Effects? Microorganisms 2022, 10, 2452. [Google Scholar] [CrossRef]
- Tuan, J.J.; Zapata, H.; Barakat, L.; Andrews, L.; Behnegar, A.; Kim, Y.W.; Kayani, J.; Mutic, S.; Ryall, L.; Turcotte, B.; et al. Long-term quantitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV (PWH). BMC Infect. Dis. 2022, 22, 744. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araujo, S.M.B.; Froz, G.M.; et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023, 42, 112189. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cui, H.; Liang, H.; Wang, X.; Yu, H.; Wang, J.; Wang, W.; Liu, D.; Zhang, Y.; Dong, E.; et al. SARS-CoV-2 spike protein acts as a beta-adrenergic receptor agonist: A potential mechanism for cardiac sequelae of long COVID. J. Intern. Med. 2024, 296, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kiatratdasakul, S.; Noisumdaeng, P.; Niyomdecha, N. Biological factors associated with long COVID and comparative analysis of SARS-CoV-2 spike protein variants: A retrospective study in Thailand. PeerJ 2024, 12, e17898. [Google Scholar] [CrossRef]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteom. Clin. Appl. 2023, 17, e2300048. [Google Scholar] [CrossRef]
- Schultheiss, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; Girndt, M.; et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28364. [Google Scholar] [CrossRef]
- Peluso, M.J.; Kelly, J.D.; Lu, S.; Goldberg, S.A.; Davidson, M.C.; Mathur, S.; Durstenfeld, M.S.; Spinelli, M.A.; Hoh, R.; Tai, V.; et al. Persistence, Magnitude, and Patterns of Postacute Symptoms and Quality of Life Following Onset of SARS-CoV-2 Infection: Cohort Description and Approaches for Measurement. Open Forum Infect. Dis. 2022, 9, ofab640. [Google Scholar] [CrossRef]
- Naito, T. A second-generation, self-amplifying COVID-19 Vaccine: World’s first approval and distribution in the Japanese market with vaccine hesitancy. Hum. Vaccines Immunother. 2025, 21, 2530291. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Nazari, A.; Ahmadi, Z.; Kouni, S.N.; de Gregorio, C.; Koniari, I.; Dousdampanis, P.; Mplani, V.; Plotas, P.; Assimakopoulos, S.; et al. The Effect of Long COVID-19 Infection and Vaccination on Male Fertility; A Narrative Review. Vaccines 2022, 10, 1982. [Google Scholar] [CrossRef]
- Tofarides, A.G.; Christaki, E.; Milionis, H.; Nikolopoulos, G.K. Effect of Vaccination against SARS-CoV-2 on Long COVID-19: A Narrative Review. Life 2022, 12, 2057. [Google Scholar] [CrossRef]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Kulzhabayeva, D.; Rodrigues, N.B.; Di Vincenzo, J.D.; Gill, H.; Subramaniapillai, M.; Lui, L.M.W.; Cao, B.; Mansur, R.B.; Ho, R.C.; et al. COVID-19 vaccination for the prevention and treatment of long COVID: A systematic review and meta-analysis. Brain Behav. Immun. 2023, 111, 211–229. [Google Scholar] [CrossRef]
- Ackerson, B.K.; Bruxvoort, K.J.; Qian, L.; Sy, L.S.; Qiu, S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; et al. Effectiveness and durability of mRNA-1273 BA.4/BA.5 bivalent vaccine (mRNA-1273.222) against SARS-CoV-2 BA.4/BA.5 and XBB sublineages. Hum. Vaccines Immunother. 2024, 20, 2335052. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Matsuda, K.; Maeda, K.; Horii, K.; Okudera, K.; Oshiro, Y.; Inamura, N.; Nemoto, T.; Takeuchi, J.S.; Li, Y.; et al. Preinfection Neutralizing Antibodies, Omicron BA.5 Breakthrough Infection, and Long COVID: A Propensity Score-Matched Analysis. J. Infect. Dis. 2023, 228, 1652–1661. [Google Scholar] [CrossRef]
- Vacharathit, V.; Pluempreecha, M.; Manopwisedjaroen, S.; Srisaowakarn, C.; Srichatrapimuk, S.; Sritipsukho, P.; Sritipsukho, N.; Thitithanyanont, A. Persistent IP-10/CXCL10 dysregulation following mild Omicron breakthrough infection: Immune network signatures across COVID-19 waves and implications for mRNA vaccine outcomes. Clin. Immunol. 2025, 278, 110507. [Google Scholar] [CrossRef]
- Matula, Z.; Beko, G.; Kiraly, V.; Gonczi, M.; Zoka, A.; Barath, A.; Uher, F.; Valyi-Nagy, I. Long-Term SARS-CoV-2-Specific Humoral and T Cell Responses after the BNT162b2 or BBIBP-CorV Booster and the Incidence of Breakthrough Infections among Healthcare Workers. Vaccines 2023, 12, 3. [Google Scholar] [CrossRef]
- Lee, K.Y.; Song, K.H.; Lee, K.H.; Baek, J.Y.; Kim, E.S.; Song, Y.G.; Kim, Y.C.; Park, Y.S.; Ahn, J.Y.; Choi, J.Y.; et al. Persistent differences in the immunogenicity of the two COVID-19 primary vaccines series, modulated by booster mRNA vaccination and breakthrough infection. Vaccine 2024, 42, 3953–3960. [Google Scholar] [CrossRef]
- Drury, R.E.; Camara, S.; Chelysheva, I.; Bibi, S.; Sanders, K.; Felle, S.; Emary, K.; Phillips, D.; Voysey, M.; Ferreira, D.M.; et al. Multi-omics analysis reveals COVID-19 vaccine induced attenuation of inflammatory responses during breakthrough disease. Nat. Commun. 2024, 15, 3402. [Google Scholar] [CrossRef]
- Bellizzi, V.; Fordellone, M.; Secondulfo, C.; Chiodini, P.; Bilancio, G. Long-Term Immuno-Response and Risk of Breakthrough Infection After SARS-CoV-2 Vaccination in Kidney Transplantation. Vaccines 2025, 13, 566. [Google Scholar] [CrossRef]
- Almanzar, G.; Koosha, K.; Vogt, T.; Stein, A.; Ziegler, L.; Asam, C.; Weps, M.; Schwagerl, V.; Richter, L.; Hepp, N.; et al. Hybrid immunity by two COVID-19 mRNA vaccinations and one breakthrough infection provides a robust and balanced cellular immune response as basic immunity against severe acute respiratory syndrome coronavirus 2. J. Med. Virol. 2024, 96, e29739. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.J.; Li, S.; Amarasena, T.H.; Reynaldi, A.; Lee, W.S.; Leeming, M.G.; O’Connor, D.H.; Nguyen, J.; Kent, H.E.; Caruso, F.; et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano 2024, 18, 27077–27089. [Google Scholar] [CrossRef]
- Mueed, A.; Shariq, A.; Ashar, M. Critical appraisal of: “expression of SARS-CoV-2 spike protein in cerebral arteries: Implications for hemorrhagic stroke post-mRNA vaccination”. J. Clin. Neurosci. 2025, 136, 111270. [Google Scholar] [CrossRef]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral Arteries: Implications for hemorrhagic stroke Post-mRNA vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2023, 12, 59. [Google Scholar] [CrossRef]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. NPJ Vaccines 2023, 8, 141. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedziela, J.; Poniedzialek, B.; Rosinska, J.; Zarebska-Michaluk, D.; Sobala-Szczygiel, B.; Flisiak, R.; Gasior, M.; Jaroszewicz, J. Humoral anti-SARS-CoV-2 response in patients with different long COVID phenotypes. Virology 2024, 596, 110118. [Google Scholar] [CrossRef]
- Gomes, S.M.R.; Brito, A.C.S.; Manfro, W.F.P.; Ribeiro-Alves, M.; Ribeiro, R.S.A.; da Cal, M.S.; Lisboa, V.D.C.; Abreu, D.P.B.; Castilho, L.D.R.; Porto, L.; et al. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients. PLoS ONE 2023, 18, e0283983. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Abellan, J.; Padilla, S.; Fernandez-Gonzalez, M.; Garcia, J.A.; Agullo, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutierrez, F.; Masia, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021, 41, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Porritt, R.A.; Rivas, M.N.; Krieger, J.M.; Ozdemir, A.B.; Garcia, G., Jr.; Arumugaswami, V.; Fries, B.C.; Arditi, M.; Bahar, I. A monoclonal antibody against staphylococcal enterotoxin B superantigen inhibits SARS-CoV-2 entry in vitro. Structure 2021, 29, 951–962.e3. [Google Scholar] [CrossRef] [PubMed]
- Porritt, R.A.; Paschold, L.; Rivas, M.N.; Cheng, M.H.; Yonker, L.M.; Chandnani, H.; Lopez, M.; Simnica, D.; Schultheiss, C.; Santiskulvong, C.; et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Investig. 2021, 131, e146614. [Google Scholar] [CrossRef]
- Dissook, S.; Umsumarng, S.; Mapoung, S.; Semmarath, W.; Arjsri, P.; Srisawad, K.; Dejkriengkraikul, P. Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID. Front. Med. 2022, 9, 1072056. [Google Scholar]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, M.; Bozzano, F.M.; Castellano, C.; Pesce, G.; Beronio, A.; Farshchi, A.H.; Limongelli, A.; Uccelli, A.; Benedetti, L.; De Maria, A. Post-SARS-CoV-2 infection and post-vaccine-related neurological complications share clinical features and the same positivity to anti-ACE2 antibodies. Front. Immunol. 2024, 15, 1398028. [Google Scholar] [CrossRef]
- Coleon, A.; Larrous, F.; Kergoat, L.; Tichit, M.; Hardy, D.; Obadia, T.; Kornobis, E.; Bourhy, H.; de Melo, G.D. Hamsters with long COVID present distinct transcriptomic profiles associated with neurodegenerative processes in brainstem. Nat. Commun. 2025, 16, 6714. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Pierce, J.; Franklin, C.; Olson, R.M.; Morrison, A.R.; Amos-Landgraf, J. Translating animal models of SARS-CoV-2 infection to vascular, neurological and gastrointestinal manifestations of COVID-19. Dis. Model. Mech. 2025, 18, dmm052086. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, J.C.; Beltrami, V.A.; Oliveira, B.D.S.; Queiroz-Junior, C.M.; Barsalini, J.; Teixeira, D.C.; de Souza-Costa, L.P.; Lima, A.L.D.; Machado, C.A.; Parreira, B.; et al. Neuropsychiatric sequelae in an experimental model of post-COVID syndrome in mice. Brain Behav. Immun. 2025, 128, 16–36. [Google Scholar] [CrossRef]
- Detrille, A.; Huvelle, S.; van Gils, M.J.; Geara, T.; Pascal, Q.; Snitselaar, J.; Bossevot, L.; Cavarelli, M.; Dereuddre-Bosquet, N.; Relouzat, F.; et al. Whole-body visualization of SARS-CoV-2 biodistribution in vivo by immunoPET imaging in non-human primates. Nat. Commun. 2025, 16, 2816. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, Y.; Luo, C.; Zhu, Y.; Zhou, X.; Wang, R.; He, J.; Guo, H.; Xu, X.; et al. Animal Models for Long COVID: Current Advances, Limitations, and Future Directions. J. Med. Virol. 2025, 97, e70237. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Diamond, M.S. Animal Models of Non-Respiratory, Post-Acute Sequelae of COVID-19. Viruses 2025, 17, 98. [Google Scholar] [CrossRef]
- Ivlev, I.; Wagner, J.; Phillips, T.; Treadwell, J.R. Interventions for Long COVID: A Narrative Review. J. Gen. Intern. Med. 2025, 40, 2005–2023. [Google Scholar] [CrossRef]
- Herbert, C.; Antar, A.A.R.; Broach, J.; Wright, C.; Stamegna, P.; Luzuriaga, K.; Hafer, N.; McManus, D.D.; Manabe, Y.C.; Soni, A. Relationship Between Acute SARS-CoV-2 Viral Clearance and Long COVID-19 (Long COVID) Symptoms: A Cohort Study. Clin. Infect. Dis. 2025, 80, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Leist, S.R.; Gralinski, L.E.; Martinez, D.R.; Winkler, E.S.; Okuda, K.; Hawkins, P.E.; Gully, K.L.; Graham, R.L.; Scobey, D.T.; et al. A Multitrait Locus Regulates Sarbecovirus Pathogenesis. mBio 2022, 13, e0145422. [Google Scholar] [CrossRef]
- Schafer, A.; Gralinski, L.E.; Leist, S.R.; Hampton, B.K.; Mooney, M.A.; Jensen, K.L.; Graham, R.L.; Agnihothram, S.; Jeng, S.; Chamberlin, S.; et al. Genetic loci regulate Sarbecovirus pathogenesis: A comparison across mice and humans. Virus Res. 2024, 344, 199357. [Google Scholar] [CrossRef] [PubMed]
- Leist, S.R.; Schafer, A.; Risemberg, E.L.; Bell, T.A.; Hock, P.; Zweigart, M.R.; Linnertz, C.L.; Miller, D.R.; Shaw, G.D.; de Villena, F.P.M.; et al. Sarbecovirus disease susceptibility is conserved across viral and host models. Virus Res. 2024, 346, 199399. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pushpakumar, S.; Bard, N.; Zheng, Y.; Homme, R.P.; Mokshagundam, S.P.L.; Tyagi, S.C. Simulation of COVID-19 symptoms in a genetically engineered mouse model: Implications for the long haulers. Mol. Cell Biochem. 2023, 478, 103–119. [Google Scholar] [CrossRef]
- Zayou, L.; Prakash, S.; Dhanushkodi, N.R.; Quadiri, A.; Ibraim, I.C.; Singer, M.; Salem, A.; Shaik, A.M.; Suzer, B.; Chilukuri, A.; et al. A multi-epitope/CXCL11 prime/pull coronavirus mucosal vaccine boosts the frequency and the function of lung-resident memory CD4(+) and CD8(+) T cells and enhanced protection against COVID-19-like symptoms and death caused by SARS-CoV-2 infection. J. Virol. 2023, 97, 1–13. [Google Scholar] [CrossRef]
- Prakash, S.; Dhanushkodi, N.R.; Zayou, L.; Ibraim, I.C.; Quadiri, A.; Coulon, P.G.; Tifrea, D.F.; Suzer, B.; Shaik, A.M.; Chilukuri, A.; et al. Cross-protection induced by highly conserved human B, CD4(+), and CD8(+) T-cell epitopes-based vaccine against severe infection, disease, and death caused by multiple SARS-CoV-2 variants of concern. Front. Immunol. 2024, 15, 1328905. [Google Scholar] [CrossRef]
- Jansen, E.B.; Orvold, S.N.; Swan, C.L.; Yourkowski, A.; Thivierge, B.M.; Francis, M.E.; Ge, A.; Rioux, M.; Darbellay, J.; Howland, J.G.; et al. After the virus has cleared-Can preclinical models be employed for Long COVID research? PLoS Pathog. 2022, 18, e1010741. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Desbonnet, L.; Konkoth, A.; Laighneach, A.; McKernan, D.; Holleran, L.; McDonald, C.; Morris, D.W.; Donohoe, G.; Kelly, J. Dual hit mouse model to examine the long-term effects of maternal immune activation and post-weaning social isolation on schizophrenia endophenotypes. Behav. Brain Res. 2022, 430, 113930. [Google Scholar] [CrossRef]
- Kendall, L.V.; Boyd, T.D.; Sillau, S.H.; Bosco-Lauth, A.; Markham, N.; Fong, D.; Clarke, P.; Tyler, K.L.; Potter, H. GM-CSF Promotes Immune Response and Survival in a Mouse Model of COVID-19. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Erickson, M.A.; Logsdon, A.F.; Rhea, E.M.; Hansen, K.M.; Holden, S.J.; Banks, W.A.; Smith, J.L.; German, C.; Farr, S.A.; Morley, J.E.; et al. Blood-brain barrier penetration of non-replicating SARS-CoV-2 and S1 variants of concern induce neuroinflammation which is accentuated in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2023, 109, 251–268. [Google Scholar] [CrossRef]
- Choi, C.Y.; Gadhave, K.; Villano, J.; Pekosz, A.; Mao, X.; Jia, H. Generation and characterization of a humanized ACE2 mouse model to study long-term impacts of SARS-CoV-2 infection. J. Med. Virol. 2024, 96, e29349. [Google Scholar] [CrossRef]
- Jeon, D.; Kim, S.H.; Kim, J.; Jeong, H.; Uhm, C.; Oh, H.; Cho, K.; Cho, Y.; Park, I.H.; Oh, J.; et al. Discovery of a new long COVID mouse model via systemic histopathological comparison of SARS-CoV-2 intranasal and inhalation infection. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167347. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, C.; Adamo, S.; Biteus, E.; Kamal, H.; Dager, L.; Miners, K.L.; Llewellyn-Lacey, S.; Ladell, K.; Amratia, P.S.; et al. Identification of soluble biomarkers that associate with distinct manifestations of long COVID. Nat. Immunol. 2025, 26, 692–705. [Google Scholar] [CrossRef]
- Russelli, G.; Pizzillo, P.; Iannolo, G.; Barbera, F.; Tuzzolino, F.; Liotta, R.; Traina, M.; Vizzini, G.; Gridelli, B.; Badami, E.; et al. HCV replication in gastrointestinal mucosa: Potential extra-hepatic viral reservoir and possible role in HCV infection recurrence after liver transplantation. PLoS ONE 2017, 12, e0181683. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, T.; Zhang, Y.; Luo, S.; Chen, H.; Chen, D.; Li, C.; Li, W. The reservoir of latent HIV. Front. Cell. Infect. Microbiol. 2022, 12, 945956. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Roman, P.; Crespo-Bermejo, C.; Valle-Millares, D.; Lara-Aguilar, V.; Arca-Lafuente, S.; Martin-Carbonero, L.; Ryan, P.; de Los Santos, I.; Lopez-Huertas, M.R.; Palladino, C.; et al. Dynamics of HIV Reservoir and HIV-1 Viral Splicing in HCV-Exposed Individuals after Elimination with DAAs or Spontaneous Clearance. J. Clin. Med. 2022, 11, 3579. [Google Scholar] [CrossRef]
- Sigal, A.; Neher, R.A.; Lessells, R.J. The consequences of SARS-CoV-2 within-host persistence. Nat. Rev. Microbiol. 2025, 23, 288–302. [Google Scholar] [CrossRef] [PubMed]
- de Assumpcao, L.; Romeo, B.G.P.; Guerra, J.C.C.; Camargo, L.F.A.; Nagaoka, M.A.; Amgarten, D.E.; Dorlass, E.G.; Petroni, R.C.; Cardoso, A.C.A.; Ruiz, R.M.; et al. Case report: Persistent COVID-19 in a patient with B cell lymphoma refractory to antiviral treatment due to resistance to Remdesivir. IDCases 2025, 40, e02199. [Google Scholar] [CrossRef]
- Haslam, A.; Prasad, V. A Systematic Review of Nirmatrelvir/Ritonavir and Molnupiravir for the Treatment of Coronavirus Disease 2019. Open Forum Infect. Dis. 2024, 11, ofae497. [Google Scholar] [CrossRef]
- Preiss, A.; Bhatia, A.; Aragon, L.V.; Baratta, J.M.; Baskaran, M.; Blancero, F.; Brannock, M.D.; Chew, R.F.; Diaz, I.; Fitzgerald, M.; et al. Effect of Paxlovid treatment during acute COVID-19 on Long COVID onset: An EHR-based target trial emulation from the N3C and RECOVER consortia. PLoS Med. 2025, 22, e1004711. [Google Scholar]
- Velati, D.; Puoti, M. Real-world experience with therapies for SARS-CoV-2: Lessons from the Italian COVID-19 studies. Infez. Med. 2025, 33, 64–75. [Google Scholar] [PubMed]
- Sweeney, D.A.; Kalil, A.C. Guidelines without borders: The case for JAK inhibitors as the first-line immunomodulator COVID-19 treatment. Lancet Respir. Med. 2025, 13, 478–480. [Google Scholar] [CrossRef]
- Gmizic, I.I.; Barac, A.; Todorovic, N.; Sabanovic, M.; Kekic, N.; Boskovic, N.; Vujovic, A.; Nikolic, N.; Knezevic, N.; Milosevic, I.; et al. Molnupiravir’s real-world effectiveness in COVID-19 outpatients at high risk of severe disease: A single-center study. J. Infect. Dev. Ctries. 2024, 18, 694–700. [Google Scholar] [CrossRef]
- Harris, V.; Holmes, J.; Gbinigie-Thompson, O.; Rahman, N.M.; Richards, D.B.; Hayward, G.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): A randomised controlled trial. Lancet Infect. Dis. 2025, 25, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tian, C.; Chu, S.; Zhou, H.; Zhang, Z.; Luo, S.; Hu, D.; Fan, H. COVID-19 patients benefit from early antiviral treatment: A comparative, retrospective study. J. Med. Virol. 2020, 92, 2675–2683. [Google Scholar] [CrossRef]
- Du, Z.; Wang, L.; Bai, Y.; Liu, Y.; Lau, E.H.Y.; Galvani, A.P.; Krug, R.M.; Cowling, B.J.; Meyers, L.A. A retrospective cohort study of Paxlovid efficacy depending on treatment time in hospitalized COVID-19 patients. Elife 2024, 13, e89801. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2023, 401, 281–293. [Google Scholar] [CrossRef]
- Group, R.C. Molnupiravir or nirmatrelvir-ritonavir plus usual care versus usual care alone in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Infect. Dis. 2025, 25, 1000–1010. [Google Scholar]
- Baldwin, K.; Wanson, A.; Gilecki, L.A.; Dalton, C.; Peters, E.; Halpape, K. Intranasal ketamine as a treatment for psychiatric complications of long COVID: A case report. Ment. Health Clin. 2023, 13, 239–243. [Google Scholar] [CrossRef]
- Denton, L.; Kapuganti, A.; Kim, S. A case report on the effects of COVID-19 on ANC monitoring in a patient on long-term clozapine treatment. Ment. Health Clin. 2023, 13, 190–192. [Google Scholar] [CrossRef]
- Taube, M. Depression and brain fog as long-COVID mental health consequences: Difficult, complex and partially successful treatment of a 72-year-old patient-A case report. Front. Psychiatry 2023, 14, 1153512. [Google Scholar] [CrossRef]
- Igarashi, S.; Okita, K.; Hayashi, D.; Yamazaki, R.; Matsuda, Y.; Noda, T.; Watanabe, K.; Kito, S. Neuroinflammatory Alterations in Treatment-Resistant Depression Secondary to Long COVID by Repetitive Transcranial Magnetic Stimulation (rTMS): A Case Report. Psychiatr. Res. Clin. Pract. 2024, 6, 63–64. [Google Scholar] [CrossRef]
- Kawalec, A.; Cichon, L.; Wilczynski, K.; Janas-Kozik, M. First onset of persistent neutropenia in patient undergoing long-term clozapine treatment after vaccination against COVID-19 and SARS-CoV-2 infection in short interval—A case report. Psychiatr. Pol. 2025, 59, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, H. Successful treatment of long-COVID postural tachycardia syndrome with epipharyngeal abrasive therapy in an adolescent patient: A case report. Medicine 2025, 104, e43333. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef]
- Li, H.; Gao, M.; You, H.; Zhang, P.; Pan, Y.; Li, N.; Qin, L.; Wang, H.; Li, D.; Li, Y.; et al. Association of Nirmatrelvir/Ritonavir Treatment on Upper Respiratory Severe Acute Respiratory Syndrome Coronavirus 2 Reverse Transcription-Polymerase Chain Reaction (SARS-Cov-2 RT-PCR) Negative Conversion Rates Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2023, 76, e148–e154. [Google Scholar] [PubMed]
- Decker, S.; Xiao, S.; Dillen, C.; Schumacher, C.M.; Milstone, A.M.; Frieman, M.; Debes, A.K. Association of Nirmatrelvir/Ritonavir Treatment and COVID-19-Neutralizing Antibody Titers in a Longitudinal Health Care Worker Cohort. Open Forum Infect. Dis. 2024, 11, ofad625. [Google Scholar] [CrossRef]
- Segura Fabrega, A.; Perez Catalan, I.; Gomez Alfaro, I.; Garcia Munoz, S.; Roig Marti, C.; Rodriguez Lozano, N.; Folgado Escudero, S.; Varea Villanueva, M.; Gascon Buj, A.; Torres Garcia, M.; et al. Association of nirmatrelvir/ritonavir and remdesivir as treatment for SARS-CoV-2 infection in immunocompromised patients with hematologic malignancies. Series of four cases. Rev. Esp. Quimioter. 2023, 36, 655–657. [Google Scholar] [CrossRef]
- Caffrey, A.R.; Appaneal, H.J.; Lopes, V.V.; Lavoie, T.; Puzniak, L.; Zasowski, E.J.; Jodar, L.; Arham, I.; LaPlante, K.L.; McLaughlin, J.M. Association between nirmatrelvir/ritonavir treatment and antibiotic prescribing in the outpatient setting among patients with COVID-19. Microbiol. Spectr. 2025, 13, e0320924. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Maslinska, M.; Dmowska-Chalaba, J.; Jakubaszek, M. The Role of IgG4 in Autoimmunity and Rheumatic Diseases. Front. Immunol. 2021, 12, 787422. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.M.; Pfannenstiel, J.J.; Alhammad, Y.M.; O’Connor, J.J.; Ghimire, R.; Shrestha, R.; Khattabi, R.; Saenjamsai, P.; Parthasarathy, S.; McDonald, P.R.; et al. Mutation of a highly conserved isoleucine residue in loop 2 of several beta-coronavirus macrodomains indicates that enhanced ADP-ribose binding is detrimental for replication. J. Virol. 2024, 98, e0131324. [Google Scholar] [CrossRef]
- Pereira Neto, T.A.; Zmasek, C.; Avalos, L.; Sidney, J.; Trevizani, R.; Phillips, E.; Mallal, S.; Frazier, A.; Tan, G.S.; Scheuermann, R.H.; et al. Highly conserved Betacoronavirus sequences are broadly recognized by human T cells. Cell 2025, S0092-8674(25)00804-9. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Nandi, A.; Nam, V.; Allen, L.J.S.; Trindade, A.A.; Kosiewicz, M.M.; Jonsson, C.B. Modeling the Immune Response for Pathogenic and Nonpathogenic Orthohantavirus Infections in Human Lung Microvasculature Endothelial Cells. Viruses 2023, 15, 1806. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Song, J. T Cells in Pathogenic Infections. Pathogens 2023, 12, 578. [Google Scholar] [CrossRef]
- Huot, N.; Rascle, P.; Tchitchek, N.; Wimmer, B.; Passaes, C.; Contreras, V.; Desjardins, D.; Stahl-Hennig, C.; Le Grand, R.; Saez-Cirion, A.; et al. Role of NKG2a/c(+)CD8(+) T cells in pathogenic versus non-pathogenic SIV infections. iScience 2021, 24, 102314. [Google Scholar] [CrossRef]
- Rathore, A.P.; St John, A.L. Protective and pathogenic roles for mast cells during viral infections. Curr. Opin. Immunol. 2020, 66, 74–81. [Google Scholar] [CrossRef]
- Shingai, M.; Welbourn, S.; Brenchley, J.M.; Acharya, P.; Miyagi, E.; Plishka, R.J.; Buckler-White, A.; Kwong, P.D.; Nishimura, Y.; Strebel, K.; et al. The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4+ Memory T Cells. PLoS Pathog. 2015, 11, e1004928. [Google Scholar] [CrossRef]
- Cremet, L.; Broquet, A.; Brulin, B.; Jacqueline, C.; Dauvergne, S.; Brion, R.; Asehnoune, K.; Corvec, S.; Heymann, D.; Caroff, N. Pathogenic potential of Escherichia coli clinical strains from orthopedic implant infections towards human osteoblastic cells. Pathog. Dis. 2015, 73, ftv065. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, M.; Uetani, T.; Sakan, H.; Maruyama, S.; Liu, F.; Sumita, K.; Asano, Y. A two-step model of T cell subset commitment: Antigen-independent commitment of T cells before encountering nominal antigen during pathogenic infections. Int. Immunol. 2002, 14, 567–575. [Google Scholar] [CrossRef]
- Igarashi, T.; Brown, C.R.; Endo, Y.; Buckler-White, A.; Plishka, R.; Bischofberger, N.; Hirsch, V.; Martin, M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 2001, 98, 658–663. [Google Scholar] [CrossRef]
- Martin, P.; Sanchez-Madrid, F. T cells in cardiac health and disease. J. Clin. Investig. 2025, 135, e185218. [Google Scholar] [CrossRef]
- Kageyama, T.; Matsuo, T.; Kurakake, R.; Sano, T. Relationship between T cells and microbiota in health and disease. Prog. Mol. Biol. Transl. Sci. 2020, 171, 95–129. [Google Scholar]
- Bystrom, J.; Clanchy, F.I.L.; Taher, T.E.; Al-Bogami, M.; Ong, V.H.; Abraham, D.J.; Williams, R.O.; Mageed, R.A. Functional and phenotypic heterogeneity of Th17 cells in health and disease. Eur. J. Clin. Investig. 2019, 49, e13032. [Google Scholar] [CrossRef] [PubMed]
- Maazi, H.; Akbari, O. Type two innate lymphoid cells: The Janus cells in health and disease. Immunol. Rev. 2017, 278, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Talwar; Arun, K.V.; Kalaivani, S. Evaluation of transcription factor that regulates T helper 17 and regulatory T cells function in periodontal health and disease. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. S2), S672–S676. [Google Scholar] [CrossRef]
- Crome, S.Q.; Wang, A.Y.; Levings, M.K. Translational mini-review series on Th17 cells: Function and regulation of human T helper 17 cells in health and disease. Clin. Exp. Immunol. 2010, 159, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Louten, J.; Boniface, K.; de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 2009, 123, 1004–1011. [Google Scholar] [CrossRef]
- Cosmi, L.; Annunziato, F.; Galli, M.I.G.; Maggi, R.M.E.; Nagata, K.; Romagnani, S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 2000, 30, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Shohrati, M.; Abedi, F.; Bagheri, M.; Sahebkar, A. Effects of curcumin on vascular smooth muscle cells: Implications for health and disease. Pharmacol. Rep. 2025, 77, 1232–1246. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Brenner, D.A. Hepatic stellate cells: Balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 481–499. [Google Scholar] [CrossRef]
- Sarmento-Cabral, A.; Fuentes-Fayos, A.C.; Ordonez, F.M.; Leon-Gonzalez, A.J.; Martinez-Fuentes, A.J.; Gahete, M.D.; Luque, R.M. From pituitary cells to prostate gland in health and disease: Direct and indirect endocrine connections. Rev. Endocr. Metab. Disord. 2025, 26, 187–203. [Google Scholar] [CrossRef]
- Royzman, D.; Dorrie, J.; Heiss, A.; Sinner, P.; Peckert-Maier, K.; Schaft, N.; Sadeghi Shermeh, A.; Strack, A.; Wild, A.; Steinkasserer, A. The Biology of Dendritic Cells: In Health and Disease. Adv. Exp. Med. Biol. 2025, 1476, 1–30. [Google Scholar]
- Ramachandran, P. Hepatic stellate cells regulate multiple aspects of hepatocyte function in health and disease. J. Hepatol. 2025, 83, 803–804. [Google Scholar] [CrossRef]
- Papa, V.; Li Pomi, F.; Di Gioacchino, M.; Mangifesta, R.; Borgia, F.; Gangemi, S. Mast Cells and Microbiome in Health and Disease. Front. Biosci. (Landmark Ed.) 2025, 30, 26283. [Google Scholar] [CrossRef] [PubMed]
- Karadima, E.; Chavakis, T.; Alexaki, V.I. Arginine metabolism in myeloid cells in health and disease. Semin. Immunopathol. 2025, 47, 11. [Google Scholar] [CrossRef]
- Hosseini, S.; Thakur, P.; Cedeno, D.L.; Fereidoni, M.; Elahdadi Salmani, M. Editorial: Glial cells in health and disease: Impacts on neural circuits and plasticity. Front. Cell Neurosci. 2025, 19, 1569725. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.C.; Sun, X.; Oudeif, F.; Corona, D.L.; He, Z.; Won, T.; Schultz, T.L.; Carruthers, V.B.; Laouar, A.; Laouar, Y. A membrane lipid signature unravels the dynamic landscape of group 1 innate lymphoid cells across the health-disease continuum. iScience 2025, 28, 112043. [Google Scholar] [CrossRef]
- Fliesser, E.; Jandl, K.; Chen, S.H.; Wang, M.T.; Schupp, J.C.; Kuebler, W.M.; Baker, A.H.; Kwapiszewska, G. Transcriptional signatures of endothelial cells shape immune responses in cardiopulmonary health and disease. JCI Insight 2025, 10, e191059. [Google Scholar] [CrossRef]
- De Backer, E.; Verdoodt, D.; Ponsaerts, P.; Pasciuto, E.; Van Rompaey, V. Cochlear T cells and their role in health and disease: A systematic review. Autoimmun. Rev. 2025, 24, 103814. [Google Scholar] [CrossRef]
- Colucci, F.; Botta, M. Innate Lymphoid Cells in Reproductive Health and Disease. Eur. J. Immunol. 2025, 55, e70007. [Google Scholar] [CrossRef]
- Chu, Y.; Setayesh, J.; Dumontet, T.; Krumeich, L.; Werner, J.; Moretti, I.F.; De Sousa, K.; Kennedy, C.; La Pensee, C.; Lerario, A.M.; et al. Adrenocortical stem cells in health and disease. Nat. Rev. Endocrinol. 2025, 21, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Checchetto, V.; Reina, S.; Garino, F.M.; Tomasello, M.F. Editorial: Molecular basis of the energy management in cells: Implications in health and disease. Front. Mol. Biosci. 2025, 12, 1578972. [Google Scholar] [CrossRef]
- Charles, N.; Blank, U. IgE-Mediated Activation of Mast Cells and Basophils in Health and Disease. Immunol. Rev. 2025, 331, e70024. [Google Scholar] [CrossRef] [PubMed]
- Blunt, M.D. NK Cells in Health and Disease. Biomedicines 2025, 13, 1312. [Google Scholar] [CrossRef]
- Biswal, S.; Borgonovo, J.E.; Freites, C.L.; Martinez-Cerdeno, V.; Mishra, R.; Maurya, S.K.; Munoz, E.M. Editorial: Community series in trends in neuroimmunology: Cross-talk between brain-resident and peripheral immune cells in both health and disease, volume II. Front. Immunol. 2025, 16, 1644278. [Google Scholar] [CrossRef]
- Bai, X.; Ihara, E.; Tanaka, Y.; Minoda, Y.; Wada, M.; Hata, Y.; Esaki, M.; Ogino, H.; Chinen, T.; Ogawa, Y. From bench to bedside: The role of gastrointestinal stem cells in health and disease. Inflamm. Regen. 2025, 45, 15. [Google Scholar] [CrossRef]
- An, C.; Jiang, C.; Pei, W.; Li, A.; Wang, M.; Wang, Y.; Wang, H.; Zuo, L. Intestinal epithelial cells in health and disease. Tissue Barriers 2025, 22, 2504744. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yang, D.; Cai, S.; Wang, Y.; Wang, L.; Li, Y.; Li, L.; Yin, T.; Diao, L. Understanding the heterogeneity of natural killer cells at the maternal-fetal interface: Implications for pregnancy health and disease. Mol. Hum. Reprod. 2024, 30, gaae040. [Google Scholar] [CrossRef]
- Medina-Arellano, A.E.; Albert-Garay, J.S.; Medina-Sanchez, T.; Fonseca, K.H.; Ruiz-Cruz, M.; Ochoa-de la Paz, L. Muller cells and retinal angiogenesis: Critical regulators in health and disease. Front. Cell Neurosci. 2024, 18, 1513686. [Google Scholar] [CrossRef] [PubMed]
- Galow, A.M.; Agriesti, F. Advances in Stem Cell Research-Insights from the Special Issue “Stem Cells in Health and Disease 2.0”. Int. J. Mol. Sci. 2024, 25, 13364. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Prakash, S.; Vahed, H.; Karan, S.; Quadiri, A.; Nesburn, A.; BenMohamed, L. A Tissue-Targeted Prime/Pull/Keep Therapeutic Herpes Simplex Virus Vaccine Protect Against Recurrent Ocular Herpes Infection and Disease in HLA-A*0201 Transgenic Rabbits. J. Virol. 2025, 345, e00135-25. [Google Scholar] [CrossRef] [PubMed]
- Quadiri, A.; Lekbach, L.; ELhoucine, E.; Prakash, S.; Vahed, H.; Karen, S.; Rehman, A.; BenMohamed, L. The Path Towards Effective Long-Lasting Tissue-Targeted Prime/Pull/Keep Herpes Simplex Therapeutic Vaccines. Vaccines 2025, 13, 908. [Google Scholar] [CrossRef]
- Hany, M.; Sheta, E.; Talha, A.; Anwar, M.; Selima, M.; Gaballah, M.; Zidan, A.; Ibrahim, M.; Agayby, A.S.S.; Abouelnasr, A.A.; et al. Incidence of persistent SARS-CoV-2 gut infection in patients with a history of COVID-19: Insights from endoscopic examination. Endosc. Int. Open 2024, 12, E11–E22. [Google Scholar] [CrossRef]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef]
- Abbasi, A.; Sharma, R.; Hansen, N.; Pirrotte, P.; Stringer, W.W. Possible long COVID biomarker: Identification of SARC-CoV-2 related protein(s) in Serum Extracellular Vesicles. Infection. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G. Re: ‘Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study’ by Swank et al. Clin. Microbiol. Infect. 2025, 31, 483. [Google Scholar] [CrossRef]
- Swank, Z.; Karlson, E.W.; Walt, D.R. ‘Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study’—Author’s reply. Clin. Microbiol. Infect. 2025, 31, 484–485. [Google Scholar] [CrossRef]
- Menezes, S.M.; Jamoulle, M.; Carletto, M.P.; Moens, L.; Meyts, I.; Maes, P.; Van Weyenbergh, J. Blood transcriptomic analyses reveal persistent SARS-CoV-2 RNA and candidate biomarkers in post-COVID-19 condition. Lancet Microbe 2024, 5, 100849. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Gattoni, C.; Iacovino, M.; Ferguson, C.; Tosolini, J.; Singh, A.; Soe, K.K.; Porszasz, J.; Lanks, C.; Rossiter, H.B.; et al. A Pilot Study on the Effects of Exercise Training on Cardiorespiratory Performance, Quality of Life, and Immunologic Variables in Long COVID. J. Clin. Med. 2024, 13, 5590. [Google Scholar] [CrossRef] [PubMed]
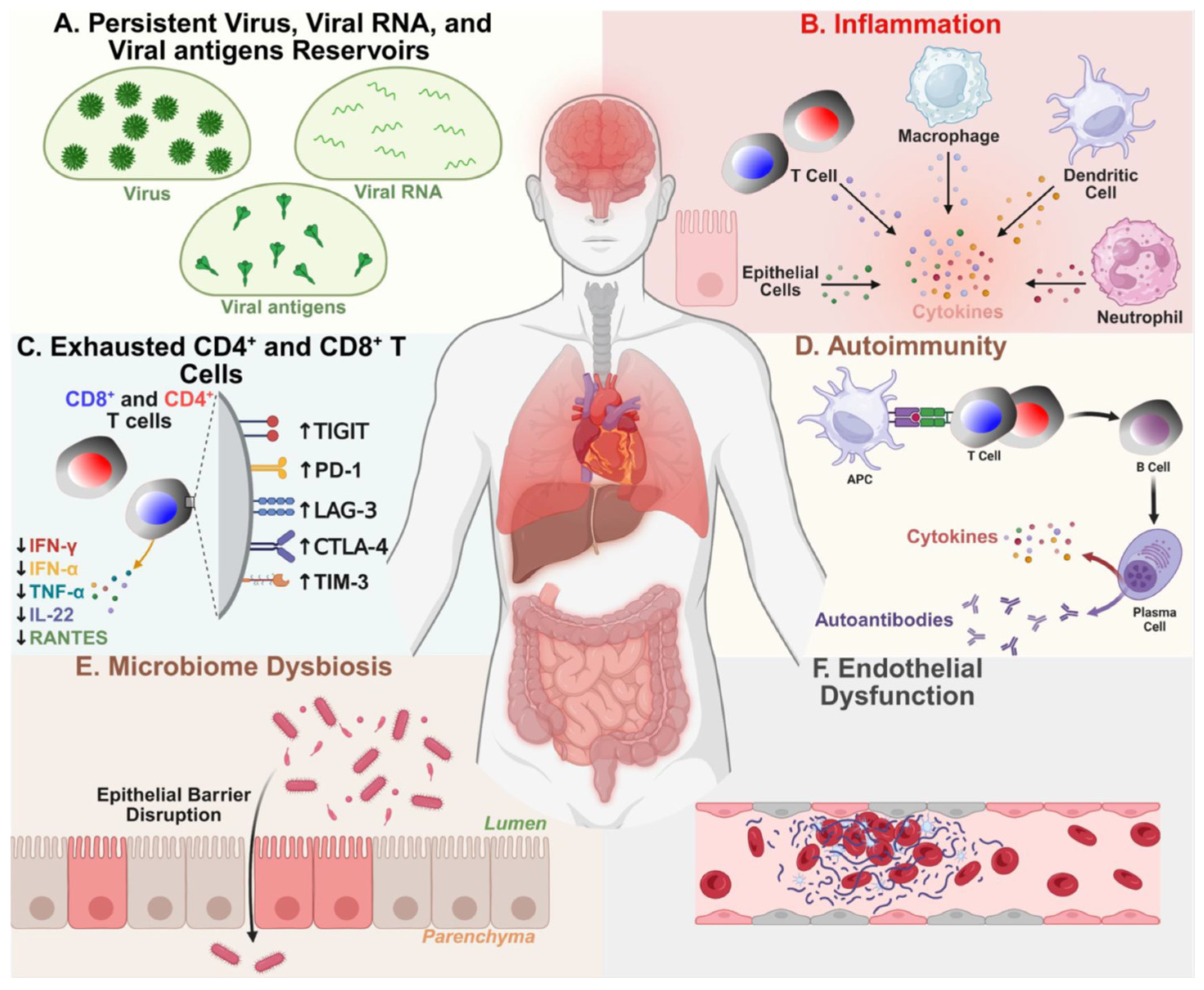
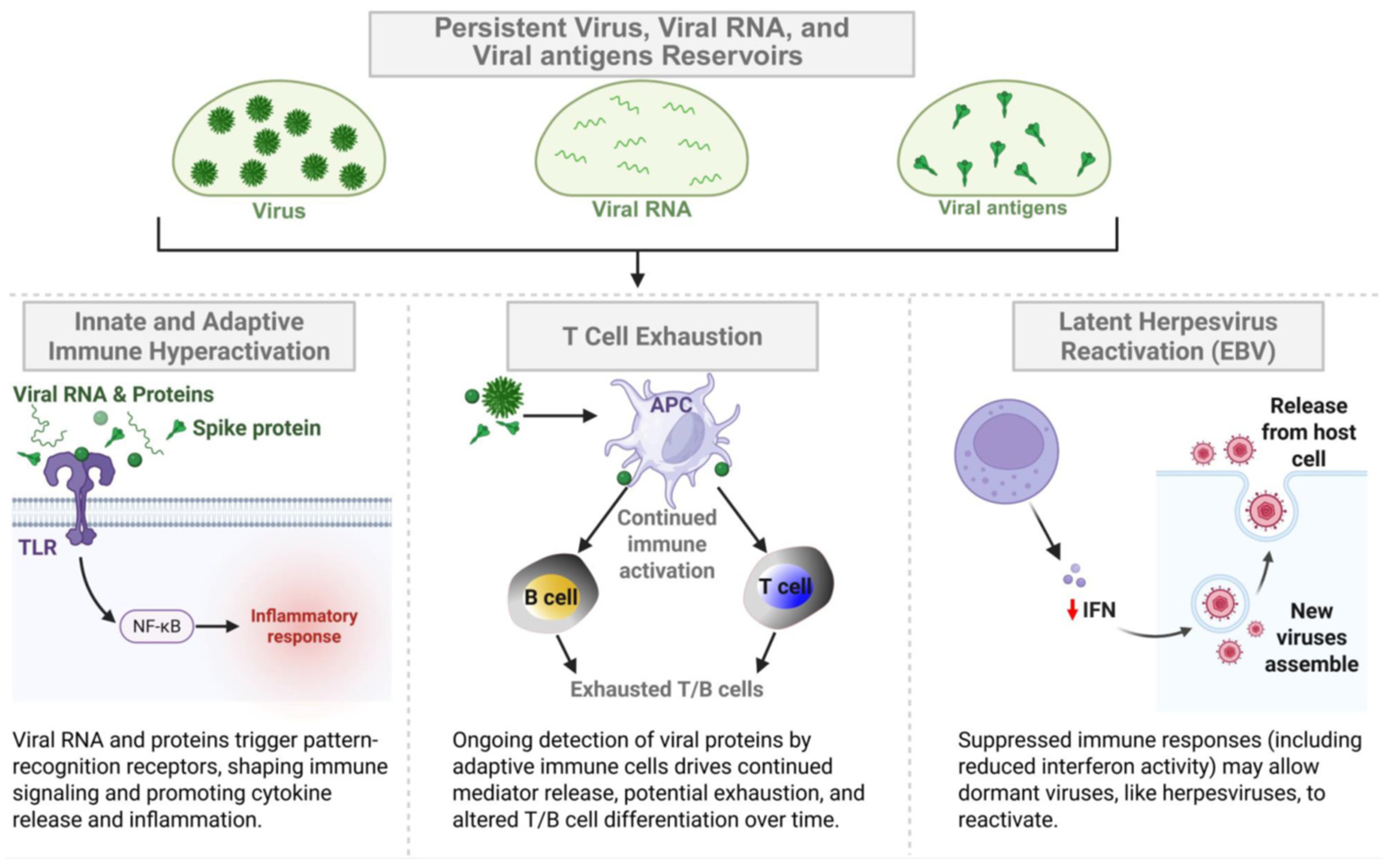
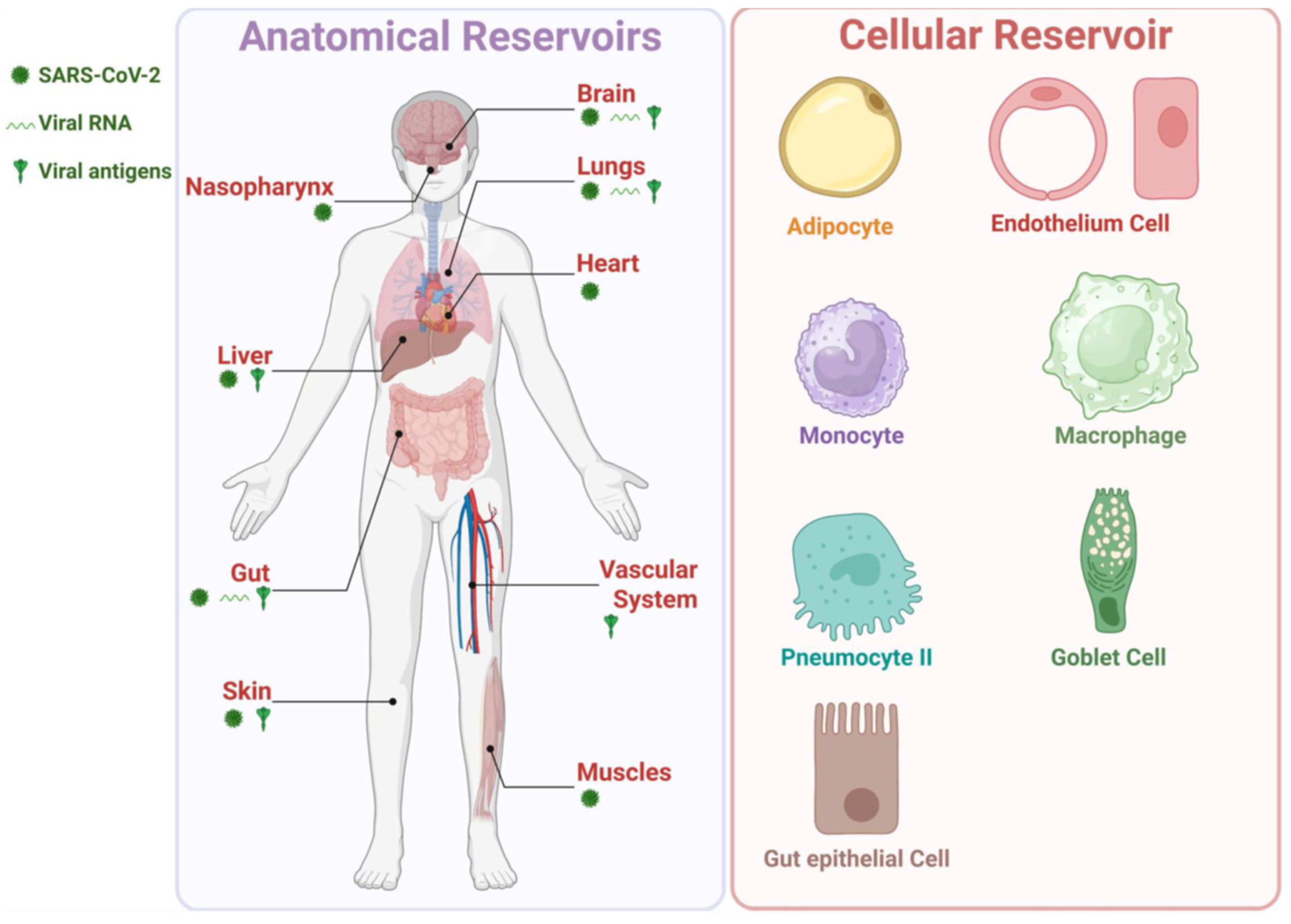
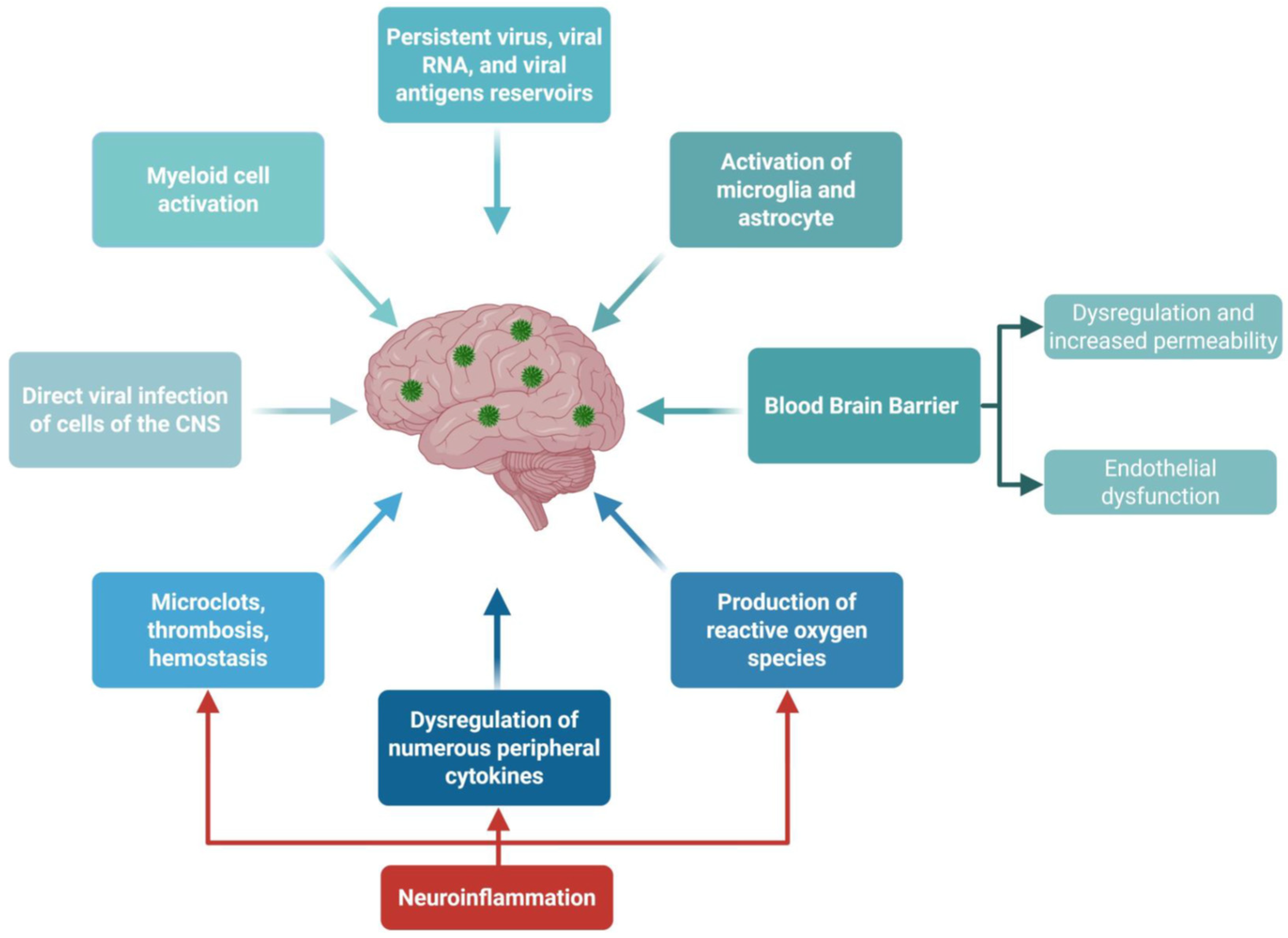
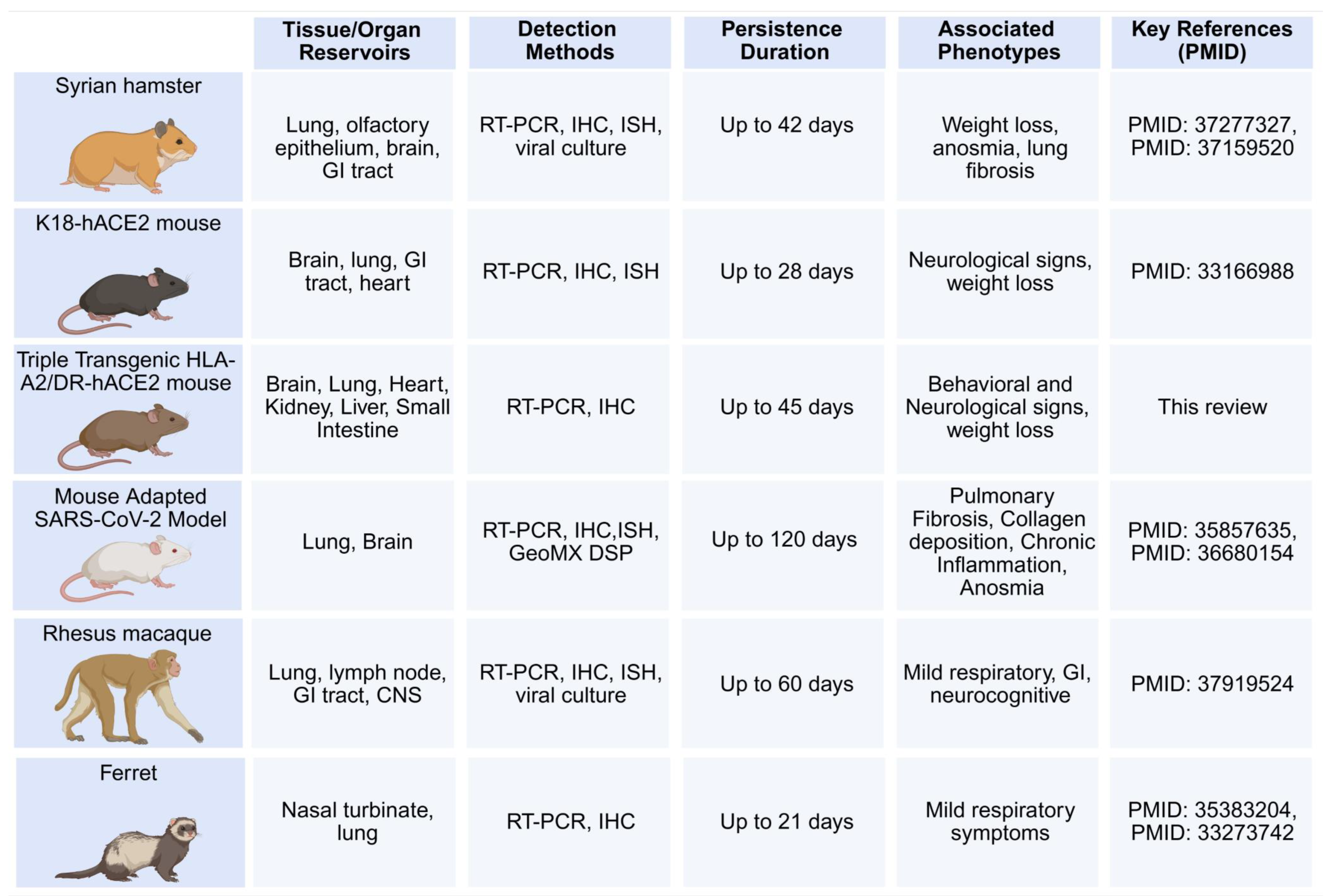
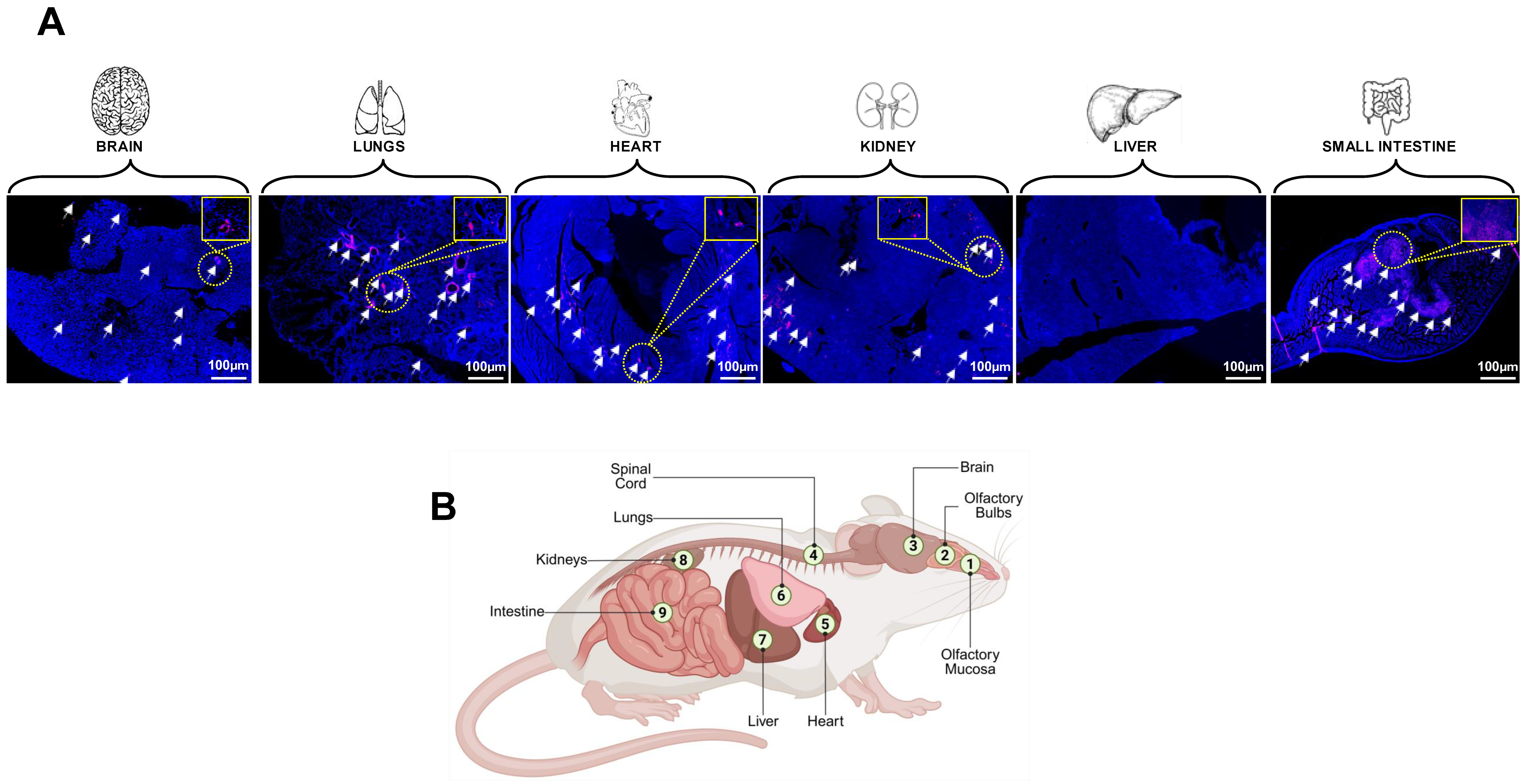
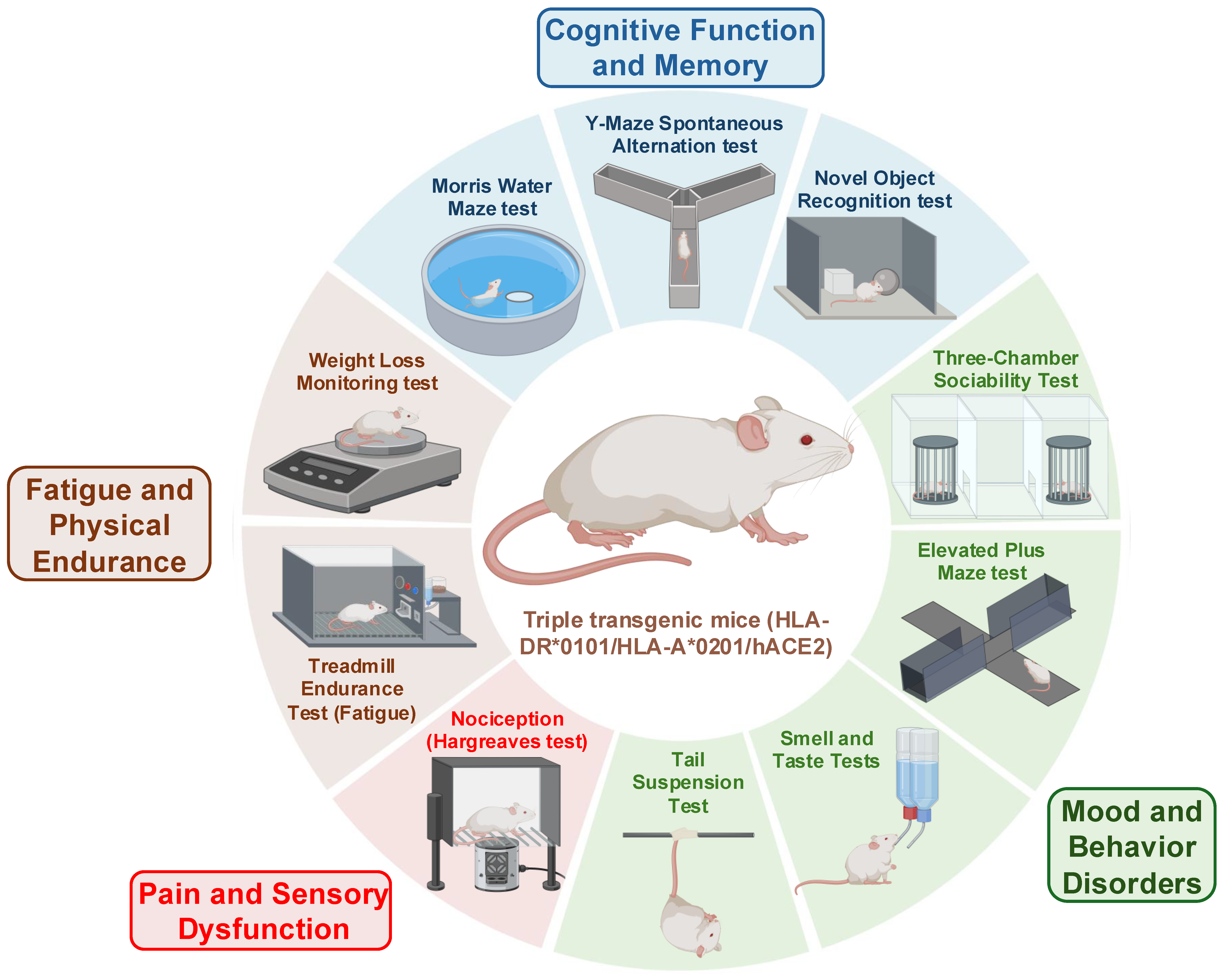
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, S.; Karan, S.; Lekbach, Y.; Tifrea, D.F.; Figueroa, C.J.; Ulmer, J.B.; Young, J.F.; Glenn, G.; Gil, D.; Jones, T.M.; et al. Insights into Persistent SARS-CoV-2 Reservoirs in Chronic Long COVID. Viruses 2025, 17, 1310. https://doi.org/10.3390/v17101310
Prakash S, Karan S, Lekbach Y, Tifrea DF, Figueroa CJ, Ulmer JB, Young JF, Glenn G, Gil D, Jones TM, et al. Insights into Persistent SARS-CoV-2 Reservoirs in Chronic Long COVID. Viruses. 2025; 17(10):1310. https://doi.org/10.3390/v17101310
Chicago/Turabian StylePrakash, Swayam, Sweta Karan, Yassir Lekbach, Delia F. Tifrea, Cesar J. Figueroa, Jeffrey B. Ulmer, James F. Young, Greg Glenn, Daniel Gil, Trevor M. Jones, and et al. 2025. "Insights into Persistent SARS-CoV-2 Reservoirs in Chronic Long COVID" Viruses 17, no. 10: 1310. https://doi.org/10.3390/v17101310
APA StylePrakash, S., Karan, S., Lekbach, Y., Tifrea, D. F., Figueroa, C. J., Ulmer, J. B., Young, J. F., Glenn, G., Gil, D., Jones, T. M., Redfield, R. R., & BenMohamed, L. (2025). Insights into Persistent SARS-CoV-2 Reservoirs in Chronic Long COVID. Viruses, 17(10), 1310. https://doi.org/10.3390/v17101310






