Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany
Abstract
1. Introduction
2. Materials and Methods
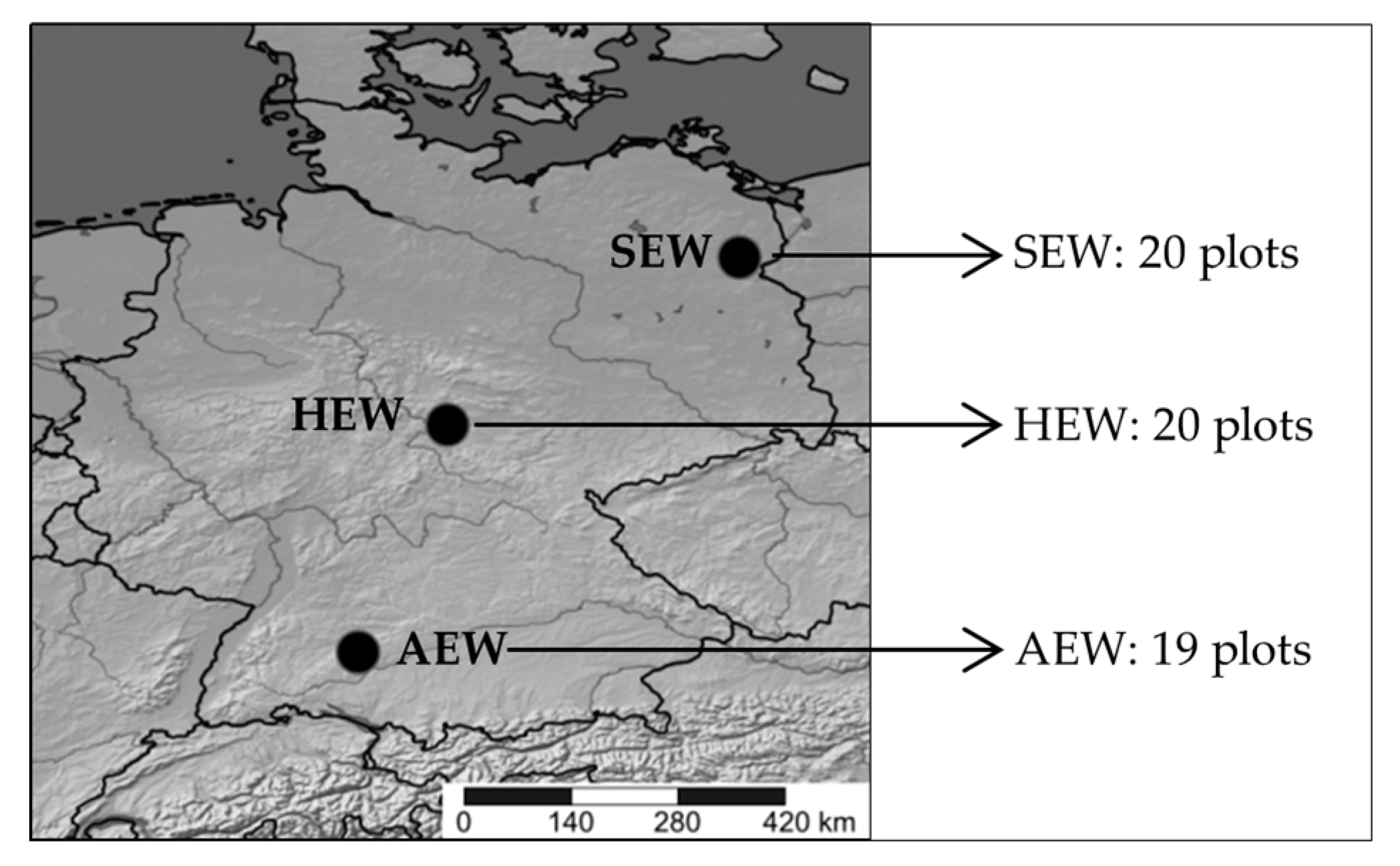
2.1. Plant Material and Study Design
2.2. Measurement of Plant Height and Recording of Plant Survival
2.3. Data Analysis
3. Results
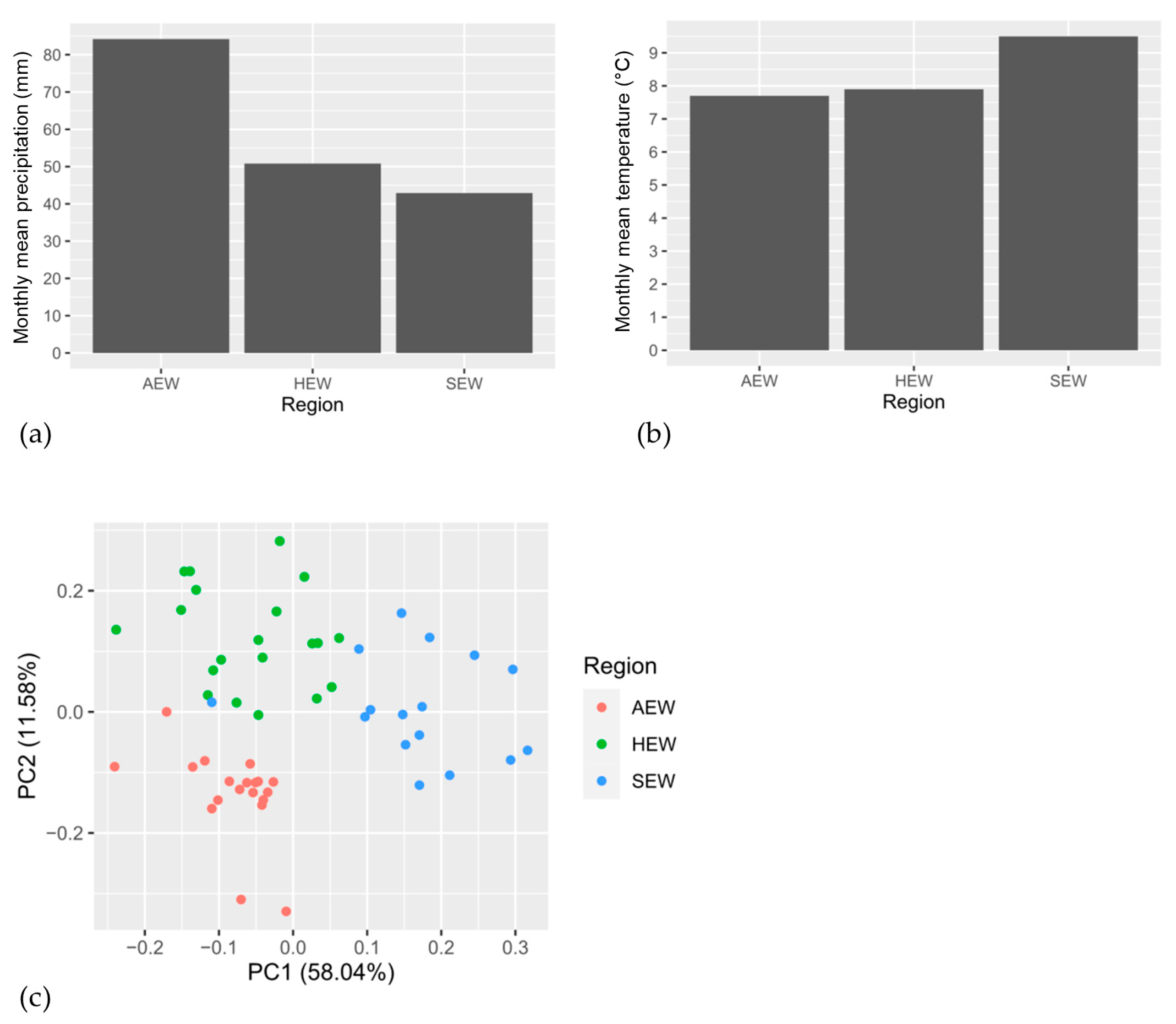
3.1. Climate Variables
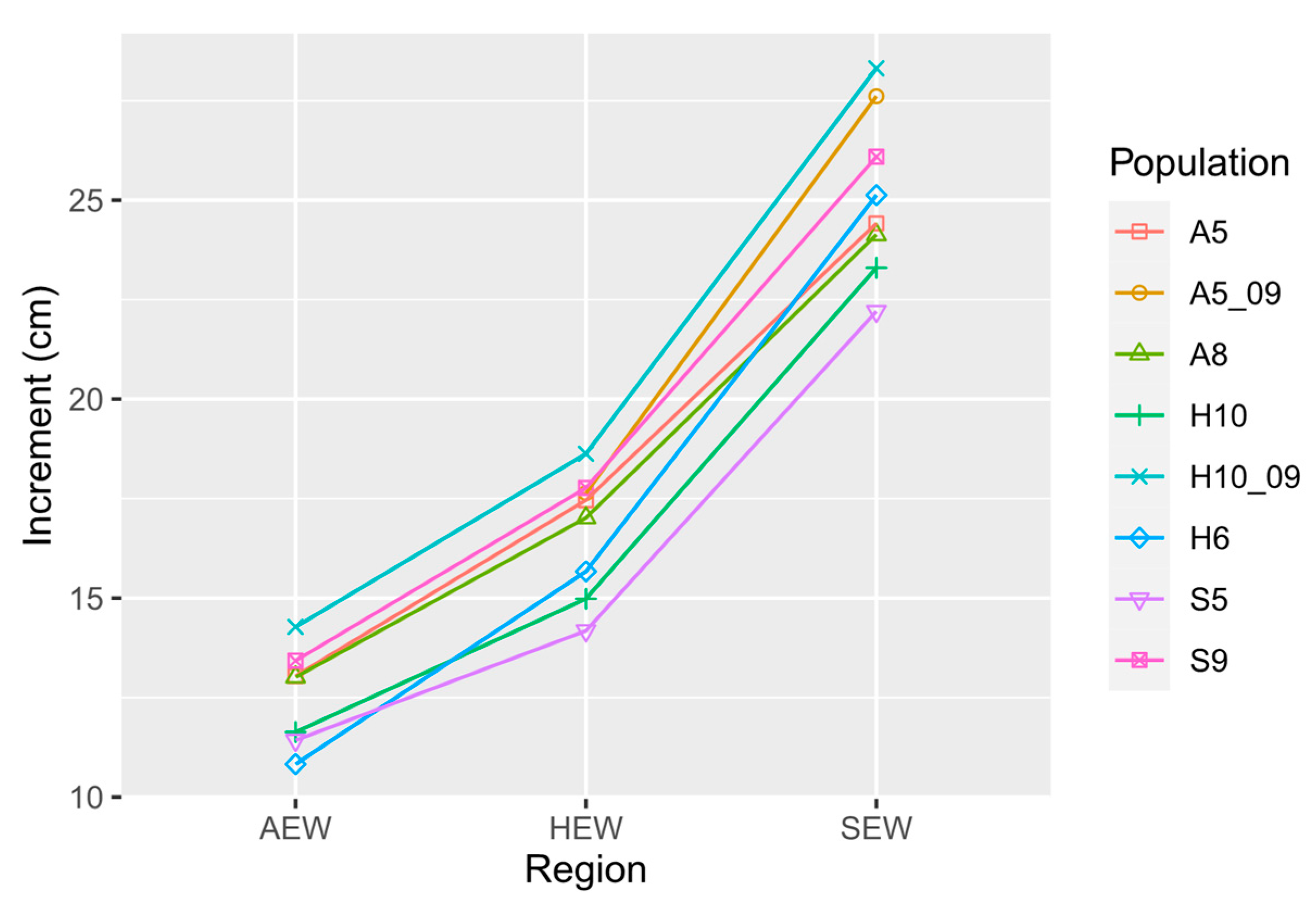
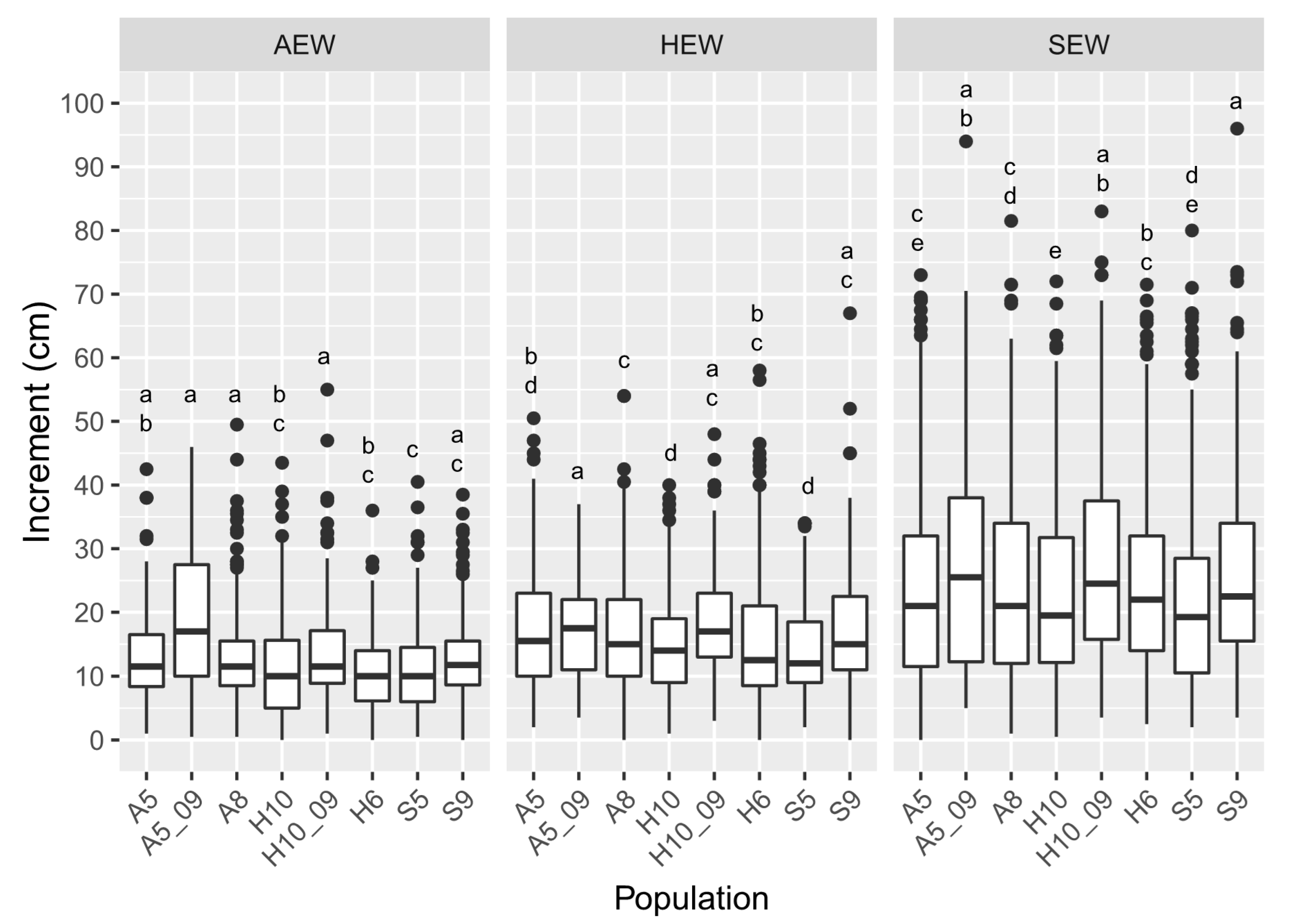
3.2. Plant Height Increment
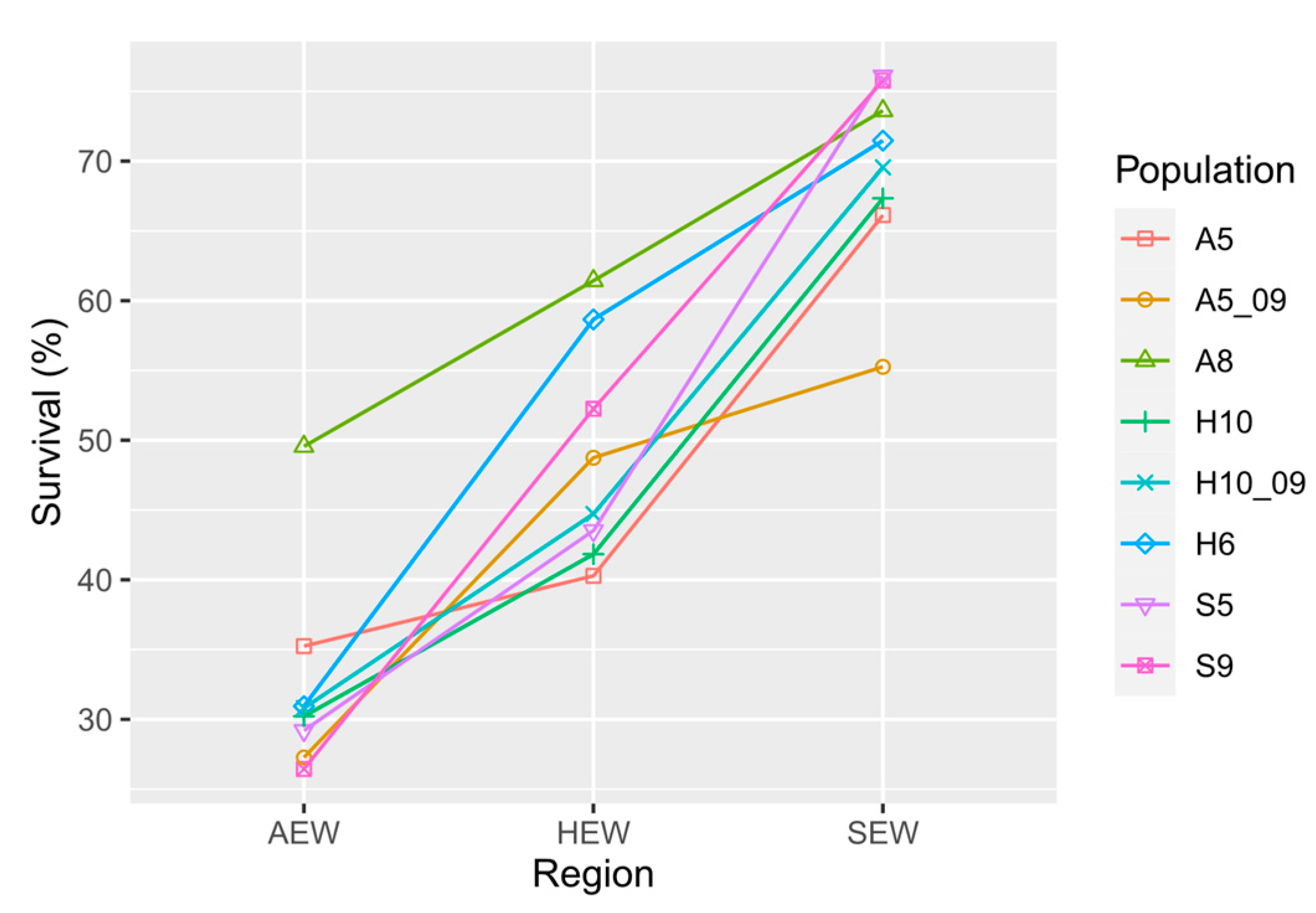
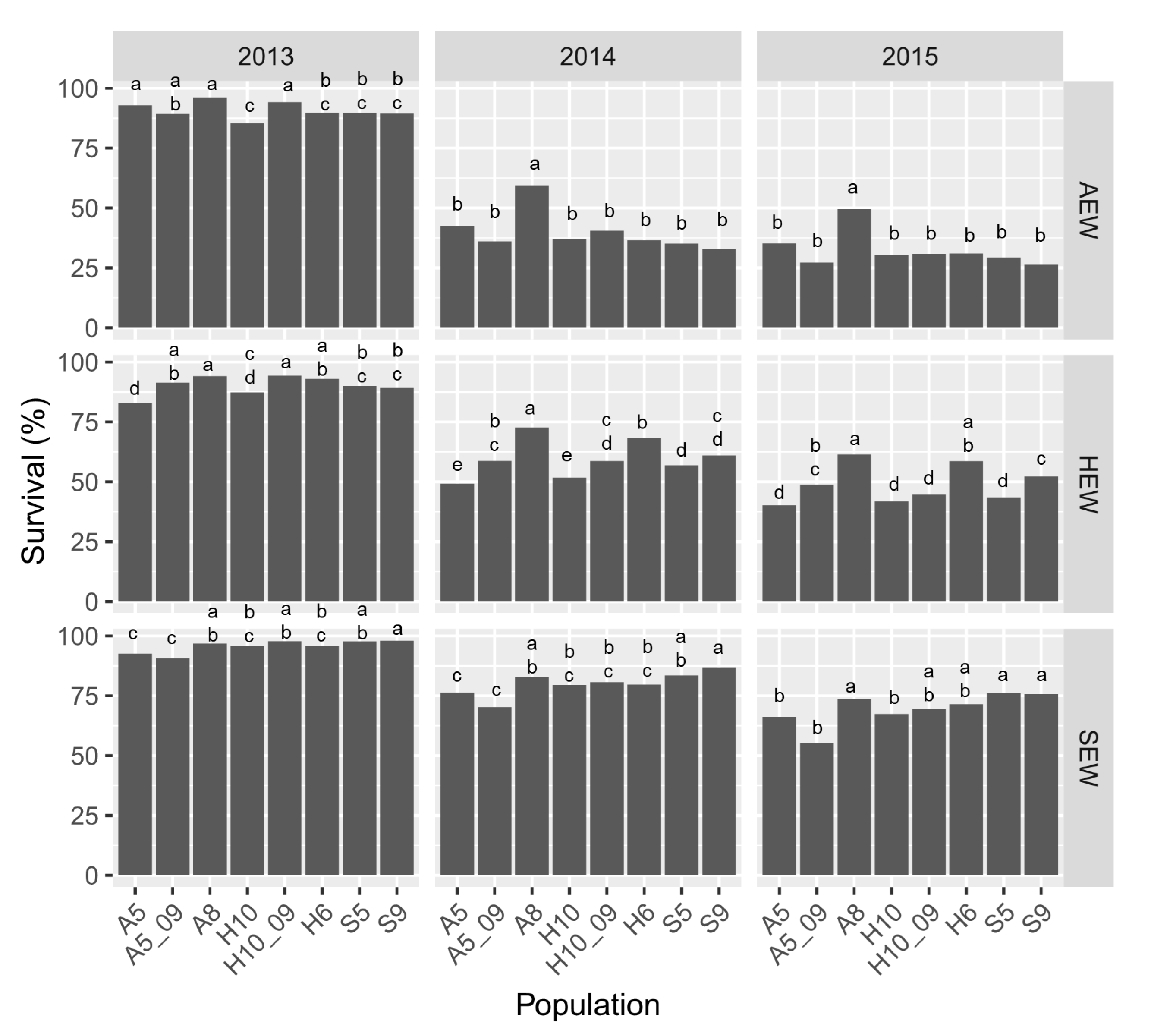
3.3. Plant Survival
3.4. Correlation Analyses
4. Discussion
4.1. Lack of Local Adaptation
4.2. Phenotypic Plasticity
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability:
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fith Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Benito Garzón, M.; Robson, T.M.; Hampe, A. ΔTraitSDMs: Species distribution models that account for local adaptation and phenotypic plasticity. New Phytol. 2019, 222, 1757–1765. [Google Scholar] [CrossRef]
- Knutzen, F.; Dulamsuren, C.; Meier, I.C.; Leuschner, C. Recent climate warming-related growth decline impairs European beech in the center of its distribution range. Ecosystems 2017, 20, 1494–1511. [Google Scholar] [CrossRef]
- NW-FVA. Komplexe Schäden an Rotbuche (Fagus sylvatica) und Auswirkungen des Trockenen und Heißen Sommers 2018 auf Ältere Bestände; Waldschutzinfo Nr. 06/2019; NW-FVA: Göttingen, Germany, 2019. [Google Scholar]
- Gennaretti, F.; Ogée, J.; Sainte-Marie, J.; Cuntz, M. Mining ecophysiological responses of European beech ecosystems to drought. Agric. For. Meteorol. 2020, 280, 107780. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate warming-related growth decline affects Fagus sylvatica, but not other broad-leaved tree species in Central European mixed forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J.A. Water relations and gas exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. in a mixed stand at their southern limit of distribution in Europe. Trees 2000, 14, 344–352. [Google Scholar] [CrossRef]
- Köcher, P.; Gebauer, T.; Horna, V.; Leuschner, C. Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann. For. Sci. 2009, 66, 101. [Google Scholar] [CrossRef]
- Bertin, R.I. Plant phenology and distribution in relation to recent climate change. J. Torrey Bot. Soc. 2008, 135, 126–146. [Google Scholar] [CrossRef]
- Kreyling, J.; Thiel, D.; Nagy, L.; Jentsch, A.; Huber, G.; Konnert, M.; Beierkuhnlein, C. Late frost sensitivity of juvenile Fagus sylvatica L. differs between southern Germany and Bulgaria and depends on preceding air temperature. Eur. J. For. Res. 2012, 131, 717–725. [Google Scholar] [CrossRef]
- Müller, M.; Gailing, O. Abiotic genetic adaptation in the Fagaceae. Plant Biol. 2019, 21, 783–795. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.M.; Menon, M.; Bolte, C.E.; Faske, T.M.; Eckert, A.J. The genomics of local adaptation in trees: Are we out of the woods yet? Tree Genet. Genomes 2018, 14, 29. [Google Scholar] [CrossRef]
- Kreyling, J.; Buhk, C.; Backhaus, S.; Hallinger, M.; Huber, G.; Huber, L.; Jentsch, A.; Konnert, M.; Thiel, D.; Wilmking, M.; et al. Local adaptations to frost in marginal and central populations of the dominant forest tree Fagus sylvatica L. as affected by temperature and extreme drought in common garden experiments. Ecol. Evol. 2014, 4, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Durka, W.; Liesebach, M.; Bruelheide, H. Intraspecific variability in frost hardiness of Fagus sylvatica L. Eur. J. For. Res. 2015, 134, 433–441. [Google Scholar] [CrossRef]
- Carsjens, C.; Nguyen Ngoc, Q.; Guzy, J.; Knutzen, F.; Meier, I.C.; Müller, M.; Finkeldey, R.; Leuschner, C.; Polle, A. Intra-specific variations in expression of stress-related genes in beech progenies are stronger than drought-induced responses. Tree Physiol. 2014, 34, 1348–1361. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are marginal beech (Fagus sylvatica L.) provenances a source for drought tolerant ecotypes? Eur. J. For. Res. 2009, 128, 335–343. [Google Scholar] [CrossRef]
- Dounavi, A.; Netzer, F.; Celepirovic, N.; Ivanković, M.; Burger, J.; Figueroa, A.G.; Schön, S.; Simon, J.; Cremer, E.; Fussi, B.; et al. Genetic and physiological differences of European beech provenances (F. sylvatica L.) exposed to drought stress. For. Ecol. Manag. 2016, 361, 226–236. [Google Scholar] [CrossRef]
- Varsamis, G.; Papageorgiou, A.C.; Merou, T.; Takos, I.; Malesios, C.; Manolis, A.; Tsiripidis, I.; Gailing, O. Adaptive diversity of beech seedlings under climate change scenarios. Front. Plant Sci. 2019, 9, 1918. [Google Scholar] [CrossRef]
- Müller, M.; Finkeldey, R. Genetic and adaptive trait variation in seedlings of European beech provenances from Northern Germany. Silvae Genet. 2016, 65, 65–73. [Google Scholar] [CrossRef]
- Baudis, M.; Ellerbrock, R.H.; Felsmann, K.; Gessler, A.; Gimbel, K.; Kayler, Z.; Puhlmann, H.; Ulrich, A.; Weiler, M.; Welk, E.; et al. Intraspecific differences in responses to rainshelter-induced drought and competition of Fagus sylvatica L. across Germany. For. Ecol. Manag. 2014, 330, 283–293. [Google Scholar] [CrossRef]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does reduced precipitation trigger physiological and morphological drought adaptations in European beech (Fagus sylvatica L.)? Comparing provenances across a precipitation gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Crispo, E. Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J. Evol. Biol. 2008, 21, 1460–1469. [Google Scholar] [CrossRef]
- Kremer, A.; Potts, B.M.; Delzon, S. Genetic divergence in forest trees: Understanding the consequences of climate change. Funct. Ecol. 2014, 28, 22–36. [Google Scholar] [CrossRef]
- Matesanz, S.; Ramírez-Valiente, J.A. A review and meta-analysis of intraspecific differences in phenotypic plasticity: Implications to forecast plant responses to climate change. Glob. Ecol. Biogeogr. 2019, 28, 1682–1694. [Google Scholar] [CrossRef]
- Schuldt, B.; Knutzen, F.; Delzon, S.; Jansen, S.; Müller-Haubold, H.; Burlett, R.; Clough, Y.; Leuschner, C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 2016, 210, 443–458. [Google Scholar] [CrossRef]
- Schüler, S.; Liesebach, M. Latitudinal population transfer reduces temperature sum requirements for bud burst of European beech. Plant Ecol. 2015, 216, 111–122. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Aranda, I.; Bahamonde, H.A.; Sanchez-Gomez, D. Intra-population variability in the drought response of a beech (Fagus sylvatica L.) population in the southwest of Europe. Tree Physiol. 2017, 37, 938–949. [Google Scholar] [CrossRef]
- Gárate-Escamilla, H.; Hampe, A.; Vizcaíno-Palomar, N.; Robson, T.M.; Benito Garzón, M. Range-wide variation in local adaptation and phenotypic plasticity of fitness-related traits in Fagus sylvatica and their implications under climate change. Glob. Ecol. Biogeogr. 2019, 28, 1336–1350. [Google Scholar] [CrossRef]
- Liu, Y.; El-Kassaby, Y.A. Phenotypic plasticity of natural Populus trichocarpa populations in response to temporally environmental change in a common garden. BMC Evol. Biol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Younginger, B.S.; Sirová, D.; Cruzan, M.B.; Ballhorn, D.J. Is biomass a reliable estimate of plant fitness? Appl. Plant Sci. 2017, 5, 1600094. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.-M.; Vaillancourt, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic effects of forest management practices: Global synthesis and perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Müller, M.; Seifert, S.; Finkeldey, R. Comparison and confirmation of SNP-bud burst associations in European beech populations in Germany. Tree Genet. Genomes 2017, 13, 59. [Google Scholar] [CrossRef]
- Rajendra, K.C.; Seifert, S.; Prinz, K.; Gailing, O.; Finkeldey, R. Subtle human impacts on neutral genetic diversity and spatial patterns of genetic variation in European beech (Fagus sylvatica). For. Ecol. Manag. 2014, 319, 138–149. [Google Scholar] [CrossRef]
- Fischer, M.; Bossdorf, O.; Gockel, S.; Hänsel, F.; Hemp, A.; Hessenmöller, D.; Korte, G.; Nieschulze, J.; Pfeiffer, S.; Prati, D.; et al. Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic Appl. Ecol. 2010, 11, 473–485. [Google Scholar] [CrossRef]
- Wöllauer, S.; Hänsel, F.; Nauss, T.; Forteva, S. Climate Data—Time Series Web Interface v1.0.19. Biodiversity Exploratories Information System. DatasetId=19007:2020. Available online: https://www.bexis.uni-jena.de (accessed on 7 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 7 April 2020).
- Tang, Y.; Horikoshi, M.; Wenxuan, L. ggfortify: Unified interface to visualize statistical result of popular R packages. R J. 2016, 8, 478–489. [Google Scholar] [CrossRef]
- Shorthouse, D. SimpleMappr, an Online Tool to Produce Publication-Quality Point Maps. Available online: http://www.simplemappr.net (accessed on 10 December 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Goral, F.; Schellenberg, J. Goeveg: Functions for Community Data and Ordinations. R Package Version 0.4.2. Available online: https://CRAN.R-project.org/package=goeveg (accessed on 7 April 2020).
- Giraudoux, P. Pgirmess: Data Analysis in Ecology. R Package Version 1.6.7. Available online: https://CRAN.R-project.org/package=pgirmess (accessed on 7 April 2020).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.2.7. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 7 April 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research. Version = 1.9.12. Available online: https://CRAN.R-project.org/package=psych (accessed on 7 April 2020).
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech a review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Röhrig, E.; Bartsch, N.; von Lüpke, B. Waldbau auf Ökologischer Grundlage; Ulmer: Stuttgart, Germany, 2006; Volume 7, p. 479. [Google Scholar]
- Kapeller, S.; Dieckmann, U.; Schueler, S. Varying selection differential throughout the climatic range of Norway spruce in Central Europe. Evol. Appl. 2017, 10, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Beloiu, M.; Stahlmann, R.; Beierkuhnlein, C. High recovery of saplings after severe drought in temperate deciduous forests. Forests 2020, 11, 546. [Google Scholar] [CrossRef]
- Konnert, M.; Behm, A. Genetische Strukturen einer Saatgutpartie—Einflussfaktoren und Einflussmöglichkeiten. Beitr. Forstwirtsch. Landsch. 1999, 33, 152–156. [Google Scholar]
- Bose, A.K.; Moser, B.; Rigling, A.; Lehmann, M.M.; Milcu, A.; Peter, M.; Rellstab, C.; Wohlgemuth, T.; Gessler, A. Memory of environmental conditions across generations affects the acclimation potential of scots pine. Plant Cell Environ. 2020, 43, 1288–1299. [Google Scholar] [CrossRef]
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.A.; Abarca, D.; et al. Epigenetic regulation of adaptive responses of forest tree species to the environment. Ecol. Evol. 2013, 3, 399–415. [Google Scholar] [CrossRef]
- Kohmann, K.; Johnsen, Ø. The timing of bud set in seedlings of Picea abies from seed crops of a cool versus a warm spring and summer. Silvae Genet. 1994, 43, 329–333. [Google Scholar]
- Li, W.; Wang, X.; Li, Y. Stability in and correlation between factors influencing genetic quality of seed lots in seed orchard of Pinus tabuliformis Carr. over a 12-year span. PLoS ONE 2011, 6, e23544. [Google Scholar] [CrossRef]
- Müller, M.; Cuervo-Alarcon, L.; Gailing, O.; Rajendra, R.; Chhetri, M.; Seifert, S.; Arend, M.; Krutovsky, K.; Finkeldey, R. Genetic variation of European beech populations and their progeny from northeast Germany to southwest Switzerland. Forests 2018, 9, 469. [Google Scholar] [CrossRef]
- Liesebach, M. Wachstum und phänotypische Variation von sechs Herkünften der Rot-Buche (Fagus sylvatica L.) an einem Standort in Schleswig-Holstein. Appl. Agric. For. Res. 2012, 62, 179–192. [Google Scholar]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Gray, L.K.; Gylander, T.; Mbogga, M.S.; Chen, P.Y.; Hamann, A. Assisted migration to address climate change: Recommendations for aspen reforestation in western Canada. Ecol. Appl. 2011, 21, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Morin, H.; Bergeron, Y.; Girona, M.M. Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Montoro Girona, M.; Morin, H. Vulnerability of conifer regeneration to spruce budworm outbreaks in the eastern Canadian boreal forest. Forests 2019, 10, 850. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Kempen, T.; Finkeldey, R.; Gailing, O. Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany. Forests 2020, 11, 1354. https://doi.org/10.3390/f11121354
Müller M, Kempen T, Finkeldey R, Gailing O. Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany. Forests. 2020; 11(12):1354. https://doi.org/10.3390/f11121354
Chicago/Turabian StyleMüller, Markus, Tanja Kempen, Reiner Finkeldey, and Oliver Gailing. 2020. "Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany" Forests 11, no. 12: 1354. https://doi.org/10.3390/f11121354
APA StyleMüller, M., Kempen, T., Finkeldey, R., & Gailing, O. (2020). Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany. Forests, 11(12), 1354. https://doi.org/10.3390/f11121354






