Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°
Abstract
1. Introduction
2. Materials and Methods
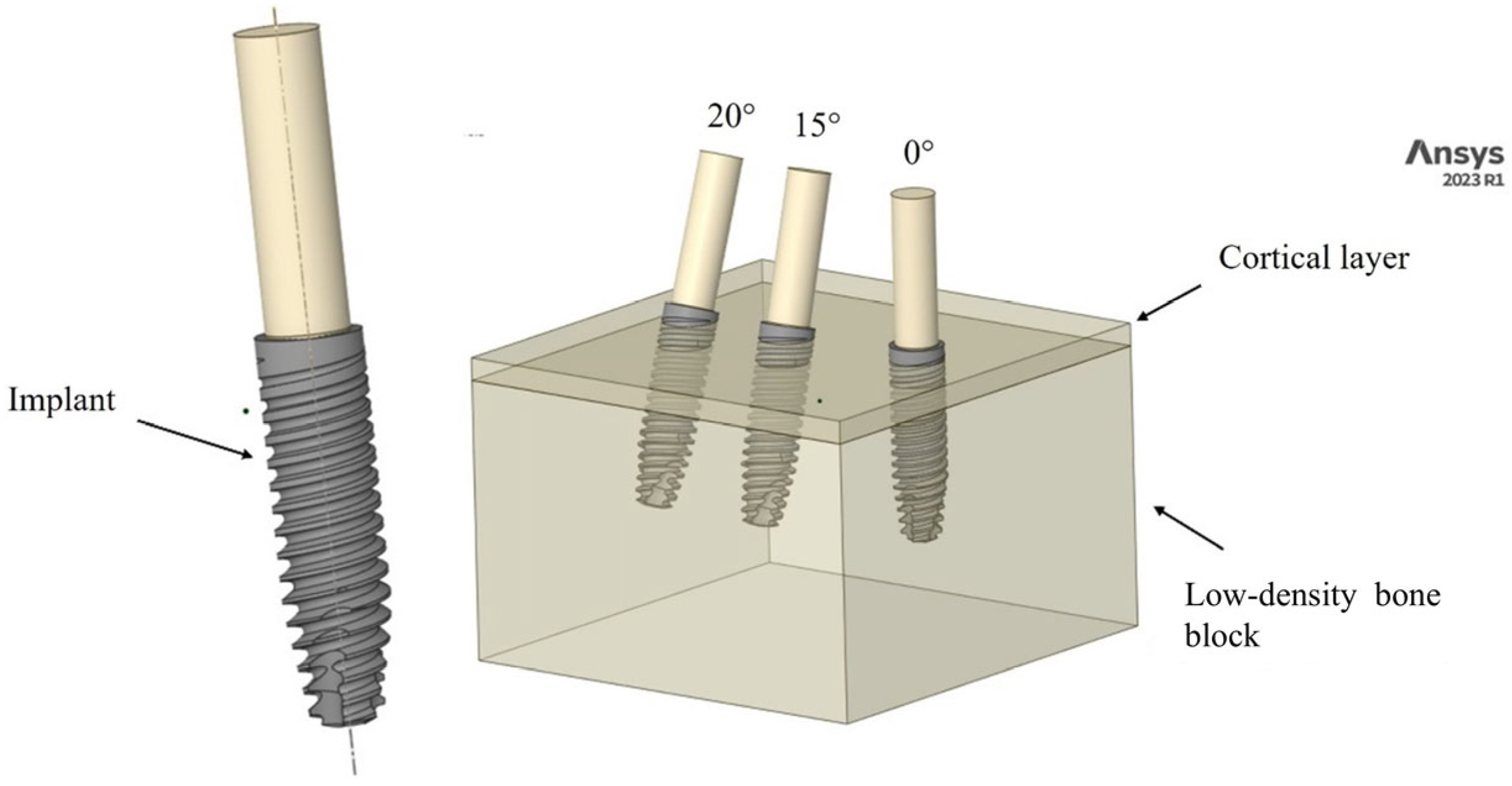
2.1. Modeling
2.2. Materials
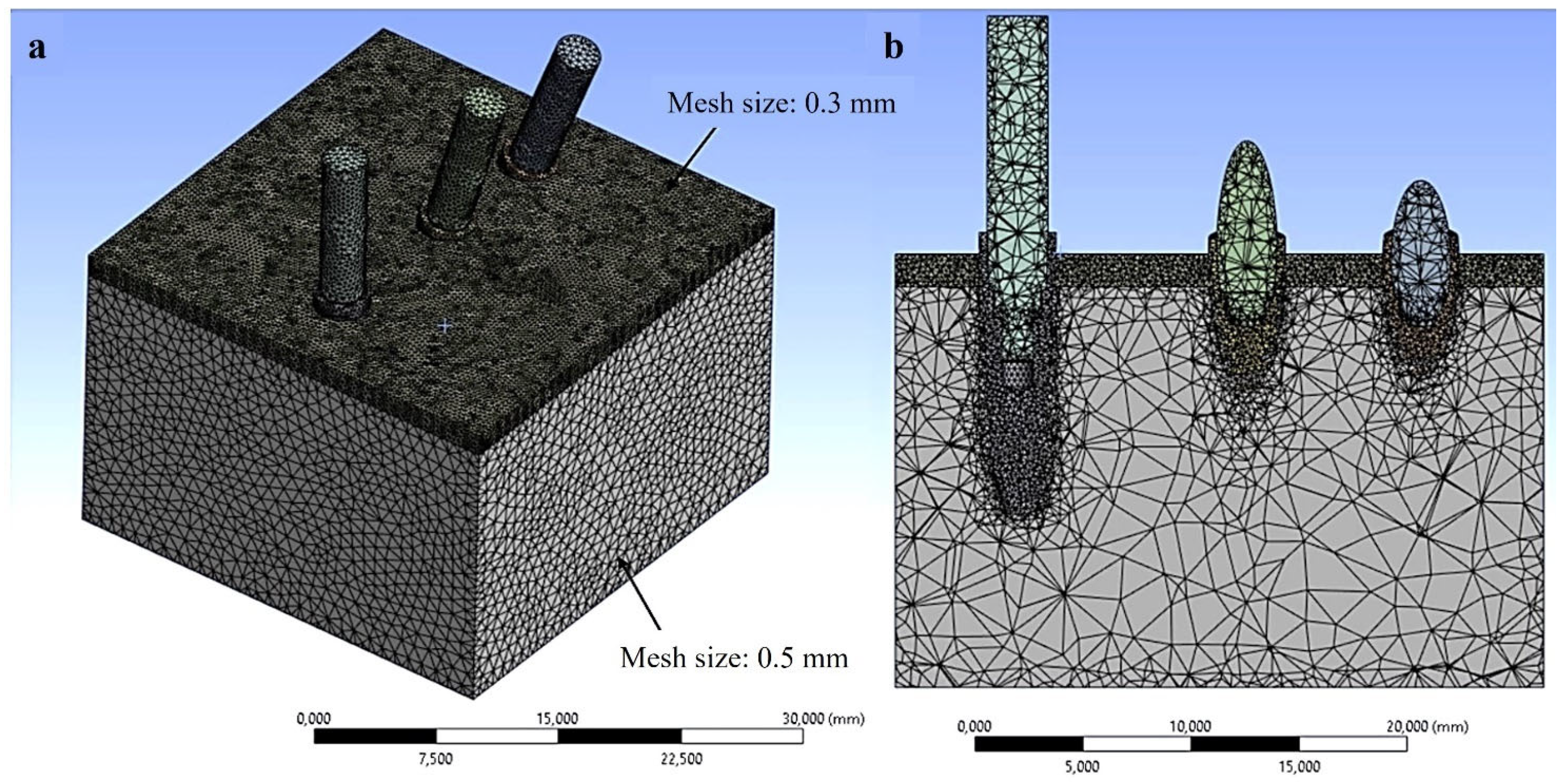
2.3. Finite Element Analysis (FEA) Simulation
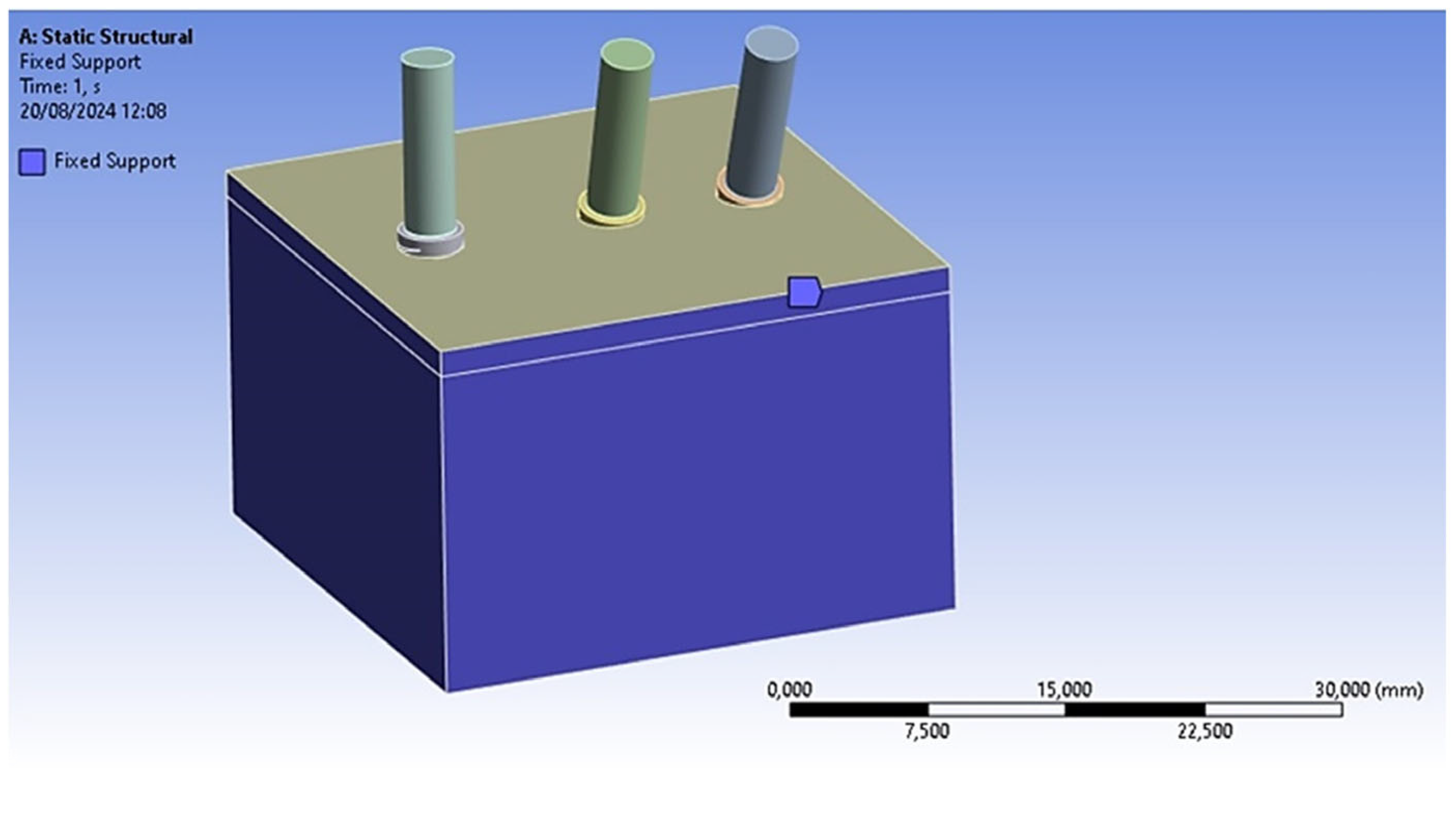
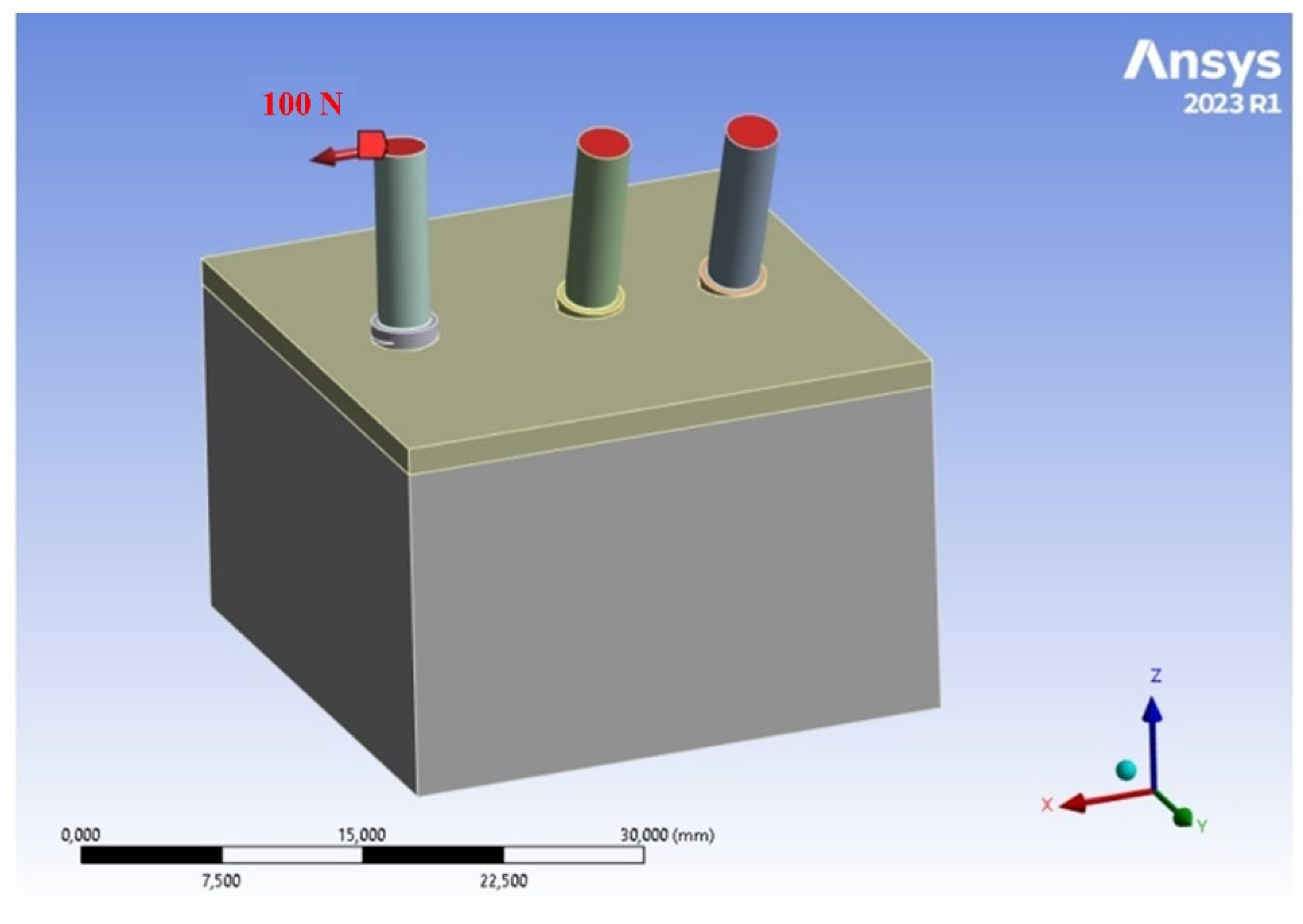
2.4. Loads and Constraints
2.5. Numerical Method for Assessing the Implant Stability Quotient (ISQ)
- A horizontal load of 100 N was applied to inclined implants embedded in the bone;
- The micro-movements of the implant were evaluated using the FEM, concentrating on the micro-movements at the neck of the implants according to the direction of the applied load;
- Subsequently, the results of the micromovement analysis were incorporated into Equation (4) [22].
3. Results
3.1. Analysis of Stress Fields in D3 Bone
3.2. ISQ Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruns, S.; Krüger, D.; Galli, S.; Wieland, D.C.F.; Hammel, J.U.; Beckmann, F.; Wennerberg, A.; Willumeit-Römer, R.; Zeller-Plumhoff, B.; Moosmann, J. On the material dependency of peri-implant morphology and stability in healing bone. Bioact. Mater. 2023, 28, 155–166. [Google Scholar] [PubMed]
- Poovarodom, P.; Rungsiyakull, C.; Suriyawanakul, J.; Li, Q.; Sasaki, K.; Yoda, N.; Rungsiyakull, P. Multi-objective optimization of custom implant abutment design for enhanced bone remodeling in single-crown implants using 3D finite element analysis. Sci. Rep. 2024, 14, 15867. [Google Scholar] [CrossRef]
- English, C.E. Dental Implant Prosthetics Carl E. Misch. Implant Dent. 2005, 14, 11–12. [Google Scholar]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmerer, P.W. How does dental implant macrogeometry affect primary implant stability? A narrative review. Int. J. Implant Dent. 2023, 9, 20. [Google Scholar]
- Huang, Y.C.; Huang, Y.C.; Ding, S.J. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef]
- Bergamo, E.T.P.; Zahoui, A.; Barrera, R.B.; Huwais, S.; Coelho, P.G.; Karateew, E.D.; Bonfante, E.A. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 317–328. [Google Scholar]
- Degidi, M.; Daprile, G. The use of an artificial intelligence-driven novel tool for the evaluation of dental implants primary stability and immediate loading feasibility: An in vitro pilot study. Int. J. Oral Maxillofac. Implant. 2025, 14, 2011. [Google Scholar]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 6170452. [Google Scholar]
- Huang, H.; Wo, G.; Hunziker, E. The clinical significance of implant stability quotient (ISQ) measurements: A literature review. J. Oral Biol. Craniofacial Res. 2020, 10, 629–638. [Google Scholar]
- Hériveaux, Y.; Vayron, R.; Fraulob, M.; Lomami, H.A.; Lenormand, C.; Haïat, G. Assessment of dental implant stability using resonance frequency analysis and quantitative ultrasound methods. J. Prosthodont. Res. 2021, 65, 421–427. [Google Scholar]
- Herrero-Climent, M.; Falcao, A.; Tondela, J.; Brizuela, A.; Rios-Carrasco, B.; Gil, J. Relevant Aspects of the Dental Implant Design on the Insertion Torque, Resonance Frequency Analysis (RFA) and Micromobility: An In Vitro Study. J. Clin. Med. 2023, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Trasarti, S.; Toti, P.; Covani, U.; Crespi, R.; Menchini-Fabris, G.B. Specific use of the implant stability quotient as a guide to improve healing for patients who had undergone rehabilitation with fixed implant-supported dental prostheses. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101528. [Google Scholar] [PubMed]
- Blazquez-Hinarejos, M.; Saka-Herrán, C.; Diez-Alonso, V.; Ayuso-Montero, R.; Velasco-Ortega, E.; Lopez-Lopez, J. Reliability and Agreement of Three Devices for Measuring Implant Stability Quotient in the Animal Ex Vivo Model. Appl. Sci. 2021, 11, 3453. [Google Scholar] [CrossRef]
- Gokmenoglu, C.; Ozmeric, N.; Erguder, I.; Elgun, S. The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed. Laser Surg. 2014, 32, 138–145. [Google Scholar] [CrossRef]
- Mello, B.F.; De Carvalho Formiga, M.; Bianchini, M.A.; Borges, I., Jr.; Coura, G.; Tumedei, M.; Fuller, R.; Petrini, M.; Romasco, T.; Vaz, P.; et al. Insertion Torque (IT) and Implant Stability Quotient (ISQ) Assessment in Dental Implants with and without Healing Chambers: A Polyurethane In Vitro Study. Appl. Sci. 2023, 13, 10215. [Google Scholar] [CrossRef]
- Romasco, T.; De Bortoli, N., Jr.; Paulo De Bortoli, J.; Jorge Jayme, S.; Piattelli, A.; Di Pietro, N. Primary stability evaluation of different morse cone implants in low-density artificial bone blocks: A comparison between high-and low-speed drilling. Heliyon 2024, 10, e35225. [Google Scholar]
- Romasco, T.; Pignatelli, P.; Tumedei, M.; Hossein, H.H.S.; Cipollina, A.; Piattelli, A.; Inchingolo, F.; Di Pietro, N. The influence of truncated-conical implant length on primary stability in maxillary and mandibular regions: An in vitro study using polyurethane blocks. Clin. Oral Investig. 2023, 28, 28. [Google Scholar]
- Comuzzi, L.; Tumedei, M.; Covani, U.; Romasco, T.; Petrini, M.; Montesani, L.; Piattelli, A.; Di Pietro, N. Primary stability assessment of conical implants in under-prepared sites: An in vitro study in low-density polyurethane foams. Appl. Sci. 2023, 10, 6041. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef]
- Wu, H.C.; Huang, H.L.; Fuh, L.J.; Tsai, M.T.; Hsu, J.T. Effect of Implant Length and Insertion Depth on Primary Stability of Short Dental Implant. Int. J. Oral Maxillofac. Implant. 2023, 38, 62–70. [Google Scholar]
- Turkyilmaz, I.; McGlumphy, E.A. Influence of bone density on implant stability parameters and implant success: A retrospective clinical study. BMC Oral Health 2008, 8, 32. [Google Scholar]
- Pammer, D. Evaluation of postoperative dental implant primary stability using 3D finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 280–287. [Google Scholar]
- Lee, H.; Jo, M.; Noh, G. Biomechanical effects of dental implant diameter, connection type, and bone density on microgap formation and fatigue failure: A finite element analysis. Comput. Methods Programs Biomed. 2021, 200, 105863. [Google Scholar]
- Comuzzi, L.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Inchingolo, A.M.; Romasco, T.; Tumedei, M.; Specchiulli, A.; Piattelli, A.; Trentadue, B. Crestal and Subcrestal Placement of Morse Cone Implant–Abutment Connection Implants: An In Vitro Finite Element Analysis (FEA) Study. Biomedicines 2023, 11, 3077. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. FEA Comparison of the Mechanical Behavior of Three Dental Crown Materials: Enamel, Ceramic, and Zirconia. Materials 2024, 17, 673. [Google Scholar] [CrossRef]
- Sciammarella, C.A.; Singh, B.; Trentadue, B.; Sciammarella, F.M. Stress analysis of weldments by holographic moiré and the finite element method. Exp. Mech. 2000, 40, 15–21. [Google Scholar]
- Tardelli, J.D.C.; da Costa Valente, M.L.; Macedo, A.P.; Dos Reis, A.C. Evaluation of biomechanical and stress distribution of different dental implant designs: Primary stability and photoelastic analysis. IRBM 2022, 43, 100–106. [Google Scholar]
- Demirbas, A.E.; Ekici, R.; Karakaya, M.; Alkan, A. Bone stress and damage distributions during dental implant insertion: A novel dynamic FEM analysis. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1381–1392. [Google Scholar]
- Cantó-Navés, O.; Marimon, X.; Ferrer, M.; Cabratosa-Termes, J. Comparison between experimental digital image processing and numerical methods for stress analysis in dental implants with different restorative materials. J. Mech. Behav. Biomed. Mater. 2021, 113, 104092. [Google Scholar]
- Teodorescu, C.; Preoteasa, E.; Preoteasa, C.T.; Murariu-Măgureanu, C.; Teodorescu, I.M. The biomechanical impact of loss of an implant in the treatment with mandibular overdentures on four nonsplinted mini dental implants: A finite element analysis. Materials 2022, 15, 8662. [Google Scholar] [CrossRef]
- Pirmoradian, M.; Naeeni, H.A.; Firouzbakht, M.; Toghraie, D.; Khabaz, M.K.; Darabi, R. Finite element analysis and experimental evaluation on stress distribution and sensitivity of dental implants to assess optimum length and thread pitch. Comput. Methods Programs Biomed. 2020, 187, 105258. [Google Scholar]
- Li, T.; Kong, L.; Wang, Y.; Hu, K.; Song, L.; Liu, B.; Li, D.; Shao, J.; Ding, Y. Selection of optimal dental implant diameter and length in type IV bone: A three dimensional Finite Element Analysis. Int. J. Oral Maxillofac. Surg. 2019, 38, 1077–1083. [Google Scholar]
- Ceddia, M.; Romasco, T.; De Bortoli, N., Jr.; Mello, B.F.; Piattelli, A.; Mijiritsky, E.; Di Pietro, N.; Trentadue, B. Biomechanical Finite Element Analysis of Two Types of Short-Angled Implants Across Various Bone Classifications. Materials 2024, 17, 5680. [Google Scholar] [CrossRef] [PubMed]
- Prados-Privado, M.; Martínez-Martínez, C.; Gehrke, S.A.; Prados-Frutos, J.C. Influence of bone definition and finite element parameters in bone and dental implants stress: A literature review. Biology 2020, 9, 224. [Google Scholar] [CrossRef]
- Milone, D.; Fiorillo, L.; Alberti, F.; Cervino, G.; Filardi, V.; Pistone, A.; Cicciù, M.; Risitano, G. Stress distribution and failure analysis comparison between Zirconia and Titanium dental implants. Procedia Struct. Integr. 2022, 41, 680–691. [Google Scholar]
- Anitua, E.; Larrazabal Saez de Ibarra, N.; Morales Martín, I.; Saracho Rotaeche, L. Influence of Implant Tilting and Length on the Biomechanics of Single-Tooth Restoration: A Finite Element Analysis in Atrophic Mandible. Dent. J. 2022, 10, 77. [Google Scholar] [CrossRef]
- Liu, C.; Xing, Y.; Li, Y.; Lin, Y.; Xu, J.; Wu, D. Bone quality effect on short implants in the edentulous mandible: A finite element study. BMC Oral Health 2022, 22, 139. [Google Scholar]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar]
- Piattelli, A. (Ed.) Bone Response to Dental Implant Materials; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Pinto, E.M.; Neves, J.R.M.; Teixeira, A.; Frada, R.; Atilano, P.; Oliveira, F.; Veigas, T.; Miranda, A. Efficacy of Hounsfield units measured by lumbar computer tomography on bone density assessment: A systematic review. Spine 2022, 47, 702–710. [Google Scholar]
- Wirtz, D.C.; Schiffers, N.; Pandorf, T.; Radermacher, K.; Weichert, D.; Forst, R. Critical evaluation of known bone material properties to realize anisotropic FE-simulation of the proximal femur. J. Biomech. 2000, 33, 1325–1330. [Google Scholar]
- Fleps, I.; Bahaloo, H.; Zysset, P.K.; Ferguson, S.J.; Pálsson, H.; Helgason, B. Empirical relationships between bone density and ultimate strength: A literature review. J. Mech. Behav. Biomed. Mater. 2020, 110, 103866. [Google Scholar]
- Moshage, S.G.; McCoy, A.M.; Kersh, M.E. Elastic modulus and its relation to apparent mineral density in juvenile equine bones of the lower limb. J. Biomech. Eng. 2023, 145, 081001. [Google Scholar]
- Wang, S.H.; Hsu, J.T.; Fuh, L.J.; Peng, S.L.; Huang, H.L.; Tsai, M.T. New classification for bone type at dental implant sites: A dental computed tomography study. BMC Oral Health 2023, 23, 324. [Google Scholar]
- Morar, L.; Băciuț, G.; Băciuț, M.; Bran, S.; Colosi, H.; Manea, A.; Almășan, O.; Dinu, C. Analysis of CBCT bone density using the Hounsfield scale. Prosthesis 2022, 4, 414–423. [Google Scholar] [CrossRef]
- Chang, C.L.; Chen, J.J.; Chen, C.S. Using optimization approach to design dental implant in three types of bone quality-A finite element analysis. J. Dent. Sci. 2025, 20, 126–136. [Google Scholar]
- Sapotnick, A.; Nackenhorst, U. A Mechanically Stimulated Fracture Healing Model Using a Finite Element Framework. In Biomedical Technology; Lenarz, T., Wriggers, P., Eds.; Lecture Notes in Applied and Computational Mechanics; Springer: Cham, Switzerland, 2015; Volume 74. [Google Scholar] [CrossRef]
- Percoco, G.; Uva, A.E.; Fiorentino, M.; Gattullo, M.; Manghisi, V.M.; Boccaccio, A. Mechanobiological Approach to Design and Optimize Bone Tissue Scaffolds 3D Printed with Fused Deposition Modeling: A Feasibility Study. Materials 2020, 13, 648. [Google Scholar] [CrossRef]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.; Maiman, D. Finite element modeling of the cervical spine: Role of the intervertebral disc under axial and eccentric loads. Med. Eng. Phys. 1999, 21, 689–700. [Google Scholar]
- Schmidt, H.; Heuer, F.; Drumm, J.; Klezl, Z.; Claes, L.; Wilke, H.J. Application of a calibration method provides more realistic results for a finite element model of a lumbar spinal segment. Clin. Biomech. 2007, 22, 377–384. [Google Scholar]
- Ezquerro, F.; Vacas, F.G.; Postigo, S.; Prado, M.; Simón, A. Calibration of the finite element model of a lumbar functional spinal unit using an optimization technique based on differential evolution. Med. Eng. Phys. 2011, 33, 89–95. [Google Scholar]
- Diken Turksayar, A.A.; Donmez, M.B. Stress behavior of an anterior single implant restored with high-performance polymer abutments under immediate and delayed loading: A 3D FEA study. J. Prosthodont. 2023, 32, 132–138. [Google Scholar]
- Bittner-Frank, M.; Reisinger, A.G.; Andriotis, O.G.; Pahr, D.H.; Thurner, P.J. Cortical and trabecular mechanical properties in the femoral neck vary differently with changes in bone mineral density. JBMR Plus 2024, 8, ziae049. [Google Scholar] [CrossRef] [PubMed]
- Aydin, T.; Korkmaz, I.H.; Sahin, A.B.; Kaymaz, I. Investigation of subcrestally placed dental implants with and without apical cortical bone anchorage under conventional or immediate loading. Eng. Sci. Technol. Int. J. 2023, 41, 101402. [Google Scholar] [CrossRef]
- Nimbalkar, S.; Dhatrak, P.; Gherde, C.; Joshi, S. A review article on factors affecting bone loss in dental implants. Mater. Today Proc. 2021, 43, 970–976. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Jeng, Y.R. Three-dimensional finite element investigation into effects of implant thread design and loading rate on stress distribution in dental implants and anisotropic bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef]
- Ellendula, Y.; Sekar, A.C.; Nalla, S.; Basany, R.B.; Sailasri, K.; Thandu, A. Biomechanical evaluation of stress distribution in equicrestal and sub-crestally placed, platform-switched Morse taper dental implants in D3 bone: Finite Element Analysis. Cureus 2022, 14, e24591. [Google Scholar] [CrossRef]
- Pagliani, L.; Sennerby, L.; Petersson, A.; Verrocchi, D.; Volpe, S.; Andersson, P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J. Oral Rehabil. 2013, 40, 221–227. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Heydari Sheikh Hossein, H.; Montesani, L.; Inchingolo, F.; Piattelli, A.; Covani, U. A Comparison of Conical and Cylindrical Implants Inserted in an In Vitro Post-Extraction Model Using Low-Density Polyurethane Foam Blocks. Materials 2023, 16, 5064. [Google Scholar] [CrossRef]
- Díaz-Sánchez, R.M.; Delgado-Muñoz, J.M.; Hita-Iglesias, P.; Pullen, K.T.; Serrera-Figallo, M.Á.; Torres-Lagares, D. Improvement in the Initial Implant Stability Quotient Through Use of a Modified Surgical Technique. J. Oral Implantol. 2017, 43, 186–193. [Google Scholar] [CrossRef]
- Deliverska, E.; Yordanov, B. Osseodensification as an alternative approach in dental implantology for implant sites with insufficient available bone. J. IMAB Annu. Proceeding Sci. Pap. 2019, 25, 2606–2610. [Google Scholar] [CrossRef]
- Comuzzi, L.; Romasco, T.; Piattelli, A.; Inchingolo, F.; Mourão, C.F.; Di Pietro, N. Comparative Evaluation of Primary Stability in Truncated Cone Implants with Different Macro-Geometries in Low-Density Polyurethane Blocks Simulating Maxillary Sinus Rehabilitations. Prosthesis 2024, 6, 923–938. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Montesani, L.; Piattelli, A.; Covani, U. Are Implant Threads Important for Implant Stability? An In Vitro Study Using Low-Density Polyurethane Sheets. Eng 2023, 4, 1167–1178. [Google Scholar] [CrossRef]
- Heydecke, G.; Mirzakhanian, C.; Behneke, A.; Behneke, N.; Fügl, A.; Zechner, W.; Baer, R.A.; Nölken, R.; Gottesman, E.; Colic, S.; et al. A prospective multicenter evaluation of immediately functionalized tapered conical connection implants for single restorations in maxillary anterior and premolar sites: 3-year results. Clin. Oral Investig. 2019, 23, 1877–1885. [Google Scholar] [PubMed]
- Comuzzi, L.; Tumedei, M.; Petrini, M.; Romasco, T.; Lorusso, F.; De Angelis, F.; Piattelli, A.; Tatullo, M.; Di Pietro, N. Clinical and radiological evaluation of a self-condensing bone implant in one-stage sinus augmentation: A 3-year follow-up retrospective study. Int. J. Environ. Res. Public Health 2023, 20, 2583. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; López-Jarana, P.; Lemos, B.F.; Gil, F.J.; Falcao, C.; Ríos-Santos, J.V.; Ríos-Carrasco, B.L.A.N.C.A. Relevant design aspects to improve the stability of titanium dental implants. Materials 2020, 13, 1910. [Google Scholar] [CrossRef]
- Yu, W.; Li, X.; Ma, X.; Xu, X. Biomechanical analysis of inclined and cantilever design with different implant framework materials in mandibular complete-arch implant restorations. J. Prosthet. Dent. 2022, 127, 783-e1. [Google Scholar] [CrossRef]
- Patil, P.G.; Seow, L.L.; Uddanwadikar, R.; Pau, A.; Ukey, P.D. Stress and strain patterns of 2-implant mandibular overdentures with different positions and angulations of implants: A 3D finite element analysis study. J. Prosthet. Dent. 2023, 130, 586–596. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takahashi, T.; Gonda, T.; Ikebe, K. Influence of Implant Distribution and Palatal Coverage on the Strain of Splinted Implant and Overdenture in Maxillary Implant Overdentures. Int. J. Oral Maxillofac. Implant. 2021, 36, 281–288. [Google Scholar]
- Guven, S.; Atalay, Y.; Asutay, F.; Ucan, M.C.; Dundar, S.; Karaman, T.; Gunes, N. Comparison of the effects of different loading locations on stresses transferred to straight and angled implant-supported zirconia frameworks: A finite element method study. Biotech. Biotechnol. Equip. 2015, 29, 766–772. [Google Scholar]
- Ibrahim, C.R.M.; Sameh, A.; Askar, O. A finite element analysis study on different angle correction designs for inclined implants in All-On-Four protocol. BMC Oral Health 2024, 24, 331. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Tealdo, T.; Menini, M.; Pera, F.; Mossolov, A.; Drago, C.; Pera, P. The influence of cantilever length and implant inclination on stress distribution in maxillary implant-supported fixed dentures. J. Prosthet. Dent. 2011, 105, 5–13. [Google Scholar]
- Wu, H.C.; Tsai, M.T.; Hsu, J.T. The effect of insertion angles and depths of dental implant on the initial stability. Appl. Sci. 2020, 10, 3112. [Google Scholar] [CrossRef]
- Liao, S.H.; Tong, R.F.; Dong, J.X. Influence of anisotropy on peri-implant stress and strain in complete mandible model from CT. Comput. Med. Imaging Graph. 2008, 32, 53–60. [Google Scholar] [PubMed]
- O’Mahony, A.M.; Williams, J.L.; Spencer, P. Anisotropic elasticity of cortical and cancellous bone in the posterior mandible increases peri-implant stress and strain under oblique loading. Clin. Oral Implant. Res. 2001, 12, 648–657. [Google Scholar]
- Geraldes, D.M.; Phillips, A.T. A comparative study of orthotropic and isotropic bone adaptation in the femur. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 873–889. [Google Scholar]
- Soleimani, K.; Ghasemloonia, A.; Sudak, L. Influence of interstitial fluid pressure, porosity, loading magnitude, and anisotropy in cortical bone adaptation. Comput. Biol. Med. 2024, 181, 109026. [Google Scholar]
- Kovács, K.; Váncsa, S.; Agócs, G.; Harnos, A.; Hegyi, P.; Weninger, V.; Baross, K.; Kovács, B.; Soós, G.; Kocsis, G. Anisotropy, Anatomical Region, and Additional Variables Influence Young’s Modulus of Bone: A Systematic Review and Meta-Analysis. J. Bone Miner. Res. Plus 2023, 7, e10835. [Google Scholar]
- Atthapreyangkul, A.; Hoffman, M.; Pearce, G. Effect of geometrical structure variations on the viscoelastic and anisotropic behaviour of cortical bone using multi-scale finite element modelling. J. Mech. Behav. Biomed. Mater. 2021, 113, 104153. [Google Scholar]
- Dorado, S.; Arias, A.; Jimenez-Octavio, J.R. Biomechanical modelling for tooth survival studies: Mechanical properties, loads and boundary conditions—A narrative review. Materials 2022, 15, 7852. [Google Scholar] [CrossRef]
- Talreja, K.S.; Rodrigues, S.J.; Pai, U.Y.; Shetty, T.; Saldanha, S.; Mahesh, M.; Hegde, P.; Shenoy, S.B.; Naik, N.; Mukherjee, S.; et al. A Nonlinear Three-Dimensional Finite Element Analysis of Stress Distribution and Microstrain Evaluation in Short Dental Implants with Three Different Implant–Abutment Connections in Single and Splinted Conditions in the Posterior Mandible. Int. J. Dent. 2023, 2023, 8851098. [Google Scholar]
- Szczepańczyk, P.; Szlachta, M.; Złocista-Szewczyk, N.; Chłopek, J.; Pielichowska, K. Recent developments in polyurethane-based materials for bone tissue engineering. Polymers 2021, 13, 946. [Google Scholar] [CrossRef]
- Strait, D.S.; Wang, Q.; Dechow, P.C.; Ross, C.F.; Richmond, B.G.; Spencer, M.A.; Patel, B.A. Modeling elastic properties in finite-element analysis: How much precision is needed to produce an accurate model? Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2005, 283, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Tekkaya, A.E.; Martins, P.A. Accuracy, reliability and validity of finite element analysis in metal forming: A user’s perspective. Eng. Comput. 2009, 26, 1026–1055. [Google Scholar]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite element analysis of fracture fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [PubMed]
- Wu, J.; Wang, D. An accuracy analysis of Galerkin meshfree methods accounting for numerical integration. Comput. Methods Appl. Mech. Eng. 2021, 375, 113631. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, H.; Ding, T. Finite element analysis on the anisotropic behavior of 3D printed concrete under compression and flexure. Addit. Manuf. 2021, 39, 101712. [Google Scholar]
- Kuang, Y.; Shen, Y.; Hu, G. Towards chemical accuracy using a multi-mesh adaptive finite element method in all-electron density functional theory. J. Comput. Phys. 2024, 518, 113312. [Google Scholar] [CrossRef]




| Mechanical Properties | Cortical Bone | D1 Bone | D2 Bone | D3 Bone | D4 Bone |
|---|---|---|---|---|---|
| Density (, kg/m3) | 1600 | 800 | 640 | 480 | 320 |
| Young’s Modulus (E, MPa) | 16,000 | 9500 | 5500 | 1600 | 690 |
| Poisson’s Ratio | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Material | ISQ (0°) | ISQ (15°) | ISQ (20°) |
|---|---|---|---|
| D2-type bone | 60.96 | 61.05 | 61.10 |
| D3-type bone | 55.68 | 55.77 | 55.90 |
| Material | Average ISQ Through FEA | Average ISQ Through In Vitro Testing [59] |
|---|---|---|
| D2-type bone | 61.10 | 62.90 |
| D3-type bone | 55.78 | 56.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Romasco, T.; Marchioli, G.; Comuzzi, L.; Cipollina, A.; Piattelli, A.; Lamberti, L.; Di Pietro, N.; Trentadue, B. Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°. Materials 2025, 18, 1625. https://doi.org/10.3390/ma18071625
Ceddia M, Romasco T, Marchioli G, Comuzzi L, Cipollina A, Piattelli A, Lamberti L, Di Pietro N, Trentadue B. Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°. Materials. 2025; 18(7):1625. https://doi.org/10.3390/ma18071625
Chicago/Turabian StyleCeddia, Mario, Tea Romasco, Giulia Marchioli, Luca Comuzzi, Alessandro Cipollina, Adriano Piattelli, Luciano Lamberti, Natalia Di Pietro, and Bartolomeo Trentadue. 2025. "Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°" Materials 18, no. 7: 1625. https://doi.org/10.3390/ma18071625
APA StyleCeddia, M., Romasco, T., Marchioli, G., Comuzzi, L., Cipollina, A., Piattelli, A., Lamberti, L., Di Pietro, N., & Trentadue, B. (2025). Finite Element Analysis of Implant Stability Quotient (ISQ) and Bone Stresses for Implant Inclinations of 0°, 15°, and 20°. Materials, 18(7), 1625. https://doi.org/10.3390/ma18071625









