Effect of Organotin on Performance of Siloxane Materials for Surface Treatment of Cementitious Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Cement Paste and Surface Treatment
2.3. Preparation of Cement Hydration Piece and Surface Treatment
2.4. Hardness Test
2.5. Water Absorption and Contact Angle Test
2.6. Characterization
3. Results and Discussion
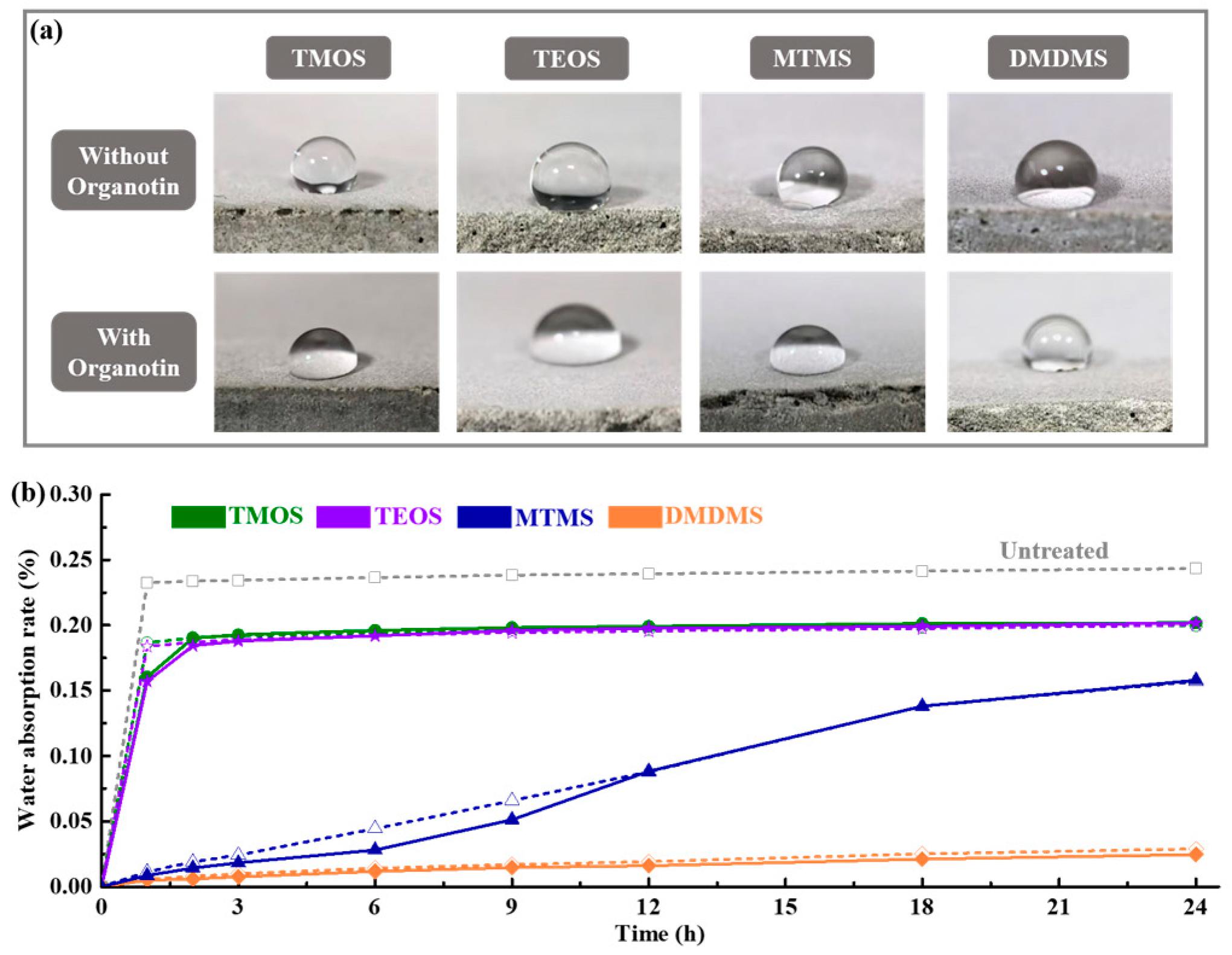
3.1. Waterproofing
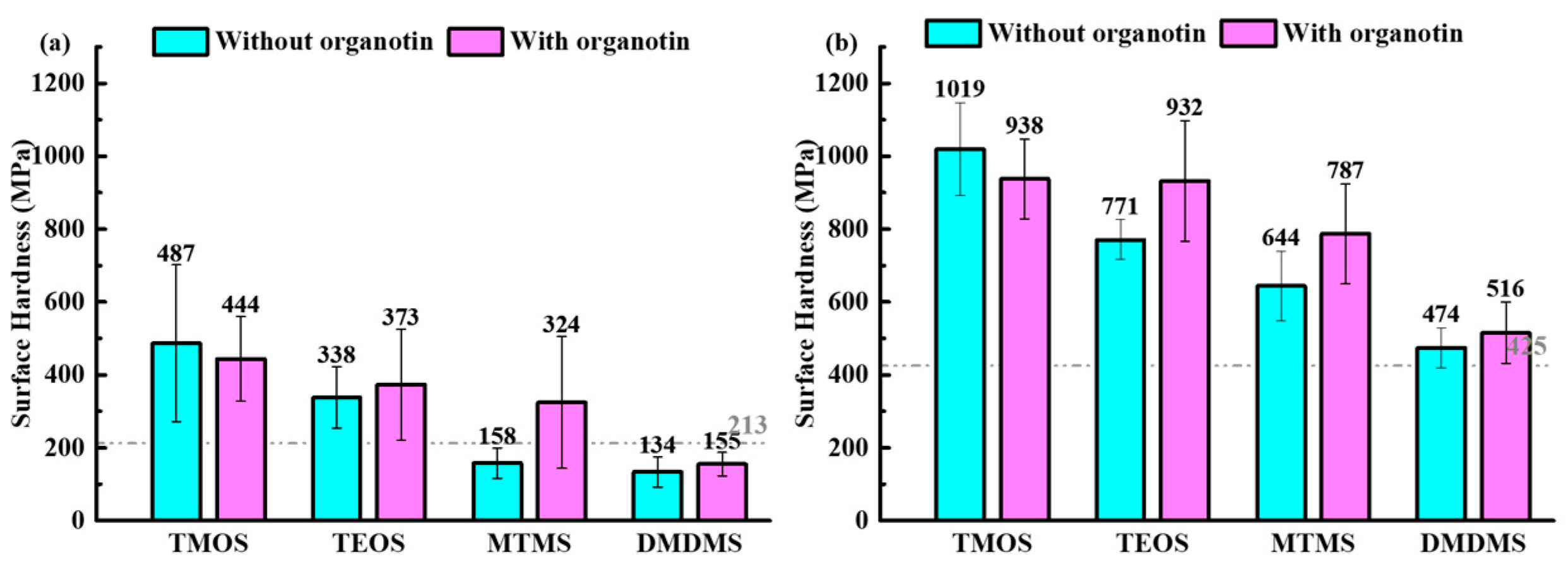
3.2. Surface Hardness
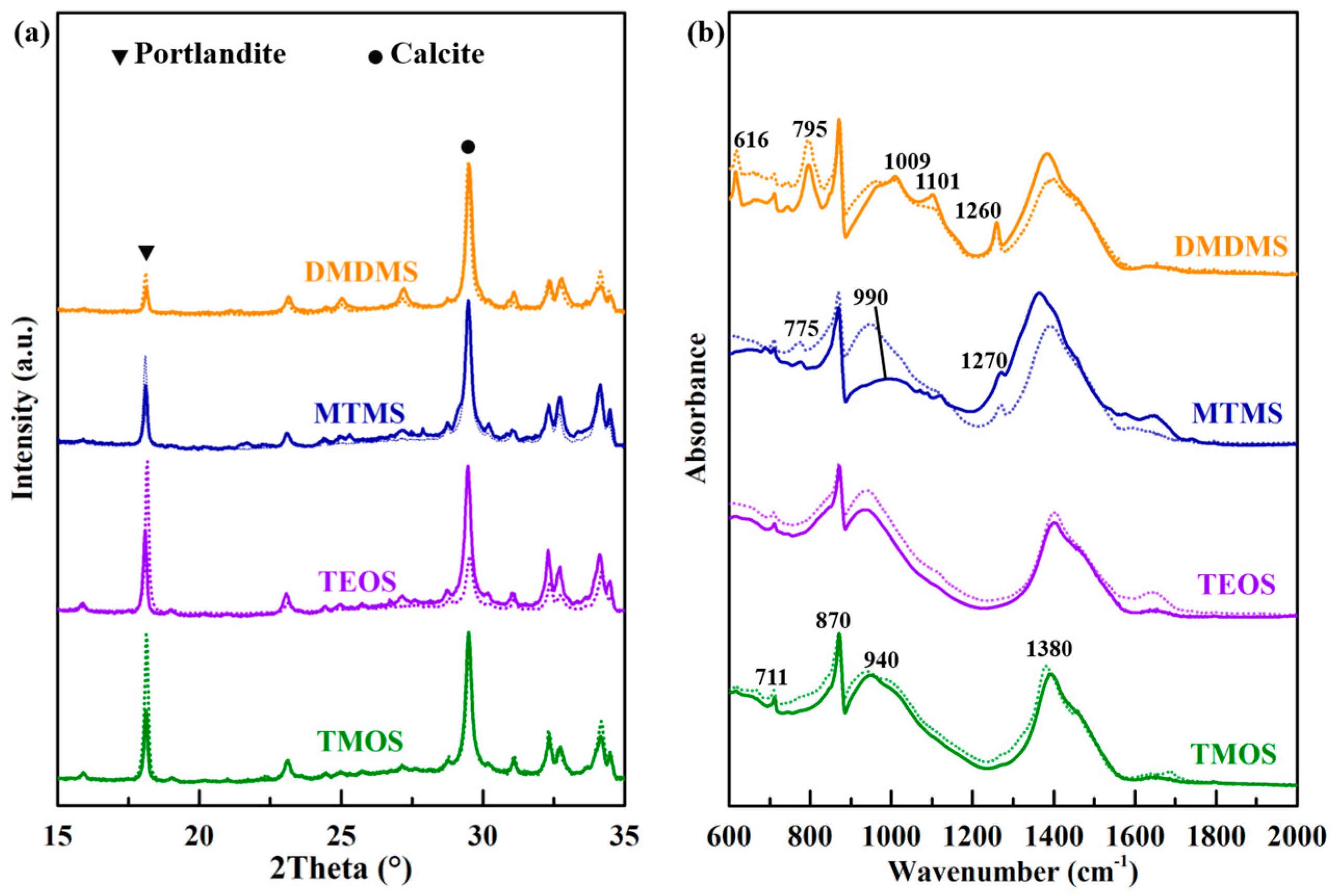
3.3. Chemical Composition
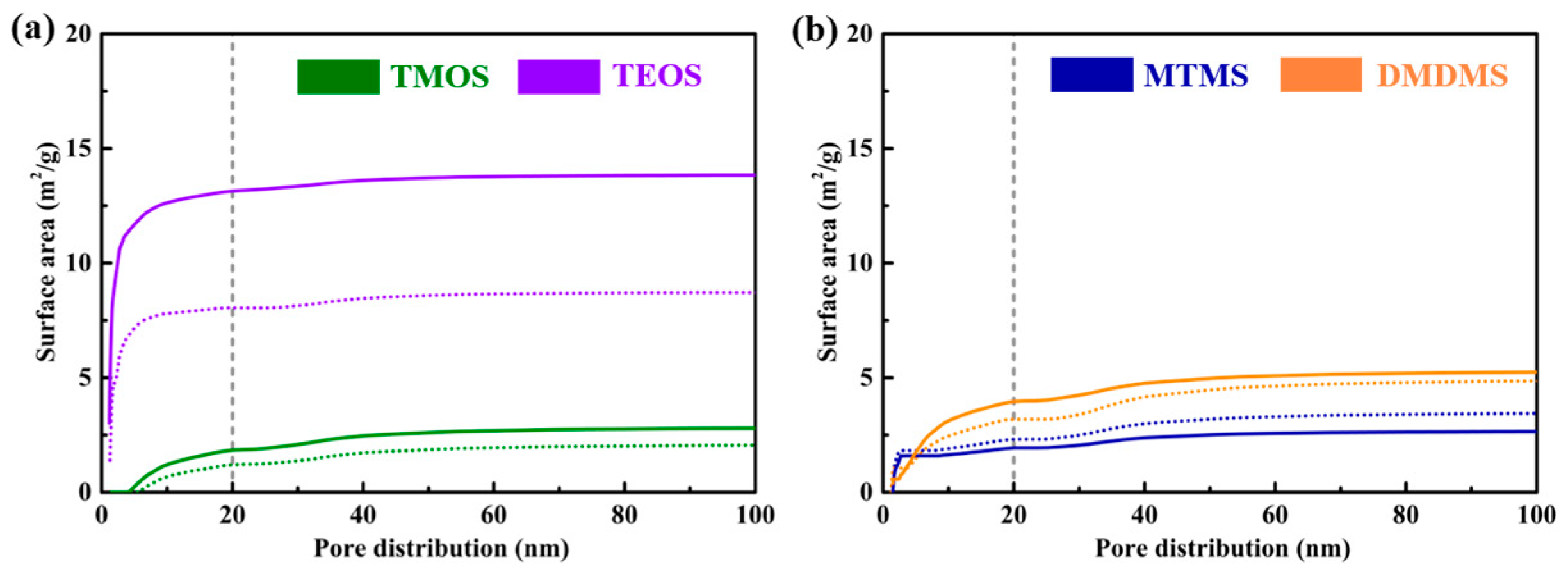
3.4. Pore Structure
3.5. Surface Layer Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, B.; Wu, C.; Hou, D.; Li, S.; Jin, Z.; Wang, M.; Wang, X. Research and application progress of nano-modified coating in improving the durability of cement-based materials. Prog. Org. Coat. 2021, 161, 106529. [Google Scholar] [CrossRef]
- Safiuddin, M. Sealer and coating systems for the protection of concrete bridge structures. Int. J. Phys. Sci. 2011, 6, 8188–8199. [Google Scholar] [CrossRef]
- Safiuddin, M. Concrete Damage in Field Conditions and Protective Sealer and Coating Systems. Coatings 2017, 7, 90. [Google Scholar] [CrossRef]
- Thompson, J.; Silsbee, M.; Gill, P.; Scheetz, B. Characterization of silicate sealers on concrete. Cem. Concr. Res. 1997, 27, 1561–1567. [Google Scholar] [CrossRef]
- Basheer, P.; Basheer, L.; Cleland, D.; Long, A. Surface treatments for concrete: Assessmentmethods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Bérubé, M.-A.; Chouinard, D.; Pigeon, M.; Frenette, J.; Rivest, M.; Vézina, D. Effectiveness of sealers in counteracting alkali-silica reaction in highway median barriers exposed to wetting and drying, freezing and thawing, and deicing salt. Can. J. Civ. Eng. 2002, 29, 329–337. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2007, 41, 59–71. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete—Part II: Characteristics and performance. Cem. Concr. Compos. 2012, 34, 313–321. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; Ley, M.T. Determining the effective service life of silane treatments in concrete bridge decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Murray, C.D.; Deschenes, R.A.; Hale, W.M. Durability of Silane Sealer in Highly Alkaline Environment. ACI Mater. J. 2016, 113, 387–393. [Google Scholar] [CrossRef][Green Version]
- Yuan, G.; Li, Q. The use of surface coating in enhancing the mechanical properties and durability of concrete exposed to elevated temperature. Constr. Build. Mater. 2015, 95, 375–383. [Google Scholar] [CrossRef]
- Pan, X.; Shi, C.; Zhang, J.; Jia, L.; Chong, L. Effect of inorganic surface treatment on surface hardness and carbonation of cement-based materials. Cem. Concr. Compos. 2018, 90, 218–224. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, X.; Zhang, W.; Yang, J.; Zhang, H.; Li, Y.; Zhang, R.; Zhang, Z.; Xu, L.; Qu, J.; et al. The investigation of factors affecting the water impermeability of inorganic sodium silicate-based concrete sealers. Constr. Build. Mater. 2015, 93, 729–736. [Google Scholar] [CrossRef]
- Song, Z.; Xue, X.; Li, Y.; Yang, J.; He, Z.; Shen, S.; Jiang, L.; Zhang, W.; Xu, L.; Zhang, H.; et al. Experimental exploration of the waterproofing mechanism of inorganic sodium silicate-based concrete sealers. Constr. Build. Mater. 2016, 104, 276–283. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Hu, X.; Wu, L. Interactions between inorganic surface treatment agents and matrix of Portland cement-based materials. Constr. Build. Mater. 2016, 113, 721–731. [Google Scholar] [CrossRef]
- Tran, P.C.; Kobayashi, K.; Asano, T.; Kojima, S. Carbonation Proofing Mechanism of Silicate-Based Surface Impregnations. J. Adv. Concr. Technol. 2018, 16, 512–521. [Google Scholar] [CrossRef]
- Li, X.; Pan, C.; Li, D.; Geng, J.; Chen, N.; He, J.; Liu, S. Design of CNS-Li2SiO3 Permeable Protective Coatings and Effects on Mortar Matrix. Materials 2020, 13, 1733. [Google Scholar] [CrossRef]
- Song, Z.; Lu, Z.; Lai, Z. The effect of lithium silicate impregnation on the compressive strength and pore structure of foam concrete. Constr. Build. Mater. 2021, 277, 122316. [Google Scholar] [CrossRef]
- Li, Z.; Shi, X. Effects of Nanomaterials on Engineering Performance of a Potassium Methyl Siliconate–Based Sealer for Cementitious Composite. J. Mater. Civ. Eng. 2022, 34, 04022006. [Google Scholar] [CrossRef]
- Yu, W.; Wenhua, Z.; Peipei, W.; Yilin, P.; Yunsheng, Z. Study on preparation and strengthening mechanism of new surface treatment agent of concrete at multi-scale. Constr. Build. Mater. 2022, 346, 128404. [Google Scholar] [CrossRef]
- Xiong, B.-B.; Gao, L.; Chen, J.-G.; Lu, X.-C.; Tian, B.; Chen, B.-F.; Li, Y.-B. Action mechanism for improving water impermeability of concrete surface based on deep penetrating sealer. Constr. Build. Mater. 2022, 322, 126424. [Google Scholar] [CrossRef]
- Dong, Q.; Cao, X.; Wang, S.; Chen, X.; Yang, B.; Xie, S.; Cheng, Z. Design and evaluation of an innovative composite silicate-based surface treatment agent of concrete. Case Stud. Constr. Mater. 2023, 18, e02207. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.-G.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Doğan, F.; Dehghanpour, H. Characterization and hydrophobic surface study of silicon-based TiO2, ZnO and recycled carbon additives on cementitious materials surface. J. Build. Eng. 2021, 40, 102689. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Qian, B.; Xiong, Y. Effect of nano-modified permeable silicone emulsion on the durability of concrete curbstone. Constr. Build. Mater. 2022, 324, 126620. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic and superamphiphobic materials for the conservation of natural stone: An overview. Constr. Build. Mater. 2022, 320, 126175. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, L.; Feng, Y. A review on silane and siloxane materials: Enhancing durability of cementitious materials through surface treatments. J. Mater. Sci. 2024, 59, 10119–10139. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, Z.; Gu, Z.; Shen, P.; Tao, Y.; Li, W.; Poon, C. Impregnate Carbonation: CO2-Guided In Situ Growth of Robust Superhydrophobic Structures on Concrete Surfaces. Adv. Mater. 2024, 36, e2405492. [Google Scholar] [CrossRef]
- Franzoni, E.; Pigino, B.; Pistolesi, C. Ethyl silicate for surface protection of concrete: Performance in comparison with other inorganic surface treatments. Cem. Concr. Compos. 2013, 44, 69–76. [Google Scholar] [CrossRef]
- Cai, Y.; Hou, P.; Duan, C.; Zhang, R.; Zhou, Z.; Cheng, X.; Shah, S. The use of tetraethyl orthosilicate silane (TEOS) for surface-treatment of hardened cement-based materials: A comparison study with normal treatment agents. Constr. Build. Mater. 2016, 117, 144–151. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.-C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Z.; Hou, P.; Fu, X.; Qu, Y.; Li, Q.; Cheng, X.; Zhu, X. The influence of surface treatment on the transport properties of hardened calcium sulfoaluminate cement-based materials. Cem. Concr. Compos. 2020, 114, 103784. [Google Scholar] [CrossRef]
- Iliushchenko, V.; Kalina, L.; Hruby, P.; Bilek, V., Jr.; Fladr, J.; Bily, P.; Bojanovsky, J. The treatment of cementitious surface by selected silicate sealers. J. Phys. Conf. Ser. 2022, 2341, 12003. [Google Scholar]
- Méndez-Vivar, J. The interaction of dibutyltin dilaureate with tetraethyl orthosilicate in sol-gel systems. J. Sol-Gel Sci. Technol. 2006, 38, 159–166. [Google Scholar] [CrossRef]
- Cervantes, J.; Zárraga, R.; Salazar-Hernández, C. Organotin catalysts in organosilicon chemistry. Appl. Organomet. Chem. 2012, 26, 157–163. [Google Scholar] [CrossRef]
- Hernández, C.S.; Hernández, M.S.; Cerritos, R.C.; Elorza, E.; Mendoza-Miranda, J.M.; Navarro, R. DBTL as neutral catalyst on TEOS/PDMS anticorrosive coating. J. Sol-Gel Sci. Technol. 2017, 81, 405–412. [Google Scholar] [CrossRef]
- Jurásková, A.; Olsen, S.M.; Skov, A.L. Reversible Dynamic Behavior of Condensation-Cured Silicone Elastomers Caused by a Catalyst. Macromol. Mater. Eng. 2023, 308, 2200615. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Raimondi, M.E.; Tetteh, J.; McKay, B.; Maschmeyer, T. Mechanistic Study of Silsesquioxane Synthesis by Mass Spectrometry and in Situ ATR FT-IR Spectroscopy. J. Phys. Chem. A 2003, 107, 8885–8892. [Google Scholar] [CrossRef]
- Chollet, M.; Horgnies, M. Analyses of the surfaces of concrete by Raman and FT-IR spectroscopies: Comparative study of hardened samples after demoulding and after organic post-treatment. Surf. Interface Anal. 2011, 43, 714–725. [Google Scholar] [CrossRef]
- John, E.; Stephan, D. Calcium silicate hydrate—In-situ development of the silicate structure followed by infrared spectroscopy. J. Am. Ceram. Soc. 2021, 104, 6611–6624. [Google Scholar] [CrossRef]
- Sato, Y.; Hayami, R.; Gunji, T. Characterization of NMR, IR, and Raman spectra for siloxanes and silsesquioxanes: A mini review. J. Sol-Gel Sci. Technol. 2022, 104, 36–52. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Zhang, J.; Li, Y.; Yang, H. Effect of Organotin on Performance of Siloxane Materials for Surface Treatment of Cementitious Materials. Materials 2025, 18, 1626. https://doi.org/10.3390/ma18071626
Huang K, Zhang J, Li Y, Yang H. Effect of Organotin on Performance of Siloxane Materials for Surface Treatment of Cementitious Materials. Materials. 2025; 18(7):1626. https://doi.org/10.3390/ma18071626
Chicago/Turabian StyleHuang, Kaiyue, Ji Zhang, Yue Li, and Hui Yang. 2025. "Effect of Organotin on Performance of Siloxane Materials for Surface Treatment of Cementitious Materials" Materials 18, no. 7: 1626. https://doi.org/10.3390/ma18071626
APA StyleHuang, K., Zhang, J., Li, Y., & Yang, H. (2025). Effect of Organotin on Performance of Siloxane Materials for Surface Treatment of Cementitious Materials. Materials, 18(7), 1626. https://doi.org/10.3390/ma18071626




