Preparation and Properties of Boron Modified Phenolic Resin for Automotive Friction Materials
Highlights
- Boron-modified phenolic resins were synthesized mainly for the characterization of heat-resistant properties.
- Semi-metallic brake pads were prepared using boron-modified phenolic resin as a binder, and their friction and wear properties were compared with those prepared with ordinary phenolic resin.
- Surface microstructure and morphology of carbon ceramic discs and brake pads were analysed and a schematic diagram proposed to to assist the analysis of thermal degradation mechanism.
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of Nano-Al2O3/BPR
2.2.2. Preparation of Nano-Al2O3/PBPR
2.2.3. Preparation of FPBPR
2.2.4. Preparation of Friction Materials
2.3. Testing and Characterization
2.3.1. Testing Methods
2.3.2. Characterization
3. Results and Discussion
3.1. Properties and Characterization of Resins
3.1.1. Structure of Modified Phenolic Resins
3.1.2. Thermogravimetric Properties of Modified Phenolic Resins
3.1.3. Curing of Modified Phenolic Resins
3.2. Characterization of Brake Pads
3.2.1. Brinell Hardness and Shear Strength
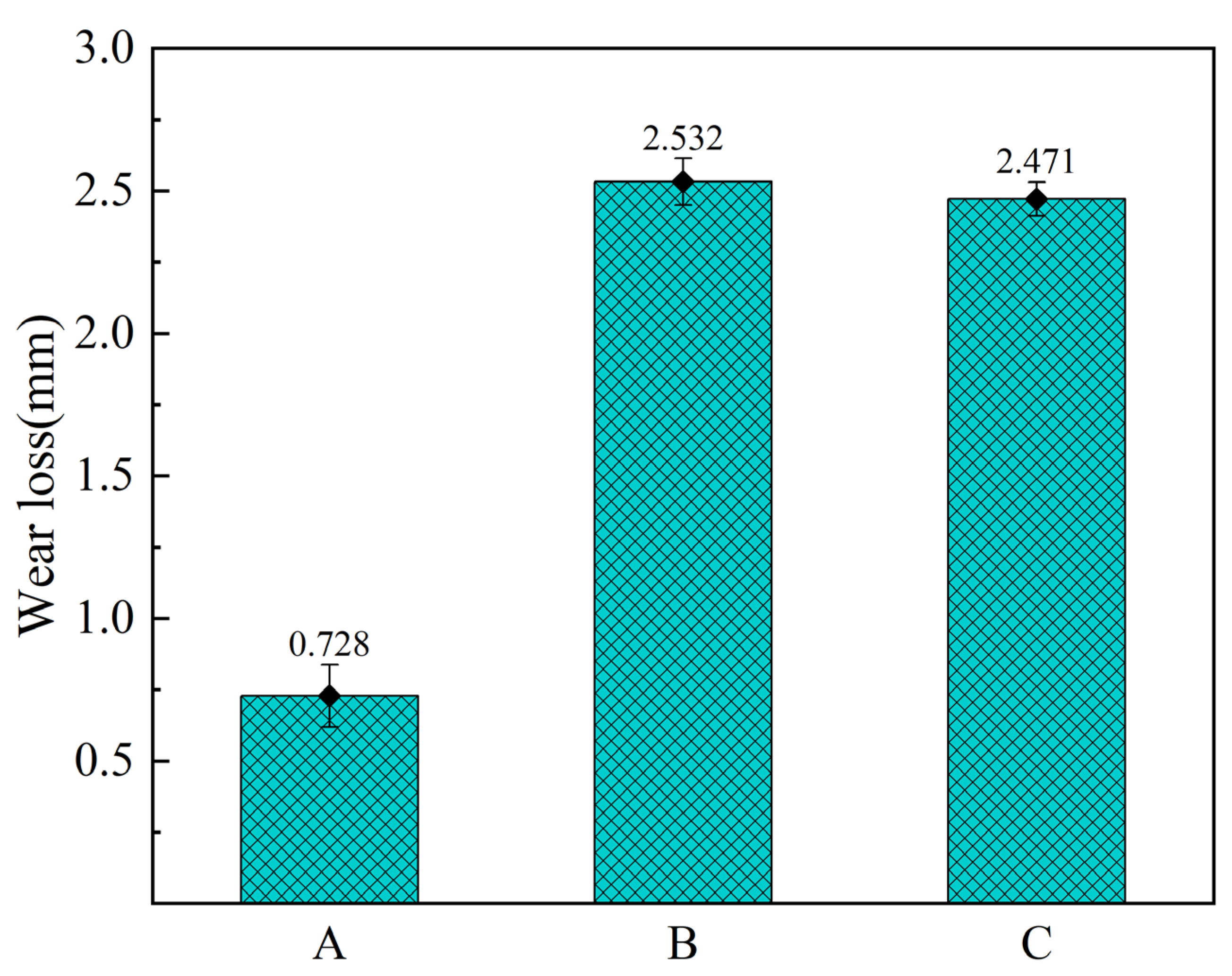
3.2.2. Friction and Wear Properties
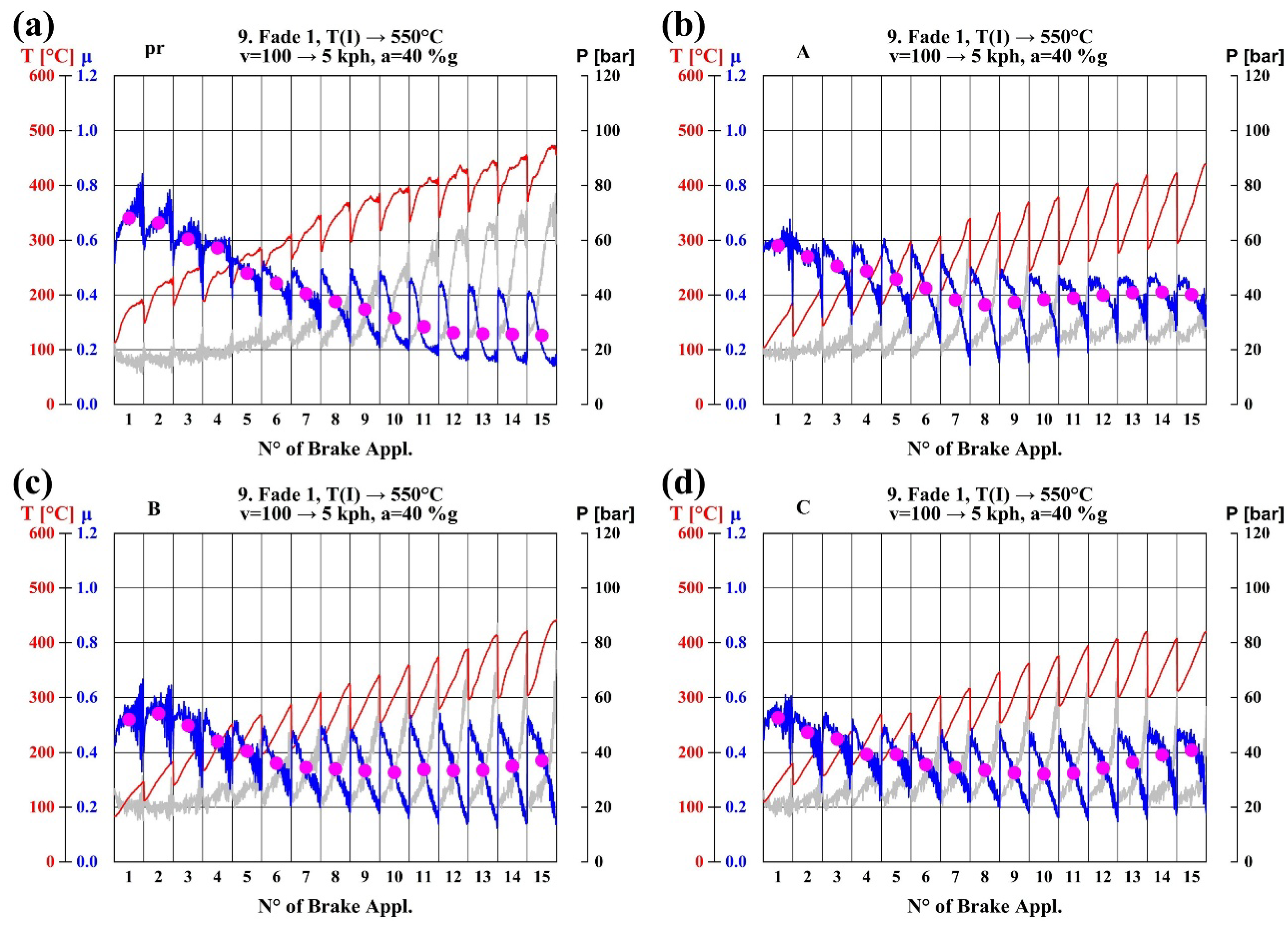
3.3. Thermal Fade
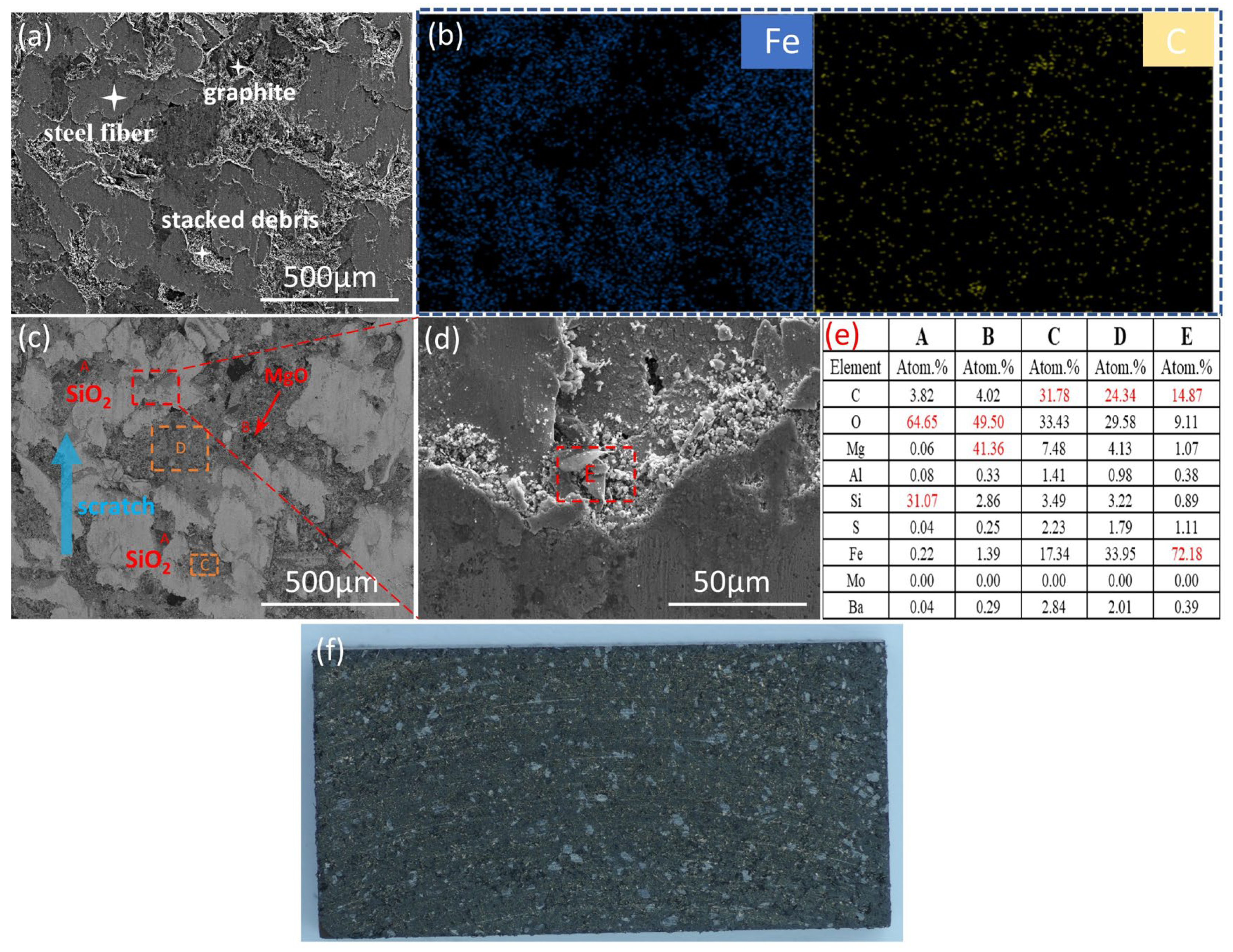
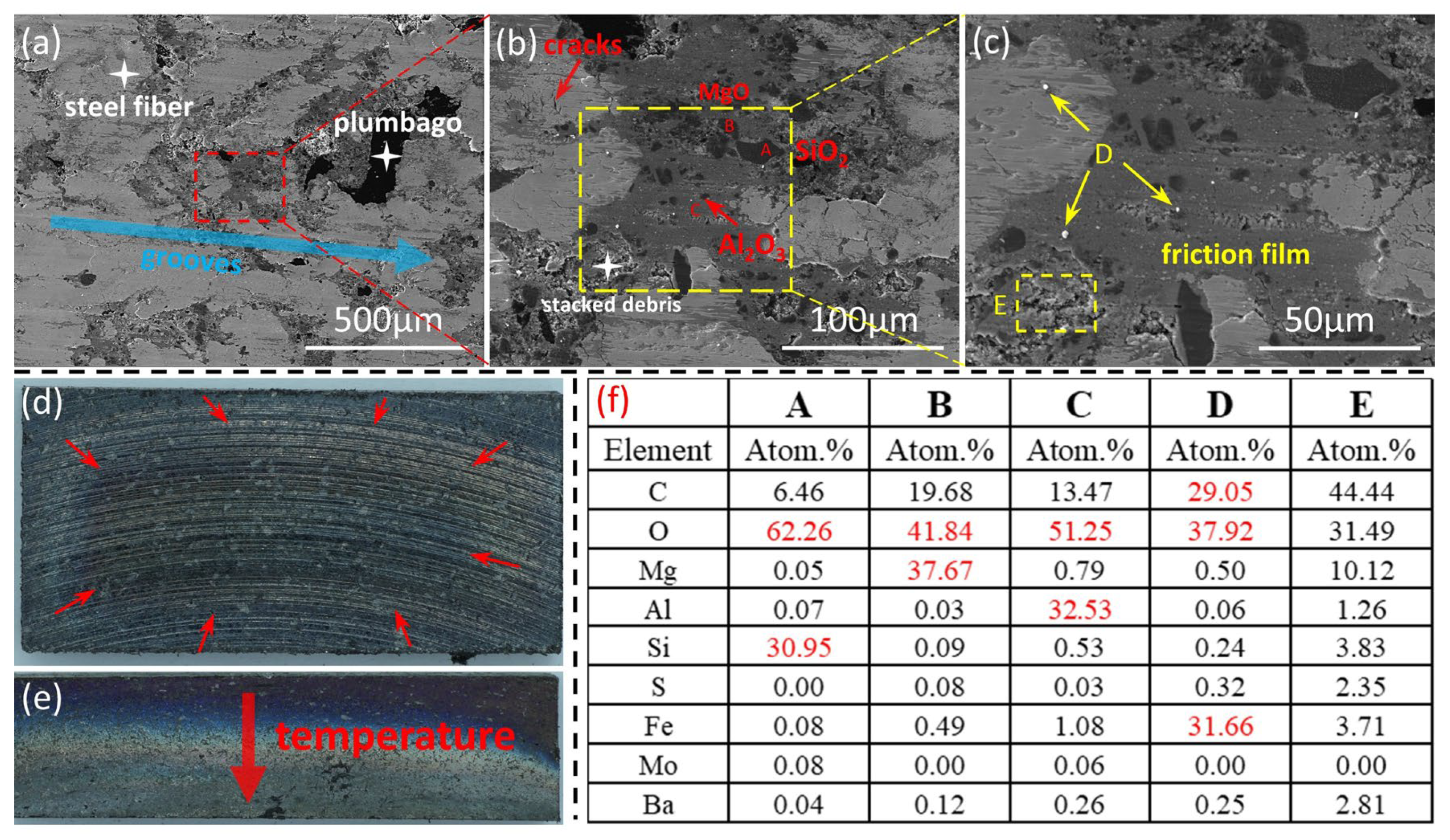
3.3.1. Surface Morphology of Brake Discs
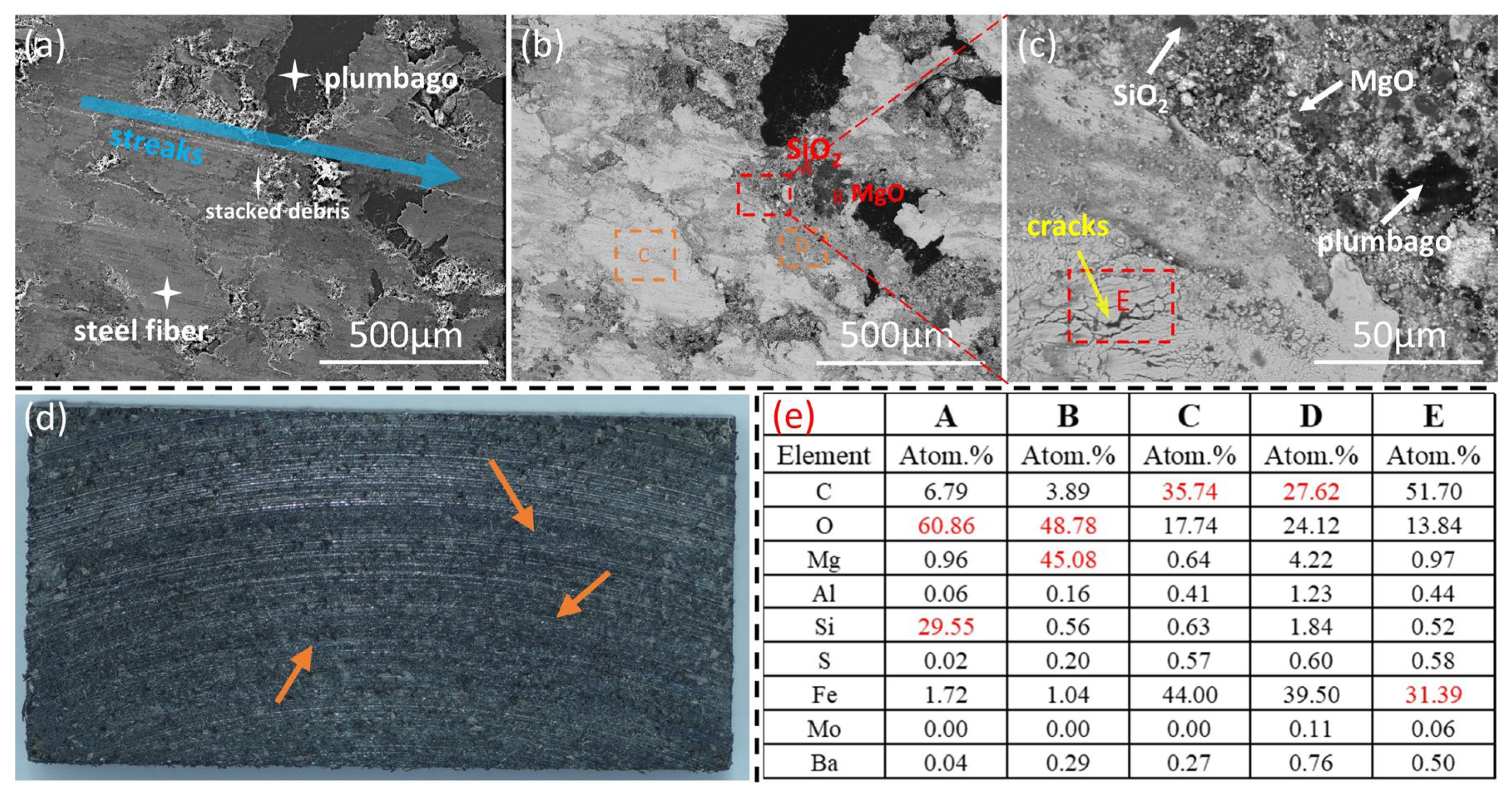
3.3.2. Surface Morphology of Brake Pads
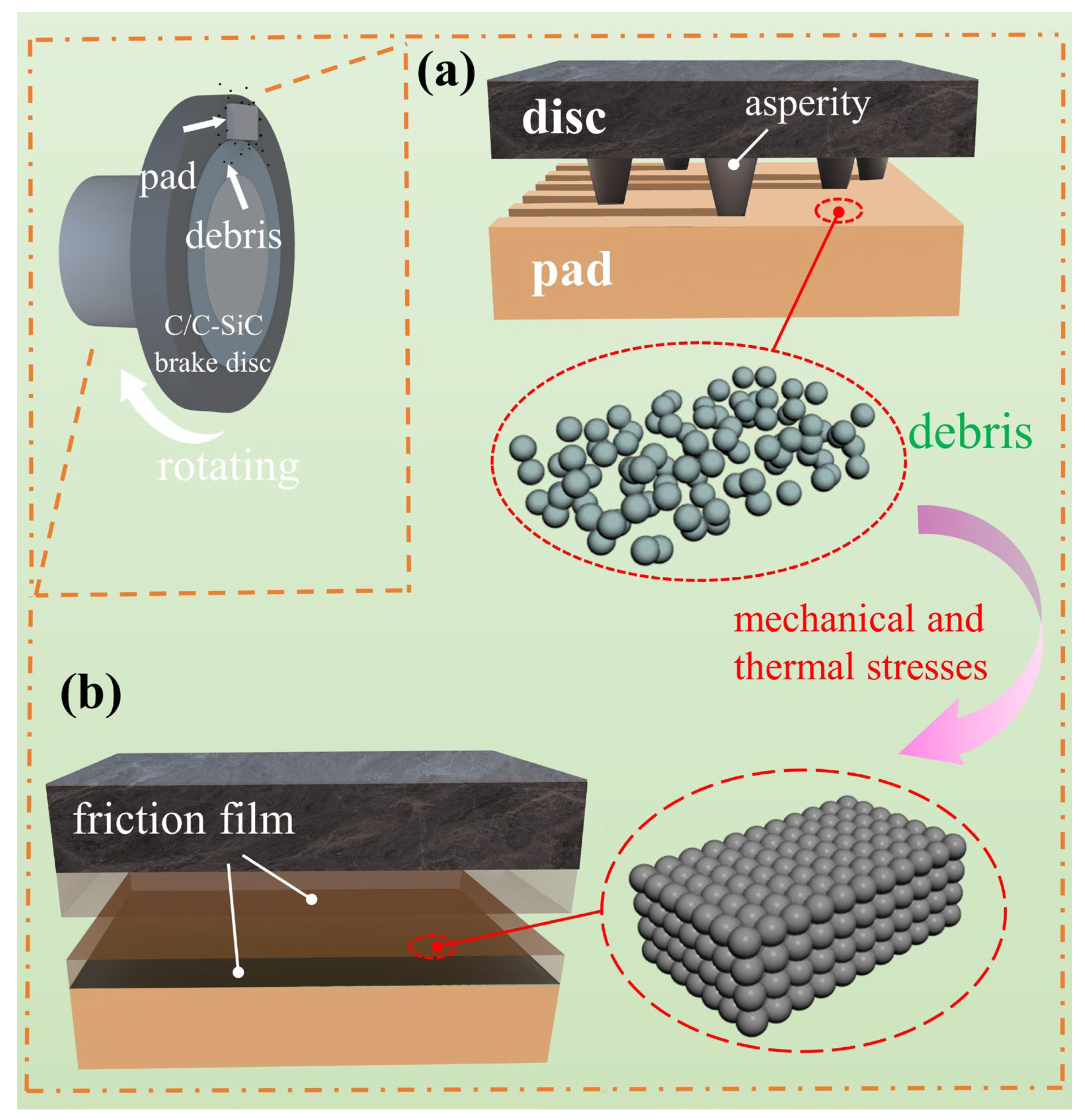
3.3.3. Analysis of Thermal Fade
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ling, J.H.; Luan, D.C.; Hu, Z.H.; Liu, L.; Ren, Y.; Zhou, X.; Wang, Z. Study on the effects of different kinds of phenolic resin binders on the properties of friction materials. China Plast. Ind. 2022, 50, 68–72. [Google Scholar]
- Awe, S.A. Developing material requirements for automotive brake disc. Mod. Concepts Mater. Sci. 2019, 2, 000531. [Google Scholar]
- Kosbe, P.; More, C. Performance of a NAO friction material for disc brake. Natl. J. Adv. Build. Sci. Mech. 2010, 1, 1–5. [Google Scholar]
- Kumar, V.V.; Kumaran, S.S. Friction material composite: Types of brake friction material formulations and effects of various ingredients on brake performance-A review. Mater. Res. Express 2019, 6, 082005. [Google Scholar]
- Dong, C.Y.; Deng, J.L.; Fan, S.W.; Kou, S.; Yang, S.; Huang, R.; Zhang, Y.; Mao, Y. Tribological performance and composition optimization of semi-metallic friction materials applied for carbon ceramic brake disc. Ceram. Int. 2024, 50, 19660–19670. [Google Scholar]
- Ma, X.; Fan, S.W.; Luan, C.H.; Li, W.; Deng, J.; Cheng, L.; Zhang, L. Effect of Cu addition on the braking performance of Fe-Si alloy modified C/CSiC brake materials. Wear 2021, 477, 203851. [Google Scholar]
- Fan, S.W.; Ning, Y.F.; Ma, X.; Yang, C.; He, L.; Deng, J.; Cheng, L.; Zhang, L. The effects of high-temperature annealing on microstructure and performance of Cf/C-SiC composites modified by FeSi2. Ceram. Int. 2018, 44, 15200–15207. [Google Scholar]
- Ma, X.; Luan, C.H.; Fan, S.W.; Deng, J.; Zhang, L.; Cheng, L. Comparison of braking behaviors between iron- and copper-based powder metallurgy brake pads that used for C/C-SiC disc. Tribol. Int. 2021, 154, 106686. [Google Scholar]
- Jiang, H.; Wang, C.X. Foreign friction material products and research progress. Adv. Mater. Ind. 2021, 3, 2–6. [Google Scholar]
- Gurunath, P.V.; Bjiwe, J. Friction and wear studies on brake-pad materials based on newly developed resin. Wear 2007, 263, 1212. [Google Scholar]
- Cai, P.; Qi, S.Q.; Leng, Y. Research progress of resin based friction materials. J. Huaiyin Inst. Technol. 2018, 27, 9–14. [Google Scholar]
- Wang, Z.F.; Zhao, L.; Shang, P. Effect of compound modified phenolic resin on the performance of brake friction materials. China Pet. Chem. Stand. Qual. 2019, 39, 121–122. [Google Scholar]
- Cong, P.H.; Wu, X.Y.; Bu, J.; Li, T.-S. Progress in research of organic friction materials for automobile and train braking. J. Tribol. 2011, 31, 88–96. [Google Scholar]
- Lai, X.H.; Wan, X.; Gan, G.L. Research status and development of automotive brake materials. Mater. Res. Appl. 2020, 14, 240–245. [Google Scholar]
- Wang, X.; Liang, X.L. Research progress on high temperature resistant phenolic resins. Chem. Adhes. 2021, 43, 304–306. [Google Scholar]
- Sun, G.X. Research progress on modification of heat resistance of phenolic resin. New Technol. New Prod. China 2016, 22, 79–80. [Google Scholar]
- Tang, K.H.; Zhang, A.L.; Ge, T.J.; Liu, X.; Tang, X.; Li, Y. Research progress on modification of phenolic resin. Mater. Today Commun. 2021, 26, 101879. [Google Scholar]
- Li, W.Q.; Yao, J.Q.; Fan, Y.X.; Yu, C. Preparation and properties of furfural-phenol-formaldehyde co-condensation resin. Plast. Sci. Technol. 2024, 52, 72–76. [Google Scholar]
- Wang, F.Y.; Huang, Z.X.; Liu, Y.; Li, Y. Novel cardanol-containing boron-modified phenolic resin composites: Non-isothermal curing kinetics, thermal properties, and ablation mechanism. High Perform. Polym. 2017, 29, 279–288. [Google Scholar]
- Wang, S.J.; Jing, X.L.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym. Degrad. Stab. 2014, 99, 1–11. [Google Scholar]
- Ma, X. Material Optimization and Friction and Wear Performance of Clamp-Type All-Carbon Ceramic Friction Sub-Materials; Northwestern Polytechnical University: Xi’an, China, 2023. [Google Scholar]
- Zhang, C.S.; Zhao, F.; Zhang, J.J.; Wang, X.Y.; Bao, G.L.; Bai, Y.B.; Li, T.J. Infrared spectroscopic study of nano-sized alumina. J. Chem. 1999, 3, 275–280. [Google Scholar]
- Zhao, G.L.; Wang, S.J.; Ling, F.X.; Wu, H.; Cui, X.; Zhang, Y. Infrared spectroscopic study of the surface structure of γ-Alumina. Contemp. Chem. Ind. 2012, 41, 661–663+667. [Google Scholar]
- Starczewski, L.; Szumniak, J. Mechanisms of transferring the matter in a friction process in a tribology system: Polymeric composite-metal. Surf. Coat. Technol. 1998, 100, 33–37. [Google Scholar] [CrossRef]
- CSDN Library. Steel Heating Temperature Vs. Tolor Mapping. 2021. Available online: https://download.csdn.net/download/zgzmdr/56419228/ (accessed on 5 December 2021).
- Dai, Y.J.; Bi, G.J. Application of low carbon steel quenching and strengthening process in chain drive industry. Heat Treat. Technol. Equip. 2024, 45, 37–41. [Google Scholar]
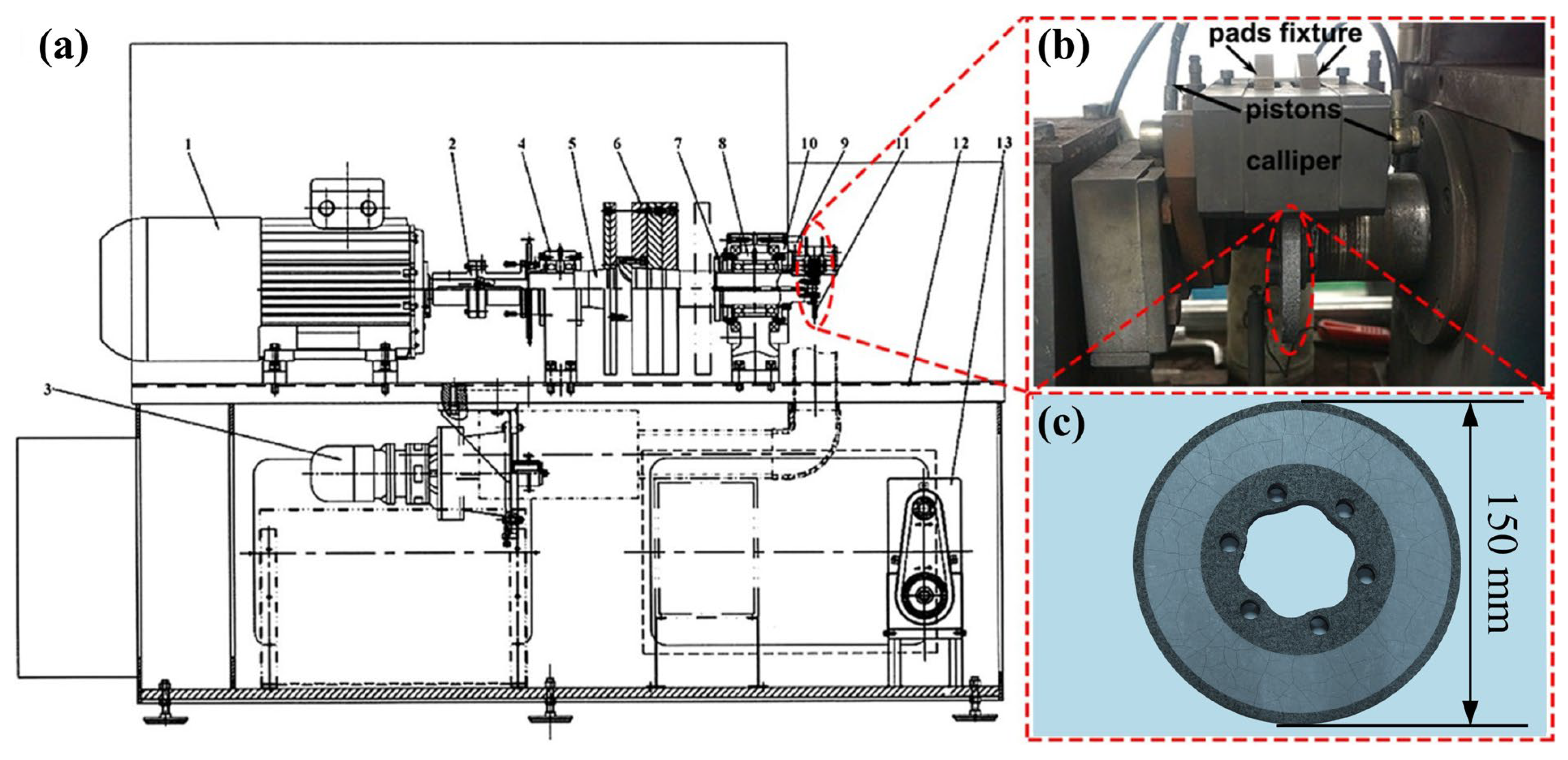
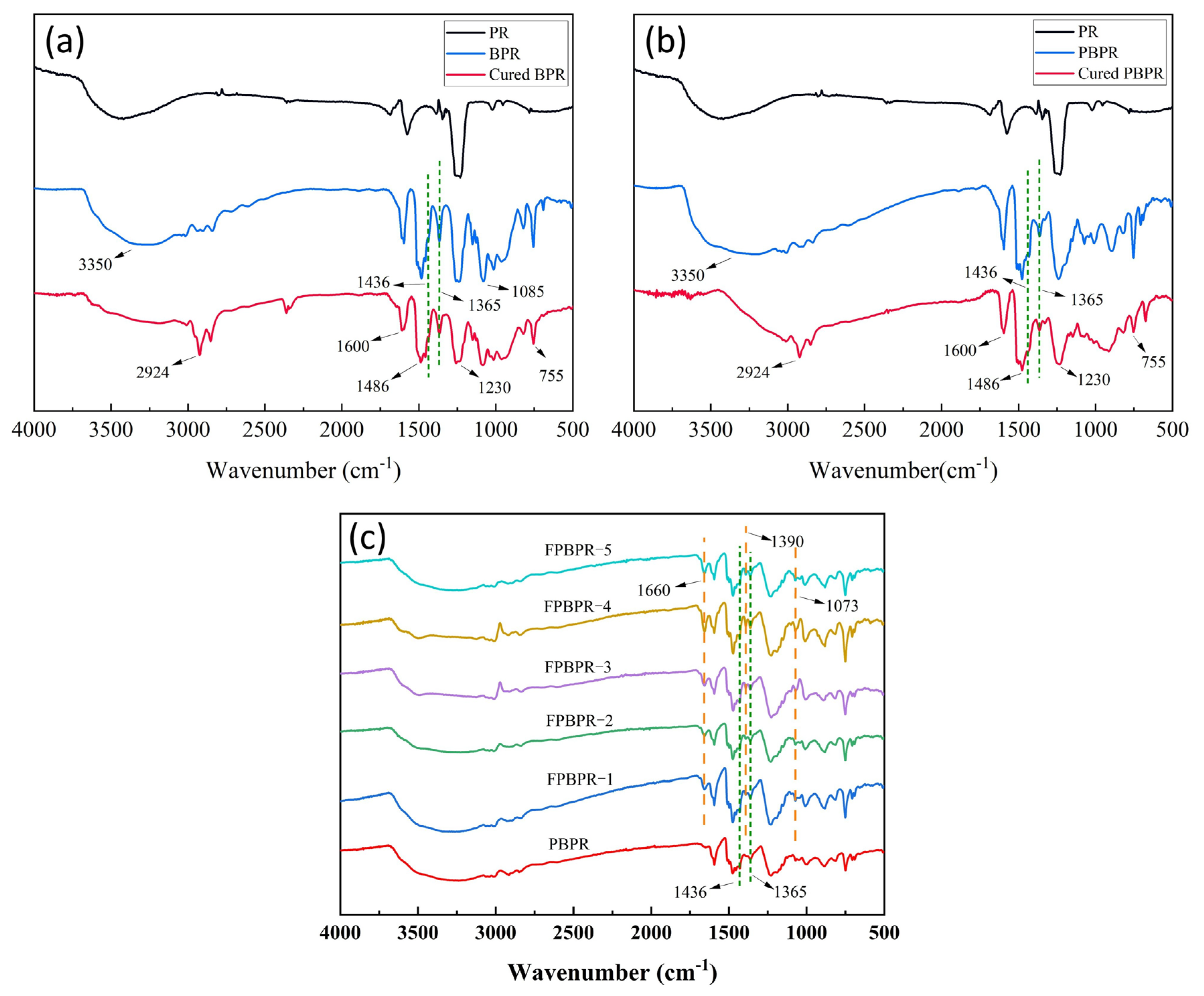

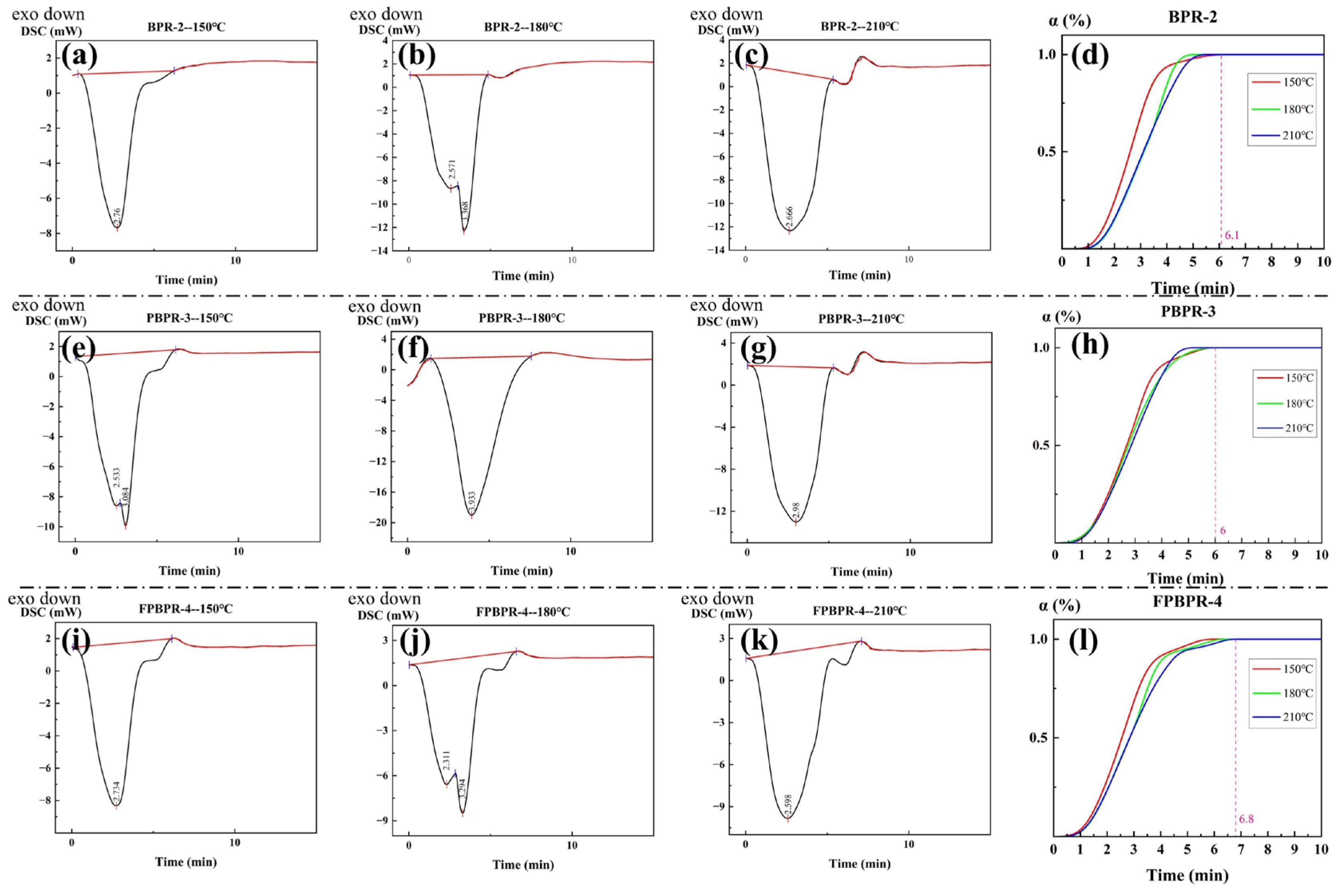
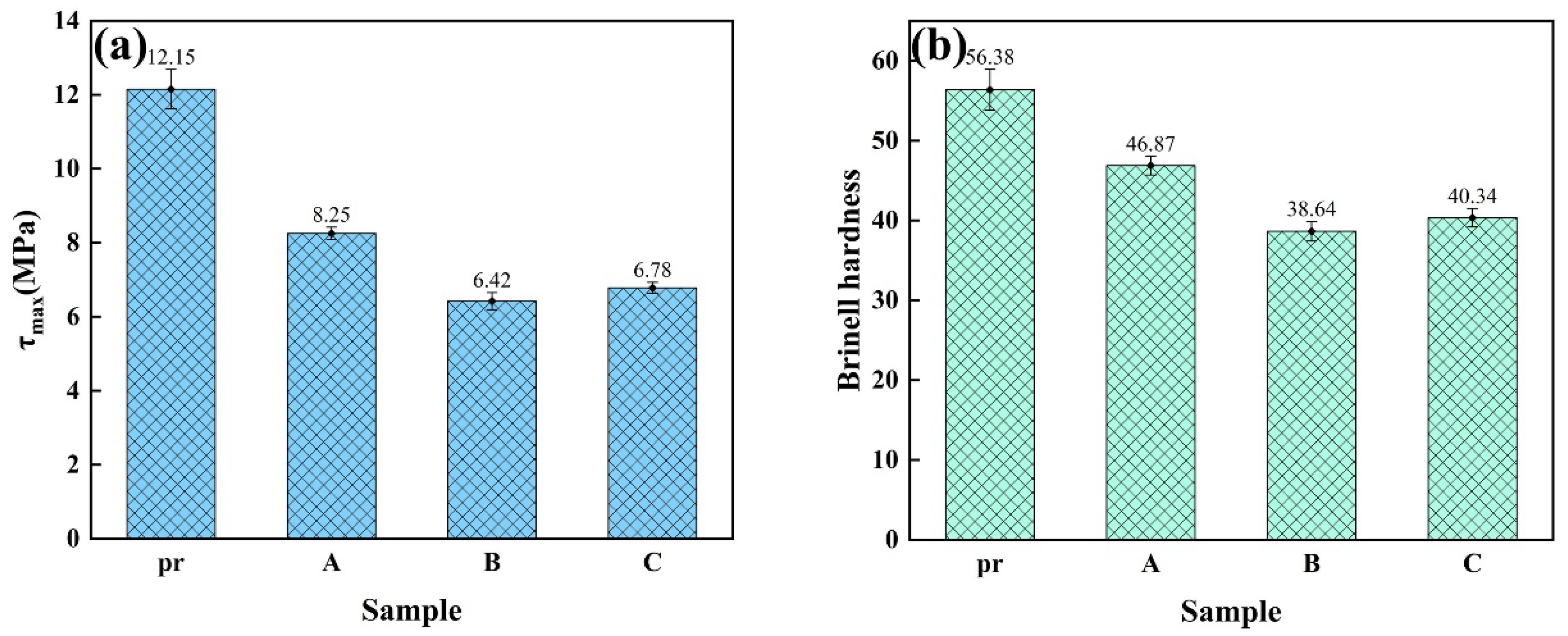

| Components | Average Size (μm) | Mohs Hardness | Source |
|---|---|---|---|
| α-alumina | 19 | 9 | Henan Fengkai Refractories Co., Ltd. (Zhenzhou, China) |
| silicon dioxide | 74 | 7 | Heyuan Wanchuan Quartz Development Co., Ltd. (Heyuan, China) |
| graphite | 250 | 2 | Jiangxi Shuobang Material Technology Co., Ltd. (Fuzhou, China) |
| magnesia | 44 | 5.5 | Shanghai Ruhua Technology Co., Ltd. (Shanghai, China) |
| others | — | — | — |
| Sections | Test Name | Conditions |
|---|---|---|
| 1 | Green μ characteristic | 80→30 km/h; 3.0 MPa; 30 stops |
| 2 | Burnish | 80→30 km/h; 1.5–5.1 MPa; 64 stops |
| 3 | Characteristic value 1 | 80→30 km/h; 3.0 MPa; 6 stops |
| 4 | Speed/pressure sensitivity | 40→5 km/h; 1.0–5.0 MPa; 5 stops |
| 5 | — | 80→40 km/h; 1.0–5.0 MPa; 5 stops |
| 6 | — | 120→80 km/h; 1.0–5.0 MPa; 5 stops |
| 7 | — | 160→130 km/h; 1.0–5.0 MPa; 5 stops |
| 8 | — | 180→150 km/h; 1.0–5.0 MPa; 5 stops |
| 9 | Characteristic value 2 | 80→30 km/h; 3.0 MPa; 6 stops |
| 10 | Cold braking | 40→5 km/h; 3.0 MPa; 1 stop |
| 11 | Motorway braking | 100→5 km/h; 40% deceleration; 1 stop |
| 160→10 km/h; 40% deceleration; 1 stop | ||
| 12 | Characteristic value 3 | 80→30 km/h; 3.0 MPa; 18 stops |
| 13 | 1st fading | 100→5 km/h; 40% deceleration; 15 stops |
| 14 | Recovery 1 | 80→30 km/h; 3.0 MPa; 18 stops |
| 15 | Temperature/pressure sensitivity 100 °C | 80→30 km/h; 1.0–5.0 MPa; 5 stops |
| 16 | Temperature/pressure sensitivity 500 °C | 80→30 km/h; 1.0–5.0 MPa; 9 stops; |
| 17 | 80→30 km/h; 1.0–5.0 MPa; 5 stops | |
| 18 | Recovery 2 | 80→30 km/h; 3.0 MPa; 18 stops |
| 19 | 2nd fading | 100→5 km/h; 40% deceleration; 15 stops |
| 20 | Recovery 2 | 80→30 km/h; 3.0 MPa; 18 stops |
| Simples | Density (g/cm3) | Open Porosity (%) |
|---|---|---|
| pr | 3.83 | 9.88 |
| A | 3.54 | 10.76 |
| B | 3.35 | 10.98 |
| C | 3.47 | 10.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Guo, J.; Deng, J.; Fan, S.; Cai, X.; Kou, S.; Yang, S. Preparation and Properties of Boron Modified Phenolic Resin for Automotive Friction Materials. Materials 2025, 18, 1624. https://doi.org/10.3390/ma18071624
Liu J, Guo J, Deng J, Fan S, Cai X, Kou S, Yang S. Preparation and Properties of Boron Modified Phenolic Resin for Automotive Friction Materials. Materials. 2025; 18(7):1624. https://doi.org/10.3390/ma18071624
Chicago/Turabian StyleLiu, Jianrong, Jialin Guo, Juanli Deng, Shangwu Fan, Xide Cai, Sijie Kou, and Shaobo Yang. 2025. "Preparation and Properties of Boron Modified Phenolic Resin for Automotive Friction Materials" Materials 18, no. 7: 1624. https://doi.org/10.3390/ma18071624
APA StyleLiu, J., Guo, J., Deng, J., Fan, S., Cai, X., Kou, S., & Yang, S. (2025). Preparation and Properties of Boron Modified Phenolic Resin for Automotive Friction Materials. Materials, 18(7), 1624. https://doi.org/10.3390/ma18071624







