Extrusion Processing of Fungal-Contaminated Cereals as a Method for Spore Reduction and Binder Development in Feed Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Moisture Content Determination
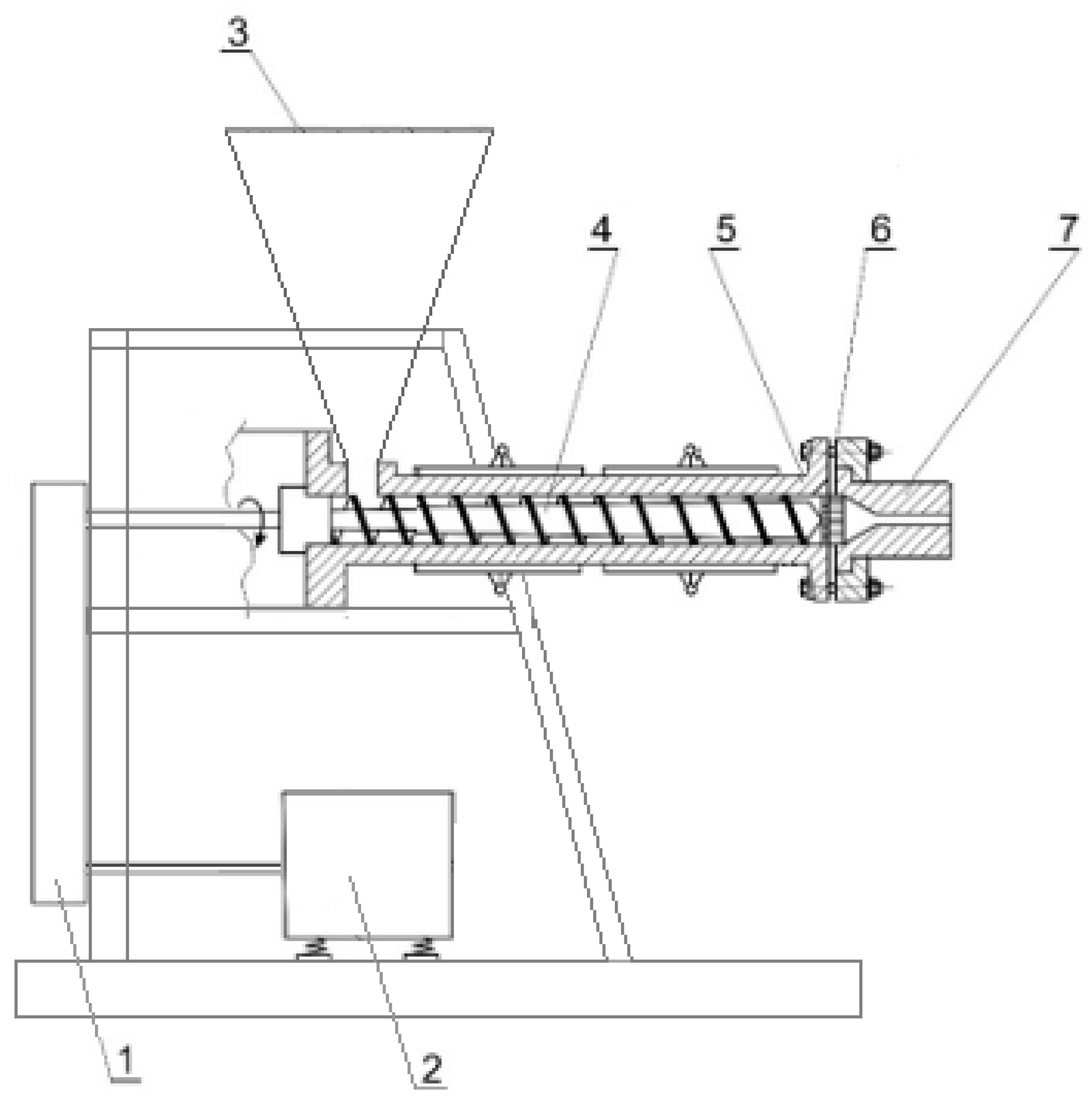
2.3. Extrusion of Contaminated Cereal
- wm = 21—mixture moisture content [%],
- d0 = 6—head diameter [mm],
- Qm = 20—mixture mass flow rate [kg·h−1],
- T = 140—temperature in the extrusion chamber [°C],
- p = 20–40—compaction pressure during pellet formation [atm],
- screw configuration: single screw,
- screw speed: 200 rpm,
- residence time: 25 s.
2.4. Pressure Agglomeration Process
- wm = 19—mixture moisture content [%],
- d0 = 6—diameter of the matrix holes [mm],
- dm = 216—matrix diameter [mm],
- hm = 32—matrix height [mm],
- Qm = 100—mixture mass flow rate [kg·h−1],
- nr = 270—rotational speed of the densifying roller system [rpm],
- hr = 0.4—gap between the rollers and the matrix [mm].
2.5. Measurement of Physical and Bulk Density of Pellets
- ρg—physical density of pellets [kg∙m−3],
- mg—mass of pellets [kg],
- Vg—volume of tested pellets [m−3].
- r—radius of pellets [m],
- h—height of pellets [m].
2.6. Determination of the Kinetic Strength of the Pellets
- Pdx—kinetic strength of pellets [%],
- m1—sample weight before test [kg],
- m2—sample weight after test [kg].
- T = 60—duration of the test [s],
- p = 7—target pressure [kPa].
2.7. Determination of Mycotoxins by LC–MS/MS
2.8. Determination of Microbial Contamination
2.9. Statistical Analysis
3. Results
3.1. Moisture Content
3.2. Effect of Extruded Fungal-Spore-Infected Grains on the Pelleting Process and Pellet Properties
3.3. Mycotoxin Reduction Efficiency of the Extrusion–Pelletisation Process
3.4. Microbiological Evaluation of Feed Mixtures
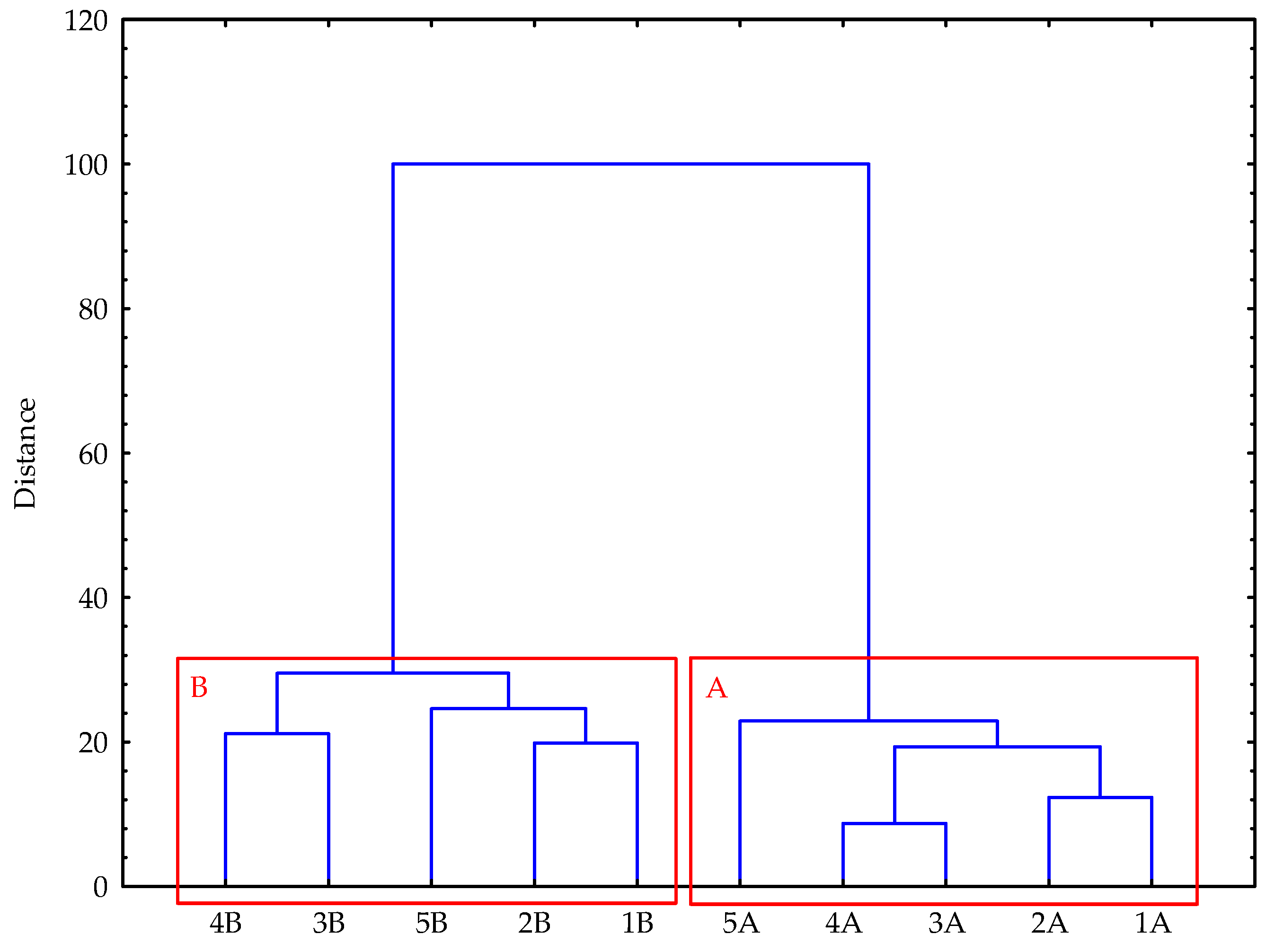
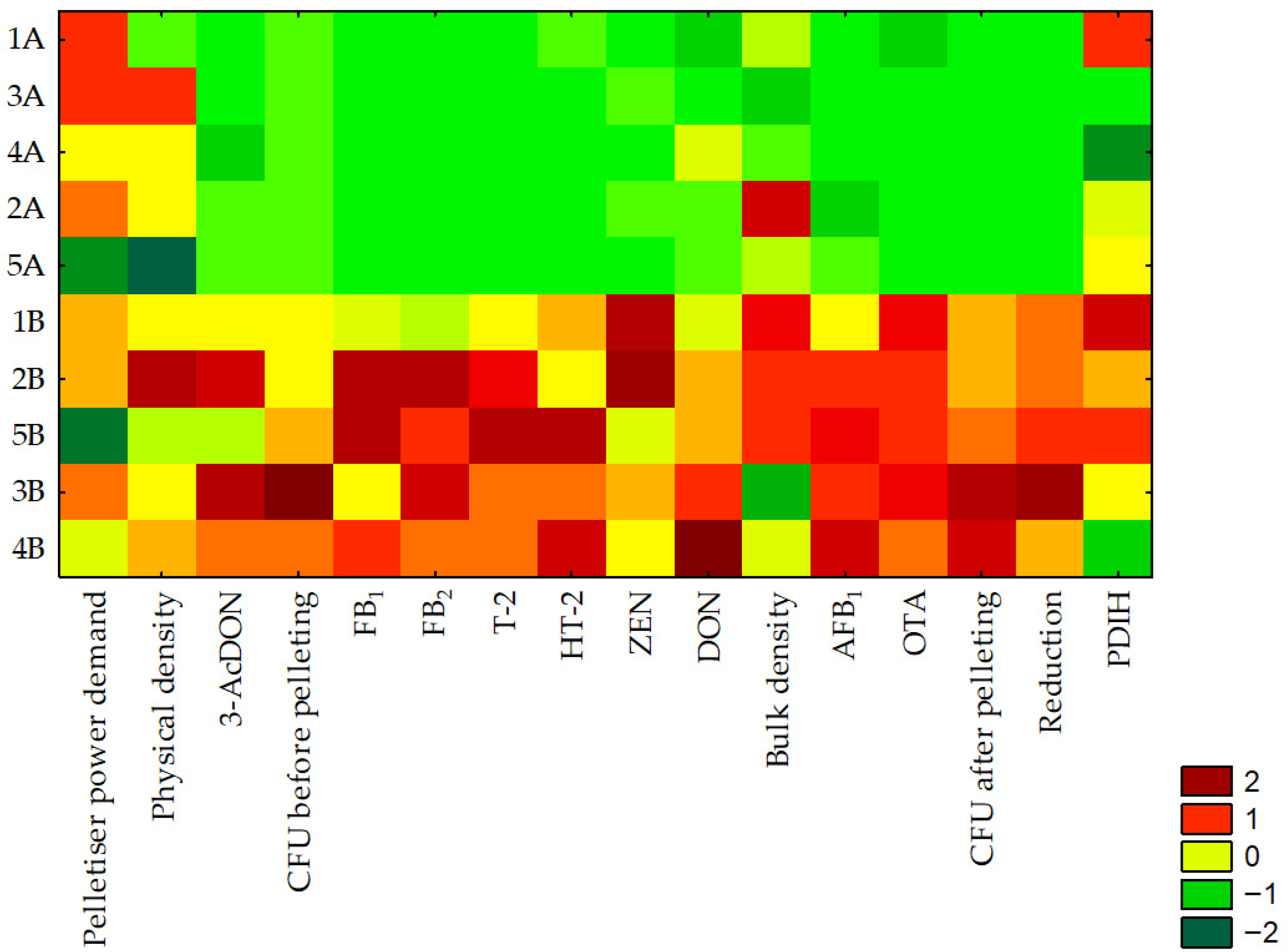
3.5. Statistical Analysis
4. Conclusions
- The use of mould-infected cereal grains at a 15% inclusion level as a feed component enabled the production of pellets with physical parameters comparable to those obtained from healthy raw materials. No significant deterioration was observed in key parameters such as physical density, bulk density, or Holmen mechanical durability.
- The physical and bulk densities of the produced pellets remained within the acceptable range for compound feeds. Variants containing mould-infected grains (B) exhibited slightly lower density values; however, these differences were not large enough to negatively affect the functional quality of the feed.
- The application of extrusion to mould-infected grains prior to pelleting increased the mechanical durability of the pellets by 4.02% compared with mixtures containing healthy grains. This result indicates that an appropriately selected thermo-mechanical processing regime can significantly enhance the mechanical properties of pellets, which is practically relevant for their transport and storage stability.
- Mycotoxin levels in the final pellets increased following the incorporation of extruded mould-infected grains compared with healthy grains. However, all values remained within the permissible limits established by the European Commission. Particular attention should be given to ochratoxin A (OTA) and T-2 toxin, which approached their respective regulatory threshold levels.
- The use of extrusion followed by pelleting represents an effective strategy for utilising mould-infected grains, reducing raw material losses and increasing the economic efficiency of feed production, provided that strict mycotoxin monitoring and control are maintained.
- The extrusion–pelleting process effectively reduced mould colony counts, with an average reduction ranging from 27% (sorghum) to 65% (maize), confirming the high efficacy of thermo-mechanical treatment in reducing microbiological contamination. The mechanism involves exposure to elevated temperature and pressure, which damages fungal spore cell structures. However, complete elimination of fungal microflora was not achieved, indicating that some spores may exhibit thermal resistance or survive in a dormant state.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A Review of Postharvest Approaches to Reduce Fungal and Mycotoxin Contamination of Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Soja, J.; Combrzyński, M.; Oniszczuk, T.; Gancarz, M.; Oniszczuk, A. Extrusion-Cooking Aspects and Physical Characteristics of Snacks Pellets with Addition of Selected Plant Pomace. Appl. Sci. 2024, 14, 8754. [Google Scholar] [CrossRef]
- Boakye, P.G.; Okyere, A.Y.; Annor, G.A. Impact of Extrusion Processing on the Nutritional and Physicochemical Properties of Intermediate Wheatgrass (Thinopyrum intermedium). Cereal Chem. 2023, 100, 628–642. [Google Scholar] [CrossRef]
- Choton, S.; Gupta, N.; Bandral, J.D.; Anjum, N.; Choudary, A. Extrusion Technology and Its Application in Food Processing: A Review. Pharma Innov. J. 2020, 9, 162–168. [Google Scholar] [CrossRef]
- Szymczak, P.; Dziadowiec, D.; Andrzejewski, J.; Szostak, M. The Efficiency Evaluation of the Reactive Extrusion Process for Polyethylene Terephthalate (PET). Monitoring of the Industrial Foil Manufacturing Process by In-Line Rheological Measurements. Appl. Sci. 2023, 13, 3434. [Google Scholar] [CrossRef]
- Kristiawan, M.; Della Valle, G.; Berzin, F. Extrusion Simulation for the Design of Cereal and Legume Foods. Foods 2022, 11, 1780. [Google Scholar] [CrossRef]
- Jain, R.; Goomer, S. Understanding Extrusion Technology for Cereal–Pulse Blends: A Review. Cogent Food Agric. 2023, 9, 2253714. [Google Scholar] [CrossRef]
- Riaz, M.N. Extruders in Food Applications; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-1-56676-779-8. [Google Scholar]
- Gulati, P.; Brahma, S.; Rose, D.J. Chapter 13—Impacts of Extrusion Processing on Nutritional Components in Cereals and Legumes: Carbohydrates, Proteins, Lipids, Vitamins, and Minerals. In Extrusion Cooking; Ganjyal, G.M., Ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 415–443. ISBN 978-0-12-815360-4. [Google Scholar]
- Castells, M.; Marín, S.; Sanchis, V.; Ramos, A.J. Fate of Mycotoxins in Cereals during Extrusion Cooking: A Review. Food Addit. Contam. 2005, 22, 150–157. [Google Scholar] [CrossRef]
- Merrettig-Bruns, U.; Sayder, B. Impact of Mycotoxins and Mouldy Maize Silage on the Biogas Process 2022. Available online: https://papers.ssrn.com/abstract=4084331 (accessed on 22 September 2025).
- Kintl, A.; Vítěz, T.; Huňady, I.; Sobotková, J.; Hammerschmiedt, T.; Vítězová, M.; Brtnický, M.; Holátko, J.; Elbl, J. Effect of Mycotoxins in Silage on Biogas Production. Bioengineering 2023, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Venslauskas, K.; Navickas, K.; Rubežius, M.; Žalys, B.; Gegeckas, A. Processing of Agricultural Residues with a High Concentration of Structural Carbohydrates into Biogas Using Selective Biological Products. Sustainability 2024, 16, 1553. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C. Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review. Agronomy 2022, 12, 3198. [Google Scholar] [CrossRef]
- Wang, J.; Xing, W.; Huang, X.; Jin, X.; Yu, H.; Wang, J.; Song, L.; Zeng, W.; Hu, Y. Smouldering of Storage Rice: Effect of Mouldy Degree and Moisture Content. Combust. Sci. Technol. 2022, 194, 1395–1407. [Google Scholar] [CrossRef]
- PN-EN ISO 18134-1:2015-11; Solid Biofuels—Determination of Moisture Content—Drying Method—Part 1: Total Mois-Ture—Reference Method. ISO: Geneva, Switzerland, 2015.
- Ekstruzja soi i Zbóż|Presoil. Available online: http://presoil.pl/product-category/urzadzenia/tloczenie-soi-i-zboz/ (accessed on 5 October 2025).
- Zasadzeń, M. Analiza niezawodności wybranych maszyn w przedsiębiorstwie produkcyjnym—Studium przypadku. In Cross-Border Exchange of Experience Production Engineering Using Principles of Mathematics: Modern Mathematical Methods in Engineering 3mi, 22.1.–24.1. 2018, Horni Lomna; VSB—Technical University of Ostrava: Ostrava, Czech Republic, 2018; ISBN 978-80-248-4136-6. [Google Scholar]
- Cwalina, P.; Obidziński, S.; Sienkiewicz, A.; Kowczyk-Sadowy, M.; Piekut, J.; Bagińska, E.; Mazur, J. Production and Quality Assessment of Fertilizer Pellets from Compost with Sewage Sludge Ash (SSA) Addition. Materials 2025, 18, 1145. [Google Scholar] [CrossRef]
- PN-EN ISO 17828:2016-02; Solid Biofuels—Determination of Bulk Density. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 17831-1:2016-02; Solid Biofuels—Determination of Mechanical Durability of Pellets and Briquettes. ISO: Geneva, Switzerland, 2016.
- PN-EN ISO 24333:2012; Ziarno Zbóż i Przetwory Zbożowe—Pobieranie Próbek. ISO: Geneva, Switzerland, 2012.
- Technologia Produkcji Pelletu—TMB Polska. Available online: https://tmbpolska.pl/produkcji-pelletu-technologia/ (accessed on 8 July 2025).
- Teixeira Netto, M.V.; Massuquetto, A.; Krabbe, E.L.; Surek, D.; Oliveira, S.G.; Maiorka, A. Effect of Conditioning Temperature on Pellet Quality, Diet Digestibility, and Broiler Performance. J. Appl. Poult. Res. 2019, 28, 963–973. [Google Scholar] [CrossRef]
- Bastiaansen, T.M.M.; de Vries, S.; Martens, B.M.J.; Benders, R.T.; Vissers, E.; Dijksman, J.A.; Hendriks, W.H.; Thomas, M.; Bosch, G. Identifying Feed Characteristics That Affect the Pellet Manufacturing of Livestock Diets Containing Different Coproducts. Clean. Circ. Bioecon. 2024, 7, 100073. [Google Scholar] [CrossRef]
- Schroeder, B.; Andretta, I.; Kipper, M.; Franceschi, C.H.; Remus, A. Empirical Modelling the Quality of Pelleted Feed for Broilers and Pigs. Anim. Feed Sci. Technol. 2020, 265, 114522. [Google Scholar] [CrossRef]
- Çitil, E.; Marakoğlu, T. Effect of Feed Materials on Pellet Properties, Capacity and Energy Consumptions Values. J. Agric. Fac. Gaziosmanpasa Univ. 2024, 40, 96–102. [Google Scholar] [CrossRef]
- Feed Safety|FEFAC. Available online: https://fefac.eu/priorities/feed-safety/ (accessed on 7 October 2025).
- Peng, F.; Xiang, R.; Fang, F.; Liu, D. Analysis of Feed Pelleting Characteristics Based on a Single Pellet Press Device. Int. J. Agric. Biol. Eng. 2022, 15, 65–70. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vladut, V.; Voicu, G.; Dinca, M.-N.; Zabava, B.-S. Influence of Biomass Moisture Content on Pellet Properties—Review. Eng. Rural Dev. 2018, 17, 1876–1883. [Google Scholar]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef]
- Rosani, U.; Ayuningsih, B.; Susilawati, I.; Hernaman, I.; Indriani, N.P.; Putri, G.N.R.; Imanda, A.R.; Begna, R. Physical and Nutritional Characteristics of Indigofera, Gamal, and Cassava-Based Pellets for Sustainable Livestock Feed. Adv. Anim. Vet. Sci. 2025, 13, 1952–1959. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Schmiele, M.; Steel, C.J. Development of Whole Grain Wheat Flour Extruded Cereal and Process Impacts on Color, Expansion, and Dry and Bowl-Life Texture. LWT 2017, 75, 261–270. [Google Scholar] [CrossRef]
- Combrzyński, M.; Wójtowicz, A.; Mitrus, M.; Oniszczuk, T.; Matwijczuk, A.; Pawelczyk, P.; Mościcki, L. Effect of Starch Type and Screw Speed on Mechanical Properties of Extrusion-Cooked Starch-Based Foams. Int. Agrophys. 2019, 33, 233–240. [Google Scholar] [CrossRef]
- Massuquetto, A.; Durau, J.F.; Schramm, V.G.; Netto, M.T.; Krabbe, E.L.; Maiorka, A. Influence of Feed Form and Conditioning Time on Pellet Quality, Performance and Ileal Nutrient Digestibility in Broilers. J. Appl. Poult. Res. 2018, 27, 51–58. [Google Scholar] [CrossRef]
- Mohammadi Ghasem Abadi, M.H.; Moravej, H.; Shivazad, M.; Karimi Torshizi, M.A.; Kim, W.K. Effect of Different Types and Levels of Fat Addition and Pellet Binders on Physical Pellet Quality of Broiler Feeds. Poult. Sci. 2019, 98, 4745–4754. [Google Scholar] [CrossRef]
- Angulo, E.; Brufau, J.; Esteve-Garcia, E. Effect of a Sepiolite Product on Pellet Durability in Pig Diets Differing in Particle Size and in Broiler Starter and Finisher Diets. Anim. Feed Sci. Technol. 1996, 63, 25–34. [Google Scholar] [CrossRef]
- Amerah, A.M.; Ravindran, V.; Lentle, R.G.; Thomas, D.G. Influence of Feed Particle Size on the Performance, Energy Utilization, Digestive Tract Development, and Digesta Parameters of Broiler Starters Fed Wheat- and Corn-Based Diets. Poult. Sci. 2008, 87, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Chewning, C.G.; Stark, C.R.; Brake, J. Effects of Particle Size and Feed Form on Broiler Performance. J. Appl. Poult. Res. 2012, 21, 830–837. [Google Scholar] [CrossRef]
- Hoffmans, Y.; Schaarschmidt, S.; Fauhl-Hassek, C.; van der Fels-Klerx, H.J. Factors during Production of Cereal-Derived Feed That Influence Mycotoxin Contents. Toxins 2022, 14, 301. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Babič, J.; Pezo, L.; Banjac, V.; Filipčev, B.; Miljanić, J.; Kos, J.; Jakovac-Strajn, B. Reduction of Alternaria Toxins via the Extrusion Processing of Whole-Grain Red Sorghum Flour. Foods 2024, 13, 255. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- Scarpino, V.; Bresciani, A.; Blandino, M. The Effects of the Extrusion Process Used for the Production of Maize Snacks and Pasta on the Free, Bound, and Total B Fumonisin Contents. LWT 2024, 198, 115977. [Google Scholar] [CrossRef]
- Rozporządzenie Komisji (UE) 2024/1038 z Dnia 9 Kwietnia 2024 r. Zmieniające Rozporządzenie (UE) 2023/915 w Odniesieniu do Najwyższych Dopuszczalnych Poziomów Toksyn T-2 i HT-2 w Żywności. 2024. Available online: https://eur-lex.europa.eu/legal-content/PL/ALL/?uri=CELEX:32024R1038 (accessed on 8 September 2025).
- Chełkowski, J. Mikotoksyny, Grzyby Toksynotwórcze i Mikotoksykozy. Available online: http://www.cropnet.pl/dbases/mycotoxins.pdf (accessed on 7 October 2025).
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, e06040. [Google Scholar] [CrossRef]
- Janić Hajnal, E.; Babič, J.; Pezo, L.; Banjac, V.; Čolović, R.; Kos, J.; Krulj, J.; Pavšič-Vrtač, K.; Jakovac-Strajn, B. Effects of Extrusion Process on Fusarium and Alternaria Mycotoxins in Whole Grain Triticale Flour. LWT 2022, 155, 112926. [Google Scholar] [CrossRef]
- Khan, G.Q.; Prestløkken, E.; Lund, P.; Hellwing, A.L.F.; Larsen, M. Effects of the Density of Extruded Pellets on Starch Digestion Kinetics, Rumen Fermentation, Fiber Digestibility and Enteric Methane Production in Dairy Cows. J. Anim. Physiol. Anim. Nutr. 2023, 107, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Jakovac-Strajn, B.; Babič, J.; Pezo, L.; Banjac, V.; Čolović, R.; Kos, J.; Miljanić, J.; Janić Hajnal, E. Mitigation of Mycotoxin Content by a Single-Screw Extruder in Triticale (x Triticosecale wittmack). Foods 2025, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium Head Blight and Mycotoxins in Wheat: Prevention and Control Strategies across the Food Chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Cortiñas Abrahantes, J.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and Co-Occurrence of Mycotoxins in Cereal-Based Feed and Food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef]
- Kukier, E.; Kwiatek, K.; Goldsztejn, M.; Grenda, T. Jakość Mikrobiologiczna Pasz. Pasze Przem. 2014, 3, s20–s26. [Google Scholar]
- LLFG Sachsen-Anhalt. Orientierungswerte zur Beurteilung der Mikrobiologischen Qualität von Futtermitteln (nach VDLUFA Methodenbuch III 28.1.4, 8. Ergänzung 2011); Landesanstalt für Landwirtschaft, Forsten und Gartenbau Sachsen-Anhalt: Bernburg-Strenzfeld, Germany, 2011; Available online: https://llfg.sachsen-anhalt.de (accessed on 10 October 2025).
- Goldsztejn, M.; Grenda, T.; Kozak, B.; Kozieł, N.; Kwiatek, K. Microbiological Contamination of Feed-Current Hazards and New Challenges. Med. Weter. 2022, 78, 57–62. [Google Scholar] [CrossRef]
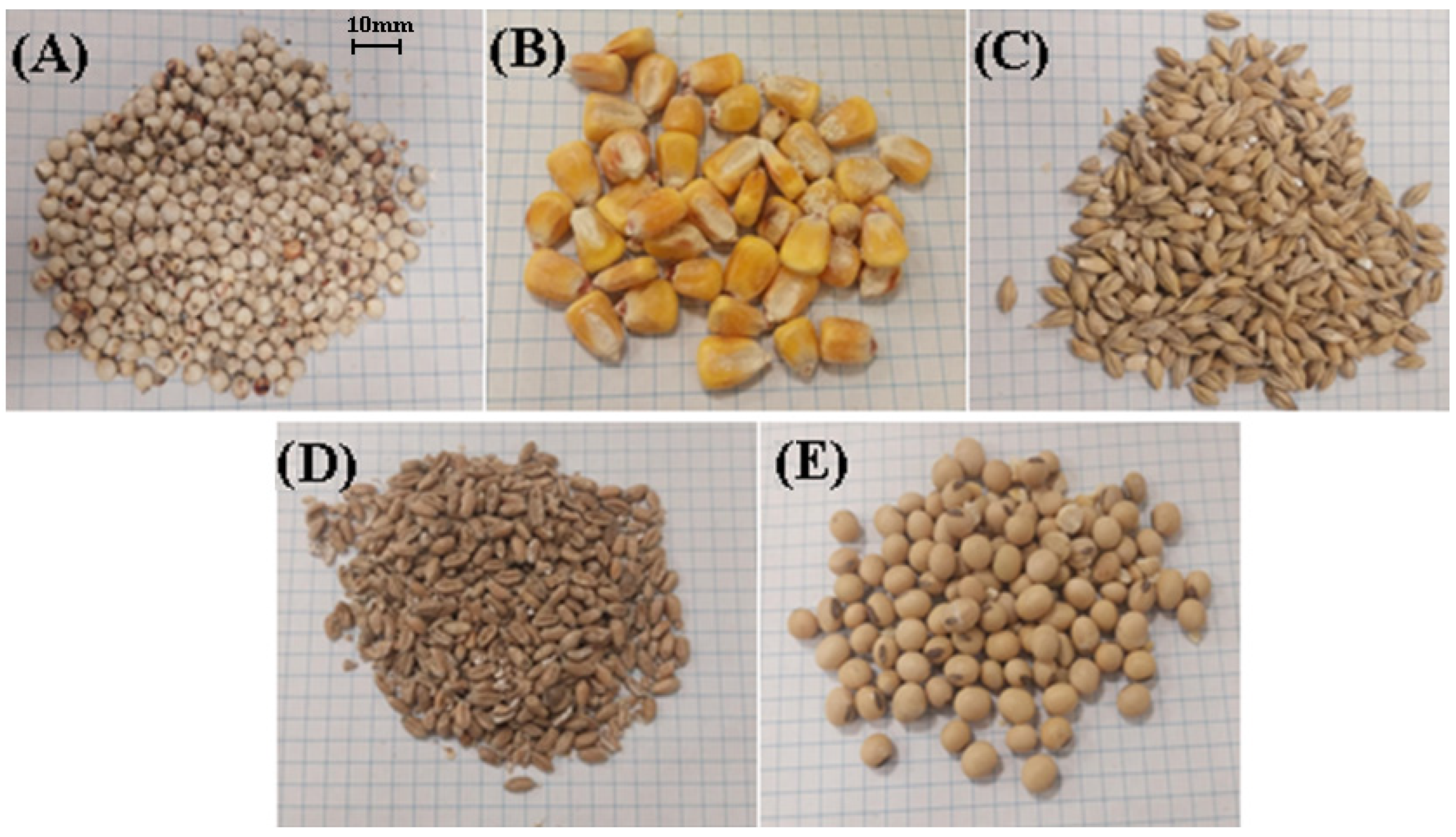
| Raw Material | Mixture Composition [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 2A | 2B | 3A | 3B | 4A | 4B | 5A | 5B | |
| Meadow hay | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Wheat bran | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Fruit pomace | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Wheat | 15 | - | - | - | - | - | - | - | - | - |
| Extruded wheat contaminated with mould spores | - | 15 | - | - | - | - | - | - | - | - |
| Barley | - | - | 15 | - | - | - | - | - | - | - |
| Extruded barley contaminated with mould spores | - | - | - | 15 | - | - | - | - | - | - |
| Maize | - | - | - | - | 15 | - | - | - | - | - |
| Extruded maize contaminated with mould spores | - | - | - | - | - | 15 | - | - | - | - |
| Sorghum | - | - | - | - | - | - | 15 | - | - | - |
| Extruded sorghum contaminated with mould spores | - | - | - | - | - | - | - | 15 | - | - |
| Soybean | - | - | - | - | - | - | - | - | 15 | - |
| Extruded soybean contaminated with mould spores | - | - | - | - | - | - | - | - | - | 15 |
| Raw Material | Moisture ± SD [%] |
|---|---|
| Meadow hay | 12.51 ± 0.19 |
| Wheat bran | 10.53 ± 0.15 |
| Fruit pomace | 9.35 ± 0.26 |
| Wheat | 11.65 ± 0.13 |
| Barley | 10.98 ± 0.11 |
| Maize | 12.76 ± 0.18 |
| Soybean | 11.47 ± 0.12 |
| Sorghum | 12.09 ± 0.21 |
| Wheat contaminated with mould spores | 19.98 ± 0.21 |
| Barley contaminated with mould spores | 20.11 ± 0.17 |
| Maize contaminated with mould spores | 22.58 ± 0.23 |
| Soybean contaminated with mould spores | 21.42 ± 0.20 |
| Sorghum contaminated with mould spores | 20.98 ± 0.25 |
| Extruded wheat contaminated with mould spores | 8.82 ± 0.08 |
| Extruded barley contaminated with mould spores | 8.16 ± 0.12 |
| Extruded maize contaminated with mould spores | 9.82 ± 0.08 |
| Extruded soybean contaminated with mould spores | 9.46 ± 0.10 |
| Extruded sorghum contaminated with mould spores | 9.28 ± 0.12 |
| Pellet Type | Pelletiser Power Demand [kW] | Specific Energy Consumption [kWh·kg−1] | Physical Density [kg·m−3] | Bulk Density [kg·m−3] | Holmen Mechanical Durability (PDIH) [%] |
|---|---|---|---|---|---|
| 1A | 2.81± 0.11 | 0.0281 | 1131.17 ± 12.11 | 402.61 ± 5.21 | 79.11 ± 1.16 |
| 1B | 2.76 ± 0.13 | 0.0276 | 1163.68 ± 18.25 | 410.82 ± 6.95 | 82.33 ± 0.99 |
| 2A | 2.79 ± 0.18 | 0.0279 | 1159.40 ± 14.52 | 412.58 ± 5.38 | 73.51 ± 1.56 |
| 2B | 2.74 ± 0.16 | 0.0274 | 1226.32 ± 9.83 | 409.27 ± 8.36 | 75.26 ± 1.18 |
| 3A | 2.83 ± 0.10 | 0.0283 | 1199.34 ± 22.09 | 399.21 ± 6.59 | 67.24 ± 1.84 |
| 3B | 2.78 ± 0.15 | 0.0278 | 1166.18 ± 25.83 | 397.47 ± 5.33 | 74.78 ± 1.53 |
| 4A | 2.72 ± 0.14 | 0.0272 | 1159.22 ± 22.61 | 401.83 ± 6.86 | 63.15 ± 1.24 |
| 4B | 2.70 ± 0.19 | 0.0270 | 1179.82 ± 29.48 | 404.02 ± 7.64 | 65.65 ± 1.49 |
| 5A | 2.49 ± 0.18 | 0.0249 | 1063.63 ± 25.72 | 402.53 ± 6.13 | 74.74 ± 1.67 |
| 5B | 2.45 ± 0.17 | 0.0245 | 1138.34 ± 24.35 | 409.89 ± 7.03 | 79.82 ± 1.26 |
| Active Substance | Concentration [μg·kg−1D.W.] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 2A | 2B | 3A | 3B | 4A | 4B | 5A | 5B | |
| 3-Acetyl-deoxynivalenol (3-AcDON) | 3.11 | 18.50 | 8.39 | 36.18 | 3.83 | 39.81 | 1.19 | 24.89 | 8.21 | 11.80 |
| Aflatoxin B1 (AFB1) | 0.83 | 2.64 | 0.38 | 3.97 | 1.03 | 4.13 | 0.99 | 4.82 | 1.15 | 4.20 |
| Deoxynivalenol (DON) | 118.12 | 315.83 | 209.58 | 418.23 | 189.63 | 538.37 | 318.90 | 842.48 | 239.11 | 430.38 |
| Fumonisin B1 (FB1) | 11.37 | 68.11 | 12.45 | 258.11 | 9.38 | 112.65 | 7.52 | 187.52 | 11.89 | 265.12 |
| Fumonisin B2 (FB2) | 8.37 | 78.38 | 16.82 | 309.65 | 12.63 | 283.19 | 9.85 | 198.82 | 8.11 | 205.83 |
| Ochratoxin A (OTA) | 3.18 | 42.19 | 5.67 | 37.74 | 4.98 | 41.52 | 5.61 | 32.98 | 5.76 | 38.17 |
| HT-2 toxin (HT-2) | 7.42 | 37.00 | 2.63 | 29.73 | 4.63 | 38.17 | 3.52 | 61.50 | 4.87 | 67.10 |
| T-2 toxin (T-2) | 17.43 | 99.10 | 14.32 | 183.00 | 18.93 | 153.00 | 11.86 | 152.10 | 18.39 | 231.00 |
| Zearalenone (ZEN) | 2.13 | 17.30 | 3.62 | 18.95 | 2.54 | 9.83 | 1.19 | 7.20 | 1.82 | 6.50 |
| Pellet Type | Total Mould Colony Count Before Pelleting [CFU·g−1] | Total Mould Colony Count After Pelleting [CFU·g−1] | Reduction [%] |
|---|---|---|---|
| 1B | 5.10 × 104 | 3.25 × 104 | 36.28 |
| 2B | 4.80 × 104 | 3.27 × 104 | 31.88 |
| 3B | 1.70 × 105 | 6.03 × 104 | 64.53 |
| 4B | 7.23 × 104 | 5.27 × 104 | 27.11 |
| 5B | 5.60 × 104 | 3.45 × 104 | 38.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwalina, P.; Obidziński, S.; Kowczyk-Sadowy, M.; Sienkiewicz, A.; Mazur, J. Extrusion Processing of Fungal-Contaminated Cereals as a Method for Spore Reduction and Binder Development in Feed Materials. Materials 2025, 18, 5117. https://doi.org/10.3390/ma18225117
Cwalina P, Obidziński S, Kowczyk-Sadowy M, Sienkiewicz A, Mazur J. Extrusion Processing of Fungal-Contaminated Cereals as a Method for Spore Reduction and Binder Development in Feed Materials. Materials. 2025; 18(22):5117. https://doi.org/10.3390/ma18225117
Chicago/Turabian StyleCwalina, Paweł, Sławomir Obidziński, Małgorzata Kowczyk-Sadowy, Aneta Sienkiewicz, and Jacek Mazur. 2025. "Extrusion Processing of Fungal-Contaminated Cereals as a Method for Spore Reduction and Binder Development in Feed Materials" Materials 18, no. 22: 5117. https://doi.org/10.3390/ma18225117
APA StyleCwalina, P., Obidziński, S., Kowczyk-Sadowy, M., Sienkiewicz, A., & Mazur, J. (2025). Extrusion Processing of Fungal-Contaminated Cereals as a Method for Spore Reduction and Binder Development in Feed Materials. Materials, 18(22), 5117. https://doi.org/10.3390/ma18225117







