Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microbiological Activity
2.2.1. Minimal Inhibitory Concentrations (MIC)
2.2.2. Anti-Biofilm Activity
2.3. Antioxidant Properties
2.4. Anti-Inflammatory Properties
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic Approaches and Materials in Restorative and Regenerative Dentistry: Review Article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The Use of Hydroxyapatite Toothpaste to Prevent Dental Caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and Repair of Enamel Surface by Biomimetic Zn-Carbonate Hydroxyapatite Containing Toothpaste: A Comparative in Vivo Study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel Remineralization and Repair Results of Biomimetic Hydroxyapatite Toothpaste on Deciduous Teeth: An Effective Option to Fluoride Toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Brezhnev, A.; Tang, F.-K.; Kwan, C.-S.; Basabrain, M.S.; Tsoi, J.K.H.; Matinlinna, J.P.; Neelakantan, P.; Leung, K.C.-F. One-Pot Preparation of Cetylpyridinium Chloride-Containing Nanoparticles for Biofilm Eradication. ACS Appl. Bio Mater. 2023, 6, 1221–1230. [Google Scholar] [CrossRef]
- Seriwatanachai, D.; Triratana, T.; Kraivaphan, P.; Huber, N.; Poth, T.; Mateo, L.R.; Stellitano, M.; Zhang, Y.-P. Effectiveness of a Novel Amine + Zinc + Fluoride Toothpaste in Reducing Plaque and Gingivitis: Results of a Six-Month Randomized Controlled Trial. BMC Oral Health 2025, 25, 188. [Google Scholar] [CrossRef]
- Stanton, K.A.; McCracken, B.A. An Activated-Zinc Oral Rinse Reduces pro-Inflammatory Cytokine Secretion and Promotes Proliferation in Porphyromonas Gingivalis LPS-Challenged Gingival Tissues—A Pilot Study. Clin. Exp. Dent. Res. 2021, 7, 995–1001. [Google Scholar] [CrossRef]
- Montesani, L.; Montesani, L.; Mateo, L.; Daep, C.; Huber, N.; Isapour, G.; Zhang, Y.-P. Antibacterial and Clinical Effectiveness of a Mouthwash with a Novel Active System of Amine + Zinc Lactate + Fluoride: A Randomized Controlled Trial. Clin. Oral. Investig. 2024, 28, 90. [Google Scholar] [CrossRef]
- Magaz, V.R.; Llovera, B.F.; Martí, M.; Garre, A. Clinical Impact and Cosmetic Acceptability of Chlorhexidineenriched Toothpaste and Mouthwash Application on Periodontal Disease: A Randomized Clinical Study. J. Contemp. Dent. Pract. 2018, 19, 1295–1300. [Google Scholar] [CrossRef]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical Use of Dexpanthenol in Skin Disorders. Am. J. Clin. Dermatol. 2002, 3, 427–433. [Google Scholar] [CrossRef]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-Microbiome Interactions Regarding Peri-Implantitis and Dental Implant Loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef]
- Benson, P.E.; Parkin, N.; Dyer, F.; Millett, D.T.; Germain, P. Fluorides for Preventing Early Tooth Decay (Demineralised Lesions) during Fixed Brace Treatment. Cochrane Database Syst. Rev. 2019, 2019, CD003809. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a Toothpaste with Microcrystalline Hydroxyapatite on the Occurrence of Early Childhood Caries: A 1-Year Randomized Clinical Trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Kunzelmann, K.-H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a Non-Fluoridated Microcrystalline Hydroxyapatite Dentifrice on Enamel Caries Progression in Highly Caries-Susceptible Orthodontic Patients: A Randomized, Controlled 6-Month Trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-Preventing Effect of a Hydroxyapatite-Toothpaste in Adults: A 18-Month Double-Blinded Randomized Clinical Trial. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ Release and Buffering Effects of Synthetic Hydroxyapatite Following Bacterial Acid Challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and Antibiofilm Activity of Selected Lamiaceae Essential Oils. FBL 2023, 28, 28. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Astaxanthin Demonstrates Moderate or Weak Activity against Bacterial and Fungal Pathogens. Food Biosci. 2025, 65, 106026. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus Mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- McCormack, M.G.; Smith, A.J.; Akram, A.N.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the Oral Cavity: An Overlooked Source of Carriage and Infection? Am. J. Infect. Control 2015, 43, 35–37. [Google Scholar] [CrossRef]
- Zaatout, N. Presence of Non-Oral Bacteria in the Oral Cavity. Arch. Microbiol. 2021, 203, 2747–2760. [Google Scholar] [CrossRef]
- Islam, A.; Hossain, N.; Hossain, S.; Khan, F.; Hossain, S.; Arup, M.R.; Chowdhury, M.A.; Rahman, M. Advances of Hydroxyapatite Nanoparticles in Dental Implant Applications. Int. Dent. J. 2025, 75, 2272–2313. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, C.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in Dentistry: A Comprehensive Review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef]
- Fan, J.; Wang, P.; Wang, S.; Li, R.; Yang, Y.; Jin, L.; Sun, Y.; Li, D. Advances in Macro-Bioactive Materials Enhancing Dentin Bonding. Discov. Nano 2025, 20, 40. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; Ma, K.; Wei, Z.; Ran, X.; Fu, X.; Zhang, C.; Zhao, C. Electrospun Nanofibrous Membranes Meet Antibacterial Nanomaterials: From Preparation Strategies to Biomedical Applications. Bioact. Mater. 2024, 42, 478–518. [Google Scholar] [CrossRef]
- Sun, T.; Huang, J.; Zhang, W.; Zheng, X.; Wang, H.; Liu, J.; Leng, H.; Yuan, W.; Song, C. Simvastatin-Hydroxyapatite Coatings Prevent Biofilm Formation and Improve Bone Formation in Implant-Associated Infections. Bioact. Mater. 2022, 21, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, A.; Yousef, N.; Askoura, M. Zinc Oxide Nanoparticles Reduce Biofilm Formation, Synergize Antibiotics Action and Attenuate Staphylococcus aureus Virulence in Host; an Important Message to Clinicians. BMC Microbiol. 2022, 22, 244. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The Anti-Inflammatory Activity of Licorice, a Widely Used Chinese Herb. Pharm. Biol. 2016, 55, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Sahar, A.; Tariq, A.; Naz, T.; Usman, M. Exploring the Role of Licorice and Its Derivatives in Cell Signaling Pathway NF-κB and MAPK. J. Nutr. Metab. 2024, 2024, 9988167. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, Y.; Bilgin Çetin, M.; Bulut, Ş.; Alptekin, N.Ö.; Börçek, P. Evaluating the Effects of a Topical Preparation with Dexpanthenol, Silbiol, Undecylenic Acid, and Lidocaine on Palatal Mucosa Wound Healing in a Rat Model. Balk. Med. J. 2019, 36, 88–95. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-Hydroxyapatite and Its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 2017, CD008676. [Google Scholar] [CrossRef]
- Lorenz, K.; Hoffmann, T.; Heumann, C.; Noack, B. Effect of Toothpaste Containing Amine Fluoride and Stannous Chloride on the Reduction of Dental Plaque and Gingival Inflammation. A Randomized Controlled 12-Week Home-Use Study. Int. J. Dent. Hyg. 2019, 17, 237–243. [Google Scholar] [CrossRef]
- Geisinger, M.L.; Geurs, N.C.; Novy, B.; Otomo-Corgel, J.; Cobb, C.M.; Jacobsen, P.L.; Takesh, T.; Wilder-Smith, P. A Randomized Double-Blind Clinical Trial Evaluating Comparative Plaque and Gingival Health Associated with Commercially Available Stannous Fluoride-Containing Dentifrices as Compared to a Sodium Fluoride Control Dentifrice. J. Periodontol. 2023, 94, 1112–1121. [Google Scholar] [CrossRef]
- Pompilio, A.; Scribano, D.; Sarshar, M.; Di Bonaventura, G.; Palamara, A.T.; Ambrosi, C. Gram-Negative Bacteria Holding Together in a Biofilm: The Acinetobacter Baumannii Way. Microorganisms 2021, 9, 1353. [Google Scholar] [CrossRef]
- Ouhayoun, J.-P. Penetrating the Plaque Biofilm: Impact of Essential Oil Mouthwash. J. Clin. Periodontol. 2003, 30, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Maziere, M.; Rompante, P.; Andrade, J.C.; Rodrigues, C.F. Are Mouthwashes Really Effective against Candida spp.? J. Fungi 2024, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Wei, S.-M.; Luo, X.-T.; Yang, Q.-Q.; Wong, K.-H.; Cheung, P.C.K.; Zhang, B.-B. How Probiotics, Prebiotics, Synbiotics, and Postbiotics Prevent Dental Caries: An Oral Microbiota Perspective. npj Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Grzegorzewski, J.; Kwiatek, J.; Leśna, M.; Cielecka-Piontek, J. Green Tea: A Novel Perspective on the Traditional Plant’s Potential in Managing Periodontal Diseases. Pharmaceuticals 2025, 18, 409. [Google Scholar] [CrossRef] [PubMed]

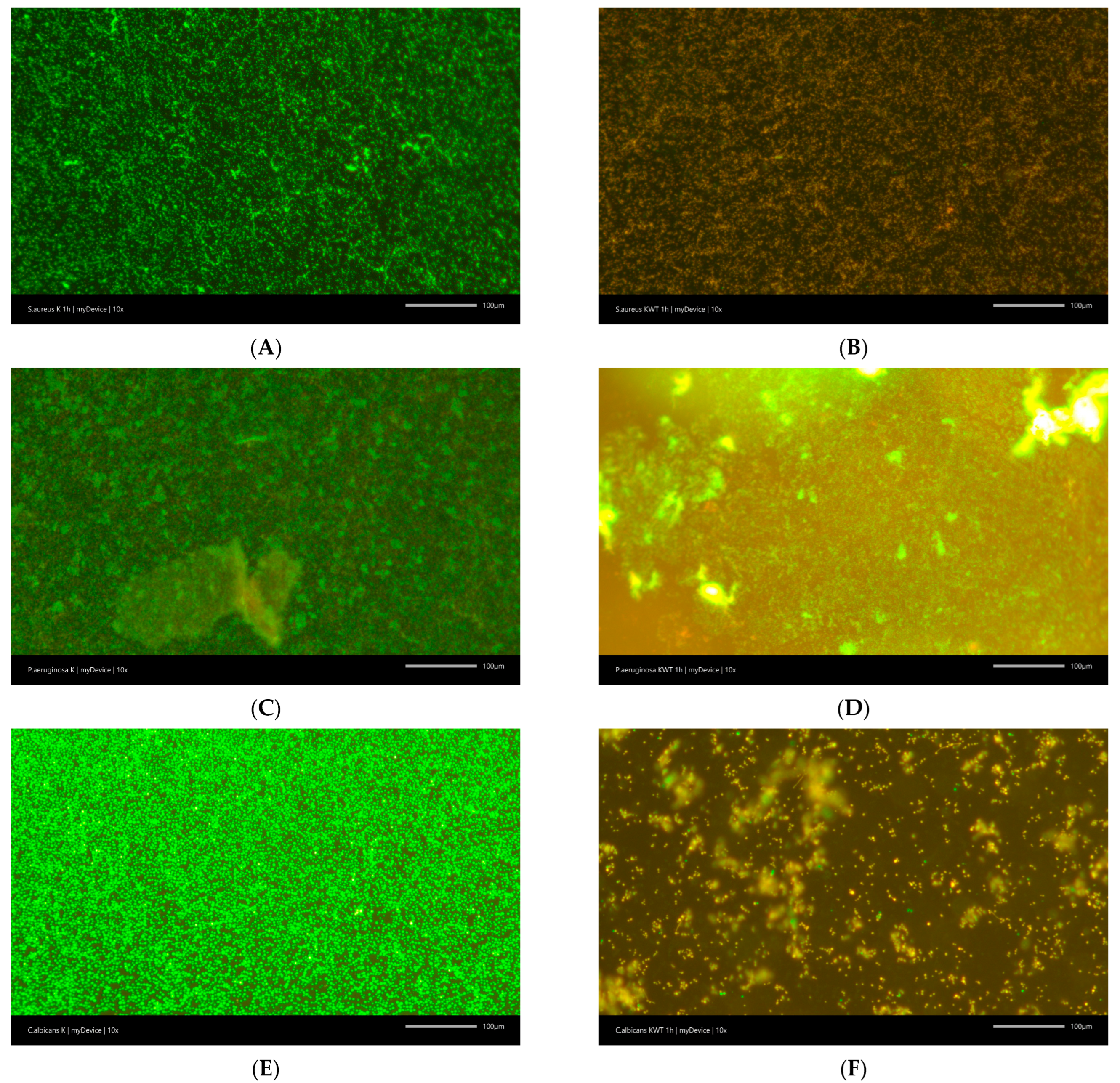


| Mouthwash | Antibiofilm Activity | Pathogens | ||
|---|---|---|---|---|
| Staphylococcus aureus | Pseudomonas aeruginosa | Candida albicans | ||
| Control | Biofilm % | 100 | 100 | 100 |
| Viability % | 98.3 ± 1.5 | 94.0 ± 1.0 | 98.3 ± 0.6 | |
| KWT0000 | Biofilm reduction % | −7.7 ± 2.5 * | −4.6 ± 2.5 | −64.7 ± 5.0 *** |
| Viability % | 31.7 ± 5.8 *** | 95.3 ± 1.2 | 40.0 ± 5.0 *** | |
| ELM | Biofilm reduction % | −6.3 ± 1.5 | −4.7 ± 2.5 | −46.7 ± 7.6 *** |
| Viability % | 38.3 ± 2.9 *** | 94.7 ± 1.2 | 50.0 ± 5.0 *** | |
| Antioxidant Properties [%] | Anti-Inflammatory Properties [%] | |
|---|---|---|
| KWT0000 | 55.33 ± 3.34 a | 23.33 ± 3.67 a |
| ELM mouthwash | 17.51 ± 1.55 b | 3.67 ± 0.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, T.M.; Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action. Materials 2025, 18, 3567. https://doi.org/10.3390/ma18153567
Karpiński TM, Paczkowska-Walendowska M, Cielecka-Piontek J. Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action. Materials. 2025; 18(15):3567. https://doi.org/10.3390/ma18153567
Chicago/Turabian StyleKarpiński, Tomasz M., Magdalena Paczkowska-Walendowska, and Judyta Cielecka-Piontek. 2025. "Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action" Materials 18, no. 15: 3567. https://doi.org/10.3390/ma18153567
APA StyleKarpiński, T. M., Paczkowska-Walendowska, M., & Cielecka-Piontek, J. (2025). Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action. Materials, 18(15), 3567. https://doi.org/10.3390/ma18153567








