The Impact of the High-Energy Grinding of Wood Ash on Its Pozzolanic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing Methods
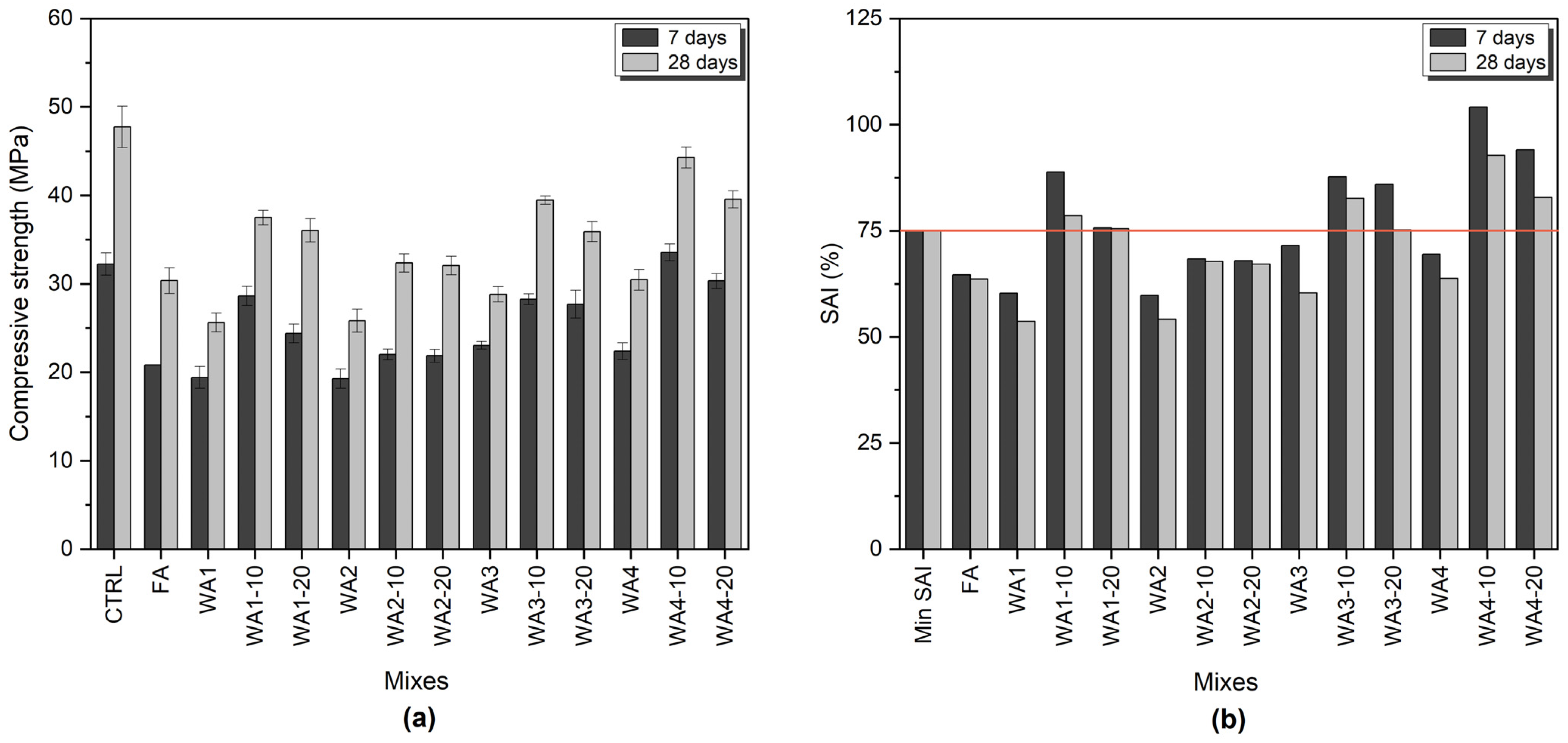
2.2.1. Strength Activity Index (SAI)
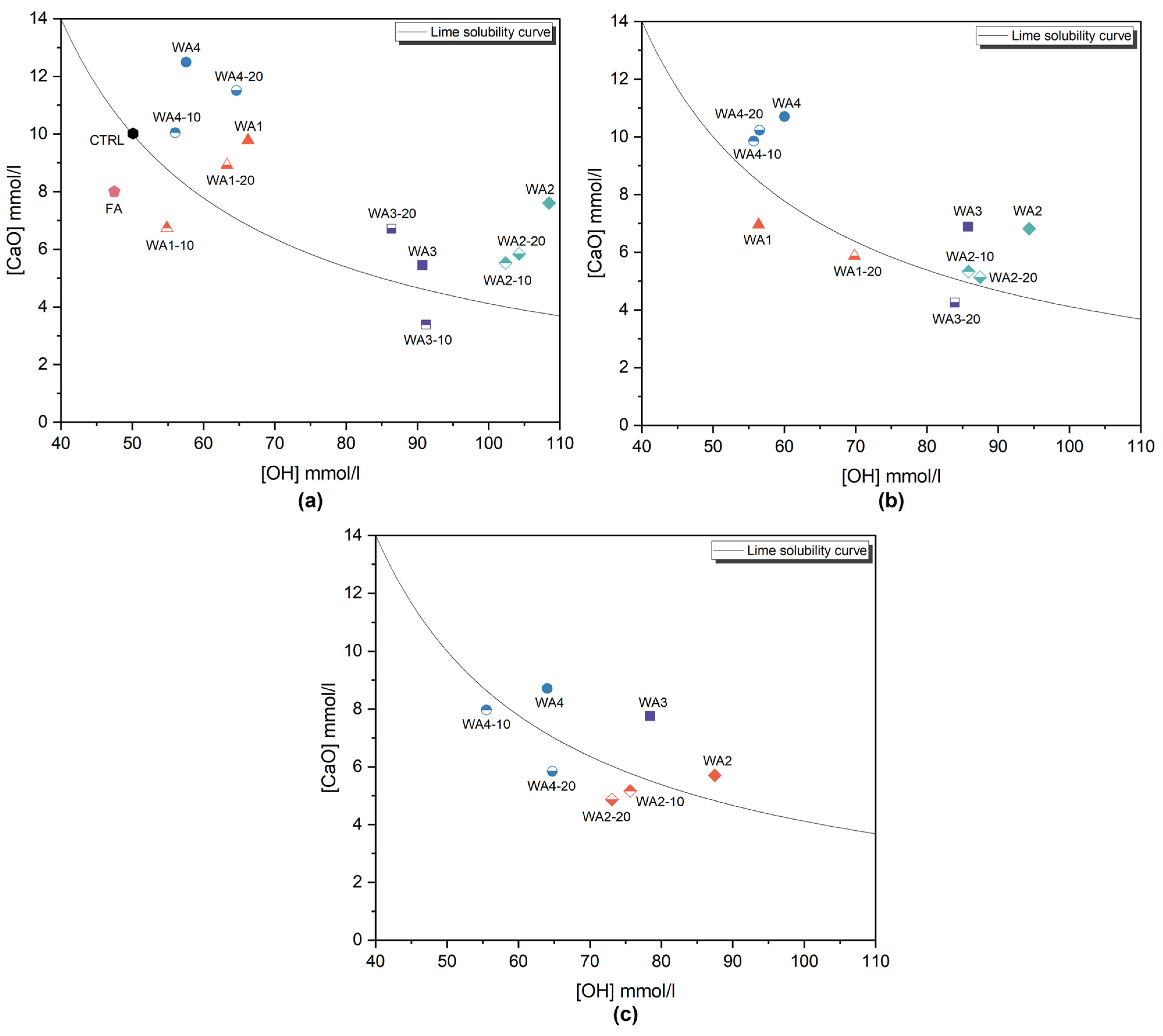
2.2.2. Frattini Test
2.2.3. R3 Test
2.2.4. X-Ray Diffraction (XRD) Analysis
2.2.5. Thermogravimetric Analysis (TGA/DTG)
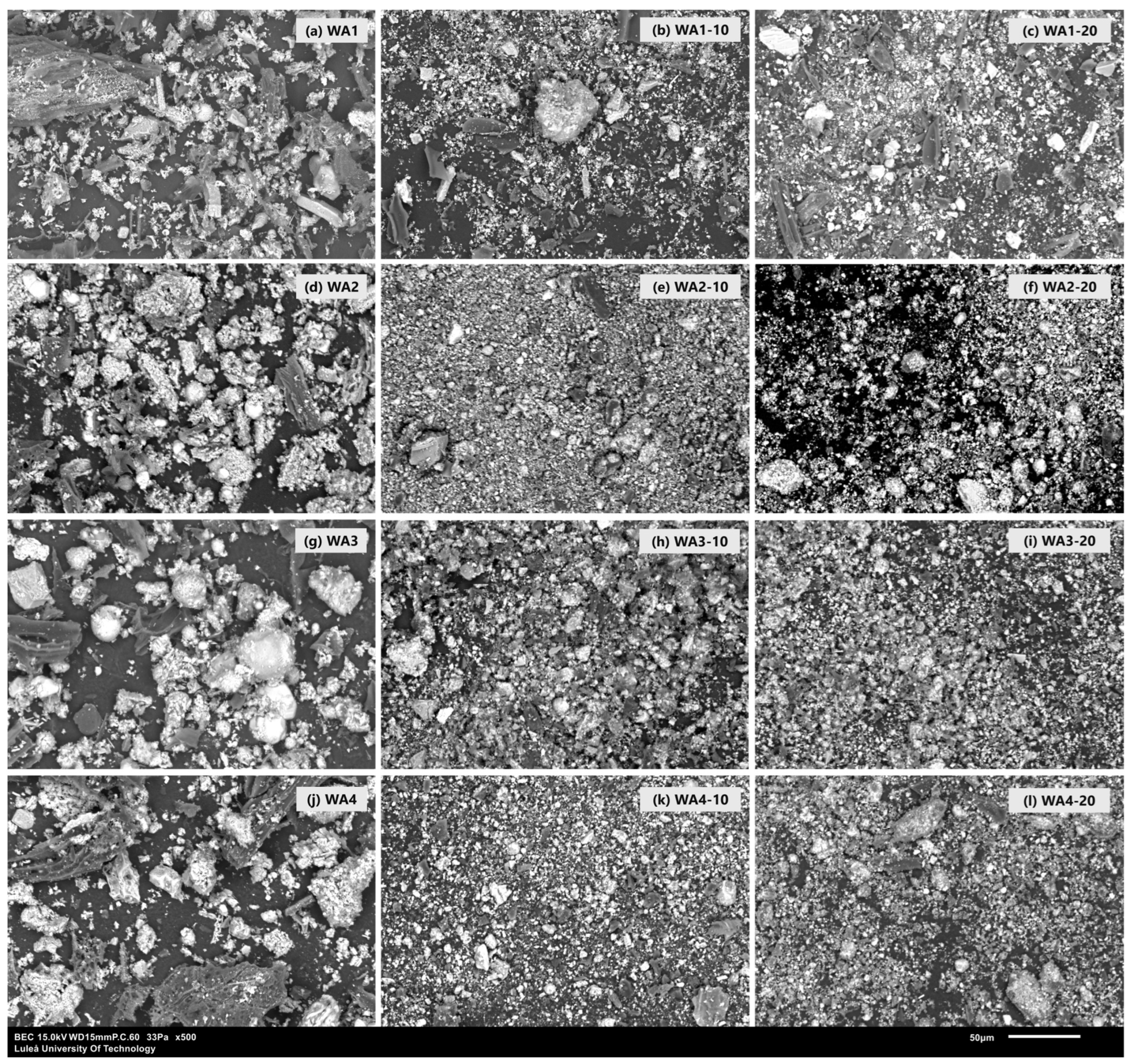
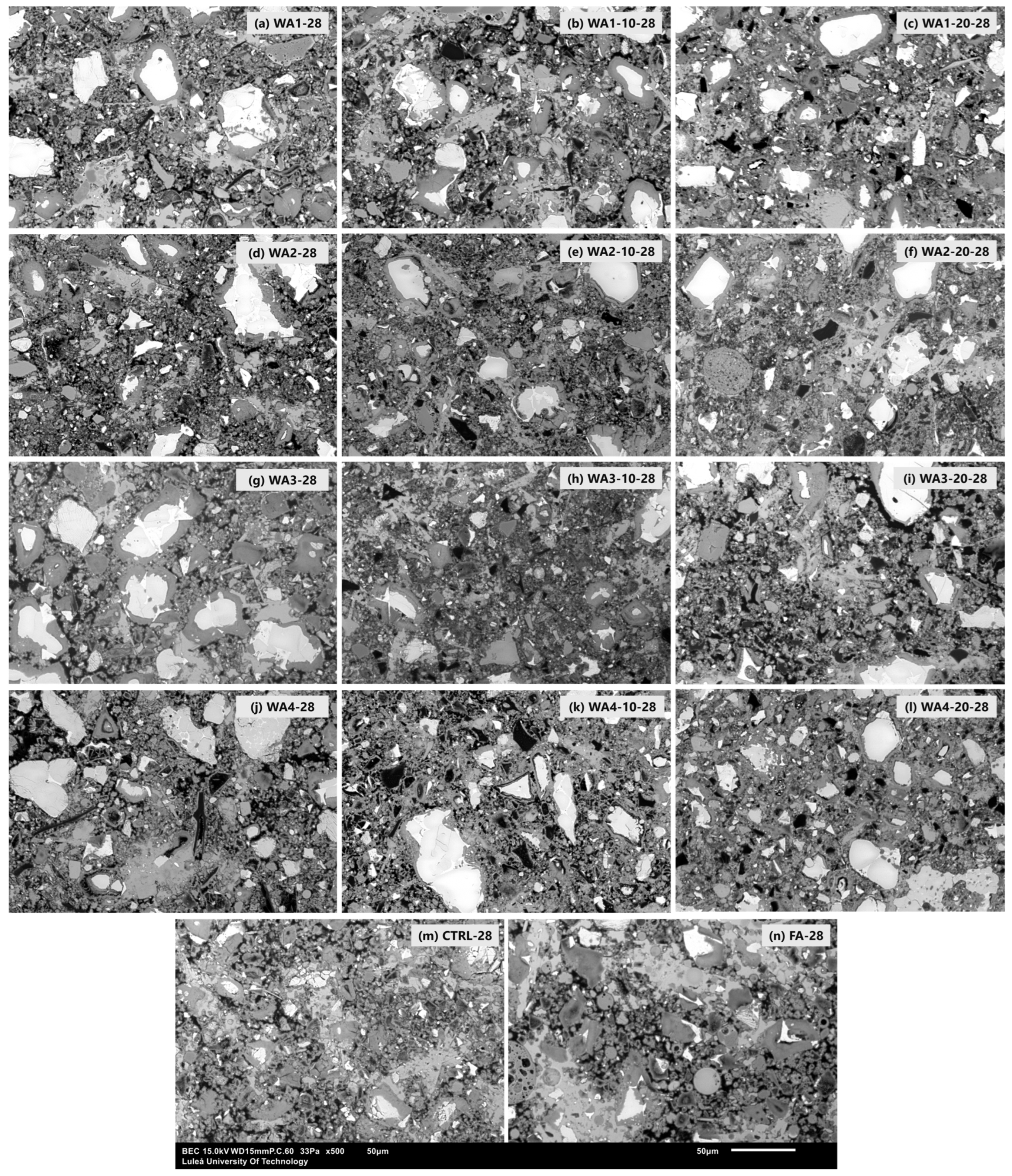
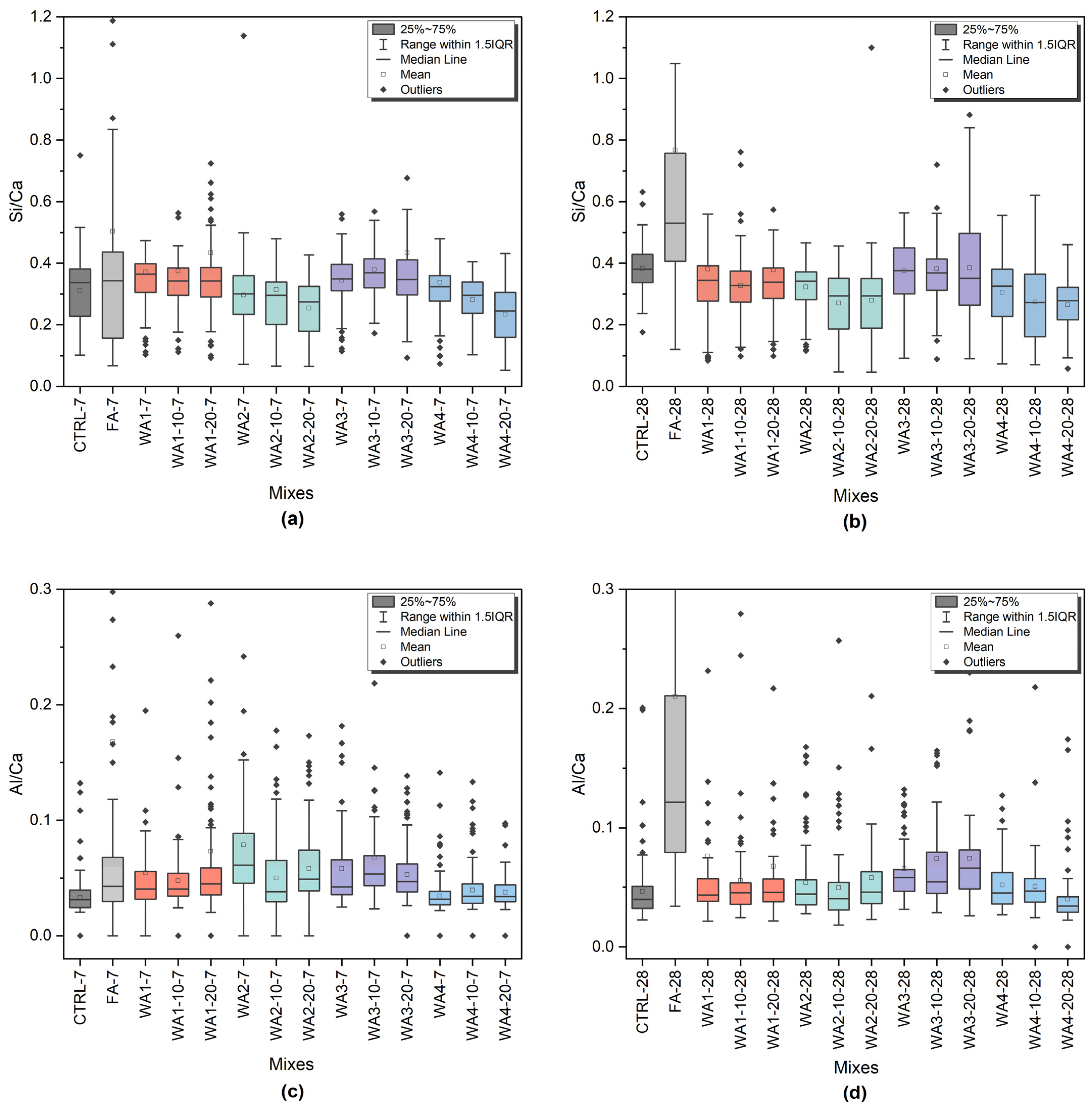
2.2.6. Microstructural Investigation
3. Results and Discussion
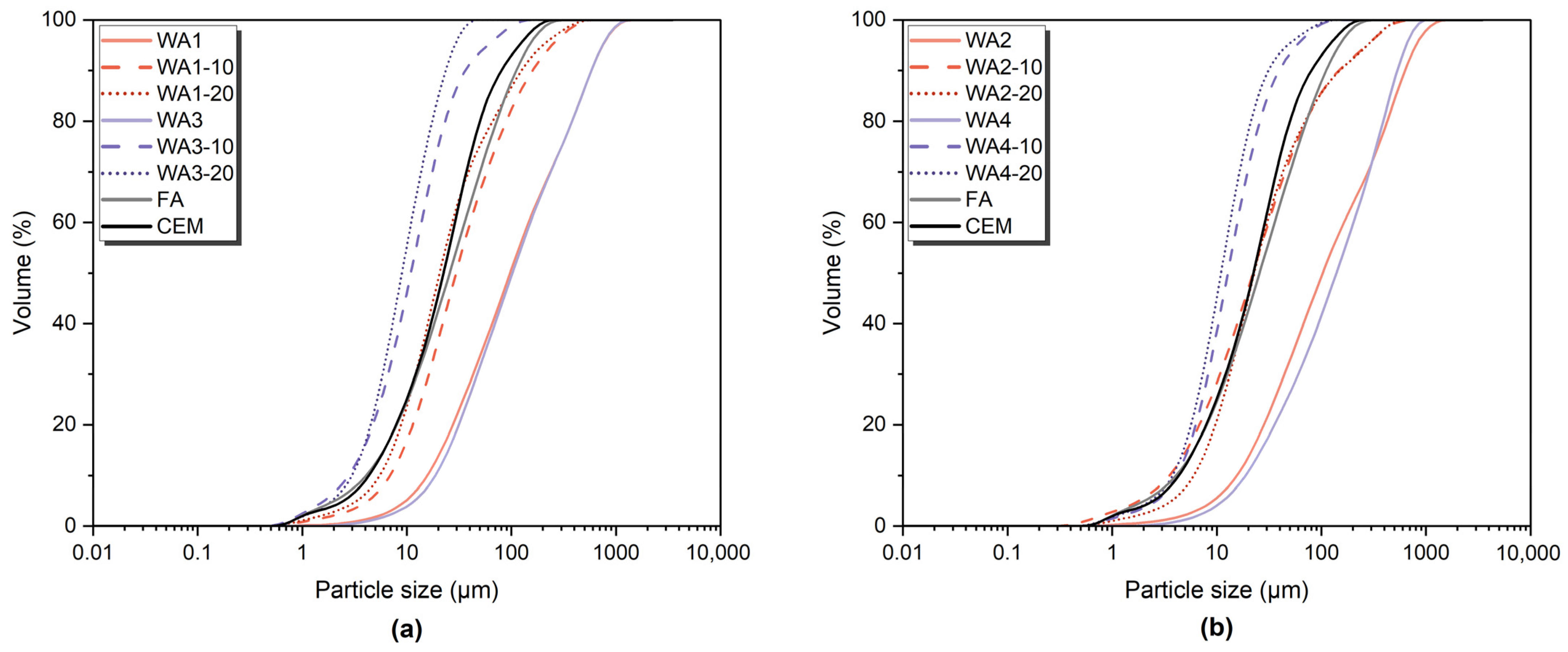
3.1. Wood Ash Characterization
3.2. Strength Activity Index (SAI)
3.3. Frattini Test
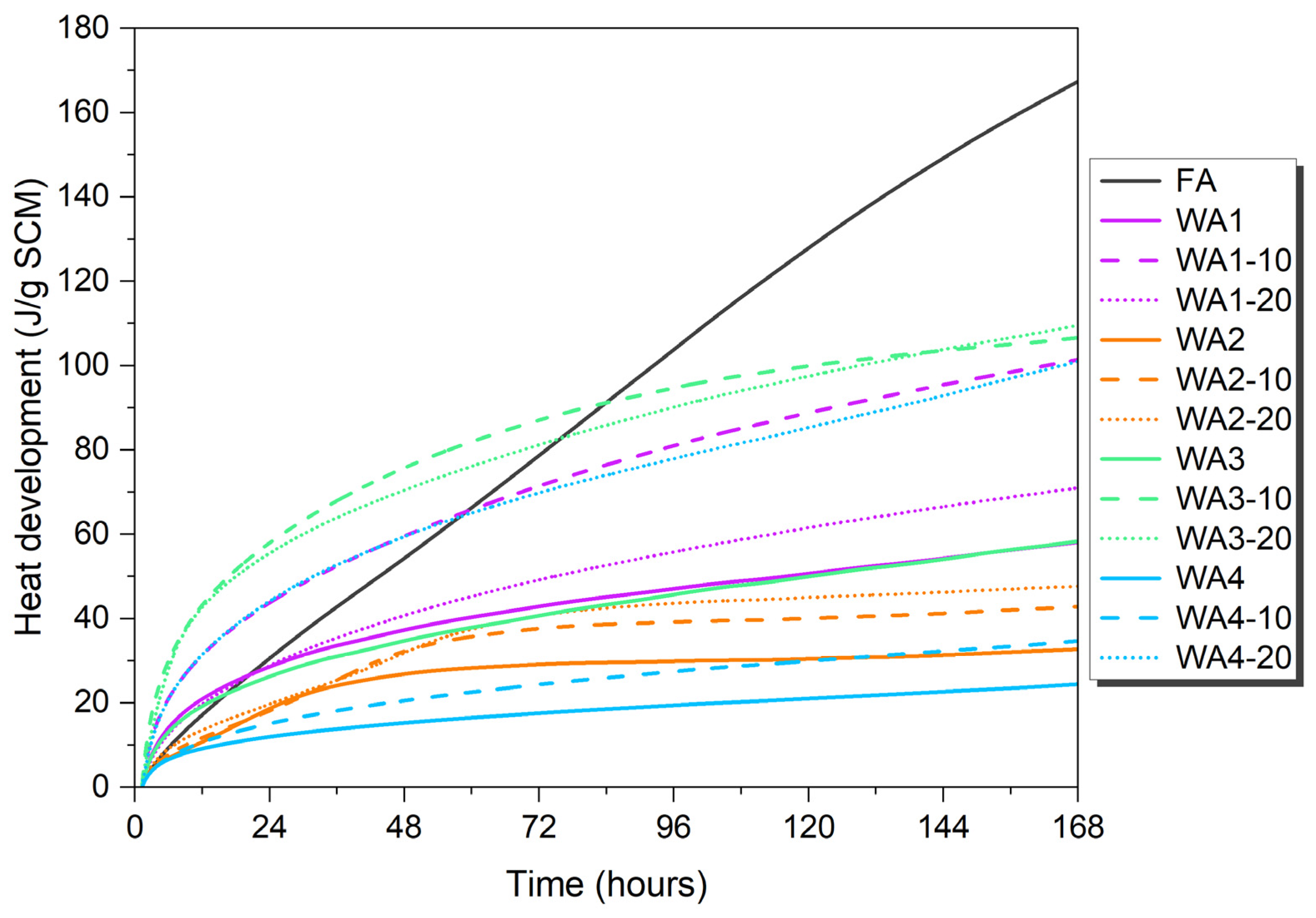
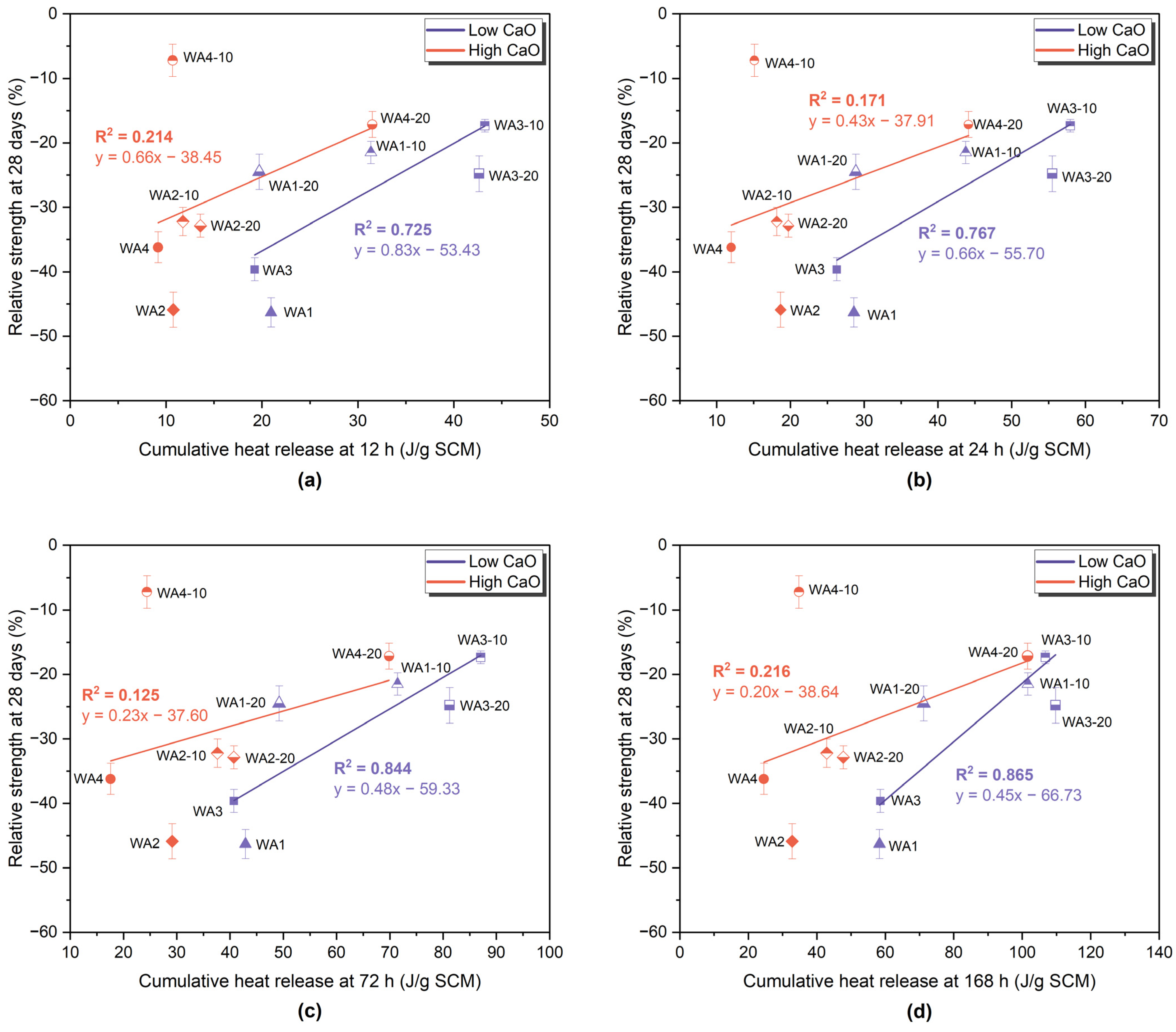
3.4. R3 Test—Isothermal Calorimetry
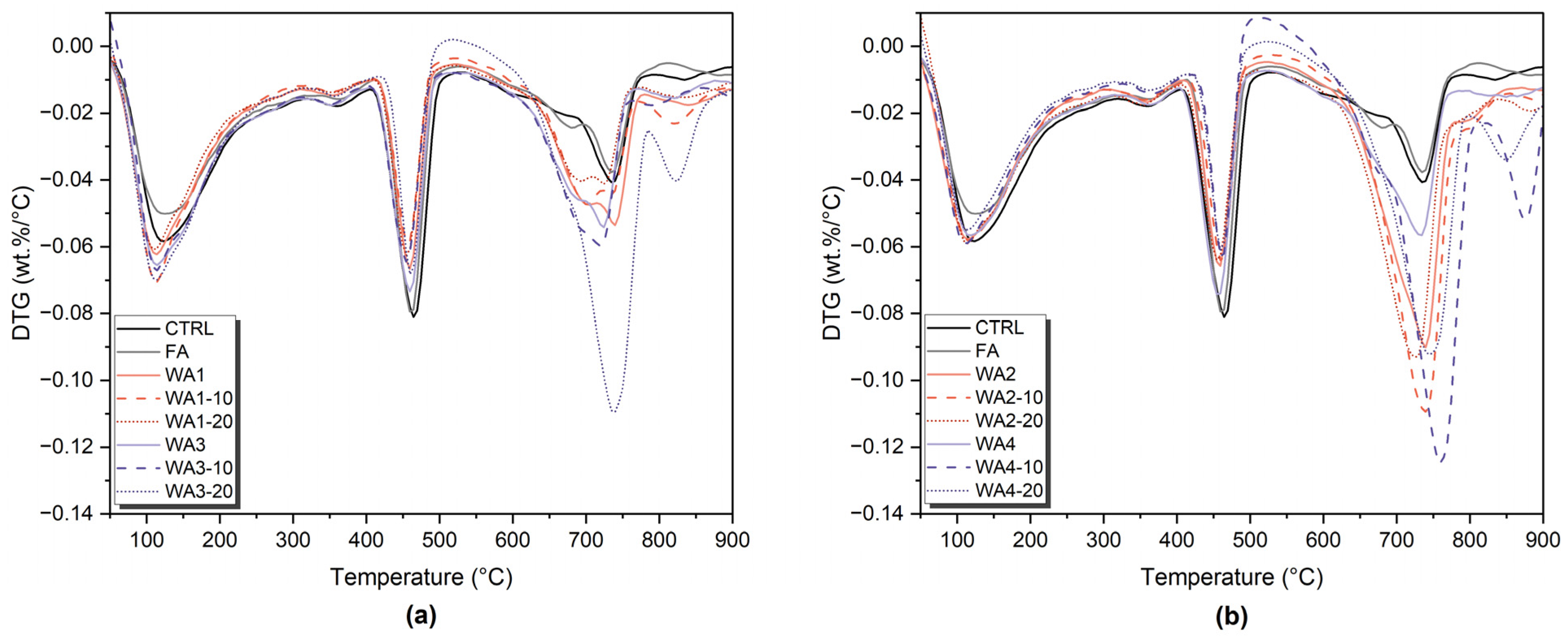
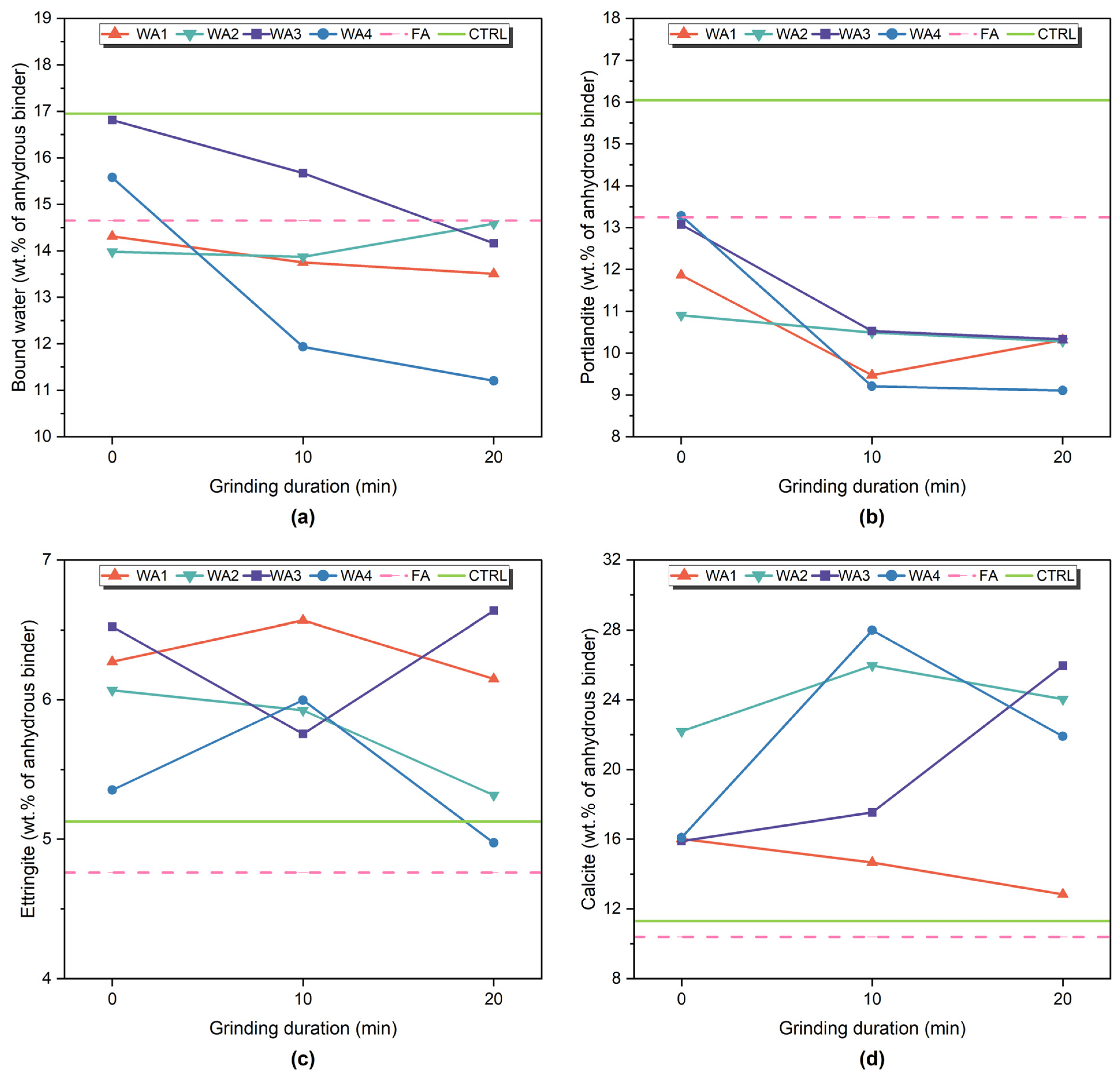
3.5. Phase Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary Cementitious Materials: New Sources, Characterization, and Performance Insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Bonifacio, A.L.; Archbold, P. The Effect of Calcination Conditions on Oat Husk Ash Pozzolanic Activity. Mater. Today Proc. 2022, 65, 622–628. [Google Scholar] [CrossRef]
- Kasaniya, M.; Alaibani, A.; Thomas, M.D.A.; Riding, K.A. Exploring the Efficacy of Emerging Reactivity Tests in Screening Pozzolanic Materials. Constr. Build. Mater. 2022, 325, 126781. [Google Scholar] [CrossRef]
- ASTM C125-21a; Standard Terminology Relating to Concrete and Concrete Aggregates. ASTM Int.: West Conshohocken, PA, USA, 2021.
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary Cementitious Materials Origin from Agricultural Wastes—A Review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of Test Methods to Assess Pozzolanic Activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Šídlová, M.; Šulc, R.; Rak, P.; Formáček, P.; Pulcová, K.; Snop, R. Comparison of Different Methods for Assessing the Pozzolanic Activity of Fly Ash and Bottom Ash. AIP Conf. Proc. 2023, 2780, 020023. [Google Scholar] [CrossRef]
- Kramar, S.; Ducman, V. Evaluation of Ash Pozzolanic Activity by Means of the Strength Activity Index Test, Frattini Test and DTA/TG Analysis. Teh. Vjesn. 2018, 25, 1746–1752. [Google Scholar] [CrossRef]
- Lazik, P.-R.; Bošnjak, J.; Cetin, E.; Kücük, A. Application of Wood Ash as a Substitute For Fly Ash And Investigation of Concrete Properties. Otto-Graf-Journal 2020, 19, 103–118. [Google Scholar]
- Siddique, R. Utilization of Wood Ash in Concrete Manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass Waste Utilisation in Low-Carbon Products: Harnessing a Major Potential Resource. Npj Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Reuse of Woody Biomass Fly Ash in Cement-Based Materials. Constr. Build. Mater. 2015, 76, 286–296. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Hansen, E.Ø.; Jensen, P.E.; Kirkelund, G.M.; Golterman, P. Wood Ash Used as Partly Sand and/or Cement Replacement in Mortar. Int. J. Sustain. Dev. Plan. 2016, 11, 781–791. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Vrbos, N.; Koenders, E.A.B. Reuse of Woody Biomass Ash Waste in Cementitious Materials. Chem. Biochem. Eng. Q. 2016, 30, 137–148. [Google Scholar] [CrossRef]
- Hamid, Z.; Rafiq, S. An Experimental Study on Behavior of Wood Ash in Concrete as Partial Replacement of Cement. Mater. Today Proc. 2021, 46, 3426–3429. [Google Scholar] [CrossRef]
- Teker Ercan, E.E.; Andreas, L.; Cwirzen, A.; Habermehl-Cwirzen, K. Wood Ash as Sustainable Alternative Raw Material for the Production of Concrete—A Review. Materials 2023, 16, 2557. [Google Scholar] [CrossRef]
- Garcia, M.D.L.; Sousa-Coutinho, J. Strength and Durability of Cement with Forest Waste Bottom Ash. Constr. Build. Mater. 2013, 41, 897–910. [Google Scholar] [CrossRef]
- Rajamma, R.; Ball, R.J.; Tarelho, L.A.C.; Allen, G.C.; Labrincha, J.A.; Ferreira, V.M. Characterisation and Use of Biomass Fly Ash in Cement-Based Materials. J. Hazard. Mater. 2009, 172, 1049–1060. [Google Scholar] [CrossRef]
- Chowdhury, S.; Maniar, A.; Suganya, O.M. Strength Development in Concrete with Wood Ash Blended Cement and Use of Soft Computing Models to Predict Strength Parameters. J. Adv. Res. 2015, 6, 907–913. [Google Scholar] [CrossRef]
- de la Grée, G.C.H.D.; Florea, M.V.A.; Keulen, A.; Brouwers, H.J.H. Contaminated Biomass Fly Ashes—Characterization and Treatment Optimization for Reuse as Building Materials. Waste Manag. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Beltrán, M.G.; López, M.; Agrela, F. Effects of Treatments on Biomass Bottom Ash Applied to the Manufacture of Cement Mortars. J. Clean. Prod. 2017, 154, 424–435. [Google Scholar] [CrossRef]
- Amaral, R.C.; Rohden, A.B.; Garcez, M.R.; Andrade, J.J.d.O. Reuse of Wood Ash from Biomass Combustion in Non-structural Concrete: Mechanical Properties, Durability, and Eco-efficiency. J. Mater. Cycles Waste Manag. Vol. 2022, 24, 2439–2454. [Google Scholar] [CrossRef]
- Elinwa, A.U.; Mahmood, Y.A. Ash from Timber Waste as Cement Replacement Material. Cem. Concr. Compos. 2002, 24, 219–222. [Google Scholar] [CrossRef]
- Jurić, K.K.; Carević, I.; Serdar, M.; Štirmer, N. Feasibility of Using Pozzolanicity Tests to Assess Reactivity of Wood Biomass Fly Ashes. J. Croat. Assoc. Civ. Eng. 2021, 72, 1145–1153. [Google Scholar] [CrossRef]
- Demis, S.; Tapali, J.G.; Papadakis, V.G. An Investigation of the Effectiveness of the Utilization of Biomass Ashes as Pozzolanic Materials. Constr. Build. Mater. 2014, 68, 291–300. [Google Scholar] [CrossRef]
- Tole, I.; Delogu, F.; Qoku, E.; Habermehl-Cwirzen, K.; Cwirzen, A. Enhancement of the Pozzolanic Activity of Natural Clays by Mechanochemical Activation. Constr. Build. Mater. 2022, 352, 128739. [Google Scholar] [CrossRef]
- Snellings, R.; Kazemi-Kamyab, H.; Nielsen, P.; Van den Abeele, L. Classification and Milling Increase Fly Ash Pozzolanic Reactivity. Front. Built Environ. 2021, 7, 670996. [Google Scholar] [CrossRef]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C.R. Effect of Milling and Acid Washing on the Pozzolanic Activity of Incinerator Sewage Sludge Ash. Cem. Concr. Compos. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Akmalaiuly, K.; Berdikul, N.; Pundienė, I.; Pranckevičienė, J. The Effect of Mechanical Activation of Fly Ash on Cement-Based Materials Hydration and Hardened State Properties. Materials 2023, 16, 2959. [Google Scholar] [CrossRef]
- Ke, X.; Baki, V.A.; Skevi, L. Mechanochemical Activation for Improving the Direct Mineral Carbonation Efficiency and Capacity of a Timber Biomass Ash. J. CO2 Util. 2023, 68, 102367. [Google Scholar] [CrossRef]
- Mucsi, G. Mechanical Activation of Power Station Fl y Ash by Grinding—A Review. Epa.—J. Silic. Based Compos. Mater. 2016, 68, 56–61. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical Activation of Fly Ash: Effect on Reaction, Structure and Properties of Resulting Geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Kaminskas, R.; Cesnauskas, V. Influence of Activated Biomass Fly Ash on Portland Cement Hydration. Ceram. Silik. 2014, 58, 260–268. [Google Scholar]
- Xu, W.; Lo, Y.T.; Ouyang, D.; Memon, S.A.; Xing, F.; Wang, W.; Yuan, X. Effect of Rice Husk Ash Fineness on Porosity and Hydration Reaction of Blended Cement Paste. Constr. Build. Mater. 2015, 89, 90–101. [Google Scholar] [CrossRef]
- Pantić, V.; Šupić, S.; Vučinić-Vasić, M.; Nemeš, T.; Malešev, M.; Lukić, I.; Radonjanin, V. Effects of Grinding Methods and Water-to-Binder Ratio on the Properties of Cement Mortars Blended with Biomass Ash and Ceramic Powder. Materials 2023, 16, 2443. [Google Scholar] [CrossRef]
- Ohenoja, K.; Tanskanen, P.; Peltosaari, O.; Wigren, V.; Österbacka, J.; Illikainen, M. Effect of Particle Size Distribution on the Self-Hardening Property of Biomass-Peat Fly Ash from a Bubbling Fluidized Bed Combustion. Fuel Process. Technol. 2016, 148, 60–66. [Google Scholar] [CrossRef]
- Šupić, S.; Malešev, M.; Radonjanin, V.; Bulatović, V.; Milović, T. Reactivity and Pozzolanic Properties of Biomass Ashes Generated by Wheat and Soybean Straw Combustion. Materials 2021, 14, 1004. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Cordeiro, G.C.; Akasaki, J.L.; Vieira, A.P.; Payá, J. Improving the Reactivity of a Former Ground Sugarcane Bagasse Ash Produced by Autogenous Combustion through Employment of Two Different Additional Grinding Procedures. Constr. Build. Mater. 2021, 270, 121471. [Google Scholar] [CrossRef]
- Teker Ercan, E.E.; Cwirzen, A.; Habermehl-Cwirzen, K. The Effects of Partial Replacement of Ground Granulated Blast Furnace Slag by Ground Wood Ash on Alkali-Activated Binder Systems. Materials 2023, 16, 5347. [Google Scholar] [CrossRef]
- ASTM C311-02; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM Int.: West Conshohocken, PA, USA, 2007.
- ASTM C618-15; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM Int.: West Conshohocken, PA, USA, 2015.
- EN 196-5; Methods of Testing Cement—Part 5—Pozzolanicity Test for Pozzolanic Cement. CEN: Brussels, Belgium, 2011.
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of Pozzolanic Activity of Different Calcined Clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- ASTM C1897-20; Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water Measurements. ASTM Int.: West Conshohocken, PA, USA, 2020; Volume 4, pp. 7–11. [CrossRef]
- Carević, I.; Serdar, M.; Štirmer, N.; Ukrainczyk, N. Preliminary Screening of Wood Biomass Ashes for Partial Resources Replacements in Cementitious Materials. J. Clean. Prod. 2019, 229, 1045–1064. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498738675. [Google Scholar]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Rec. 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Geiker, M.R.; Ottosen, L.M. Phase Development and Mechanical Response of Low-Level Cement Replacements with Wood Ash and Washed Wood Ash. Constr. Build. Mater. 2021, 269, 121234. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Geiker, M.R.; Ottosen, L.M. Reaction Mechanisms of Wood Ash for Use as a Partial Cement Replacement. Constr. Build. Mater. 2021, 286, 122889. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial Use of Limestone Filler with Calcium Sulphoaluminate Cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Humad, A.M.; Habermehl-Cwirzen, K.; Cwirzen, A. Effects of Fineness and Chemical Composition of Blast Furnace Slag on Properties of Alkali-Activated Binder. Materials 2019, 12, 3447. [Google Scholar] [CrossRef]
- Rossen, J.E.; Scrivener, K.L. Optimization of SEM-EDS to Determine the C–A–S–H Composition in Matured Cement Paste Samples. Mater. Charact. 2017, 123, 294–306. [Google Scholar] [CrossRef]
- Ikumapayi, O.M.; Akinlabi, E.T.; Adedeji, P.A.; Akinlabi, S.A. Comparative Analysis of Milling Time on the Particle Sizes of Coal Fly Ash and Wood Fly Ash Using Otsu Method for Thresholding. J. Phys. Conf. Ser. 2019, 1378, 042075. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Bouchenafa, O.; Guessasma, S.; Leklou, N.; Bouaziz, A. The Sequel of Modified Fly Ashes Using High Energy Ball Milling on Mechanical Performance of Substituted Past Cement. Mater. Des. 2016, 90, 29–37. [Google Scholar] [CrossRef]
- Kato, K.; Xin, Y.; Hitomi, T.; Shirai, T. Surface Modification of Fly Ash by Mechano-Chemical Treatment. Ceram. Int. 2019, 45, 849–853. [Google Scholar] [CrossRef]
- Rissanen, J.; Ohenoja, K.; Kinnunen, P.; Romagnoli, M.; Illikainen, M. Milling of Peat-Wood Fly Ash: Effect on Water Demand of Mortar and Rheology of Cement Paste. Constr. Build. Mater. 2018, 180, 143–153. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Ni, X.; Zhu, Z.C.; Yu, Z.F.; Yin, Z.X.; Li, T.Q.; Liu, S.Y.; Zhao, L.L.; Xu, J. Friction and Wear of Liner and Grinding Ball in Iron Ore Ball Mill. Tribol. Int. 2017, 115, 506–517. [Google Scholar] [CrossRef]
- EN 450-1; Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria. CEN: Brussels, Belgium, 2012; Volume 50.
- Ban, C.C.; Ken, P.W.; Ramli, M. Mechanical and Durability Performance of Novel Self-Activating Geopolymer Mortars. Procedia Eng. 2017, 171, 564–571. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. CEN: Brussels, Belgium, 2011.
- Sigvardsen, N.M.; Kirkelund, G.M.; Jensen, P.E.; Geiker, M.R.; Ottosen, L.M. Impact of Production Parameters on Physiochemical Characteristics of Wood Ash for Possible Utilisation in Cement-Based Materials. Resour. Conserv. Recycl. 2019, 145, 230–240. [Google Scholar] [CrossRef]
- Fořt, J.; Šál, J.; Žák, J.; Černý, R. Assessment of Wood-Based Fly Ash as Alternative Cement Replacement. Sustainability 2020, 12, 9580. [Google Scholar] [CrossRef]
- Silvestro, L.; Scolaro, T.P.; Ruviaro, A.S.; Lima, G.T.d.S.; Gleize, P.J.P.; Pelisser, F. Use of Biomass Wood Ash to Produce Sustainable Geopolymeric Pastes. Constr. Build. Mater. 2023, 370, 130641. [Google Scholar] [CrossRef]
- Carević, I.; Baričević, A.; Štirmer, N.; Šantek Bajto, J. Correlation between Physical and Chemical Properties of Wood Biomass Ash and Cement Composites Performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Sklivaniti, V.; Tsakiridis, P.E.; Katsiotis, N.S.; Velissariou, D.; Pistofidis, N.; Papageorgiou, D.; Beazi, M. Valorisation of Woody Biomass Bottom Ash in Portland Cement: A Characterization and Hydration Study. J. Environ. Chem. Eng. 2017, 5, 205–213. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Sanna, A.L.; Pia, G.; Delogu, F. Kinetics of Grain Size Reduction in Minerals Undergoing Ball Milling. Trans. Indian Inst. Met. 2023, 77, 4161–4168. [Google Scholar] [CrossRef]
- Makó, É.; Frost, R.L.; Kristóf, J.; Horváth, E. The Effect of Quartz Content on the Mechanochemical Activation of Kaolinite. J. Colloid Interface Sci. 2001, 244, 359–364. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, J.; Sheng, J. Dry Grinding Effect on Pyrophyllite-Quartz Natural Mixture and Its Influence on the Structural Alternation of Pyrophyllite. Micron 2015, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Avet, F.; Snellings, R.; Diaz, A.A.; Ben Haha, M.; Scrivener, K. Development of a New Rapid, Relevant and Reliable (R3) Test Method to Evaluate the Pozzolanic Reactivity of Calcined Kaolinitic Clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Parashar, A.; Bishnoi, S. A Comparison of Test Methods to Assess the Strength Potential of Plain and Blended Supplementary Cementitious Materials. Constr. Build. Mater. 2020, 256, 119292. [Google Scholar] [CrossRef]
- Suraneni, P. Recent Developments in Reactivity Testing of Supplementary Cementitious Materials. RILEM Tech. Lett. 2021, 6, 131–139. [Google Scholar] [CrossRef]
- Kalina, R.D.; Al-Shmaisani, S.; Ferron, R.D.; Juenger, M.C.G. False Positives in ASTM C618 Specifications for Natural Pozzolans. ACI Mater. J. 2019, 116, 165–172. [Google Scholar] [CrossRef]
- Vu, V.A.; Cloutier, A.; Bissonnette, B.; Blanchet, P.; Duchesne, J. The Effect of Wood Ash as a Partial Cement Replacement Material for Making Wood-Cement Panels. Materials 2019, 12, 2766. [Google Scholar] [CrossRef]
- Ban, C.C. Properties of High Calcium Wood Ash and Densified Silica Fume Blended Cement. Int. J. Phys. Sci. 2011, 6, 6596–6606. [Google Scholar] [CrossRef]
- Koufany, I.; De la Torre, A.G.; Redondo-Soto, C.; Santacruz, I.; Cuesta, A.; Gastaldi, D.; Canonico, F.; Mazanec, O.; Aranda, M.A.G. General Correlation between R3 Test Results and Compressive Strengths for Five Families of Supplementary Cementitious Materials. Cem. Concr. Compos. 2025, 163, 106166. [Google Scholar] [CrossRef]
- Londono-Zuluaga, D.; Gholizadeh-Vayghan, A.; Winnefeld, F.; Avet, F.; Ben Haha, M.; Bernal, S.A.; Cizer, Ö.; Cyr, M.; Dolenec, S.; Durdzinski, P.; et al. Report of RILEM TC 267-TRM Phase 3: Validation of the R3 Reactivity Test across a Wide Range of Materials. Mater. Struct. Constr. 2022, 55, 142. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; T. Telford: London, UK, 1997; ISBN 0727725920. [Google Scholar]
- Suraneni, P.; Weiss, J. Examining the Pozzolanicity of Supplementary Cementitious Materials Using Isothermal Calorimetry and Thermogravimetric Analysis. Cem. Concr. Compos. 2017, 83, 273–278. [Google Scholar] [CrossRef]
- Wang, Y.; Ramanathan, S.; Burris, L.; Hooton, R.D.; Shearer, C.R.; Suraneni, P. Reactivity of Unconventional Fly Ashes, SCMs, and Fillers: Effects of Sulfates, Carbonates, and Temperature. Adv. Civ. Eng. Mater. 2022, 11, 639–657. [Google Scholar] [CrossRef]
- Avet, F.; Li, X.; Haha, M.B.; Bernal, S.A.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; Dolenec, S.; Durdzinski, P.; Haufe, J.; et al. Report of RILEM TC 267-TRM Phase 2: Optimization and Testing of the Robustness of the R3 Reactivity Tests for Supplementary Cementitious Materials. Mater. Struct. Constr. 2022, 55, 92. [Google Scholar] [CrossRef]


| Wood Ash | Biofuel Composition | Combustion Technology | Combustion Temperature (°C) |
|---|---|---|---|
| WA1 | 60% bark, 30% sawdust, and 10% dry wood chips | Grate combustion | 1050 |
| WA2 | 60% bark, 30% sawdust, and 10% dry wood chips | Grate combustion | 1050 |
| WA3 | 60% bark, 30% sawdust, and 10% dry wood chips | Grate combustion | 1050 |
| WA4 | 30% bark and 70% wood shavings | Grate combustion | 800–900 |
| Chemical Composition (%) | WA1 | WA2 | WA3 | WA4 | FA | CEM I |
|---|---|---|---|---|---|---|
| SiO2 | 31.7 | 6.63 | 22.4 | 0.75 | 60.20 | 21.20 |
| Al2O3 | 7.25 | 3.48 | 6.75 | 0.37 | 17.70 | 3.40 |
| Fe2O3 | 4.19 | 1.11 | 2.62 | 0.17 | 2.78 | 4.10 |
| CaO | 14.7 | 22 | 15.1 | 29 | 1.37 | 63.30 |
| K2O | 5.14 | 9.10 | 8.25 | 1.20 | 1.61 | 0.56 |
| MgO | 2.08 | 3.43 | 2.69 | 2.52 | 0.43 | 2.20 |
| MnO | 0.61 | 1.22 | 0.80 | 1.80 | 0.05 | - |
| P2O5 | 1.39 | 2.63 | 2.81 | 2.11 | 0.07 | - |
| TiO2 | 0.33 | 0.12 | 0.3 | 0.01 | 0.71 | - |
| Na2O | 1.53 | 0.37 | 1.46 | 0.03 | 0.60 | 0.18 |
| SO3 | - | - | - | - | - | 2.70 |
| LOI (1000 °C) | 34.5 | 43.7 | 29.7 | 56.7 | 1.80 | 2.50 |
| SiO2 + Al2O3 + Fe2O3 | 43.14 | 11.22 | 31.77 | 1.29 | 80.68 | 28.70 |
| CaO/SiO2 | 0.46 | 3.32 | 0.67 | 38.67 | 0.02 | 2.98 |
| (CaO + MgO)/SiO2 | 0.53 | 3.84 | 0.79 | 42.03 | 0.02 | 3.08 |
| Phase | Temperature Range (°C) | Measured (wt.%) | Anhydrous (wt.% of Anhydrous Binder) |
|---|---|---|---|
| Bound Water | 50–550 | ||
| Portlandite (Ca(OH)2) | 400–550 | ||
| Ettringite (C6As3H32) (estimated) | 50–120 | ||
| Calcite (CaCO3) | 600–800 |
| Test | Sample Type | Mix Design | Description |
|---|---|---|---|
| SAI [41] | Mortar | 0 wt.%, 20 wt.% WA/FA w/b ≈ 0.49 (0 wt.); 0.84 (20 wt.%) | Compressive strength of 50 mm cubes at 7 and 28 days |
| Frattini Test [42] | Paste | 0 wt.%, 20 wt.% WA/FA | Cement + SCM + distilled water; 100 mL, sealed at 40 °C; filtrate titrated at 8, 15, 28 days |
| R3 Test [44] | R3 Paste | 100 wt.% WA/FA | SCM + Ca(OH)2 + CaCO3 (1:3:0.5) in K+ solution; L/S = 1.2; sealed ampoules, 40 °C for 7 days |
| TGA | Paste | 0 wt.%, 20 wt.% WA/FA w/b = 0.4 | 28-day hardened pastes; solvent-exchanged and analyzed between 20 and 1000 °C under Ar |
| XRD | Powder | WAs, FA, CEM I | Raw materials analyzed to identify original crystalline phases |
| Paste | 0 wt.%, 20 wt.% WA/FA w/b = 0.4 | 7- and 28-day hardened pastes analyzed for hydration products | |
| SEM/EDS | Powder | WAs | Wood ash powders on conductive tape, morphology analysis |
| Paste | 0 wt.%, 20 wt.% WA/FA w/b = 0.4 | 7- and 28-day hardened pastes, BSE imaging and EDS analysis of C-S-H |
| Material | Grinding Duration (min) | D10 (µm) | D50 (µm) | D90 (µm) | SSA (m2/kg) |
|---|---|---|---|---|---|
| WA1 | 0 | 15.7 | 97.2 | 581 | 172.4 |
| WA1-10 | 10 | 6.91 | 29.5 | 159 | 444.3 |
| WA1-20 | 20 | 5.49 | 20.6 | 125 | 566.5 |
| WA2 | 0 | 15.6 | 103 | 629 | 186.6 |
| WA2-10 | 10 | 3.53 | 22.2 | 147 | 829.6 |
| WA2-20 | 20 | 6.03 | 22.8 | 150 | 519.3 |
| WA3 | 0 | 18.7 | 102 | 581 | 143 |
| WA3-10 | 10 | 2.77 | 10.9 | 36.3 | 1021 |
| WA3-20 | 20 | 2.98 | 8.98 | 23.5 | 1048 |
| WA4 | 0 | 18.8 | 139 | 532 | 131.9 |
| WA4-10 | 10 | 4.16 | 12.7 | 39.5 | 782.1 |
| WA4-20 | 20 | 3.78 | 11 | 31.1 | 882.9 |
| FA | 0 | 4.09 | 25.6 | 111 | 666.6 |
| CEM I | 0 | 4.34 | 22.4 | 80.3 | 672.9 |
| Sample | Days | [OH] (mmol/L) | [CaO] (mmol/L) | Theoretical Max. [CaO] (mmol/L) | [CaO] Reduction (%) |
|---|---|---|---|---|---|
| CTRL | 8 | 50.1 | 10.0 | 10.0 | 0 (−0.5) |
| FA | 8 | 47.5 | 8.0 | 10.8 | 25.7 |
| WA1 | 8 | 66.2 | 9.8 | 6.8 | 0 (−43.2) |
| 15 | 56.4 | 7.0 | 8.5 | 17.8 | |
| WA1-10 | 8 | 54.9 | 6.7 | 8.8 | 23.3 |
| WA1-20 | 8 | 63.3 | 8.9 | 7.2 | 0 (−23.1) |
| 15 | 69.8 | 5.9 | 6.4 | 8.0 | |
| WA2 | 8 | 108.5 | 7.6 | 3.7 | 0 (−103.1) |
| 15 | 94.4 | 6.8 | 4.4 | 0 (−54.6) | |
| 28 | 87.5 | 5.7 | 4.8 | 0 (−18.1) | |
| WA2-10 | 8 | 102.4 | 5.5 | 4.0 | 0 (−38.0) |
| 15 | 85.8 | 5.3 | 4.9 | 0 (−7.9) | |
| 28 | 75.6 | 5.1 | 5.8 | 10.5 | |
| WA2-20 | 8 | 104.3 | 5.8 | 3.9 | 0 (−48.9) |
| 15 | 87.5 | 5.1 | 5.8 | 0 (−6.2) | |
| 28 | 73.0 | 4.8 | 6.0 | 19.3 | |
| WA3 | 8 | 90.7 | 5.5 | 4.6 | 0 (−17.9) |
| 15 | 85.8 | 6.9 | 4.9 | 0 (−39.2) | |
| 28 | 78.4 | 7.8 | 5.5 | 0 (−40.7) | |
| WA3-10 | 8 | 91.2 | 3.4 | 4.6 | 26.3 |
| WA3-20 | 8 | 86.4 | 6.7 | 4.9 | 0 (−36.9) |
| 15 | 83.9 | 4.3 | 5.1 | 16.3 | |
| WA4 | 8 | 57.6 | 12.5 | 8.2 | 0 (−51.8) |
| 15 | 60.0 | 10.7 | 7.8 | 0 (−37.6) | |
| 28 | 64.0 | 8.7 | 7.1 | 0 (−22.0) | |
| WA4-10 | 8 | 56.0 | 10.0 | 8.5 | 0 (−17.8) |
| 15 | 55.7 | 9.9 | 8.6 | 0 (−14.6) | |
| 28 | 55.5 | 8.0 | 8.6 | 7.8 | |
| WA4-20 | 8 | 64.6 | 11.5 | 7.1 | 0 (−63.2) |
| 15 | 56.5 | 10.2 | 8.4 | 0 (−21.4) | |
| 28 | 64.7 | 5.8 | 7.0 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teker Ercan, E.E.; Panek, R.; Szeląg, M.; Cwirzen, A.; Habermehl-Cwirzen, K. The Impact of the High-Energy Grinding of Wood Ash on Its Pozzolanic Activity. Materials 2025, 18, 3100. https://doi.org/10.3390/ma18133100
Teker Ercan EE, Panek R, Szeląg M, Cwirzen A, Habermehl-Cwirzen K. The Impact of the High-Energy Grinding of Wood Ash on Its Pozzolanic Activity. Materials. 2025; 18(13):3100. https://doi.org/10.3390/ma18133100
Chicago/Turabian StyleTeker Ercan, Ece Ezgi, Rafał Panek, Maciej Szeląg, Andrzej Cwirzen, and Karin Habermehl-Cwirzen. 2025. "The Impact of the High-Energy Grinding of Wood Ash on Its Pozzolanic Activity" Materials 18, no. 13: 3100. https://doi.org/10.3390/ma18133100
APA StyleTeker Ercan, E. E., Panek, R., Szeląg, M., Cwirzen, A., & Habermehl-Cwirzen, K. (2025). The Impact of the High-Energy Grinding of Wood Ash on Its Pozzolanic Activity. Materials, 18(13), 3100. https://doi.org/10.3390/ma18133100









