Green Design and Life Cycle Assessment of Novel Thiophene-Based Surfactants to Balance Their Synthesis Performance and Environmental Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
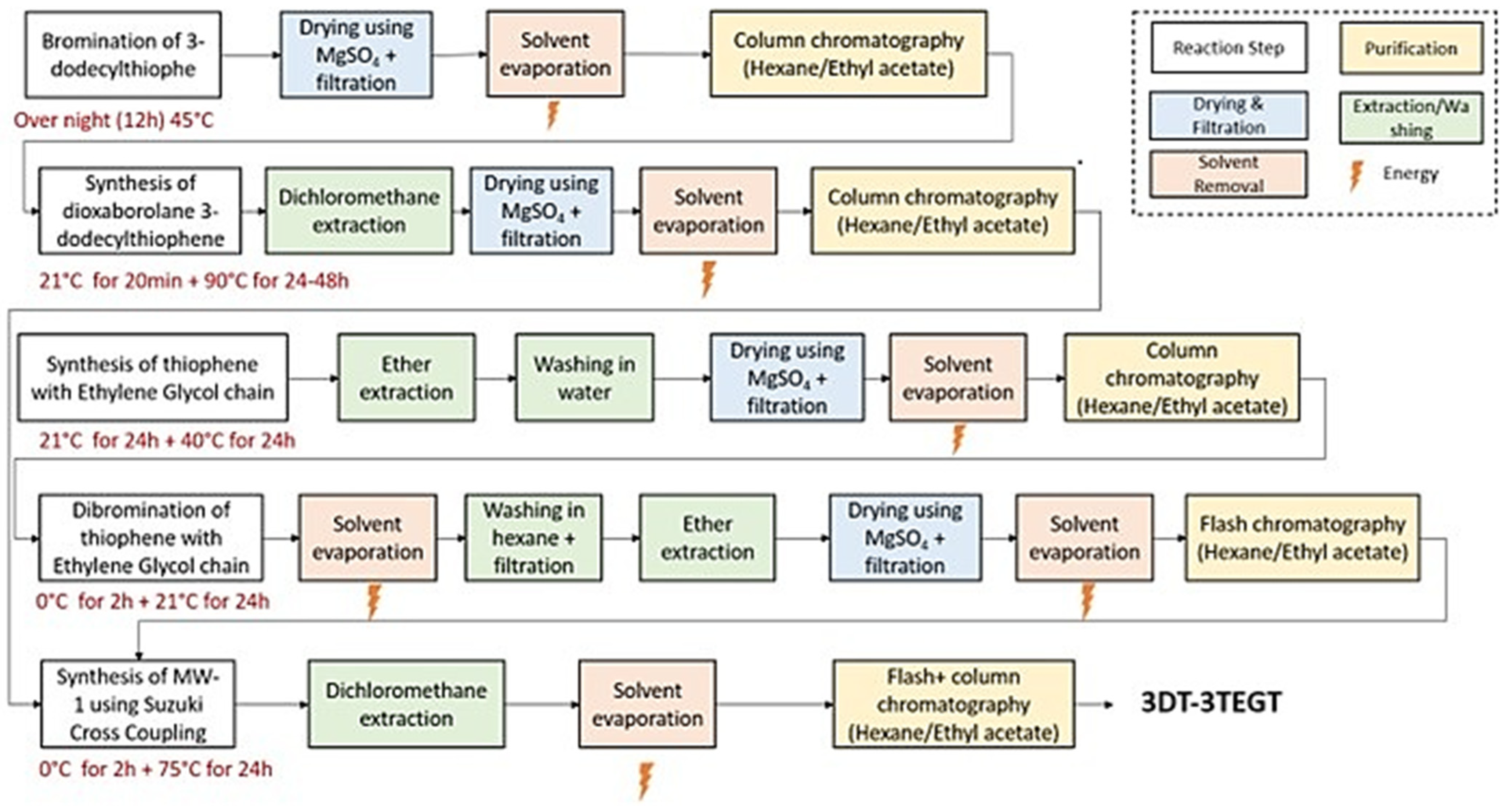
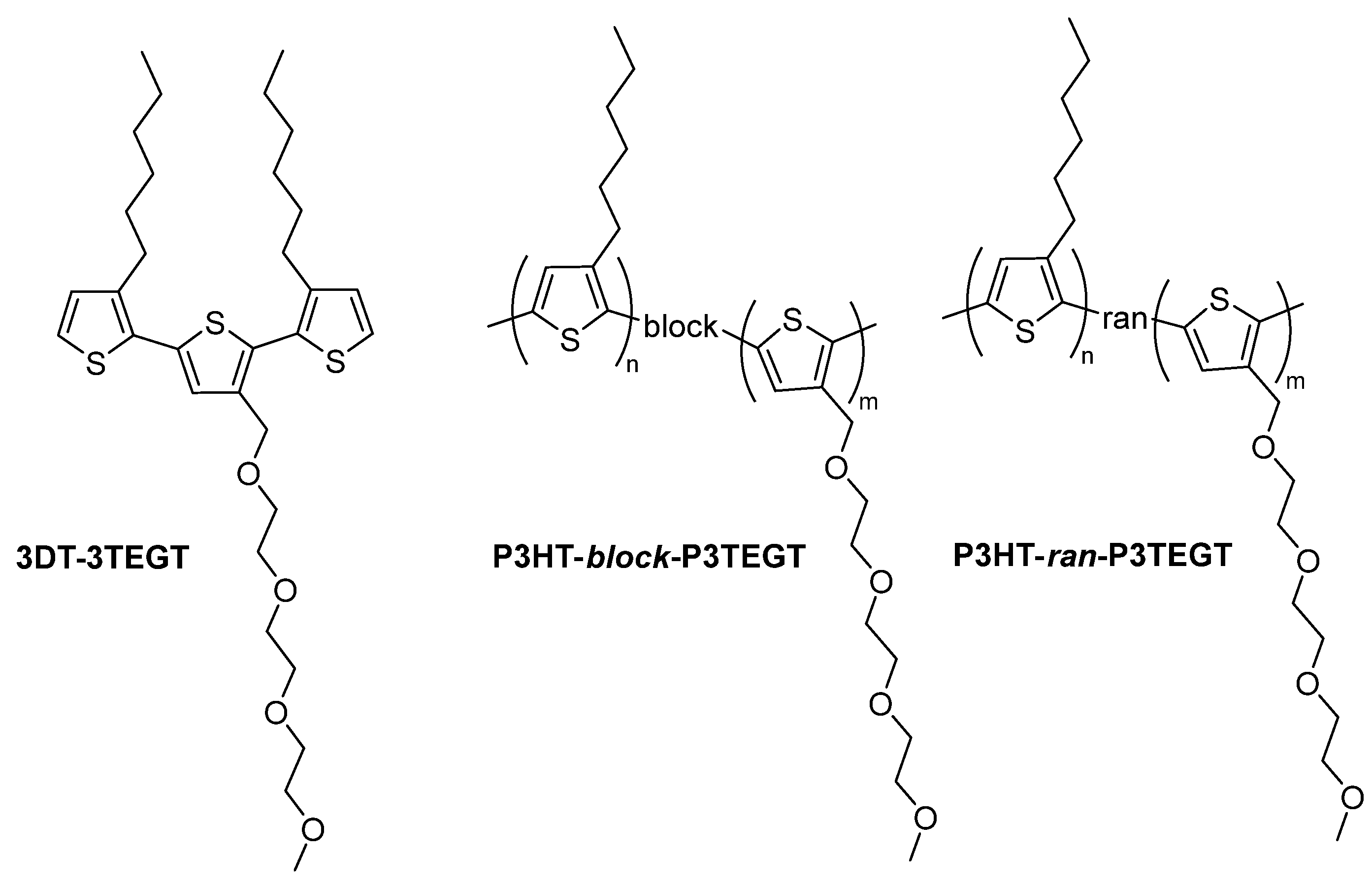
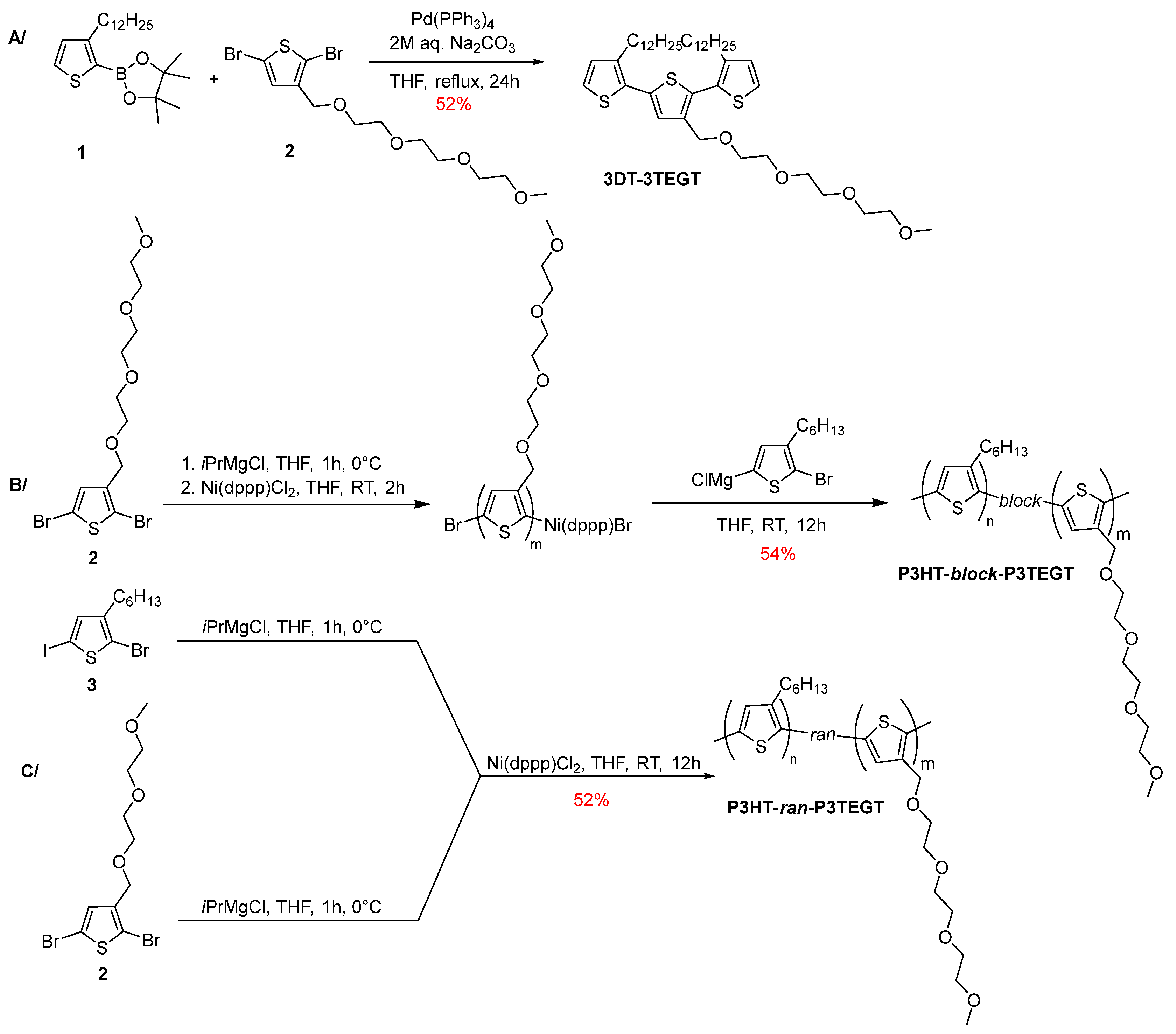
2.3. Synthesis of Oligomeric and Polymeric Surfactants
2.4. Dynamic Light Scattering (DLS)
2.5. Biodegradation of Oligomeric and Polymeric Surfactants
2.6. Bacterial Growth Inhibition Tests
2.7. Non-Ionic Surfactant Detection
2.8. Life Cycle Assessment of Surfactants Synthesis Process
3. Results and Discussions
3.1. Synthesis of Thiophene-Based Surfactants
3.2. Biodegradability Potential of Surfactant Compounds
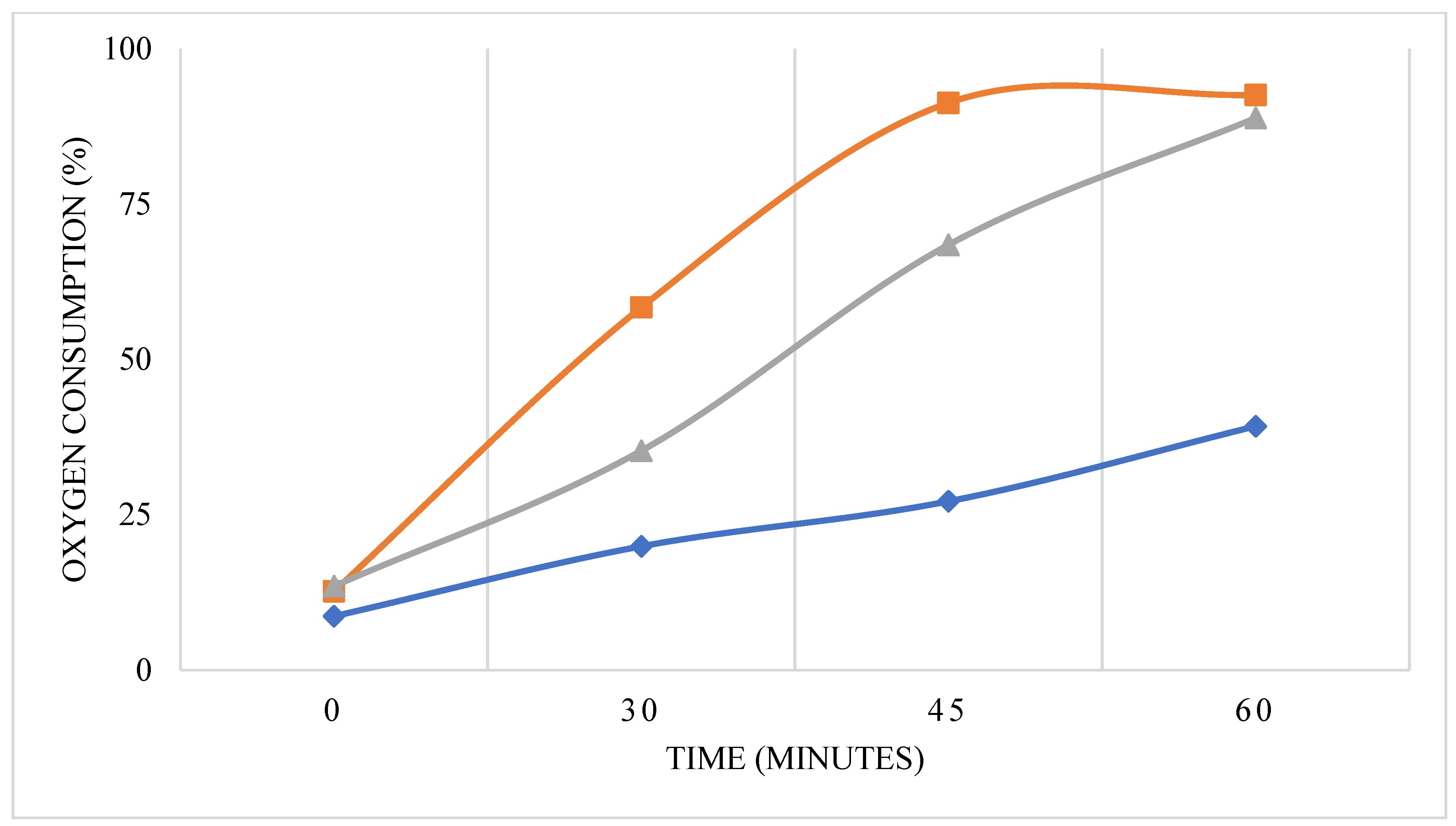
3.3. Bacterial Growth Inhibition
3.4. Life Cycle Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Free, M.L. Introduction to surfactants, Chapter 1. In Surfactants in Precision Cleaning. Removal of Contaminants at the Micro and Nanoscale; Kohli, R., Mittal, K.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Farias, C.; Almeida, F.; Silva, I.; Souza, T.; Meira, H.; da Silva, R.S.; Luna, J.; Santos, V.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. The use of surfactants in enhanced oil recovery: A review of recent advances. Energy Rep. 2020, 6, 3150–3178. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2005; ISBN 3-527-30743-5. [Google Scholar]
- Liu, Y.; Zhang, H.; Yu, H.; Liao, Z.; Paasch, S.; Xu, S.; Zhao, R.; Brunner, E.; Bonn, M.; Wang, H.I.; et al. A thiophene backbone enables two-dimensional poly(arylene vinylene)s with high charge carrier mobility. Angew. Chem. Int. 2023, 62, e202305978. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, X. Thiophene-Based Polymers: Synthesis and Applications; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Volkert, C.; Colucci, R.; Berger, R.; Besenius, P.; Blom, P.W.M.; Kraft, U. Transfer-printing of patterned PEDOT:PSS structures for bendable, stretchable and biodegradable electronics. J. Mater. Chem. C 2024, 12, 3865–3872. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, X.; Xing, D. Recent advances in water-soluble polythiophenes for biomedical applications. Eur. Polym. J. 2024, 213, 113096. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, T.; Sun, T.; Li, T.; Chi, H.; Niu, Q. A colorimetric and fluorometric oligothiophene-indenedione-based sensor for rapid and highly sensitive detection of cyanide in real samples and bioimaging in living cells. Dye. Pigment. 2019, 163, 667–674. [Google Scholar] [CrossRef]
- Liu, Q.; Mukherjee, S.; Huang, R.; Liu, K.; Liu, T.; Liu, K.; Miao, R.; Peng, H.; Fang, Y.; Liu, Q. Naphthyl End-Capped Terthiophene-Based Chemiresistive Sensors for Biogenic Amine Detection and Meat Spoilage Monitoring. Chem. Asian J. 2019, 14, 2751–2758. [Google Scholar] [CrossRef]
- Preya, U.H.; Lee, K.-T.; Jang, D.S.; Kim, N.-J.; Lee, J.-Y.; Choi, J.-H. The natural terthiophene α-terthienylmethanol induces S phase cell cycle arrest of human ovarian cancer cells via the generation of ROS stress. Chem. Biol. Interact. 2017, 272, 72–79. [Google Scholar] [CrossRef]
- Das, S.; Routh, P.; Ghosh, R.; Chatterjee, D.P.; Nandi, A.K. Water-soluble ionic polythiophenes for biological and analytical applications. Polym. Int. 2016, 66, 623–639. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, D.P.; Ghosh, R.; Nandi, A.K. Water soluble polythiophenes: Preparation and applications. RSC Adv. 2015, 5, 20160–20177. [Google Scholar] [CrossRef]
- Chow, C.-F. Two-photon induced emissive thiophene donor-acceptor systems as molecular probes for in vitro bio-imaging: Synthesis, crystal structure, and spectroscopic properties. RSC Adv. 2013, 3, 18835–18845. [Google Scholar] [CrossRef]
- Braga, J.K.; Varesche, B.A. Commercial laundry water characterization. Am. J. Anal. Chem. 2014, 5, 8–16. [Google Scholar] [CrossRef]
- Paun, I.; Mitru, D.; Covaliu, C.I.; Paraschiv, G.; Nechifor, G.; Moga, I.C.; Datcu-Manea, A.; Nita-Lazar, M. Biodegradation of anionic and cationic surfactants using bacterial strains from activated sludge. Int. J. Conserv. Sci. 2021, 12, 1171–1178. [Google Scholar]
- Wu, Q.; Zhao, L.; Song, R.; Ma, A. Research progress of surfactant biodegradation. IOP Conf. Ser. Earth Environ. Sci. 2019, 227, 5. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial Biodegradation of perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability 2023, 15, 14000. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Stoica, C.; Paun, I.; Pirvu, F.; Galaon, T.; Nita-Lazar, M. Biodegradation of two organic ultraviolet-filters by single bacterial strains. Int. J. Environ. Sci. Technol. 2023, 20, 9065–9076. [Google Scholar] [CrossRef]
- Jurado, E.; Fernandez-Serrano, M.; Nunez-Olea, J.; Luzon, G.; Lechuga, M. Acute toxicity and relationship between metabolites and ecotoxicity during the biodegradation process of non-ionic surfactants: Fatty-alcohol ethoxylates, nonylphenol polyethoxylate, and alkylpolyglucosides. Water Sci. Technol. 2009, 59, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Wyrwas, B.; Kruszelnicka, I.; Ginter-Kramarczyk, D. Effects of selected anionic and nonionic surfactants on the operation of activated sludge. Chem. Ind. 2011, 90, 613–619. [Google Scholar]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behavior and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Renaud, F.; Warnau, M.; Oberhänsli, F.; Teyssié, J.L.; Temara, A.; Rouleau, C.; Metian, M. Bioconcentration of the anionic surfactant linear alkylbenzene sulfonate (LAS) in the marine shrimp Palaemonetes varians: A radiotracer study. Mar. Pollut. Bull. 2014, 85, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Ranjan, A.; Chauhan, A.; Biswas, R.; Rajput, V.D.; Sushkova, S.; Mandzhieva, S.; Minkina, T.; Jindal, T. Surfactant pollution, an emerging threat to ecosystem: Approaches for effective bacterial degradation. J. Appl. Microbiol. 2022, 133, 1229–1244. [Google Scholar] [CrossRef]
- Gheorghe, S.; Stan, M.S.; Mitroi, D.N.; Staicu, A.C.; Cicirma, M.; Lucaciu, I.E.; Nita-Lazar, M.; Dinischiotu, A. Oxidative Stress and Histopathological Changes in Gills and Kidneys of Cyprinus carpio following Exposure to Benzethonium Chloride, a Cationic Surfactant. Toxics 2022, 10, 227. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Chatterjee, A.; Chatterjee, S.; Saha, N. Oxidative stress in benthic oligochaete worm, Tubifex tubifex induced by sublethal exposure to a cationic surfactant cetylpyridinium chloride and an anionic surfactant sodium dodecyl sulfate. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108906. [Google Scholar] [CrossRef]
- Sobrino-Figueroa, A. Toxic effect of commercial detergents on organisms from different trophic levels. Environ. Sci. Pollut. Res. 2018, 25, 13283–13291. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the Environment. Arch. Ind. Hyg. Toxicol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 648/2004 of the European Parliament and of the Council of 31 March 2004 on Detergents. Available online: http://data.europa.eu/eli/reg/2004/648/oj (accessed on 6 February 2025).
- Regulation (EC) No 66/2010 of the European Parliament and of the Council of 25 November 2009 on the EU Ecolabel. Available online: http://data.europa.eu/eli/reg/2010/66/oj (accessed on 6 February 2025).
- Gheorghe, S.; Petre, J.; Lucaciu, I.; Stoica, C.; Nita-Lazar, M. Risk screening of pharmaceutical compounds in Romanian aquatic environment. Environ. Monit. Assess. 2016, 188, 379. [Google Scholar] [CrossRef] [PubMed]
- Szklarek, S.; Stolarska, M.; Wagner, I.; Mankiewicz-Boczek, E. The microbiotest battery as an important component in the assessment of snowmelt toxicity in urban watercourse—Preliminary studies. J. Environ. Monit. Assess. 2015, 187, 1–11. [Google Scholar] [CrossRef]
- Stoica, C.; Gheorghe, S.; Lucaciu, E.I.; Stanescu, E.; Paun, C.I.; Niculescu, L.D. The impact of chemical compounds on benthic invertebrates from the Danube and Danube Delta systems. Soil Sediment Contam. 2014, 23, 763–778. [Google Scholar] [CrossRef]
- Cucurachi, S.; van der Giesen, C.; Guinée, J.; Guineé, J.B. Ex-ante LCA of emerging technologies. CIRP Procedia 2018, 69, 463–468. [Google Scholar] [CrossRef]
- Guinée, J.B.; Cucurachi, S.; Henriksson, P.J.G.; Heijungs, R. Digesting the alphabet soup of LCA. Int. J. Life Cycle Assess. 2018, 23, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Parvatker, A.G.; Eckelman, M.J. Comparative evaluation of chemical life cycle inventory generation methods and implications for life cycle assessment results. ACS Sustain. Chem. Eng. 2019, 7, 350–367. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From laboratory to industrial scale: A scale-up framework for chemical processes in life cycle assessment studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Bennett, J.A.; Campbell, Z.S.; Abolhasani, M. Role of continuous flow processes in green manufacturing of pharmaceuticals and specialty chemicals. Curr. Opin. Chem. Eng. 2019, 26, 9–19. [Google Scholar] [CrossRef]
- Coulson, D.R.; Satek, L.C.; Grim, S.O. Tetrakis(Triphenylphosphine) Palladium(0). In Inorganic Syntheses; John Wiley & Sons Ltd.: Hoboken, USA, 1972; pp. 121–124. [Google Scholar] [CrossRef]
- Kong, H.; Jung, Y.K.; Cho, N.S.; Kang, I.-N.; Park, J.-H.; Cho, S.; Shim, H.-K. New semiconducting polymers containing 3,6-dimethyl(Thieno [3,2-b]Thiophene or Selenophene[3,2-b]Selenophene) for organic thin-film transistors. Chem. Mater. 2009, 21, 2650–2660. [Google Scholar] [CrossRef]
- Lee, E.; Hammer, B.; Kim, J.-K.; Page, Z.; Emrick, T.; Hayward, R.C. Hierarchical helical assembly of conjugated poly(3-hexylthiophene)-block-poly(3-triethylene glycol thiophene) diblock copolymers. J. Am. Chem. Soc. 2011, 133, 10390–10393. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.; Albert, S.; Bae, C.; Poyac, L.; Gerbier, P.; Bonhomme, O.; Lai-Kee-Him, J.; Ancelin, A.; Richeter, S.; Sen, I.; et al. Molecular Assemblies of Amphiphilic Oligothiophenes at the Air–Water Interface. Langmuir 2025, 41, 12287–12300. [Google Scholar] [CrossRef]
- SR EN ISO 5815-1; Water Quality. Determination of Biochemical Oxygen Demand After n Days (BODn). Part 1: Dilution and Seeding Method with Allylthiourea Addition. Romanian Standardization Association (ASRO): București, Romania.
- SR ISO 15705:2022; Water Quality-Determination of the Chemical Oxygen Demand Index (ST-COD)–Small-Scale Sealed-Tube Method. Romanian Standardization Association (ASRO): București, Romania.
- Rudaru, D.; Lucaciu, I.; Fulgheci, A. Correlation between BOD5 and COD—Biodegradability indicator of wastewater. Rom. J. Ecol. Environ. Chem. 2022, 4, 80–86. [Google Scholar] [CrossRef]
- SR EN ISO 8192:2008; Water Quality. Test for Inhibition of Oxygen Consumption by Activated Sludge for Carbonaceous and Ammonium Oxidation. Romanian Standardization Association (ASRO): București, Romania.
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Rijn, P.; Janeliunas, D.; Brizard, A.; Stuart, M.; Koper, G.; Eelkema, R.; van Esch, J. Self-assembly behaviour of conjugated terthiophene surfactants in water. New J. Chem. 2011, 35, 558–567. [Google Scholar] [CrossRef]
- Thomas, A.; Houston, J.E.; Van den Brande, N.; De Winter, J.; Chevrier, M.; Heenan, R.K.; Terry, A.E.; Richeter, S.; Mehdi, A.; Van Mele, B.; et al. Polymers. Polym. Chem. 2014, 5, 3352–3362. [Google Scholar] [CrossRef]
- Reitzel, N.; Greve, D.R.; Kjaer, K.; Howes, P.B.; Jayaraman, M.; Savoy, S.; McCullough, R.D.; McDevitt, J.T.; Bjørnholm, T. Self-assembly of conjugated polymers at the air/water interface. Structure and properties of Langmuir and Langmuir−Blodgett films of amphiphilic regio-regular polythiophenes. J. Am. Chem. Soc. 2000, 122, 5788–5800. [Google Scholar] [CrossRef]
- Brustolin, F.; Goldoni, F.; Meijer, E.W.; Sommerdijk, N.A.J.M. Highly ordered structures of amphiphilic polythiophenes in aqueous media. Macromolecules 2002, 35, 1054–1059. [Google Scholar] [CrossRef]
- Winchell, K.J.; Yee, P.Y.; Li, Y.L.; Simafranca, A.F.; Chang, J.; Beren, C.; Liu, X.; Garcia Vidales, D.; Thompson, R.J.; Salamat, C.Z.; et al. Designing amphiphilic conjugated polyelectrolytes for self-assembly into straight-chain rod-like micelles. Macromolecules 2023, 56, 3160–3170. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Cheng, C.-C.; Lin, Y.-C.; Huang, C.-W.; Lu, F.-H.; Chang, F.-C.; Kuo, S.-W. Synthesis and self-assembly of water-soluble polythiophene-graft-poly(ethylene oxide) copolymers. RSC Adv. 2014, 4, 21830–21839. [Google Scholar] [CrossRef]
- Janeliunas, D.; Eelkema, R.; Nieto-Ortega, B.; Ramírez Aguilar, F.J.; López Navarrete, J.T.; van der Mee, L.; Stuart, M.C.A.; Casado, J.; van Esch, J.H. Designing new symmetrical facial oligothiophene amphiphiles. Org. Biomol. Chem. 2013, 11, 8435–8442. [Google Scholar] [CrossRef] [PubMed]
- Houston, J.E.; Chevrier, M.; Appavou, M.-S.; King, S.M.; Clément, S.; Evans, R.C. A self-assembly toolbox for thiophene-based conjugated polyelectrolytes: Surfactants, solvent and copolymerisation. Nanoscale 2017, 9, 17481–17493. [Google Scholar] [CrossRef]
- Ding, L.; Olesik, S. Dispersion polymerization of MMA in supercritical CO2 in the presence of copolymers of perfluorooctylethylene methacrylate and poly(propylene glycol) methacrylate. Macromolecules 2003, 36, 4779–4785. [Google Scholar] [CrossRef]
- Deniz, S.; Baran, N.; Akgun, M.; Uzun, I.N.; Dincer, S. Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide using a silicone-containing fluoroacrylate stabilizer. Polym. Int. 2005, 54, 1660–1668. [Google Scholar] [CrossRef]
- Yan, X.; Xu, W.; Shao, R.; Tang, L.; Shi, W. Random copolymer as polymeric surfactant in seed emulsion polymerization. Tenside Surfactants Deterg. 2015, 52, 323–328. [Google Scholar] [CrossRef]
- Zhang, C.; Lessard, B.; Maric, M. Synthesis and characterization of benzyl methacrylate/styrene random copolymers prepared by NMP. Macromol. React. Eng. 2010, 4, 415. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morishima, Y. Effect of hydrophobe content on intra- and interpolymer self-associations of hydrophobically modified poly(sodium 2-(acrylamido)-2-methylpropanesulfonate) in water. Macromolecules 1999, 32, 7469–7475. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, P.; Lee, J.; Choi, C.; Seo, Y.; Park, S.Y.; Kim, K.; Park, C.; Cho, K.; Moon, H.C.; et al. End-on chain orientation of poly(3-alkylthiophene)s on a substrate by microphase separation of lamellar forming amphiphilic diblock copolymer. Macromolecules 2019, 52, 6734–6740. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Galaon, T.; Banciu, A.; Paun, I.; Stoica, C.; Lucaciu, I. Screening of various harmful compounds in a new bacterial biological model. J. Environ. Prot. Ecol. 2016, 17, 237–247. [Google Scholar]
- Nita-Lazar, M.; Gheorghe, S.; Anghelache, A.; Banciu, A.; Stoica, C.; Lucaciu, I. Modulation of the bacterial defense mechanisms by various chemical structures. Rev. Chim. 2016, 67, 1454–1457. [Google Scholar]
- Zhang, C.; Tezel, U.; Li, K.; Liu, D.; Ren, R.; Du, J.; Pavlostathis, S.G. Evaluation and modeling of benzalkonium chloride inhibition and biodegradation in activated sludge. Water Res. 2011, 45, 1238–1246. [Google Scholar] [CrossRef]
- Evans, K.A.; Dubey, S.T.; Kravetz, L.; Evetts, S.W.; Dzidic, I.; Dooyema, C.C. Quantitation of alcohol ethoxylate surfactants in environmental samples by electrospray mass spectrometry. J. Am. Oil Chem. Soc. 1997, 74, 765–773. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, J.; Zhang, X.; Li, Z.; Tang, Y. Cationic Oligo(thiophene ethynylene) with Broad-Spectrum and High Antibacterial Efficiency under White Light and Specific Biocidal Activity against, S. aureus in Dark. ACS Appl. Mater. Interfaces 2016, 8, 1019–1024. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Lobato-Guarnido, I.; Fernández-Serrano, M. Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum. Toxics 2023, 11, 344. [Google Scholar] [CrossRef]
- Sutormin, O.S.; Kolosova, E.M.; Torgashina, I.G.; Kratasyuk, V.A.; Kudryasheva, N.S.; Kinstler, J.S.; Stom, D.I. Toxicity of different types of surfactants via cellular and enzymatic assay systems. Int. J. Mol. Sci. 2023, 24, 515. [Google Scholar] [CrossRef]
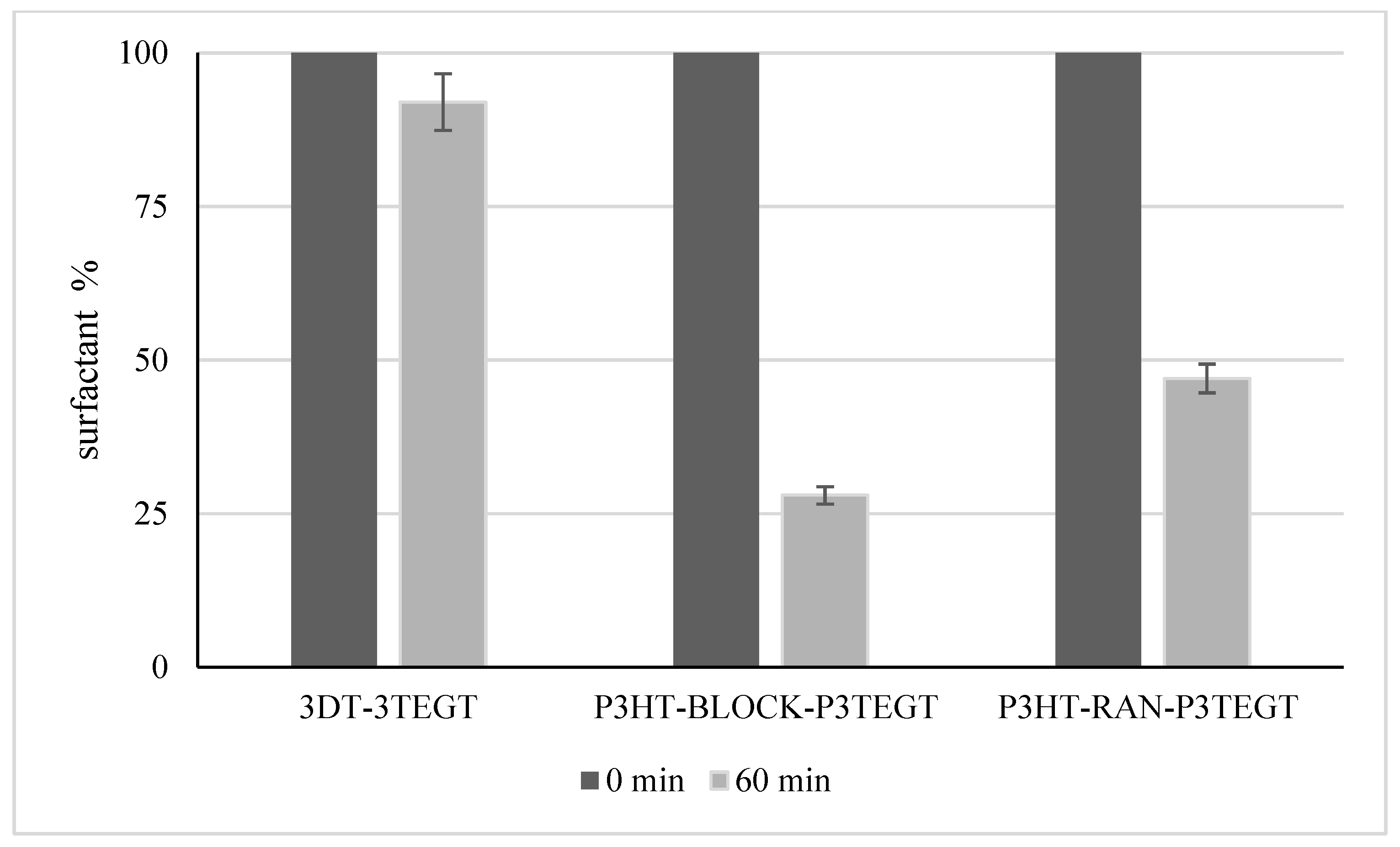
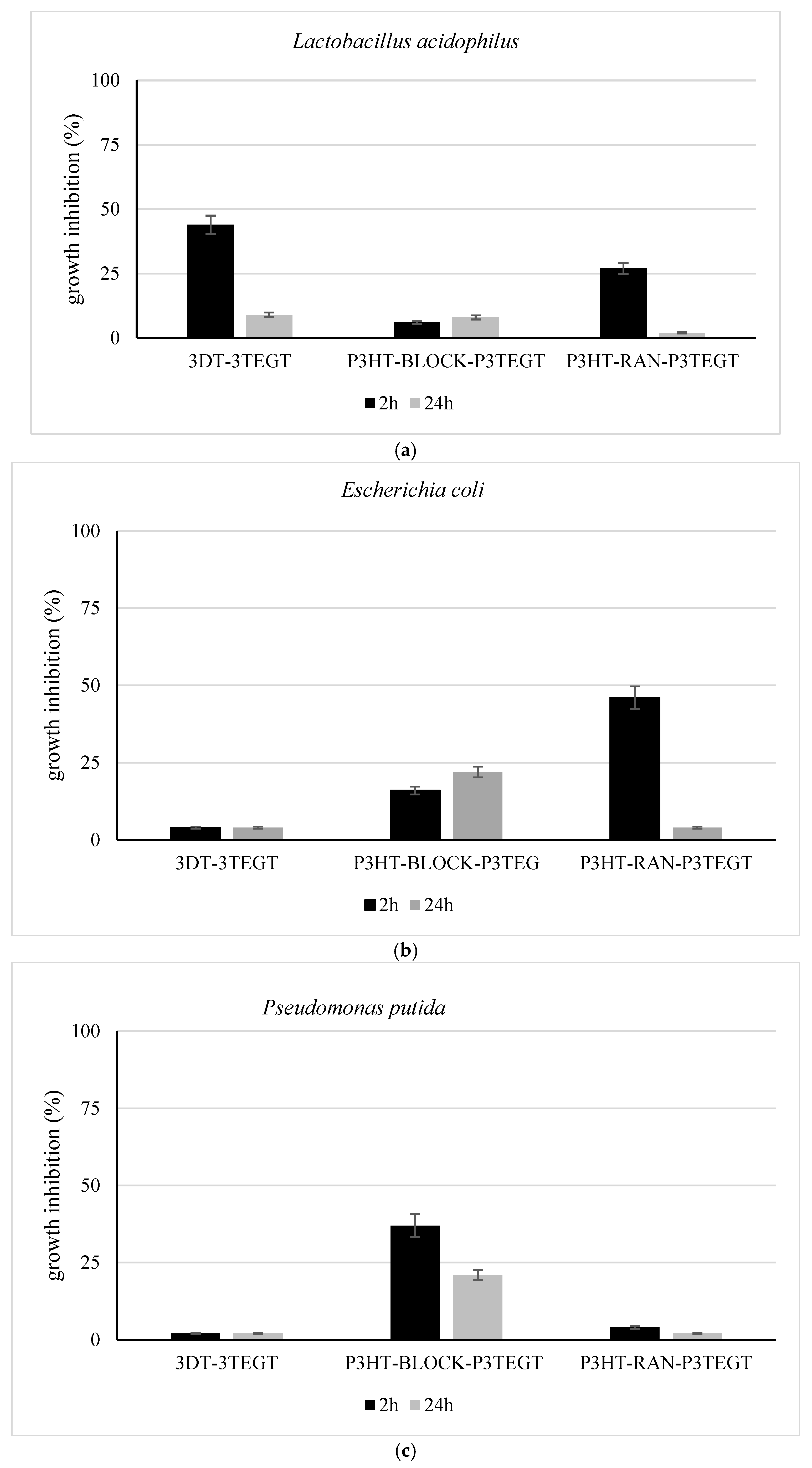
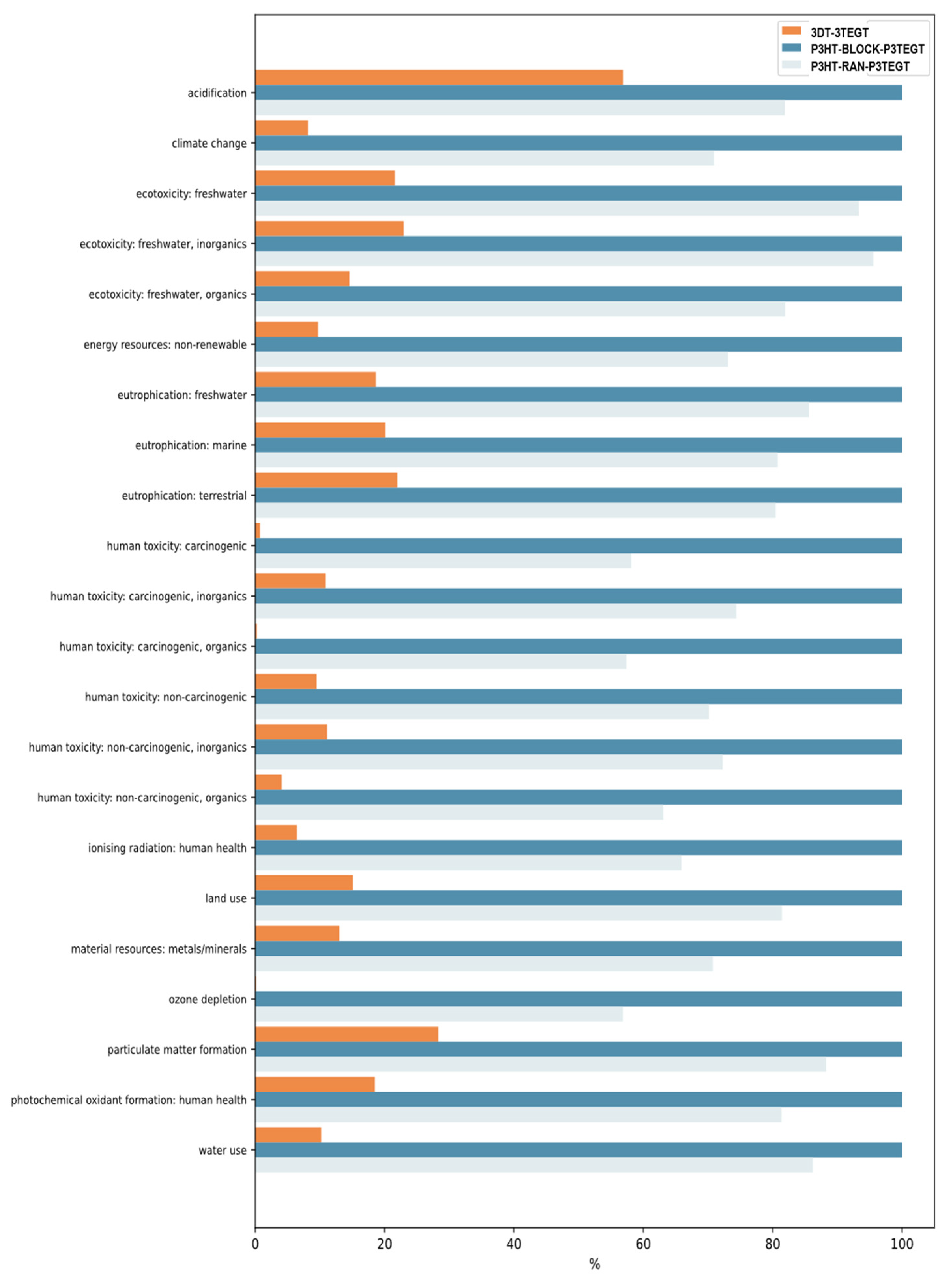
| BOD/COD Ratio | |
|---|---|
| <0.2 | Non-biodegradable |
| 0.2–0.5 | Medium biodegradable |
| >0.5 | Highly biodegradable |
| Compounds/Parameters | COD | BOD5 | BOD5/COD |
|---|---|---|---|
| mg O2/L | mg O2/L | ||
| 3DT-3TEGT | 2049 | 717 | 0.35 |
| P3HT-block-P3TEGT | 2091 | 1505 | 0.72 |
| P3HT-ran-P3TEGT | 2003 | 1121 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, C.; Banciu, A.R.; Idriss, H.; Lian, J.Z.; Patrascu, A.-M.; Cucurachi, S.; Richeter, S.; Clément, S.; Nita-Lazar, M. Green Design and Life Cycle Assessment of Novel Thiophene-Based Surfactants to Balance Their Synthesis Performance and Environmental Impact. Materials 2025, 18, 2701. https://doi.org/10.3390/ma18122701
Stoica C, Banciu AR, Idriss H, Lian JZ, Patrascu A-M, Cucurachi S, Richeter S, Clément S, Nita-Lazar M. Green Design and Life Cycle Assessment of Novel Thiophene-Based Surfactants to Balance Their Synthesis Performance and Environmental Impact. Materials. 2025; 18(12):2701. https://doi.org/10.3390/ma18122701
Chicago/Turabian StyleStoica, Catalina, Alina Roxana Banciu, Hisham Idriss, Justin Z. Lian, Anca-Maria Patrascu, Stefano Cucurachi, Sébastien Richeter, Sébastien Clément, and Mihai Nita-Lazar. 2025. "Green Design and Life Cycle Assessment of Novel Thiophene-Based Surfactants to Balance Their Synthesis Performance and Environmental Impact" Materials 18, no. 12: 2701. https://doi.org/10.3390/ma18122701
APA StyleStoica, C., Banciu, A. R., Idriss, H., Lian, J. Z., Patrascu, A.-M., Cucurachi, S., Richeter, S., Clément, S., & Nita-Lazar, M. (2025). Green Design and Life Cycle Assessment of Novel Thiophene-Based Surfactants to Balance Their Synthesis Performance and Environmental Impact. Materials, 18(12), 2701. https://doi.org/10.3390/ma18122701









