On the Origin of Thermally Enhanced Upconversion Luminescence in Lanthanide-Doped Nanosized Fluoride Phosphors
Abstract
1. Introduction
2. A Brief Survey of the Typical Results and Explanations of the Thermal Enhancement of UCL
3. Potential Issues with Some Experimental Observations and Associated Explanations About Thermal Enhancement of UCL
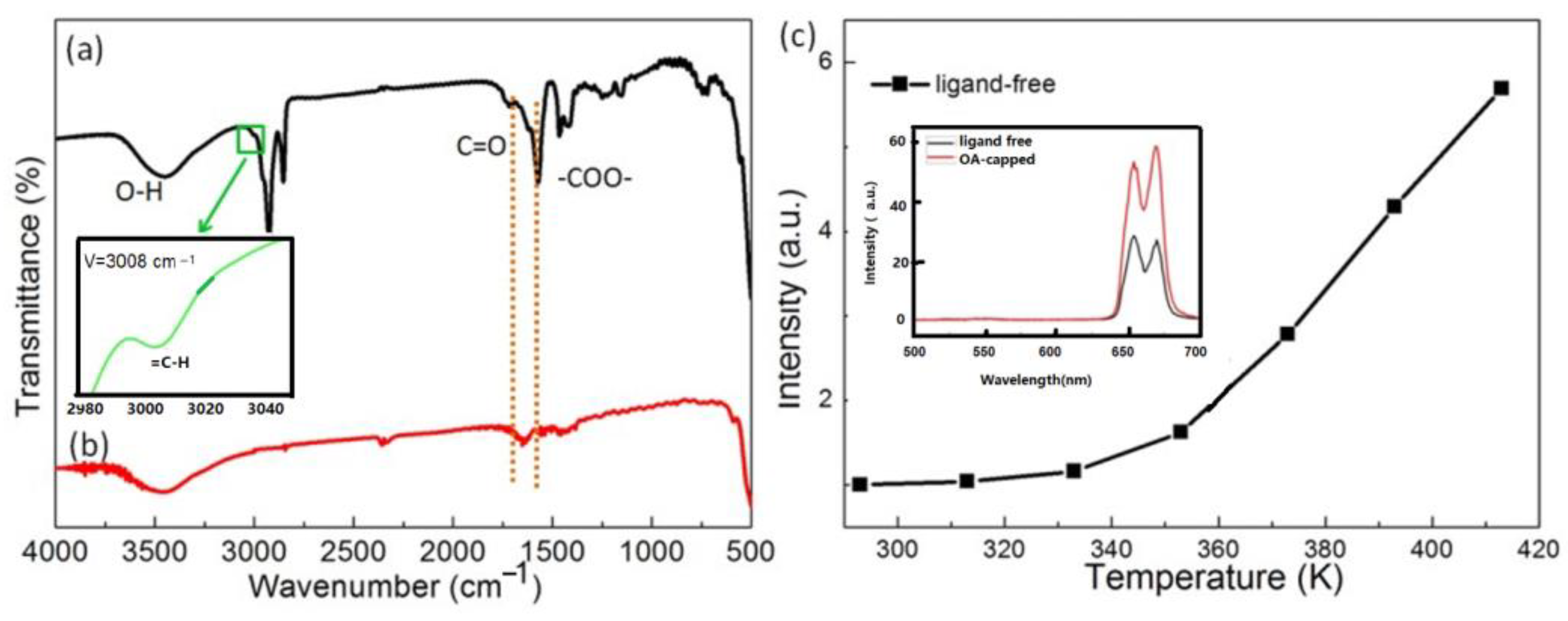
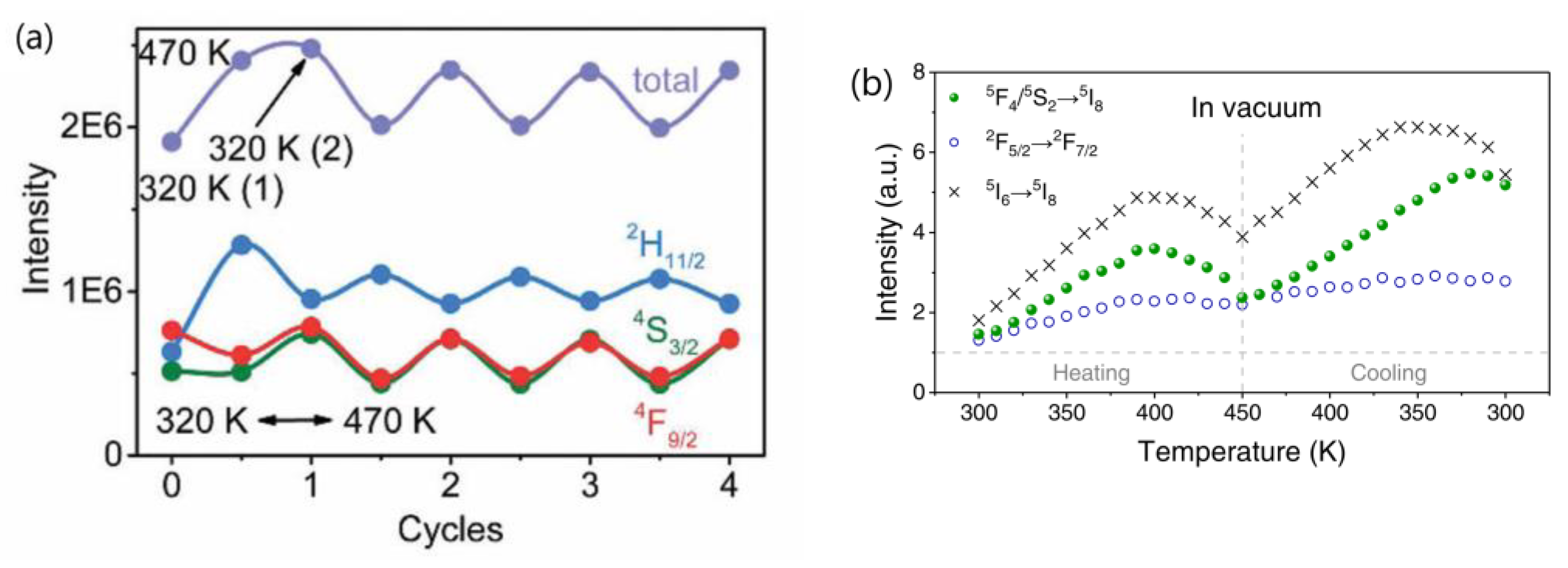
- The research conducted by Shao’s group indicated that the thermal enhancement of UCL above room temperature is a size-, morphology-, and architecture-dependent property of Yb3+/Ln3+ co-doped UCNP. This phenomenon was observed in core-only or active-core/active-shell structured UCNP, but not in their bulk and nanowire counterparts or UCNP with an active core/inert-shell architecture. Smaller UCNP exhibited a more significant enhancement factor. Shao’s group proposed that the thermal enhancement of UCL arises from the heat-induced recovery (or alleviation) of surface-related quenching, specifically the desorption of water molecules from the UCNP at elevated temperatures, which alleviates surface quenching and results in UCL enhancement [44,45,48,53,54]. Other researchers have also attributed thermally enhanced UCL to recovery of quenching due to the desorption of surface-adsorbed water [28,55,56,57]. However, if this mechanism holds true, one would expect the thermal enhancement factor to be associated with the desorption amount of water interacting with Ln3+ ions in the UCNP, which is dependent upon the specific surface area (surface-to-volume ratio) of the UCNP, but is independent of the experimental atmosphere. Contrary to this expectation, Shao’s group found that thermal enhancement of UCL was not observed when the ~10 nm sized core-only UCNP was dispersed in ODE or when the spectral measurement was conducted in an Ar atmosphere, as shown in Figure 1b,c. It is unclear why the desorption of water from the surface of the core-only UCNP did not occur upon heating in Ar or dispersing the UCNP in ODE. If the desorption of water could occur at elevated temperatures, it is perplexing why thermal enhancement was not observed in these conditions [44,51,52]. Measurements of the temperature-dependent spectra in controlled environments by Meijerink’s group and Zhang’s group showed that the thermal enhancements of UCL in 10 nm sized NaY(WO4)2:49%Yb3+/1%Er3+ and 42.7 nm sized NaYF4:2%Ho,20%Yb@NaYF4:40%Yb UCNP were still observed in the first heating cycle, even when the temperature-dependent spectral measurements were conducted in N2 or in a vacuum, respectively, as depicted in Figure 3 [28,55]. On the other hand, multiple independent researchers have demonstrated that the thermal enhancement of UCL can also be observed in active-core/inert-shell-structured UCNP [21,47,59,60,71]. The thermal enhancement of UCL in these UCNP cannot be attributed to the alleviation of surface quenching induced by the desorption of surface-adsorbed water, as the inert shell substantially suppresses surface-related quenching, as evidenced by the significantly higher QY values of core/shell UCNP compared to the core-only ones [10,13,14,15]. Furthermore, the alleviation of surface quenching induced by the desorption of surface adsorbed water can hardly explain why the thermal enhancement of UCL was not observed in nanowires and nanorods. Moreover, it is noteworthy that UCL thermal enhancement above room temperature was also observed in bulk materials like glass, ceramics, and microcrystals, even at temperatures of 800 K, substantially exceeding the threshold necessary for water desorption [34,35,36,37,72].
- 2.
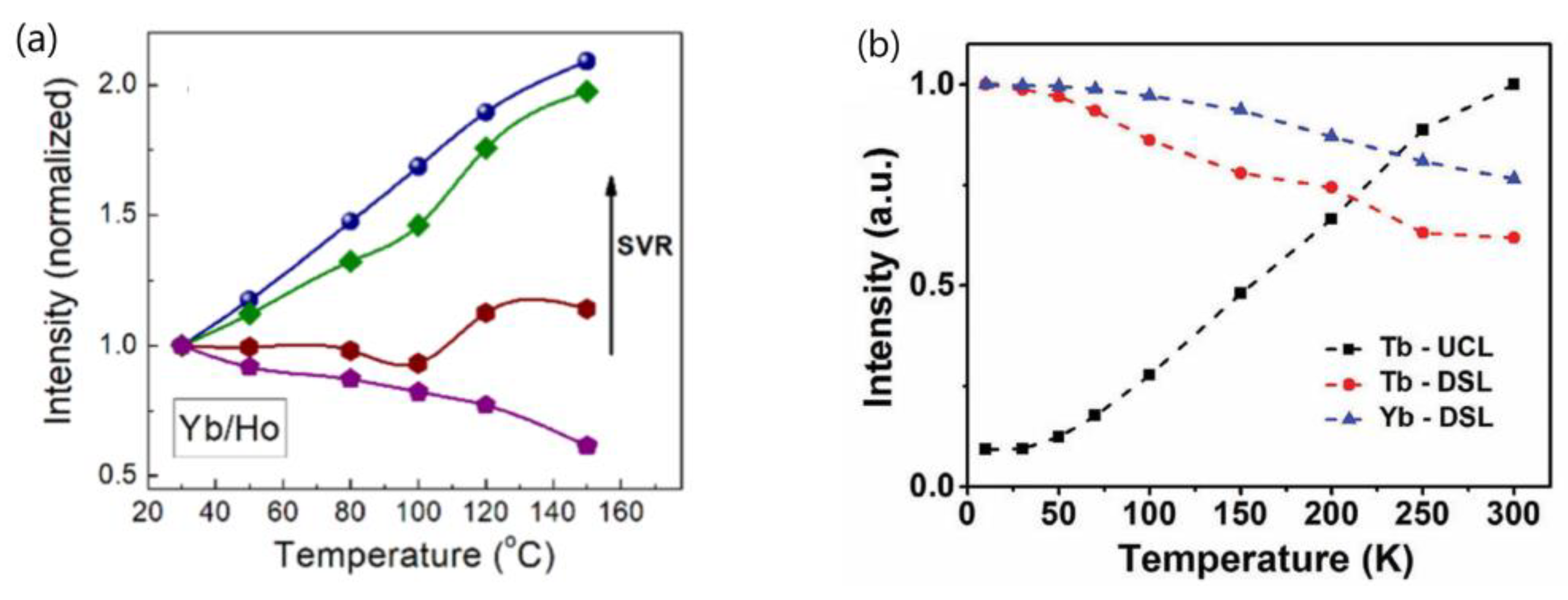
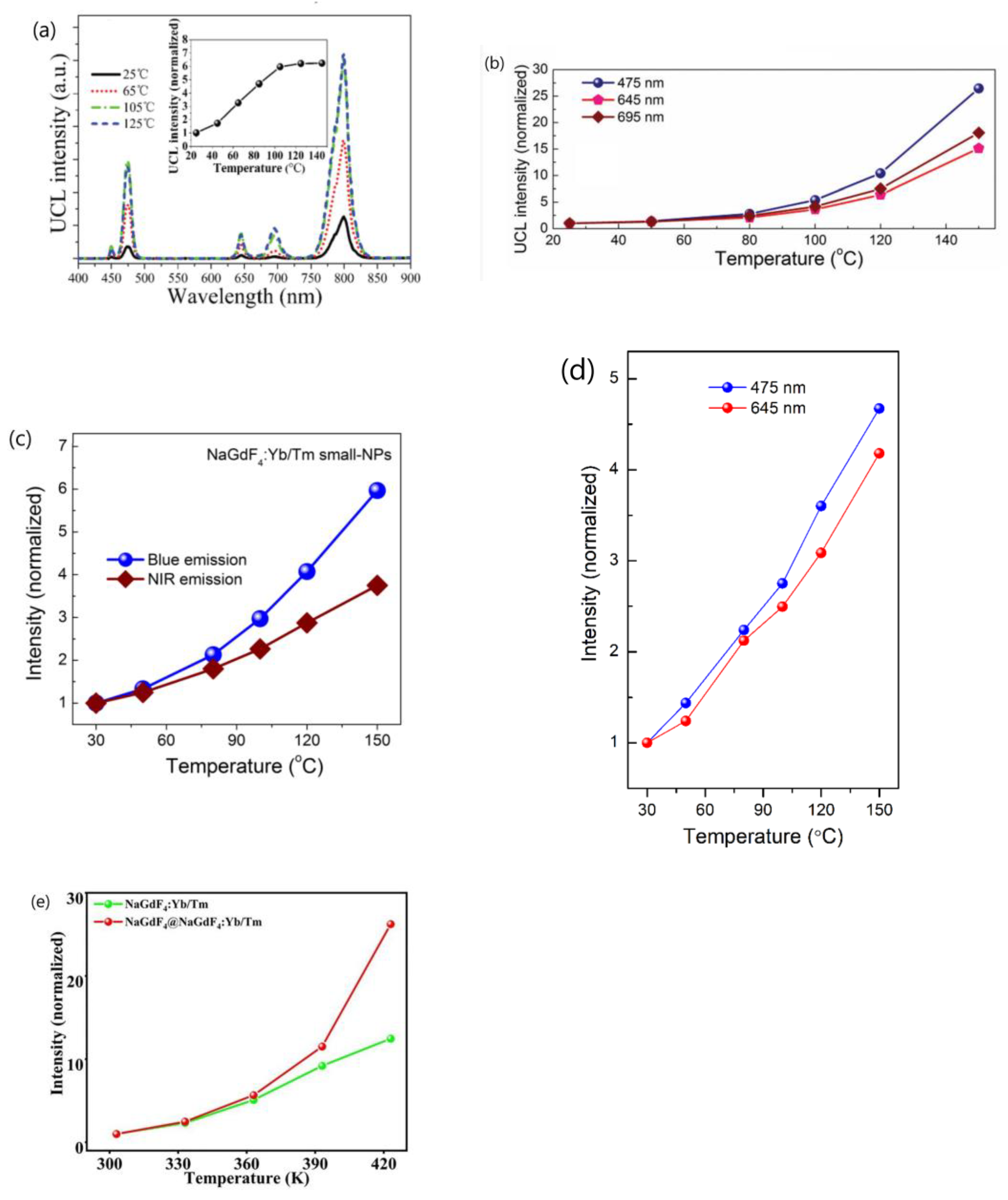
- Wang’s group conducted a comprehensive investigation into the temperature-dependent emission intensity of both DSL of Yb3+ and UCL of Eu3+ and Tm3+ in NaGdF4:20%Yb3+/Eu3+ and NaGdF4@ NaGdF4:20%Yb3+/1%Tm3+ UCNP, respectively. Their experimental findings revealed that, under 980 nm excitation, both the DSL intensity of Yb3+ at 1020 nm and UCL of intensity of Eu3+ and Tm3+ increased as the temperature rose from 303 to 423 K [25,58]. Shao et al. and Li et al. also reported the thermally enhanced intensities of both the DSL of Yb3+ at ~1050 nm and UCL of Ho3+, Er3+ and Tm3+ in NaGdF4:Yb3+/Ln3+ (Ln = Er, Tm, Ho) UCNP, as exemplified in Figure 4a [44,50,51,53,56]. Similar results were also noted in Al2(WO4)3:Yb3+/Er3+ phosphors by Liao et al. [79]. Conversely, Chen and colleagues observed a different variation trend of DSL and UCL when heating LiYbF4:30%Tb3+@LiYF4 core/shell UCNP from 10 K to 300 K; the integrated UCL intensity of Tb3+ increased by approximately one order of magnitude, while the integrated DSL intensities of Tb3+ and Yb3+ decreased with rising temperatures, as illustrated in Figure 4b [26]. The variability in temperature dependencies of DSL and UCL reported by different authors poses a challenge for our understanding. Furthermore, it is well-established that after the absorption of a 980 nm photon, the Yb3+ is excited from the 2F7/2 to 2F5/2 level. The absorbed energy by Yb3+ ion can be depleted through two pathways: transfer to a neighboring Ln3+ (Ln = Ho, Er, Tm, Eu) ion, leading to UCL; or radiative decay accompanied with DSL emission at 1020 nm. In essence, the DSL of Yb3+ and UCL of Ln3+ are competing processes that both depopulate the intermediate excited state of Yb3+ (2F5/2). An efficient DSL may hinder the achievement of high UCL [44,80]. Given the conservation of energy, it is perplexing how the enhancement of both Yb3+ DSL and UCL intensities of Ln3+ (Eu3+, Ho3+, Er3+, Tm3+) could occur simultaneously in these phosphors under the condition that absorbed energy maintains constant.
- 3.
- Shao’s group conducted a comparative analysis of the thermal enhancement factors associated with UCL in β-NaYF4 UCNP doped with various ion-couples, namely Yb3+/Ln3+(Ln = Ho, Er, Tm). Their findings revealed that the Yb3+/Ho3+ co-doped phosphor exhibited the highest thermal enhancement factor, demonstrating a remarkable 12.6-fold enhancement compared to the 4-fold enhancement of Yb3+/Tm3+ co-doped variant within the temperature range of 303–423 K [44]. Intriguingly, a similar ranking of thermal enhancement factors among these ion-couples was also noted in Yb3+/Ln3+(Ln = Ho, Tm) co-doped β-NaGdF4 UCNP by the same research group [48,51]. However, Jin’s group conducted an investigation into the ion-pair-dependent thermal enhancement factors of UCL in β-NaYF4 49%Yb3+/1%Ln3+ (Ln = Ho, Er, Tm) UCNP and reported contrasting results. They found that the Yb3+/Tm3+co-doped phosphor demonstrated the highest thermal enhancement factor, with a 16-fold enhancement, while Yb3+/Ho3+ and Yb3+/Er3+ co-doped phosphors exhibited only 7-fold and 2-fold enhancements, respectively [27]. The significant discrepancy in the reported ion-couple-dependent thermal enhancement factors of UCL for the same phosphor by different research groups poses a challenge in our understanding of the underlying physical mechanisms responsible for these variations.
- 4.
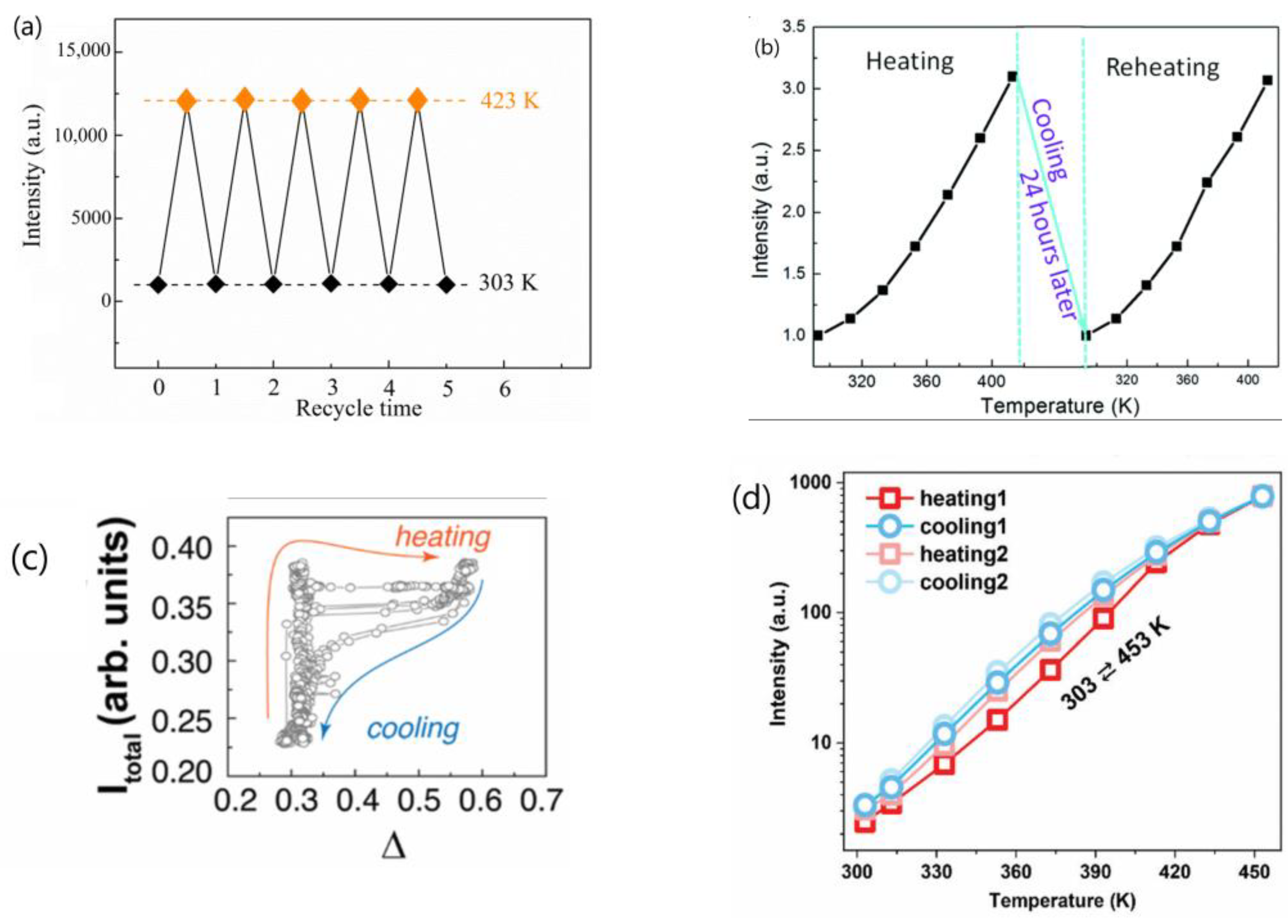
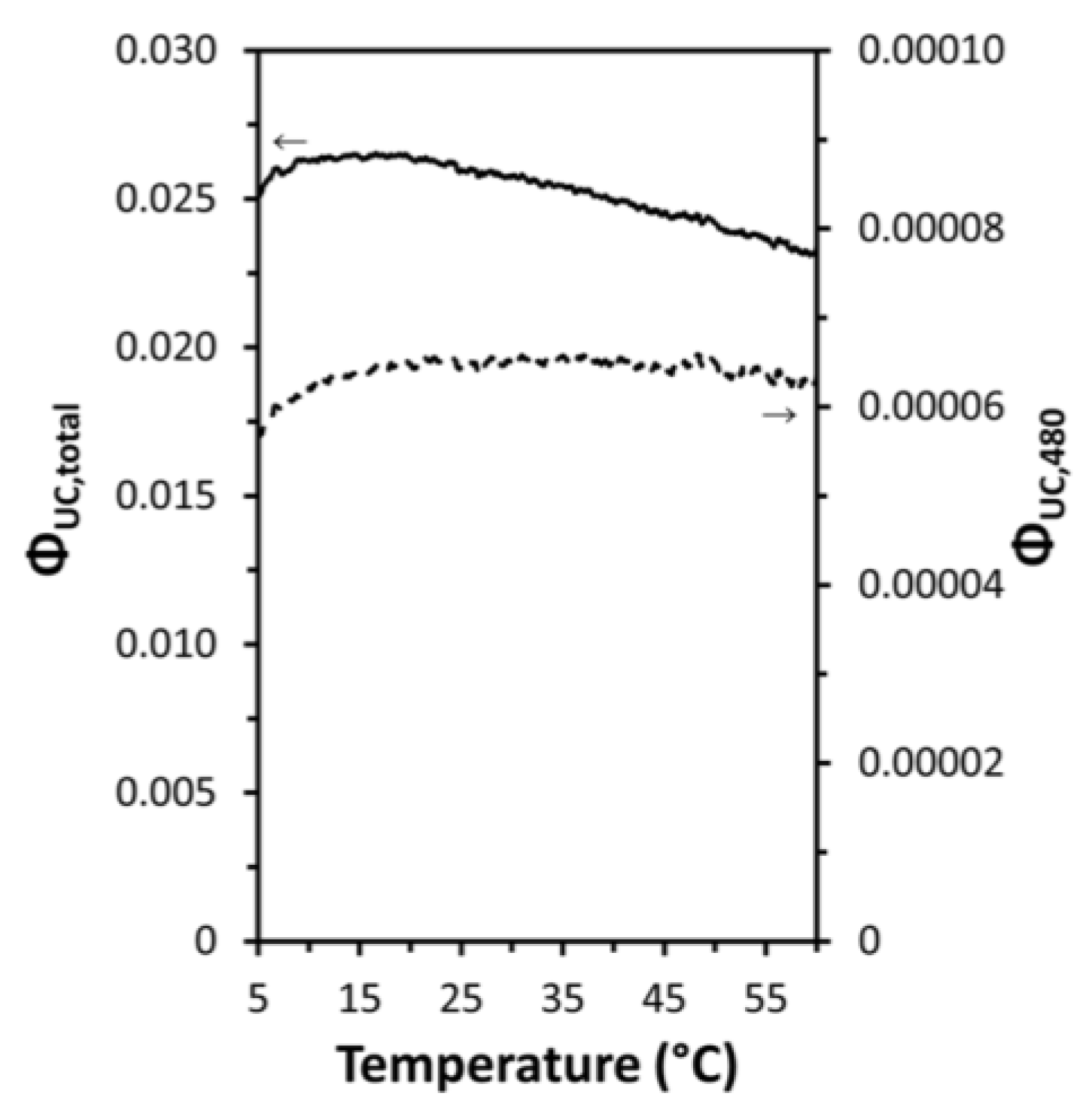
- Many authors have asserted that the thermal enhancement of UCL is reversible, as evidenced in their studies, as exemplified in Figure 5a,b [22,23,25,28,54,65,79,81]. However, a contrasting viewpoint has been put forward by multiple independent researchers who have reported that the thermal enhancement of UCL is non-reversible, as exemplified in Figure 5c,d [24,57,60,82,83,84]. Even for the same bulk phosphor, Yb2W3O12:6%Er3+, the reversibility of thermal enhancement of UCL and DSL were distinct [65,84]. It is difficult to understand why such contrasting phenomena could take place for the thermal enhancement of UCL.
4. Assessment of the Credibility of the Experimental Observations and Associated Explanations About the Thermal Enhancement of UCL in Lanthanide-Doped Fluoride Nanophosphors
4.1. Is the Thermal Enhancement of UCL an Intrinsic Property of Lanthanide-Doped Fluoride-Based UCNP?
- The majority of the researchers examined the temperature-dependent UCL spectra of solid powder samples directly [27,44,55,58,59,114]. Alternatively, some authors investigated the temperature-dependent UCL spectra of the UCNP colloidal solutions in organic solvents [42,51,81,112]. Notably, a significant thermal enhancement of UCL above room temperature was exclusively observed when the measurement was conducted using solid powder UCNP. Conversely, no any thermal enhancement was observed when the measurement was conducted on the colloidal solutions of both core-only and core/shell UCNP, and the luminescence intensities decreased at a higher temperature with the core-only UCNP decreasing more strongly [81,112].
- The temperature range where the thermal enhancement of UCL was observed varied considerably between papers by different authors. Some authors reported that the thermal enhancement of UCL was observed only at cryogenic temperatures, with thermal quenching initiating already at low temperatures [41,42]. However, other authors reported that the thermal enhancement of UCL was observed in the range from room temperature to 450 K [27,55,56], and even to 473 K [59].
- The reported critical size of UCNP, beyond which UCL intensity changes from thermal enhancement to thermal quenching, varied significantly among different authors or different publications of the same research group. Shao and colleagues observed thermal enhancement exclusively in small-sized NaYF4:Yb3+/Er3+ UCNP (<30 nm), noting that the increase in UCL intensity with temperature for 32 nm UCNP was almost negligible [21]. In another paper authored by Shao and colleagues, strong thermal enhancement of UCL was still observed in NaYF4:20%Yb3+/2%Ho3+@NaYF4: 20%Yb3+ with a size of ~37.8 nm (core-size ~32.6 nm) [54]. Wang and co-workers reported that the critical size of β-NaGdF4:Yb3+/Ln3+ (Ln = Eu/Tb/Er/Tm) UCNP at which this abnormal thermo-enhanced UCL phenomenon ceased was 50 nm [25]. Conversely, Jin and co-workers found that the thermal enhancement of the UCL could occur even in NaYF4:49%Yb3+/1%Tm3+ UCNP with sizes of 57 nm [27]. On the other hand, Ji and colleagues reported that the thermal enhancement of UCL was observed in OA-removed NaYbF4:0.5%Tm3+ UCNP with sizes as large as 152 nm [118].
- The architecture of UCNP exhibiting thermal enhancement of UCL was also considerable different in the reports. Shao and colleagues observed thermal enhancement only in core-only and active-core/active-shell UCNP, while thermal quenching of UCL was observed in UCNP with active-core/inert-shell architectures [44,46,51,53]. However, Jin et al. and Rettori et al. reported that thermal enhancement of UCL was also observed in the nanocrystals passivated with an inert shell [22,60,71].
- The critical shell thickness in active-core/inert-shell-architectured UCNP, beyond which UCL intensity switches from thermal enhancement to thermal quenching, has been reported to span a wide range by the same research group in various publications. In 2014, Shao and co-workers observed UCL enhancement in NaYF4:Yb3+,Er3+ @NaYF4 core/shell UCNP with a core diameter of ~24 nm and a shell thickness of 2 nm, as illustrated in Figure 7a [21]. However, in subsequent reports in 2017 and 2018, they found no thermal enhancement in NaGdF4:Yb/Tm@NaGdF4 core/shell UCNP with a core diameter of ~8 nm and a shell thickness of 3.5 nm [51] or in NaGdF4:Yb3+/Er3+@NaGdF4 core/shell UCNP with a core diameter of ~7 nm and a shell thickness of 3.5 nm [44], as depicted in Figure 7b. In 2019, Shao and colleagues reported a critical “shell thickness (ST)” for thermal enhancement of UCL in NaGdF4:20%Yb3+/2%Er3+@NaGdF4 core/shell UCNP of 5.4 nm [47], as illustrated in Figure 7c, which was 1.5 times larger than the value reported in 2017 and 2018. More recently, in 2022, Shao’s group reported a “critical shell thickness” for the thermal enhancement of UCL in NaGdF4:20%Yb3+/2%Er3+@NaGdF4 of 4.9 nm, as shown in Figure 7d [49]. This value was evidently different from those reported previously.
- 7.
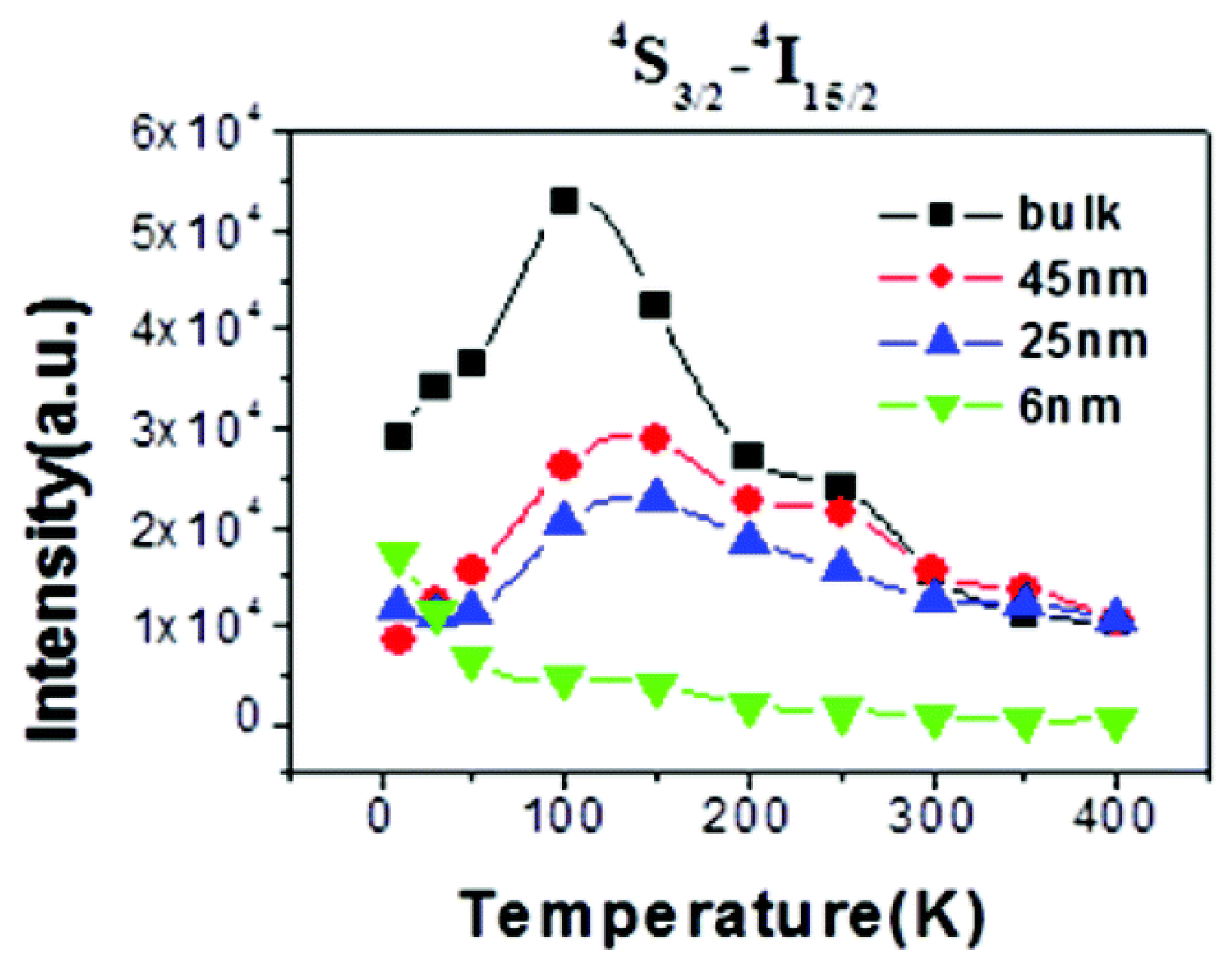
- The variation trends of UCL intensity with temperature for fluoride UCNP with a similar composition, particle size, and architecture, as reported by various research groups, exhibit notable discrepancies. Specifically, some researchers have documented that the UCL intensity of core-only NaYF4:Yb3+/Er3+ UCNP with a particle size of 30 nm [21,25] increases monotonously as the temperature rises above room temperature, as illustrated in Figure 7a. Conversely, other researchers have observed a steady decline in UCL intensity for NaYF4:Yb3+/Er3+ UCNP with particle sizes of 30–40 nm decreased steadily with increasing temperature [82,111,112]. In some cases, thermal quenching is evident even from cryogenic temperatures [41,109], as exemplified in Figure 8. Distinct from these two monotonic patterns, Tong et al. reported that the UCL intensity of α-NaYF4:Yb3+/Er3+UCNP with sizes of 75 nm decreased with an increase in the temperature from 303 to 483 K, but subsequently increased with further temperature elevation from 483 to 573 K [83].
4.2. Can the Proposed Mechanisms Explaining the Enhancement Be Unequivocally Supported by the Existing Literature?
- i.
- ii.
- iii.
- iv.
- v.
- (i)
- (ii)
- (iii)
- (1)
- At a specified elevated temperature, such as 90 °C, the moisture content in the UCNP during heating is expected to be greater than that during cooling. This is because water desorbed from the phosphor’s surface at the temperatures above 90 °C cannot be re-adsorbed reversibly at this temperature; re-adsorption typically occurs at lower temperatures. A TGA experiment by Meijerink’s group on Yb3+/Ln3+ (Ln = Tm, Ho, Er) co-doped NaY(WO4)2 UCNP during the heating, cooling, and reheating cycles in air within a temperature range of 300–570 K indicated that, during cooling from 570 to 300 K, there was a significant weight increase due to water adsorption below 370 K. However, during reheating, continuous weight loss due to moisture desorption occurred even above 400 K [28], as demonstrated in Figure 11b.
- (2)
- The UCNP powder samples used in the temperature-variable emission spectral measurement is generally around 1–2 mm in depth [48,51,82,119]. Considering the variation in excitation light penetration depth based on the activator doping concentration, for the UCNP powder with a particle size of, for example, 50 nm, at least 10 layers of the sample may be involved in the measurement and contribute to the emission spectral intensity recorded. During the cooling in heating and cooling cycles, moisture re-adsorption on the top surface layer of UCNP may easily occur. However, achieving equilibrium in moisture re-adsorption on deeper layers of densely packed powders during cooling is challenging. This can be demonstrated by the room temperature FTIR spectrum of the OH-coated NaGdF4:20%Yb3+,0.5%Tm3+ UCNP before and after temperature-dependent spectral measurement reported by Qiu et al., as displayed in Figure 11c [24]. It can be seen in Figure 11c that intensity bands at OH absorption bands between 1640 cm−1 and 3200–3700 cm−1 are notably reduced after temperature-dependent spectral measurement.
- (iv)
- (1)
- Wang and co-workers raised concerns about the existence of a critical temperature threshold beyond which efficient [Yb···O]-Ln3+ pairs start to form, since the surface vibration could only result in the optically inactive Yb3+ and Ln3+ ions within the dark layer at room temperature according to the experimental results [30]. Furthermore, Wang and colleagues stressed that the surface-phonon-assisted energy-transfer mechanism failed to explain the reported increase in intensity accompanied with the prolonged lifetime of Yb3+ DSL with temperature [25,44,50,51,56,58,79]. Since the DSL (Yb3+) and UCL (Ln3+) processes directly compete with the nonradiative transition of Yb3+ ions, an increase in energy transfer from Yb3+ to activator Ln3+ at higher temperatures should decrease the intensity and decay time of Yb3+ DSL [30].
- (2)
- Meijerink and co-workers pointed out that surface-phonon-assisted energy-transfer mechanism could not explain the luminescence thermal quenching of OA ligand-stabilized Yb3+/Ln3+ co-doped UCNP when the temperature variable spectral measurement was conducted in dry Ar and N2 atmosphere [28,44]. It also failed to explain the thermal quenching of UCL observed in the bulk NaY(WO4)2:Yb3+/Ln3+ (Er3+, Ho3+, Tm3+) samples [28]. Since the Yb–O bond was embedded in the oxidic host like NaY(WO4)2 [28,44].
- (3)
- In addition, multiple research groups have reported temperature-variable spectral measurements using colloidal suspensions of UCNP, showing luminescence thermal quenching of OA ligand-stabilized Yb3+/Ln3+ co-doped UCNP [51,81,112]. The surface-phonon-assisted energy-transfer mechanism encounters difficulties in explaining this phenomenon, as the interaction between Yb3+ and surface OA ligands was maintained in organic solvent during the spectral measurements.
- (4)
- The surface-phonon-assisted energy-transfer mechanism could hardly give a reasonable explanation for the observed thermal enhancement of UCL in ligand-free and inert-shell coated UCNP, as exemplified in Figure 2 [22,47,60,71,118]. This is attributed to the absence or inhibition of the interactions between Yb and surface ligands due to their protective inert shells.
- (v)
5. Discussion
5.1. Factors Contributing to Changes in UCL Intensity with Temperature
- Absorption of excitation light by the adsorbed water and its influence on the excitation power density at different temperatures
- 2.
- Laser-induced local heating and temperature rise during measurement and associated issues
- 3.
- Lattice thermal expansion-induced changes in sample volume and measurement geometry
- (1)
- The moisture present on UCNP exhibits strong absorption in the NIR region, which can divert the power density of the excitation light incident to the sensitizer, thereby influencing the intensity of the UCL. Furthermore, the moisture on UCNP can retard the laser-induced temperature rise. Given that the quantity of moisture adsorbed at room temperature depends on the surface-to-volume ratio of the UCNP, the moisture-related influence on the temperature-dependent spectral intensity is inherently size-dependent.
- (2)
- Laser-induced local heating is another factor influencing the reliability of spectral intensity. The magnitude of the laser-induced temperature rise is contingent upon the doping-concentration of Yb3+ ions and the QY of the UCNP under the constant excitation power density. Given that the QY of UCNP is dependent on particle size and decreases as particle size diminishes, it is anticipated that the impact of laser-induced temperature rise on the emission spectral intensity will also exhibit a size-dependent behavior.
- (3)
- Changes in sample volume and surface state due to lattice thermal expansion can lead to alterations in the excitation power density and geometrical configuration of the measurement, ultimately influencing the spectral intensity recorded. The extent of these changes, while changes in sample packing density are not considered, depends on both the thermal expansion coefficient and the temperature increase. Given that both the thermal expansion coefficient and the laser-induced temperature rise exhibit size-dependence and tend to increase with decreasing particle size, it is expected that the influence of the lattice thermal expansion on the emission spectral intensity will also be size-dependent.
5.2. Factors Influencing Quantum Yield in Yb3+-Sensitized Upconversion Luminescence Phosphors and Challenges in Its Measurement
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auzel, F. Upconversion and anti-Stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Schäfer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles:design,nanochemistry,and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Wilhelm, S. Perspectives for upconverting nanoparticles. ACS Nano 2017, 11, 10644–10653. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, J.C.; Fischer, S. Upconversion for photovoltaics–a review of materials, devices and concepts for Performance Enhancement. Adv. Optical Mater. 2015, 3, 510–535. [Google Scholar] [CrossRef]
- Deng, T.; Yan, S.; Hu, J. Effect of calcination temperature on up-conversion photoluminescence of the GdAlO3: Er3+,Yb3+ phosphor. ECS J. Solid State Sci. Technol. 2015, 4, R48–R53. [Google Scholar] [CrossRef]
- Liu, G. Advances in the theoretical understanding of photon upconversion in rare-earth activated nanophosphors. Chem. Soc. Rev. 2015, 44, 1635–1652. [Google Scholar] [CrossRef]
- Nadort, A.; Zhao, J.; Goldys, E.M. Lanthanide upconversion luminescence at thenanoscale: Fundamentals and optical properties. Nanoscale 2016, 8, 13099–13130. [Google Scholar] [CrossRef]
- Tanner, P.A.; Zhou, L.; Duan, C.; Wong, K.-L. Misconceptions in electronic energy transfer: Bridging the gap between chemistry and physics. Chem. Soc. Rev. 2018, 47, 5234–5265. [Google Scholar] [CrossRef]
- Rabouw, F.T.; Prins, P.T.; Villanueva-Delgado, P.; Castelijns, M.; Geitenbeek, R.G.; Meijerink, A. Quenching pathways in NaYF4:Er3+,Yb3+upconversion nanocrystals. ACS Nano 2018, 12, 4812–4823. [Google Scholar] [CrossRef]
- Wang, Z.; Meijerink, A. Concentration quenching in upconversion nanocrystals. J. Phys. Chem. C 2018, 122, 26298–26306. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, J.; Wu, Y.; Liu, X. Energy-transfer editing in lanthanide-activated upconversion nanocrystals: A toolbox for emerging applications. ACS Cent. Sci. 2019, 5, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924–936. [Google Scholar] [CrossRef]
- Shi, R.; Brites, C.D.S.; Carlos, L.D. Understanding the shell passivation in Ln3+-doped luminescent nanocrystals. Small Struct. 2022, 3, 2100194. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Meijer, M.S.; Howard, I.A.; Rojas-Gutierrez, P.A.; Busko, D.; Frenzel, F.; Würth, C.; Richards, B.S.; Bonnet, S. Absolute upconversion quantum yields of blue-emitting LiYF4:Yb3+,Tm3+ upconverting nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 22556–22562. [Google Scholar] [CrossRef]
- Würth, C.; Geißler, D.; Behnke, T.; Kaiser, M.; Resch-Genger, U. Critical review of the determination of photoluminescence quantum yields of luminescent reporters. Anal. Bioanal. Chem. 2015, 407, 59–78. [Google Scholar] [CrossRef]
- May, P.S.; Baride, A.; Hossan, M.Y.; Berry, M. Measuring the internal quantum yield of upconversion luminescence for ytterbium sensitized upconversion phosphors using the ytterbium (III) emission as an internal standard. Nanoscale 2018, 10, 17212–17226. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmeier, B.C. Luminescent Materials, 1st ed.; Springer: Berlin, Germany, 1994. [Google Scholar]
- Li, D.; Shao, Q.; Dong, Y.; Jiang, J. Anomalous temperature-dependent upconversion luminescence of small-sized NaYF4:Yb3+, Er3+ nanoparticles. J. Phys. Chem. C 2014, 118, 22807–22813. [Google Scholar] [CrossRef]
- Lei, L.; Chen, D.; Li, C.; Huang, F.; Zhang, J.; Xu, S. Inverse thermal quenching effect in lanthanide-doped upconversion nanocrystals for anti-counterfeiting. J. Mater. Chem. C 2018, 6, 5427–5433. [Google Scholar] [CrossRef]
- Liao, J.; Wang, M.; Lin, F.; Han, Z.; Fu, B.; Tu, D.; Chen, X.; Qiu, B.; Wen, H.-R. Thermally boosted upconversion and downshifting luminescence in Sc2(MoO4)3:Yb/Er with two-dimensional negative thermal expansion. Nat. Common. 2022, 13, 2090. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, W.; Liu, X.; Jiang, C.; Qiu, J. Discovery of non-reversible thermally enhanced upconversion luminescence behavior in rare-earth doped nanoparticles. J. Mater. Chem. C 2019, 7, 4336–4343. [Google Scholar] [CrossRef]
- Cui, X.; Cheng, Y.; Lin, H.; Huang, F.; Wu, Q.; Wang, Y. Size-dependent abnormal thermo-enhanced luminescence of ytterbium-doped nanoparticles. Nanoscale 2017, 9, 13794–13799. [Google Scholar] [CrossRef]
- Zou, Q.; Huang, P.; Zheng, W.; You, W.; Li, R.; Tu, D.; Xu, J.; Chen, X. Cooperative and non-cooperative sensitization upconversion in lanthanide-doped LiYbF4 nanoparticles. Nanoscale 2017, 9, 6521–6528. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, S.; Liao, J.; Clarke, C.S.; Tawfik, A.; Ren, W.; Mi, C.; Wang, F.; Jin, D. Activation of the surface dark-layer to enhance upconversion in a thermal field. Nat. Photonics 2018, 12, 154–158. [Google Scholar] [CrossRef]
- Wang, Z.; Christiansen, J.; Wezendonk, D.; Xie, X.; Huis, M.A.; Meijerink, A. Thermal enhancement and quenching of upconversion emission in nanocrystals. Nanoscale 2019, 11, 12188–12197. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Zhang, Z.; Guo, C. Ultra-sensitive optical nano-thermometer LaPO4:Yb3+/Nd3+ based on thermo-enhanced NIR-to-NIR emissions. Chem. Eng. J. 2020, 389, 12450. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Huang, Q.; Xu, J.; Lin, H.; Wang, Y. Abnormal thermally enhanced upconversion luminescence of lanthanide-doped phosphors: Proposed mechanisms and potential applications. J. Mater. Chem. C 2021, 9, 2220–2230. [Google Scholar] [CrossRef]
- Shi, R.; Martinez, E.D.; Brites, C.D.S.; Carlos, L.D. Thermal enhancement of upconversion emission in nanocrystals: A comprehensive summary. Phys. Chem. Chem. Phys. 2021, 23, 20–42. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Wang, F. Overcoming thermal quenching in upconversion Nanoparticles. Nanoscale 2021, 13, 3454–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Y.; Suo, H.; Li, L.-P.; Guo, C.-F. Recent advances in rare-earth doped upconverison materials with thermally-enhanced emissions. Chin. J. Lumin. 2021, 11, 1673–1685. (in Chinese). [Google Scholar] [CrossRef]
- dos Santos, P.V.; Gouveia, E.A.; de Araujo, M.T.; Gouveia-Neto, A.S.; Sombra, A.S.B.; Medeiros Neto, J.A. Thermally induced threefold upconversion emission enhancement in nonresonant excited Yb3+/Er3+-codoped chalcogenide glass. Appl. Phys. Lett. 1999, 74, 3607–3609. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Y.; Tang, F.; Zhao, B.; Lv, S. Large negative thermal quenching of upconversion luminescence through selective excitation in a YAG:Yb/Er transparent ceramics. J. Lumin. 2023, 263, 120075. [Google Scholar] [CrossRef]
- Gao, G.; Busko, D.; Kauffmann-Weiss, S.; Turshatov, A.; Howard, I.A.; Richards, B.S. Wide-range non-contact fluorescence intensity ratio thermometer based on Yb3+/Nd3+ co-doped La2O3 microcrystals operating from 290 to 1230 K. J. Mater. Chem. C 2018, 6, 4163–4170. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, H.; Zhang, Z.; Cao, W. Highly sensitive optical thermometry through thermally enhanced near infrared emissions from Nd3+/Yb3+ codoped oxyfluoride glass ceramic. Sens. Actuator B 2013, 178, 520–524. [Google Scholar] [CrossRef]
- Peng, T.; Cao, Y.; Cui, H.; Li, Y.; Zhang, Y.; Li, L.; Zhang, J.; Zhang, X.; Che, B. Upconversion NIR luminescence negative thermal quenching and temperature sensing in LiYGeO4:Yb3+/Nd3+ phosphors. Mater. Chem. Phys. 2023, 309, 12830. [Google Scholar] [CrossRef]
- Pu, L.; Wang, Y.; Zhao, J.; Jin, M.; Li, L.; Li, P.; Wang, Z.; Guo, C.; Suo, H. Multi-mode ratiometric thermometry using thermo-intensified NIR emission. Chem. Eng. J. 2022, 449, 137890. [Google Scholar] [CrossRef]
- Suyver, J.F.; Grimm, J.; Kramer, K.W.; Gudel, H.-U. Highly efficient near-infrared to visible up-conversion process in NaYF4: Er3+,Yb3+. J. Lumin. 2005, 114, 53–59. [Google Scholar] [CrossRef]
- Yu, W.; Xu, W.; Song, H.; Zhang, S. Temperature-dependent upconversion luminescence and dynamics of NaYF4:Yb3+/Er3+ nanocrystals: Influence of particle size and crystalline phase. Dalton Trans. 2014, 43, 6139–6147. [Google Scholar] [CrossRef]
- Wu, K.; Cui, J.; Kong, X.; Wang, Y. Temperature dependent upconversion luminescence of Yb/Er codoped NaYF4 nanocrystals. J. Appl. Phys. 2011, 110, 053510. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion luminescence properties of NaYF4:Yb:Er nanoparticles codoped with Gd3+. J. Phys. Chem. C 2015, 119, 3363–3373. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Zhang, P.; Dong, Y.; Fang, F.; Jiang, J. Mechanistic investigations on the dramatic thermally induced luminescence enhancement in upconversion nanocrystals. J. Phys. Chem. C 2018, 122, 26142–26152. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Deng, X.; Jiang, J. Thermal-responsive multicolor emission of single NaGdF4:Yb/Ce/Ho upconversion nanocrystals for anticounterfeiting application. Nanophotonics 2020, 9, 2879–2885. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Deng, X.; Han, S.; Song, D.; Jiang, J. Core/shell upconversion nanocrystal hybrids with temperature-dependent emission color changes for multilevel anticounterfeiting applications. Adv. Mater. Technol. 2019, 4, 1800498. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Dong, Y.; Jiang, J. Energy loss mechanism of upconversion core/shell nanocrystals. J. Phys. Chem. C 2019, 123, 22674–22679. [Google Scholar] [CrossRef]
- Li, D.; Shao, Q.; Dong, Y.; Fang, F.; Jiang, J. Ho3+(or Tm3+)-activated upconversion nanomaterials: Anomalous temperature dependence of upconversion luminescence and applications in multicolor temperature indicating and security. Part. Part. Syst. Charact. 2015, 32, 728–733. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.; Shao, Q.; Li, C.M. Theory of competition between surface quenching and scattering effect in core/shell upconversion nanocrystals. J. Phys. Chem. C 2022, 126, 18796–18801. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.; Deng, X.; Zhou, J.; Zhang, R.; Shao, Q. Opposite luminescence thermal behavior of upconversion core/shell nanocrystals for anticounterfeiting. Nanoscale 2023, 15, 15552–15557. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, G.; Ouyang, L.; Hu, Y.; Dong, Y.; Jiang, J. Emission color tuning of core/shell upconversion nanoparticles through modulation of laser power or temperature. Nanoscale 2017, 9, 12132–12141. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Deng, X.; Song, D.; Han, S.; Dong, Y.; Jiang, J. Thermally induced multicolor emissions of upconversion hybrids with large color shifts for anticounterfeiting applications. J. Mater. Chem. C 2019, 7, 11770–11775. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.; Zhao, M.; Shao, Q. Investigation of the energy loss in upconversion luminescence of lanthanide-doped nanocrystals for anticounterfeiting and a nanoheater. ACS Appl. Nano Mater. 2022, 5, 14256–14262. [Google Scholar] [CrossRef]
- Nie, J.; Shao, Q.; Yu, S.; Shi, M.; Dong, Y.; Jiang, J. Distinct luminescent thermal behaviors of Yb3+- and Nd3+-sensitized core/shell upconversion nanocrystals. J. Phys. Chem. C 2023, 127, 7552–7559. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Pan, G.-H.; Zhang, L.; Wu, H.; Hao, Z.; Liu, F.; Zhang, J. Vacuum-assisted strong luminescence thermal enhancement in NaYF4:Ho3+/Yb3+ upconverting nanocrystals: A conclusive evidence for the effect of water desorption. ACS Sustain. Chem. Eng. 2022, 10, 16862–16870. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, S.; Zhu, K.; Gao, L.; Wang, L.; Chen, G.; Li, A.-H. Experimental evidence for thermally enhanced energy transfer in Yb3+/Tm3+ codoped nanocrystals. Laser Photonics Rev. 2024, 18, 240061. [Google Scholar] [CrossRef]
- Martínez, E.D.; Brites, C.D.S.; García-Flores, A.A.N.; Carneiro Neto, L.D.; Carlos, R.R.; Urbano, C.; Rettori, C. Controlling the thermal switching in upconverting nanoparticles through surface chemistry. Nanoscale 2021, 13, 16267–16276. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Xu, J.; Lin, H.; Wang, Y. Thermo-enhanced upconversion luminescence in inert-core/active-shell UCNPs: The inert core matters. Nanoscale 2021, 13, 6569–6576. [Google Scholar] [CrossRef]
- Chen, B.; Kong, W.; Wang, N.; Zhu, G.; Wang, F. Oleylamine-mediated synthesis of small NaYbF4 nanoparticles with tunable size. Chem. Mater. 2019, 31, 4779–4786. [Google Scholar] [CrossRef]
- Mi, C.; Zhou, J.; Wang, F.; Jin, D. Thermally enhanced NIR–NIR anti-Stokes emission in rare earth doped nanocrystals. Nanoscale 2019, 11, 12547–12552. [Google Scholar] [CrossRef]
- Mei, S.; Zhou, J.; Sun, H.-T.; Cai, Y.; Sun, L.-D.; Jin, D.; Yan, C.-H. Networking state of ytterbium ions probing the origin of luminescence quenching and activation in nanocrystals. Adv. Sci 2021, 8, 2003325. [Google Scholar] [CrossRef]
- Liang, L.; Liu, X. Nanocrystals feel the heat. Nat. Photonics 2018, 12, 124–125. [Google Scholar] [CrossRef]
- Lei, L.; Xia, J.; Cheng, Y.; Wang, Y.; Bai, G.; Xia, H.; Xu, S. Enhancing negative thermal quenching effect via low-valence doping in two-dimensional confined core–shell upconversion nanocrystals. J. Mater. Chem. C 2018, 6, 11587–11592. [Google Scholar] [CrossRef]
- Wang, R.; Gao, W.; Yan, S.; Hu, J.; Balfourd, E.A.; Fu, H. Defect level induced negative thermal quenching of β-Ba3LuAl2O7.5: Yb3+, Er3+ phosphor. J. Alloys Compd. 2024, 1004, 175852. [Google Scholar] [CrossRef]
- Zou, H.; Yang, X.; Chen, B.; Du, Y.; Ren, B.; Sun, X.; Qiao, X.; Zhang, Q.; Wang, F. Thermal enhancement of upconversion by negative lattice expansion in orthorhombic Yb2W3O12. Angew. Chem. Int. Ed. 2019, 58, 17255–17259. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, B.; Hu, Y.; Zhang, Q.; Wang, X.; Wang, F. Simultaneous enhancement and modulation of upconversion by thermal stimulation in Sc2Mo3O12 crystals. J. Phys. Chem. Lett. 2020, 11, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yan, H.; Li, R.; Yu, Y.; Sun, Y.; Liao, J. Thermal enhanced near-infrared upconversion-luminescence in Y2Mo4O15:Yb/Nd with uniaxial negative thermal expansion. Inorg. Chem. 2025, 64, 295–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, M.; Chen, W.; Wu, Z.; Li, Z.; Guo, C. Thermally enhanced NIR up-conversion fluorescence multimode thermometry based on Y2Mo3O12:Nd3+,Yb3+. J. Mater. Chem. C 2024, 12, 7588–7595. [Google Scholar] [CrossRef]
- Dai, M.; Li, Y.; Wang, Z.; Li, A.; Sheng, T.; Xu, H.; Li, K.; Fu, Z. Thermally boosted upconversion luminescence and high-performance thermometry in ScF3:Yb3+/Tm3+ nanorods with negative thermal expansion. J. Lumin. 2024, 265, 120219. [Google Scholar] [CrossRef]
- Wang, Y.; An, Z.; Tao, Z.; Zhang, S.; Yang, X.; Kuang, X.; Ye, S. Thermodynamics and kinetics accounting for antithermal quenching of luminescence in Sc2(MoO4)3:Yb/Er: Perspective beyond negative thermal expansion. J. Phys. Chem. Lett. 2022, 13, 12032–12040. [Google Scholar] [CrossRef]
- Martínez, E.D.; Brites, C.D.S.; Carlos, L.D.; García-Flores, A.F.; Urbano, R.R.; Rettori, C. Electrochromic switch devices mixing small- and large-sized upconverting nanocrystals. Adv. Funct. Mater. 2019, 29, 1807758. [Google Scholar] [CrossRef]
- Pan, E.; Bai, G.; Ma, B.; Lei, L.; Huang, L.; Xu, S. Reversible enhanced upconversion luminescence by thermal and electric fields in lanthanide ions doped ferroelectric nanocomposites. Sci. China Mater. 2020, 63, 110–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Liu, J.; Zhang, Z.; Sun, B.; Liu, H. Anti-thermal quenching of luminescence in Y2W3O12:Yb3+/RE3+ (RE = Er/Ho/Tm) and its temperature sensing application. Dalton Trans. 2024, 53, 2575–2590. [Google Scholar] [CrossRef]
- Zi, Y.; Cun, Y.; Bai, X.; Xu, Z.; Haider, A.A.; Qiu, J.; Song, Z.; Huang, A.; Zhu, J.; Yang, Z. Negative lattice expansion-induced upconversion luminescence thermal enhancement in novel Na2MoO4:Yb3+, Er3+ transparent glass ceramics for temperature sensing applications. J. Mater. Chem. C 2023, 11, 1541–1549. [Google Scholar] [CrossRef]
- Jin, X.; Sun, P.; Yang, W.; Wang, Y.; Xiao, Z. Thermal enhancement of upconversion luminescence in negative-thermal-expansion Ho3+-doped Yb2−xW3O12 phosphors. J. Electron. Mater. 2024, 53, 4929–4938. [Google Scholar] [CrossRef]
- Lv, H.; Du, P.; Luo, L.; Li, W. Negative thermal expansion triggered anomalous thermal upconversion luminescence behaviors in Er3+/Yb3+-codoped Y2Mo3O12 microparticles for highly sensitive thermometry. Mater. Adv. 2021, 2, 2642–2648. [Google Scholar] [CrossRef]
- Ren, B.; Chen, B.; Zhao, J.; Guo, Y.; Zhang, X.; Chen, X.; Du, Y.; Deng, Z.; Zhu, G.; Wang, F. Synthesis of core−shell ScF3 nanoparticles for thermal enhancement of upconversion. Chem. Mater. 2021, 33, 158–163. [Google Scholar] [CrossRef]
- Lu, H.; Lu, Y.; Lin, D.; Zhu, J.; Du, Y.; Zou, H. Giant thermal enhancement of upconversion photoluminescence in Y2W3O12. Ceram. Int. 2024, 50, 51733–51737. [Google Scholar] [CrossRef]
- Yan, H.; Li, R.; Feng, L.; Yu, Y.; Gong, G.; Huang, H.; Wen, H.-R.; Liao, J. Simultaneous negative thermal quenching luminescence of upconversion and downshifting processes in Al2(WO4)3:Yb/Er phosphors with low thermal expansion. J. Mater. Chem. C 2024, 12, 12353–12362. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Gorris, H.H. Perspectives and challenges of photon-upconversion nanoparticles–Part I: Routes to brighter particles and quantitative spectroscopic studies. Anal. Bioanal. Chem. 2017, 409, 5855–5874. [Google Scholar] [CrossRef]
- Liu, D.; Xu, X.; Wang, F.; Zhou, J.; Mi, C.; Zhang, L.; Lu, Y.; Ma, C.; Goldys, E.; Lince, J.; et al. Emission stability and reversibility of upconversion nanocrystals. J. Mater. Chem. C 2016, 4, 9227–9234. [Google Scholar] [CrossRef]
- Shan, J.; Kong, W.; Wei, R.; Yao, N.; Ju, Y. An investigation of the thermal sensitivity and stability of the β-NaYF4:Yb,Er upconversion nanophosphors. J. Appl. Phys. 2010, 107, 054901. [Google Scholar] [CrossRef]
- Tong, L.; Li, X.; Hua, R.; Peng, T.; Wang, Y.; Zhang, X.; Chen, B. Anomalous temperature-dependent upconversion luminescence of α-NaYF4:Yb3+/Er3+ nanocrystals synthesized by a microwave assisted hydrothermal method. J. Nanosci. Nanotechnol. 2016, 16, 816–821. [Google Scholar] [CrossRef]
- Chen, Z.; Cun, Y.; Yan, S.; Zi, Y.; Zhu, B.; Ruan, K.; Ding, B.; Yang, Z.; Haider, A.A.; Khan, I.; et al. Negative and positive thermal expansion effects regulate the upconversion and near-infrared downshift luminescence for multiparametric temperature sensing. Sci. China Mater. 2023, 66, 4742–4748. [Google Scholar] [CrossRef]
- Levitus, M. Tutorial: Measurement of fluorescence spectra and determination of relative fluorescence quantum yields of transparent samples. Methods Appl. Fluoresc. 2020, 8, 033001. [Google Scholar] [CrossRef] [PubMed]
- Yan, S. Negative thermal quenching of photoluminescence: An evaluation from the macroscopic viewpoint. Materials 2024, 17, 586. [Google Scholar] [CrossRef]
- Yan, S. On the validity of the defect- induced negative thermal quenching of Eu2+-doped phosphors. ECS J. Solid State Sci. Technol. 2023, 12, 016001. [Google Scholar] [CrossRef]
- Yan, S. On the anomalous thermal quenching of Mn4+ luminescence in A2XF6:Mn4+ (A = K, Na, Rb or Cs; X = Si, Ti, Ge, Sn, Zr orHf). ECS J. Solid State Sci. Technol. 2020, 9, 106004. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
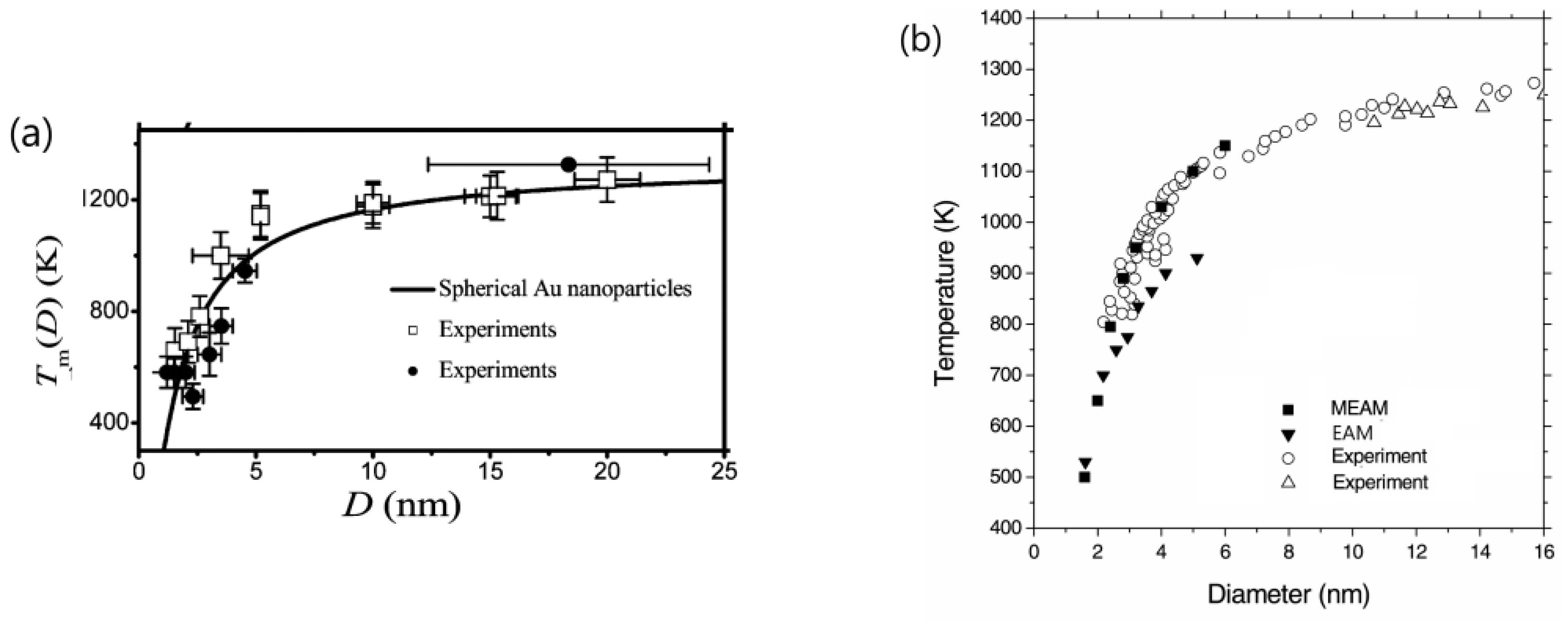
- Nanda, K.K. Size-dependent melting of nanoparticles: Hundred years of thermodynamic model. Pramana 2009, 72, 617–628. [Google Scholar] [CrossRef]
- Pawlow, P. The dependency of the melting point on the surface energy of a solid body. Z. Phys. Chem. 1909, 65, 545–548. [Google Scholar] [CrossRef]
- Takagi, M. Electron-diffraction study of liquid-solid transition of thin metal films. J. Phys. Soc. Jpn. 1954, 9, 359–363. [Google Scholar] [CrossRef]
- Schmidt, M.; Kusche, R.; Issendorff, B.; Haberland, H. Irregular variations in the melting point of size-selected atomic clusters. Nature 1998, 393, 238–240. [Google Scholar] [CrossRef]
- Li, Z.H.; Truhlar, D.G. Nanothermodynamics of metal nanoparticles. Chem. Sci. 2014, 5, 2605–2624. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef]
- Castro, T.; Reifenberger, R.; Choi, E.; Andres, R.P. Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys. Rev. B 1990, 42, 8548–8556. [Google Scholar] [CrossRef]
- Buffat, P.; Borel, J.-P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]
- Sambles, J.R. An electron microscope study of evaporating gold particles: The Kelvin equation for liquid gold and the lowering of the melting point of solid gold particles. Proc. Roy. Soc. Lond. A 1971, 324, 339–351. [Google Scholar]
- Lee, J.; Lee, J.; Tanaka, T.; Mori, H. In situ atomic-scale observation of melting point suppression in nanometer-sized gold particles. Nanotechnology 2009, 20, 475706. [Google Scholar] [CrossRef]
- Schlexer, P.; Andersen, A.B.; Sebok, B.; Chorkendorff, I.; Schiøtz, J.; Hansen, T.W. Size-dependence of the melting temperature of individual Au nanoparticles. Part. Part. Syst. Charact. 2019, 36, 1800480. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Wu, D.; Deng, Y.; Shao, J.; Chen, L.; Fang, D. Size and shape dependent melting temperature of metallic nanomaterials. J. Phys. Condens. Matter 2019, 31, 075701. [Google Scholar] [CrossRef]
- Ercolessi, F.; Andreoni, W.; Tosatti, E. Melting of small gold particles: Mechanism and size effects. Phys. Rev. Lett. 1991, 66, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.J.; Jensen, P.; Barrat, J.-L. Melting, freezing, and coalescence of gold nanoclusters. Phys. Rev. B 1997, 56, 2248–2257. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, B.-J.; Cho, Y.W. Thermal stability of unsupported gold nanoparticle: A molecular dynamics study. Surf. Sci. 2002, 512, 262–268. [Google Scholar] [CrossRef]
- Lu, H.M.; Li, P.Y.; Cao, Z.H.; Meng, X.K. Size-, shape-, and dimensionality- dependent melting temperatures of nanocrystals. J. Phys. Chem. C 2009, 113, 7598–7602. [Google Scholar] [CrossRef]
- Lee, B.J.; Shim, J.H.; Baskes, M.I. Semiempirical atomic potentials for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, Al, and Pb based on first and second nearest-neighbor modified embedded atom method. Phys. Rev. B 2003, 68, 399–404. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Lian, J.S.; Jian, Q. Modeling of the melting point, Debye temperature, thermal expansion coefficient, and the specific heat of nanostructured materials. J. Phys. Chem. C 2009, 113, 16896–16900. [Google Scholar] [CrossRef]
- Foster, D.M.; Pavloudis, T.; Kioseoglou, J.; Palmer, R.E. Atomic-resolution imaging of surface and core melting in individual size-selected Au nanoclusters on carbon. Nat. Comm. 2019, 10, 2583. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Zeng, Q.; Song, K.; Wang, X.; Kong, X. Temperature-dependent upconversion luminescence of NaYF4:Yb3+,Er3+ nanoparticles. Chem. Lett. 2013, 42, 310–312. [Google Scholar] [CrossRef]
- Xi, J.; Ding, M.; Dai, J.; Pan, Y.; Chen, D.; Ji, Z. Comparison of upconversion luminescent properties and temperature sensing behaviors of β-NaYF4:Yb3+/Er3+ nano/microcrystals prepared by various synthetic methods. J. Mater. Sci. Mater. Electron 2016, 27, 8254–8270. [Google Scholar] [CrossRef]
- Li, L.; Qin, F.; Zhou, Y.; Zheng, Y.; Zhao, H.; Zhang, Z. Temperature sensing based on the 4F7/2/4S3/2−4I15/2 upconversion luminescence intensity ratio in NaYF4:Er3+/Yb3+ nanocrystals. J. Lumin. 2019, 206, 335–341. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion NaYF4:Yb:Er nanoparticles co-doped with Gd3+ and Nd3+ for thermometry on the nanoscale. RSC Adv. 2015, 5, 67149–67156. [Google Scholar] [CrossRef]
- Tu, L.; Wu, K.; Luo, Y.; Wang, E.; Yuan, J.; Zuo, J.; Zhou, D.; Li, B.; Zhou, J.; Jin, D.; et al. Significant enhancement of the upconversion emission in highly Er-doped nanoparticles at cryogenic temperatures. Angew. Chem. Int. Ed. 2023, 62, e202217100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, L.; Ye, R.; Jia, G.; Hua, Y.; Deng, D.; Xu, S. Integrating positive and negative thermal quenching effect for ultrasensitive ratiometric temperature sensing and anticounterfeiting. ACS Appl. Mater. Interfaces 2021, 13, 23951–23959. [Google Scholar] [CrossRef]
- Xia, J.; Lei, L.; Xia, H.; Xu, S. Improved negative thermal quenching effect via high sensitizer doping content in NaGdF4 based active-core/active-shell architecture. Optics Comm. 2019, 444, 131–136. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, J.; Song, H.; Yuan, Z.; Huang, X.; Liu, J.; Zhou, J.; Li, C.; Wang, H.; Wu, S.; et al. Antithermal quenching upconversion luminescence via suppressed multiphonon relaxation in positive/negative thermal expansion core/shell NaYF4:Yb/Ho@ScF3 nanoparticles. J. Am. Chem. Soc. 2024, 146, 6530–6535. [Google Scholar] [CrossRef]
- Xu, W.; Qi, H.; Zheng, L.; Zhang, Z.; Cao, W. Multifunctional nanoparticles based on the Nd3+/Yb3+ codoped NaYF4. Opt. Lett. 2015, 40, 5678–5681. [Google Scholar] [CrossRef]
- Ji, H.; Dai, X.; Zheng, W.; Zhuang, C.; Dong, W.; Chen, D.; Ling, S.; Qiao, X.; Wang, Z.; Fan, X.; et al. Negative thermal quenching effect of NaYbF4:Tm nanoparticles: Towards high performance LIR temperature sensing. J. Alloys Compd. 2025, 1013, 178513. [Google Scholar] [CrossRef]
- Jones, C.M.S.; Biner, D.; Misopoulos, S.; Krämer, K.W.; Marques-Hueso, J. Optimized photoluminescence quantum yield in upconversion composites considering the scattering, inner-filter effects, thickness, self-absorption, and temperature. Sci. Rep. 2021, 11, 13910. [Google Scholar] [CrossRef]
- Solé, J.G.; Bausá, L.E.; Jaque, D. An Introduction to the Optical Spectroscopy of Inorganic Solids; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Kaiser, M.; Würth, C.; Kraft, M.; Hyppänen, I.; Soukk, T.; Resch-Genger, U. Power-dependent upconversion quantum yield of NaYF4:Yb3+,Er3+ nano- and micrometer-sized particles- measurements and simulations. Nanoscale 2017, 9, 10051–10058. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.T.; Lindgren, D.; Xie, H.; Thomas, D.; Gundlach, C.; Andersson-Engels, S. Balancing power density based quantum yield characterization of upconverting nanoparticles for arbitrary excitation intensities. Nanoscale 2013, 5, 4770–4775. [Google Scholar] [CrossRef]
- Joseph, R.E.; Busko, D.; Hudry, D.; Gao, G.; Biner, D.; Krämer, K.; Turshatov, A.; Richards, B.S.; Howard, I.A. A method for correcting the excitation power density dependence of upconversion emission due to laser-induced heating. Opt. Mater. 2018, 82, 65–70. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Wawrzynczyk, D.; Gagor, A.; Kepinski, L.; Kurnatowska, M.; Krajczyk, L.; Nyk, M.; Samoc, M.; Strek, W. Giant enhancement of upconversion in ultra-small Er3+/Yb3+:NaYF4 nanoparticles via laser annealing. Nanotechnology 2012, 23, 145705. [Google Scholar] [CrossRef]
- Wang, H.; Yin, X.; Xing, M.; Fu, Y.; Tian, Y.; Feng, X.; Jiang, T.; Luo, X. Investigation on the thermal effects of NaYF4:Er under 1550 nm irradiation. Phys. Chem. Chem. Phys. 2017, 19, 8465–8470. [Google Scholar] [CrossRef] [PubMed]
- Balabhadra, S.; Debasua, M.L.; Brites, C.D.S.; Ferreira, R.A.S.; Carlos, L.D. A cost-effective quantum yield measurement setup for upconverting nanoparticles. J. Lumin. 2017, 189, 64–70. [Google Scholar] [CrossRef]
- Hest, J.J.H.A.; Blab, G.A.; Gerritsen, H.C.; Mello Donega, C.; Meijerink, A. The role of a phonon bottleneck in relaxation processes for Ln doped NaYF4 nanocrystals. J. Phys. Chem. C 2018, 122, 3985–3993. [Google Scholar] [CrossRef]
- Jones, L.E.A.; Liebermann, R.C. Elastic and thermal properties of fluoride and oxide analogues in the rocksalt, fluorite, rutile and perovskite structures. Phys. Earth Planet. Inter. 1974, 9, 101–107. [Google Scholar] [CrossRef]
- George, N.C.; Pell, A.J.; Dantelle, G.; Page, K.; Llobet, A.; Balasubramanian, M.; Pintacuda, G.; Chmelka, B.F.; Seshadri, R. Local environments of dilute activator ions in the solid-state lighting phosphor Y3–xCexAl5O12. Chem. Mater. 2013, 25, 3979–3995. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Lu, K. Grain-size dependence of thermal properties of nanocrystalline elemental selenium studied by x-ray diffraction. Phys. Rev. B 1997, 56, 14330–14337. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Yan, S. Comment on “Tunable thermal quenching properties of Na3Sc2(PO4)3:Eu2+ phosphors tailored by phase transformation details” by Liu et al., Dalton Trans. 2020, 49, 3915. Dalton Trans. 2020, 49, 11772–11774. [Google Scholar] [CrossRef]
- Yan, S. Luminescence thermal quenching of M2SiO4:Eu2+ (M = Sr, Ba) phosphors. J. Rare Earths 2020, 38, 113–123. [Google Scholar] [CrossRef]
- Yan, S. On the origin of diminishing radiative lifetime of Mn4+ in complex fluoride phosphors with temperature. ECS J. Solid State Sci. Technol. 2021, 10, 086005. [Google Scholar] [CrossRef]
- Yan, S. On the origin of temperature dependence of the emission maxima of Eu2+and Ce3+- activated phosphors. Opt. Mat. 2018, 79, 172–185. [Google Scholar] [CrossRef]
- Yan, S. Comment on “Investigation on anomalous thermal quenching of Mn4+ luminescence in A2XF6:Mn4+” [ECS J. Solid State Sci. Technol. 10, 076007 (2021)]. ECS J. Solid State Sci. Technol. 2021, 10, 120001. [Google Scholar] [CrossRef]
- Terraschke, H.; Wickleder, C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+-Doped Nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef]
- MacDougall, S.K.W.; Ivaturi, A.; Marques-Hueso, J.; Richards, B.S. Measurement procedure for absolute broadband infrared upconversion photoluminescent quantum yields: Correcting for absorption/re-emission. Rev. Sci. Instrum. 2014, 85, 063109. [Google Scholar] [CrossRef]
- Jonesa, C.M.S.; Gakamsky, A.; Marques-Hueso, J. The upconversion quantum yield (UCQY): A review to standardize the measurement methodology, improve comparability, and define efficiency standards. Sci. Technol. Adv. Mater. 2021, 22, 810–848. [Google Scholar] [CrossRef]





| Phosphor | IX | Imax(T)/I(T0) | ∆T (K) | ΔI/ΔT (%·K−1) | Exc. Power (Density), Wavelength * | Size and Architecture ** | Ref. |
|---|---|---|---|---|---|---|---|
| β-NaYF4:20%Yb3+/2%Er3+ | Iintegrated | 2 | 298–358 | 3.33 | 0.178 W·cm−2, 975 nm | 24 nm, core-only | [21] |
| β-NaYF4:18%Yb3+/2%Er3+ | Iintegrated | 2 | 10–150 | 1.4 | 290 W·cm−2, 980 nm | 25 nm, core-only | [41] |
| α-NaYF4:18%Yb3+/2%Er3+ | Iintegrated | <1 | 10–400 | 290 W·cm−2, 980 nm | 6 nm, core-only | ||
| NaYF4:10%Yb3+/0.72%Er3+ | I540nm | ~40% | 10–120 | 3.33 | 700 mW, 980 nm (in organic solvent) | 120 nm length, 50 nm width | [42] |
| β-NaYF4:20%Yb3+/2%Er3+ | Iintegrated | 3 | 303–423 | 2.5 | 1.6 W·cm−2, 980 nm | ~7 nm, core-only | [44] |
| β-NaYF4:20%Yb3+/2%Er3+ | Iintegrated | <1 | 283–353 | 200 mW, 980 nm (in cyclohexane) | 24.1 nm, core-only | [81] | |
| β-NaYF4:20%Yb3+/2%Er3+@NaYF4 | Iintegrated | <1 | 283–353 | 200 mW, 980 nm (in cyclohexane) | 27.2 nm, 28.9 nm, 33.2 nm and 40.2 nm (core/shell) | ||
| α-NaYF4:20%Yb3+/2%Er3+ | I545nm | <1 | 303–483 | 2 W·cm−2, 980 nm | 75 nm, core-only | [83] | |
| α-NaYF4:10%Yb3+/1%Er3+ | Iintegrated | <1 | 84–364 | 980 nm | 40 nm, core-only | [109] | |
| β-NaYF4:20%Yb3+/2%Er3+ | Iintegrated | <1 | 303–523 | 20 W·cm−2, 980 nm | 52 nm, core-only | [110] | |
| β-NaYF4:40%Yb3+/2%Er3+ | I540nm | <1 | 303–483 | 30 mW, 980 nm | 29 nm, core-only | [111] | |
| OA-capped β-NaYF4:Yb3+:Gd3+:Er3+ | I545nm I660nm | <1 | 288–328 | 130 mW, 976 nm (in cyclohexane) | 30.2 nm,core-only | [112] | |
| NaYF4:20%Yb3+/2%Er3+@NaYF4 | Iintegrated | <1 | 160–300 | 0.1 W·cm−2, 980 nm | 30 nm, core/inert shell | [113] | |
| NaYF4:20%Yb3+/2%Er3+@ NaYF4 | Iintegrated | <1 | 313–553 | 980 nm | 23.5 nm, core/inert shell | [114] | |
| β-NaGdF4:20%Yb3+/2%Er3+ | Iintegrated | 3.3 | 298–338 | 8.25 | 0.178 W·cm−2, 975 nm | 7 nm, core-only | [21] |
| β-NaGdF4:20%Yb3+/0.5%Er3+ | Igreen | 11 | 303–393 | 12.22 | 1.0 W, 980 nm | 10 nm, core-only | [25] |
| β-NaGdF4:20%Yb3+/2%Er3+ | Iintegrated | 4 | 303–423 | 3.33 | 1.6 W·cm−2, 980 nm | 19.3 nm, core-only | [49] |
| β-NaGdF4:39%Yb3+/1%Er3+ | Iintegrated | 2.1 | 303–423 | 1.75 | 5.0 W·cm−2, 976 nm | 13.1 nm, core-only | [56] |
| NaGdF4:20%Yb3+/2%Er3+ | Iintegrated | >1 | 483–573 | 980 nm | 11–12 nm, core-only | [63] | |
| ~2.2 | 293–413 | 1.83 | |||||
| NaGdF4:20%Yb3+/2%Er3+@NaYF4 | Iintegrated | 3 | 300–400 | 3 | 1.3 W, 976 nm | 9.0 ± 2.0 nm, core/inert shell | [71] |
| NaGdF4@NaGdF4:20%Ca2+/20%Yb3+/2%Er3+ | Iintegrated | ~10.9 | 293–413 | 9.08 | 980 nm | 11 nm, core/shell | [115] |
| NaGdF4:20%Ca2+/20%Yb3+/2%Er3+ | Iintegrated | ~5.3 | 293–413 | 4.42 | 980 nm | 14 nm, core-only | |
| NaGdF4@ NaGdF4:20%Yb3+/2%Er3+ | Iintegrated | ~3.44 | 293–413 | 2.87 | 980 nm | 10 nm, core/shell | |
| NaGdF4:40%Yb3+@ NaGdF4:60%Yb3+/2%Er3+ | Iintegrated | ~8.24 | 293–413 | 6.87 | 980 nm | 13 nm, core/shell | [46] |
| NaGdF4:20%Yb2%/Er@NaGdF4:20%Yb3+ | Igreen | 6.8 | 303–423 | 5.67 | 1.6 W·cm−2, 975 nm | 14.3 nm, core/active shell | |
| NaGdF4:20%Yb3+/2%Er3+@NaGdF4 | Igreen | <1 | 303–423 | 1.6 W·cm−2, 975 nm | 14.9 nm, core/inert shell | [47] | |
| NaGdF4:20%Yb3+/2%Er3+@NaGdF4 | Igreen | 7.7 | 303–423 | 6.42 | 1.6 W·cm−2, 975 nm | 8 nm, core/inert shell | |
| NaGdF4:20%Yb3+/2%Er3+@NaGdF4 | Ired | 5.1 | 303–423 | 4.25 | 1.6 W·cm−2, 975 nm | 16.6 nm, core/inert shell | [47] |
| Iintegrated | <1 | ||||||
| NaGdF4:20%Yb3+/2%Er3+@NaYbF4 | Igreen | 13 | 303–423 | 10.8 | 1.2 W·cm−2, 975 nm | ~7.3 nm core/active shell | [52] |
| OA-capped NaYbF4:2%Er3+@NaLuF4:25%Yb3+ | I542nm | 4.74 | 298– 473 | 2.71 | 36 W·cm−2, 980 nm | ~40 nm, core/inert shell | [59] |
| β-NaGdF4:20%Yb3+/2%Er3+@NaGdF4 | Iintegrated | <1 | 303–423 | 1.6 W·cm−2, 980 nm | ~14 nm, core/inert shell | [44] | |
| NaGdF4@NaGdF4:20%Yb3+/2% Er3+ | Iintegrated | 7 | 303–423 | 5.83 | 1.1 W·cm−2, 980 nm | ~12 nm (~9@3 nm) inert-core/active-shell | [58] |
| β-NaYF4:49%Yb3+/1%Tm3+ | I475nm I450nm | 300 2000 | 300–453 300–453 | 196.1 1307.2 | 10 W·cm−2, 980 nm 10 W·cm−2, 980 nm | ~10 nm, core-only | [27] |
| NaYF4:20%Yb3+/1%Tm3+ | Iintegrated | 2.9 | 298–398 | 2.9 | 975 nm | ~22.1 nm, core-only | [48] |
| NaYF4:18%Yb3+/2%Tm3+ | I475nm | 16 | 300–453 | 10.45 | 0.5 W·cm−2, 980 nm | 42 nm, core-only | [116] |
| I800nm | <1 | 80–298 | 1.2 W·cm−2, 980 nm | 6.1 nm, core-only | |||
| NaGdF4:20%Yb3+/0.2%Tm3+ | I800nm | 9 | 303–423 | 7.5 | 1.0 W, 980 nm | 10 nm, core-only | [25] |
| NaGdF4:20%Yb3+/0.5%Tm3+ | I475nm | 24.2 | 343–463 | 201.6 | 980 nm | 11.6 ± 1.6 nm, core-only | [24] |
| β-NaGdF4:20%Yb3+/2%Tm3+ | Iintegrated | 4 | 303–423 | 3.33 | 1.6 W·cm−2, 980 nm | ~7 nm, core-only | [44] |
| NaGdF4:20%Yb 3+/1%Tm3+ | Iintegrated | 6.2 | 298–398 | 6.2 | 975 nm | 8.4 nm, core-only | [48] |
| NaGdF4:20%Yb 3+/1%Tm3+ | I475nm | 4.5 | 303–423 | 3.75 | 975 nm | ~8.5 nm, core-only | [50] |
| β-NaGdF4:39%Yb3+/1%Tm3+ | Iintegrated | 17 | 303–453 | 11.33 | 5.0 W·cm−2, 976 nm | 12.5 nm, core-only | [56] |
| NaGdF4:20%Yb3+/1%Tm3+ | Iintegrated | 10 | 303–423 | 8.33 | 3.4 W·cm−2, 980 nm | 9.5 nm, core-only | [51] |
| I475nm | 25 | 298–423 | 20 | 4.7 W·cm−2, 980 nm | |||
| NaGdF4:30%Yb3+/5%Tm3+@NaYF4 | I476nm | 8.3 | 300–415 | 7.22 | 1.3 W, 976 nm | 6.6 ± 0.8 nm, core/inert shell | [71] |
| NaGdF4@NaGdF4:20%Yb3+/1%Tm3+ | Iintegral | 29 | 303–423 | 24.17 | 1.1 W·cm−2, 980 nm | ~12 nm ~9@3 nm, inert-core/active-shell | [58] |
| NaGdF4:20%Yb3+/1%Tm3+ | Iintegrated | 11 | 303–423 | 9.17 | 1.1 W·cm−2, 980 nm | ~12 nm, core-only | [27] |
| OA-capped β-NaYF4: 49%Yb3+/1%Ho3+ | I545nm | 7 | 303–403 | 7 | 0.5 W·cm−2, 980 nm | 30 nm, core-only | |
| NaGdF4:20%Yb3+/2%Ho3+ | Iintegrated | 12.6 | 303–423 | 10.5 | 1.6 W·cm−2, 980 nm | ~7 nm, core-only | [44] |
| NaYF4:2%Ho3+/20%Yb3+@NaYF4:40%Yb3+ | I540nm | 3 | 300–450 | 2.0 | 0.56 W·cm−2, 980 nm | 42.7 ± 2.3 nm, core/active shell | [55] |
| NaGdF4:20%Yb3+/1%Ho3+ | I540nm | 52 | 298–423 | 41.6 | 4.7 W·cm−2, 970 nm | 9.5 nm, core-only | [51] |
| NaGdF4:20%Yb3+/2%Ho3+ | Iintegrated | 52.1 | 298–398 | 52.1 | 975 nm | 7.5 nm, core-only | [48] |
| β-NaGdF4:30%Ce3+/20%Yb3+/2%Ho3+ | Iintegrated | 7.5 | 298–398 | 7.5 | 975 nm | 24.2 nm, core-only | [45] |
| Igreen | 10.2 | 303–423 | 8.5 | 1.6 W·cm−2, 975 nm | 11.2 nm, core-only | ||
| β-NaYF4:20%Yb3+/6%Nd3+ | I803nm | 136 | 298–433 | 100.74 | 0.13 W·cm−2, 980 nm | 25 nm, core-only | [60] |
| β-NaYF4:20%Yb3+/6%Nd3+ | I803nm | 55 | 298–413 | 47.82 | 1 W·cm−2, 980 nm | 20 nm, core-only | |
| β-NaYF4:20%Yb3+/6%Nd3+@NaYF4 | I803nm | 13 | 298–413 | 11.30 | 1 W·cm−2, 980 nm | 30 nm, core/inert shell | |
| β-NaYF4:20%Yb3+/2%Nd3+ | Iintegrated | 1.8 | 297–420 | 1.46 | 10 W·cm−2, 980 nm | 20 nm, core-only | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S. On the Origin of Thermally Enhanced Upconversion Luminescence in Lanthanide-Doped Nanosized Fluoride Phosphors. Materials 2025, 18, 2700. https://doi.org/10.3390/ma18122700
Yan S. On the Origin of Thermally Enhanced Upconversion Luminescence in Lanthanide-Doped Nanosized Fluoride Phosphors. Materials. 2025; 18(12):2700. https://doi.org/10.3390/ma18122700
Chicago/Turabian StyleYan, Shirun. 2025. "On the Origin of Thermally Enhanced Upconversion Luminescence in Lanthanide-Doped Nanosized Fluoride Phosphors" Materials 18, no. 12: 2700. https://doi.org/10.3390/ma18122700
APA StyleYan, S. (2025). On the Origin of Thermally Enhanced Upconversion Luminescence in Lanthanide-Doped Nanosized Fluoride Phosphors. Materials, 18(12), 2700. https://doi.org/10.3390/ma18122700






