High-Efficiency Deep-Blue Solution-Processed OLED Devices Enabled by New Dopant Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
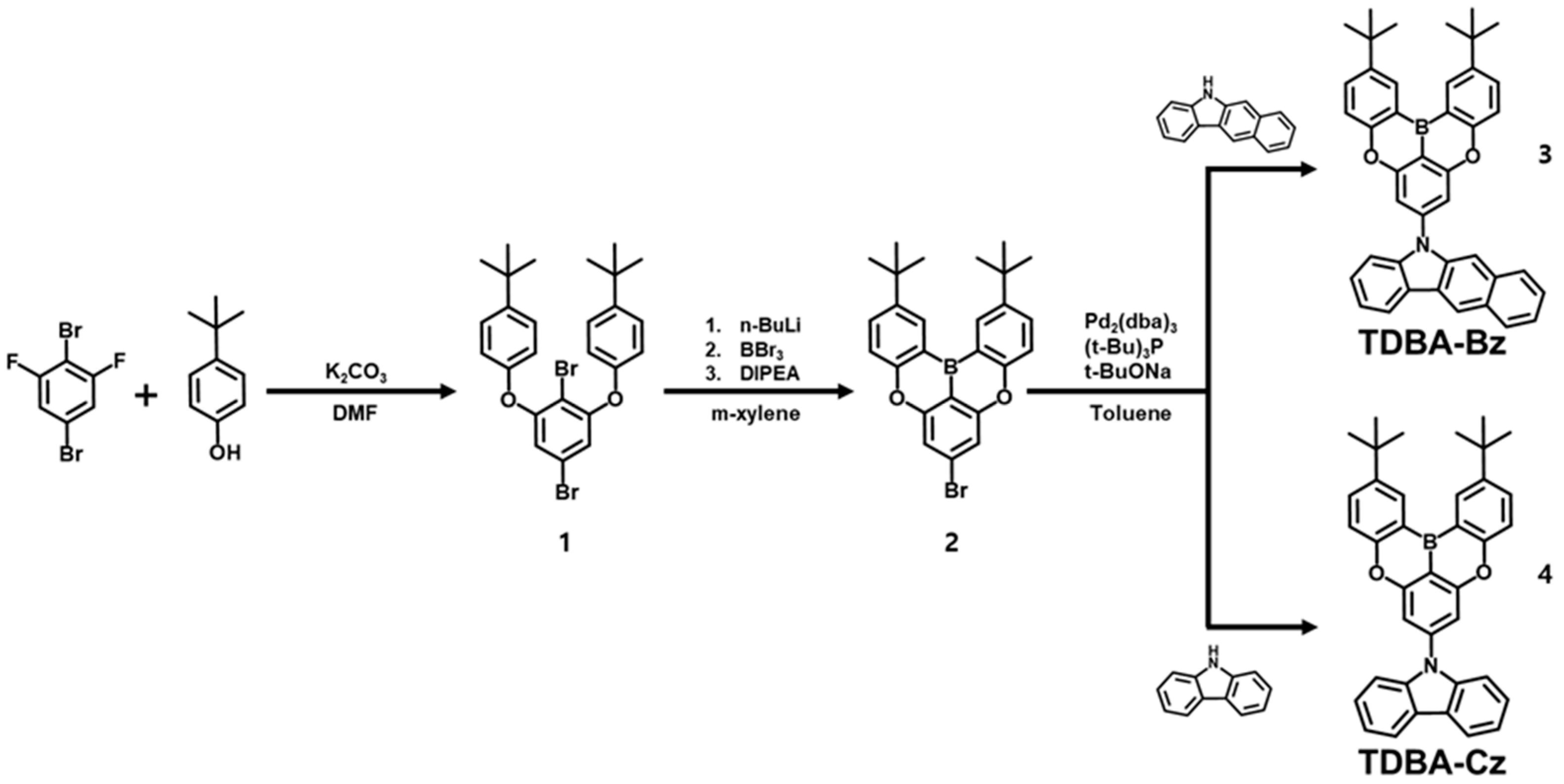
2.1.1. Synthesis of 4,4′-((2,5-Dibromo-1,3-phenylene)bis(oxy))bis(tert-butylbenzene) (1)
2.1.2. Synthesis of 7-Bromo-2,12-di-tert-butyl-5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (2)
2.1.3. Synthesis of 5-(2,12-Di-tert-butyl-5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracen-7-yl)-5H-benzo[b]carbazole (TDBA-Bz) (3)
2.1.4. Synthesis of 9-(2,12-Di-tert-butyl-5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracen-7-yl)-9H-carbazole (TDBA-Cz) (4)
3. Results and Discussion
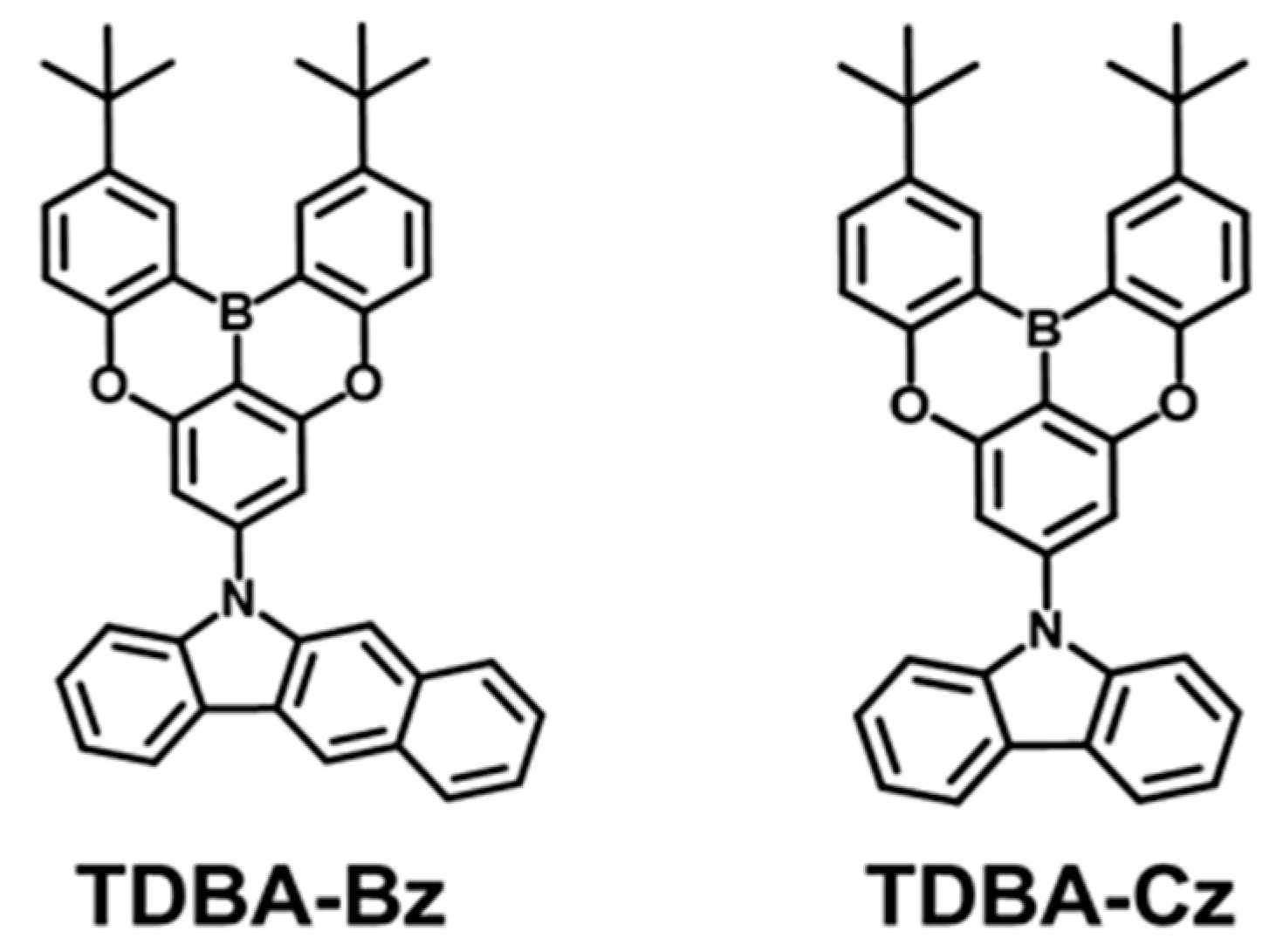
3.1. Molecular Design, Synthesis, and Characterization
3.2. Photophysical Properties
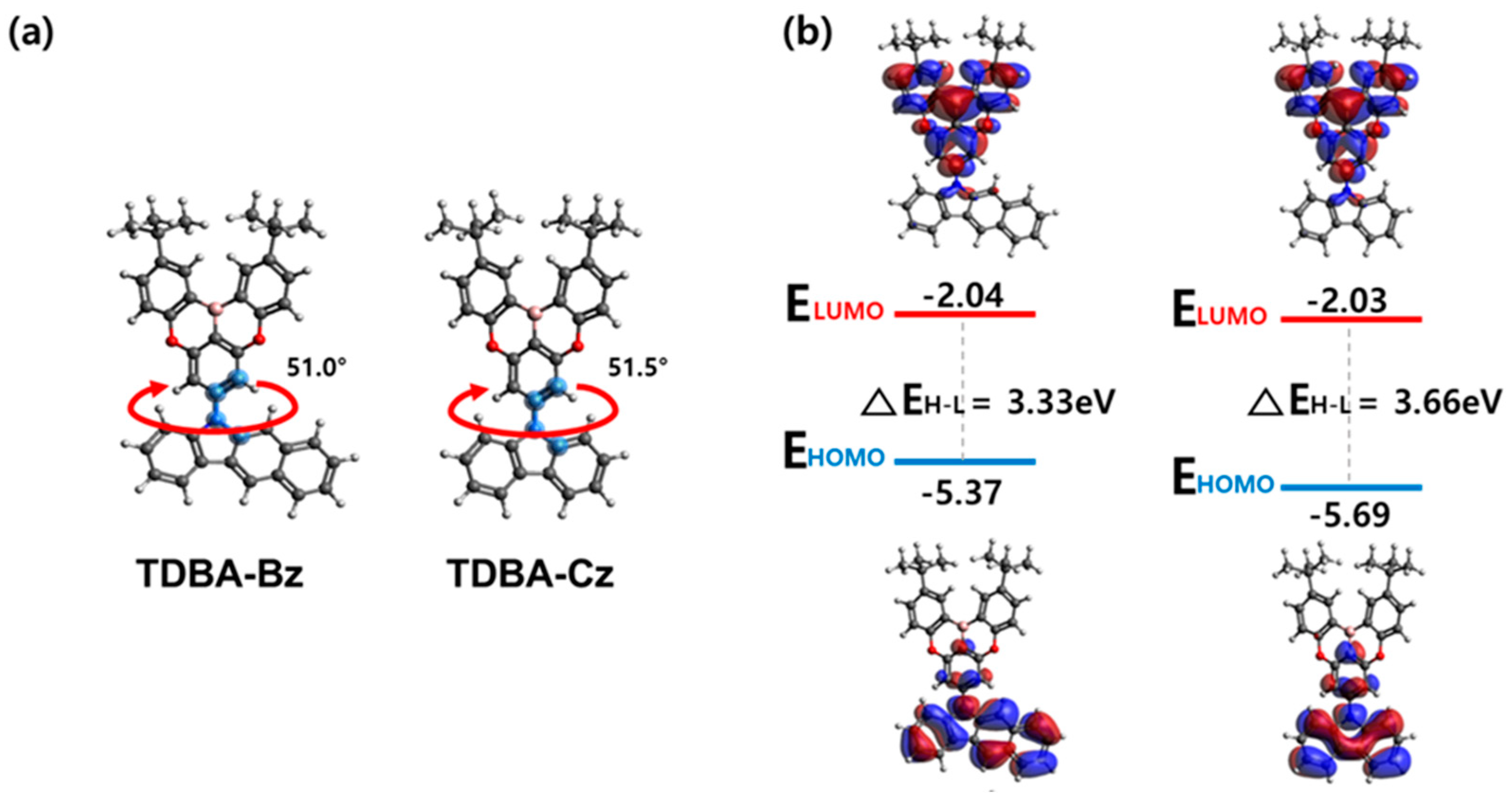
3.3. Theoretical Calculation
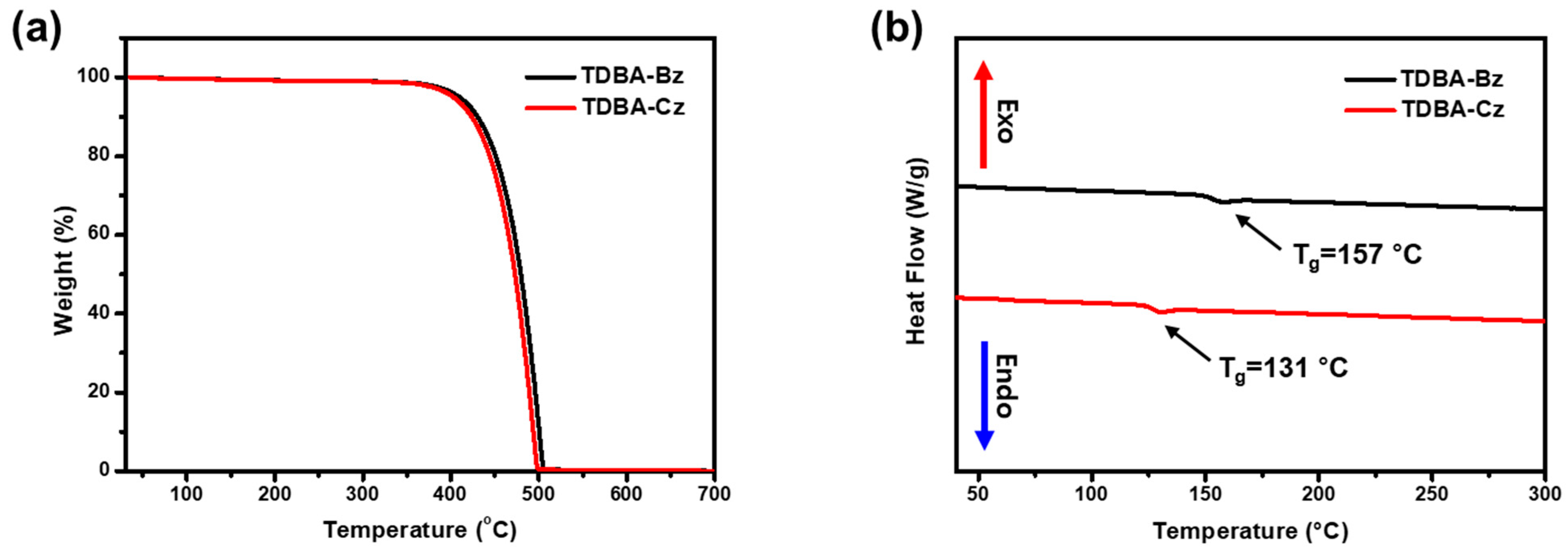
3.4. Thermal Properties
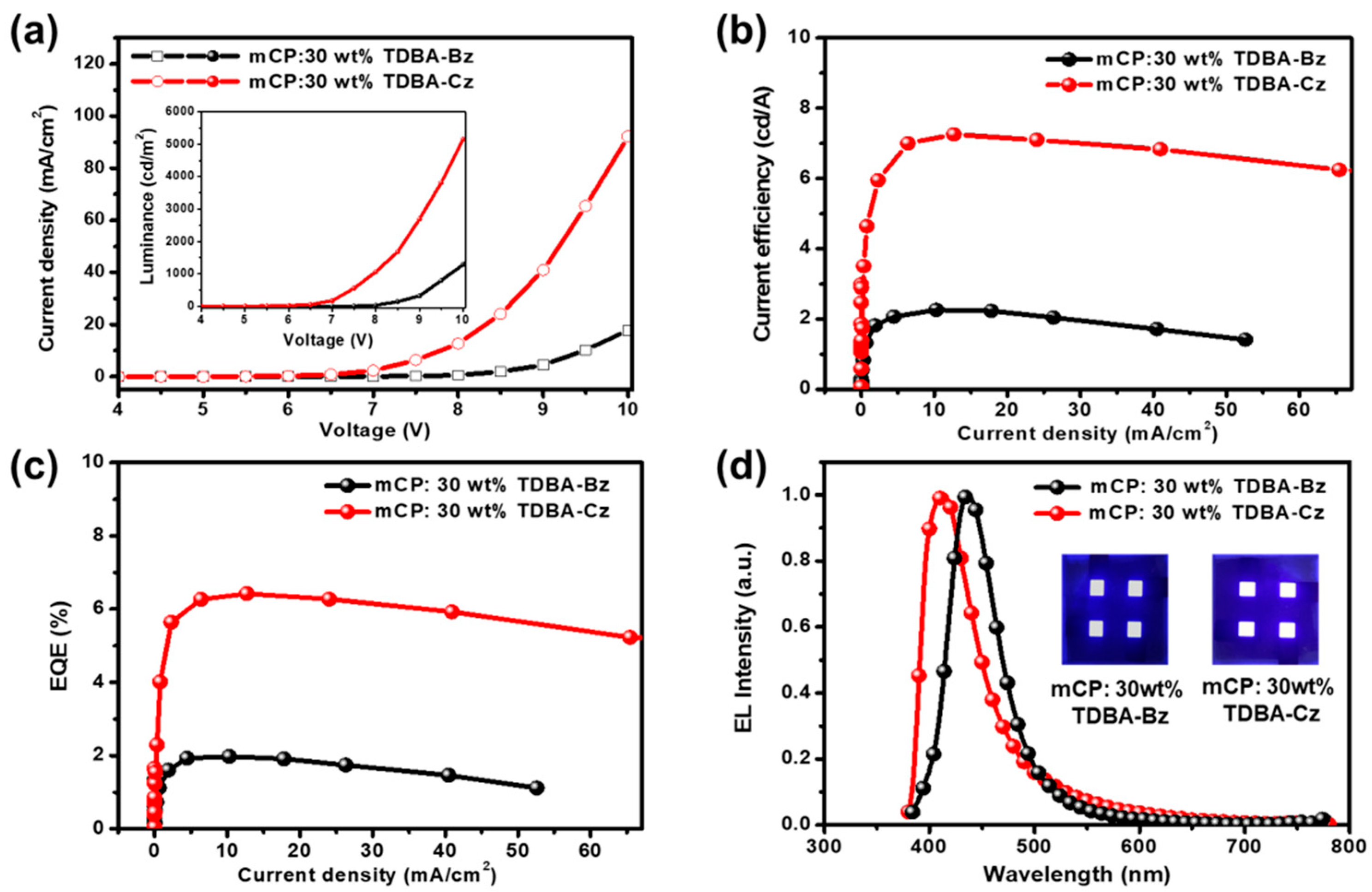
3.5. Electroluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konidena, R.K.; Oh, S.; Kang, S.; Park, S.S.; Lee, H.; Park, J. Indolo[3,2,1-jk]carbazole-Derived Narrowband Violet-Blue Fluorophores: Tuning the Optical and Electroluminescence Properties by Chromophore Juggling. J. Org. Chem. 2022, 87, 6668–6679. [Google Scholar] [CrossRef]
- Mamada, M.; Aoyama, A.; Uchida, R.; Ochi, J.; Oda, S.; Kondo, Y.; Kondo, M.; Hatakeyama, T. Efficient Deep-Blue Multiple-Resonance Emitters Based on Azepine-Decorated ν-DABNA for CIE below 0.06. Adv. Mater. 2024, 36, 2402905. [Google Scholar] [CrossRef]
- Ochi, J.; Yamasaki, Y.; Tanaka, K.; Kondo, Y.; Isayama, K.; Oda, S.; Kondo, M.; Hatakeyama, T. Highly efficient multi-resonance thermally activated delayed fluorescence material toward a BT.2020 deep-blue emitter. Nat. Commun. 2024, 15, 2361. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Fu, X.-F.; Zhang, D.-H.; Sun, Y.-F.; Lu, X.; Lin, F.-L.; Meng, L.; Chen, X.-L.; Lu, C.-Z. Thermally Activated Delayed Fluorescence with Nanosecond Emission Lifetimes and Minor Concentration Quenching: Achieving High-Performance Nondoped and Doped Blue OLEDs. Adv. Mater. 2024, 36, 2401724. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kang, M.J.; Lee, H.J.; Park, J.Y.; Kwak, H.; Park, C.Y.; Ahn, H.J.; Kim, J.-Y.; Baek, J.-H.; Kim, H.Y.; et al. Enhancing Narrowband Blue TADF OLED Performance with Adamantane Group-Integrated Spatially Hindered 1,3-Bis(N-Carbazolyl)Benzene-Based Host. Adv. Funct. Mater. 2024, 34, 2408491. [Google Scholar] [CrossRef]
- Kuang, C.; Li, S.; Murtaza, I.; Meng, Z.; Li, H.; Zhang, X.; Wu, C.; Tong, K.-N.; Shang, Y.; He, Y.; et al. Enhanced Horizontal Dipole Orientation by Novel Penta-Helicene Anthracene-Based Host for Efficient Blue Fluorescent OLEDs. Small 2024, 20, 2311114. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Liang, H.-C.; Chen, C.-H.; Chiu, C.-H.; Huang, L.-C.; Lee, Y.-T.; Dzeng, Y.-C.; Chen, C.; Lin, B.-Y.; Lee, J.-H.; et al. High efficiency in blue TADF OLED using favorable horizontal oriented host. Chem. Eng. J. 2024, 498, 155553. [Google Scholar] [CrossRef]
- Dhineshkumar, E.; Arumugam, N.; Manikandan, E.; Maaza, M.; Mandal, A. Fabrication of high performance based deep-blue OLED with benzodioxin-6-amine-styryl-triphenylamine and carbazole hosts as electroluminescent materials. Sci. Rep. 2024, 14, 2432. [Google Scholar] [CrossRef]
- Jeon, S.O.; Lee, K.H.; Kim, J.S.; Ihn, S.-G.; Chung, Y.S.; Kim, J.W.; Lee, H.; Kim, S.; Choi, H.; Lee, J.Y. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photonics 2021, 15, 208–215. [Google Scholar] [CrossRef]
- Lv, J.; Song, S.; Li, J.; Peng, L.; Li, Y.; Liu, Y.; Ma, D.; Ying, S.; Yan, S. High and Balanced Bipolar-Transporting Deep-Blue HLCT Material for Efficient Monochrome and White OLEDs based on a Simple Phenanthroimidazole-Dibenzothiophene Derivative. Adv. Opt. Mater. 2024, 12, 2301413. [Google Scholar] [CrossRef]
- Peethani, N.; Kwon, N.Y.; Koh, C.W.; Park, S.H.; Ha, J.M.; Cho, M.J.; Woo, H.Y.; Park, S.; Choi, D.H. Rational Design of a TADF Emitter with Steric Shielding and Multiple Resonance for Narrowband Solution-Processed OLEDs. Adv. Opt. Mater. 2024, 12, 2301217. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.Y.; Koh, C.W.; Park, J.Y.; Kang, M.J.; Kwak, H.; Park, C.Y.; Park, S.; Cho, M.J.; Choi, D.H. Eco-friendly solution-processed narrowband OLEDs using non-halogenated aliphatic solvent systems. Chem. Eng. J. 2024, 481, 148484. [Google Scholar] [CrossRef]
- Park, C.Y.; Park, S.H.; Kwon, N.Y.; Park, J.Y.; Kang, M.J.; Kwak, H.; Son, J.H.; Woo, H.Y.; Hong, C.S.; Cho, M.J.; et al. Polymer Hosts Containing Carbazole-Dibenzothiophene-Based Pendants for Application in High-Performance Solution-Processed TADF-OLEDs. ACS Appl. Mater. Interfaces 2024, 16, 45242–45251. [Google Scholar] [CrossRef]
- Niikura, H.; Légaré, F.; Hasbani, R.; Ivanov, M.Y.; Villeneuve, D.M.; Corkum, P.B. Probing molecular dynamics with attosecond resolution using correlated wave packet pairs. Nature 2003, 421, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Chen, Z.; Wang, W.; Wang, Q.; Chen, S.; Xu, Y.; Wong, W.-Y. Efficient deep red and NIR OLEDs based on Ir(III) complexes fabricated by evaporation and solution processing. Chem. Eng. J. 2023, 451, 138632. [Google Scholar] [CrossRef]
- Li, B.; Lou, J.; Zhang, B.; Liu, L.; He, X.; Xu, H.; Feng, X.; Zhang, H.; Wang, Z.; Tang, B.Z. Modulating electronic confinement and structural distortion of multiple resonance emitters enables high-performance ultrapure blue OLED. Chem. Eng. J. 2024, 482, 148876. [Google Scholar] [CrossRef]
- Cai, X.; Pan, Y.; Li, C.; Li, L.; Pu, Y.; Wu, Y.; Wang, Y. Nitrogen-Embedding Strategy for Short-Range Charge Transfer Excited States and Efficient Narrowband Deep-Blue Organic Light Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2024, 63, e202408522. [Google Scholar] [CrossRef]
- Chen, B.; Liu, B.; Zeng, J.; Nie, H.; Xiong, Y.; Zou, J.; Ning, H.; Wang, Z.; Zhao, Z.; Tang, B.Z. Efficient Bipolar Blue AIEgens for High-Performance Nondoped Blue OLEDs and Hybrid White OLEDs. Adv. Funct. Mater. 2018, 28, 1803369. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, S.; Zhang, Y.; Fan, S.; Zhao, X.; Liu, H.; Dong, X.; Wang, S.; Li, X. Achieving non-doped deep-blue OLEDs by applying bipolar imidazole derivatives. Org. Electron. 2019, 69, 289–296. [Google Scholar] [CrossRef]
- Xu, J.; Liu, H.; Li, J.; Zhao, Z.; Tang, B.Z. Multifunctional Bipolar Materials Serving as Emitters for Efficient Deep-Blue Fluorescent OLEDs and as Hosts for Phosphorescent and White OLEDs. Adv. Opt. Mater. 2021, 9, 2001840. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, D.; Wang, F.; Zhang, S. Bipolar host materials for high-efficiency blue phosphorescent and delayed-fluorescence OLEDs. J. Mater. Chem. C 2015, 3, 12529–12538. [Google Scholar] [CrossRef]
- Chen, J.; Shi, C.; Fu, Q.; Zhao, F.; Hu, Y.; Feng, Y.; Ma, D. Solution-processable small molecules as efficient universal bipolar host for blue, green and red phosphorescent inverted OLEDs. J. Mater. Chem. 2012, 22, 5164–5170. [Google Scholar] [CrossRef]
- Woo, J.Y.; Park, M.-H.; Jeong, S.-H.; Kim, Y.-H.; Kim, B.; Lee, T.-W.; Han, T.-H. Advances in Solution-Processed OLEDs and their Prospects for Use in Displays. Adv. Mater. 2023, 35, 2207454. [Google Scholar] [CrossRef]
- Hwang, J.; Koh, C.W.; Ha, J.M.; Woo, H.Y.; Park, S.; Cho, M.J.; Choi, D.H. Aryl-Annulated [3,2-a] Carbazole-Based Deep-Blue Soluble Emitters for High-Efficiency Solution-Processed Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes with CIEy <0.1. ACS Appl. Mater. Interfaces 2021, 13, 61454–61462. [Google Scholar] [CrossRef]
- Jiang, K.; Chang, X.; Zhu, J.; Zhu, T.; Yu, J.; Wang, Y.; Zhang, Y.; Ma, D.; Zhu, W. High-Performance Solution-Processable Organic Light-Emitting Diode Based on a Narrowband Near-Ultraviolet Emitter and a Hot Exciton Strategy. Angew. Chem. Int. Ed. 2025, 64, e202421520. [Google Scholar] [CrossRef]
- Pan, J.-H.; Chiu, H.-L.; Wang, B.-C. Theoretical investigation of carbazole derivatives as hole-transporting materials in OLEDs. J. Mol. Struct. THEOCHEM 2005, 725, 89–95. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, H.W.; Ko, I.J.; Qiong, W.; Nguyen, Q.P.B.; Jayashantha, P.G.S.; Kwon, J.H.; Chai, K.Y. Thermally stable efficient hole transporting materials based on carbazole and triphenylamine core for red phosphorescent OLEDs. Org. Electron. 2017, 51, 463–470. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, S.U.; Tak, S.H.; Joung, K.S.; Yu, J.-W. Effect of arylamino-carbazole containing hole transport materials on the device performance and lifetime of OLED. Org. Electron. 2022, 100, 106394. [Google Scholar] [CrossRef]
- Sun, J.; Ahn, H.; Kang, S.; Ko, S.-B.; Song, D.; Um, H.A.; Kim, S.; Lee, Y.; Jeon, P.; Hwang, S.-H.; et al. Exceptionally stable blue phosphorescent organic light-emitting diodes. Nat. Photonics 2022, 16, 212–218. [Google Scholar] [CrossRef]
- Weerasinghe, R.W.; Madayanad Suresh, S.; Hall, D.; Matulaitis, T.; Slawin, A.M.Z.; Warriner, S.; Lee, Y.-T.; Chan, C.-Y.; Tsuchiya, Y.; Zysman-Colman, E.; et al. A Boron, Nitrogen, and Oxygen Doped π-Extended Helical Pure Blue Multiresonant Thermally Activated Delayed Fluorescent Emitter for Organic Light Emitting Diodes That Shows Fast kRISC Without the Use of Heavy Atoms. Adv. Mater. 2024, 36, 2402289. [Google Scholar] [CrossRef]
- Ha, T.H.; Kang, S.W.; Lee, C.W. Highly reduced efficiency roll-off with 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracen-containing unit on bipolar host for improved OLEDs device lifespan. Org. Electron. 2024, 124, 106960. [Google Scholar] [CrossRef]
- Park, J.; Moon, J.; Jo, U.; Han, S.; Lee, D.R.; Ahn, H.J.; Kim, J.Y.; Baek, J.-H.; Lee, J.Y. Boron- and Silane-Based Electron Transport–Type Host Materials for Long-Lifetime Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Opt. Mater. 2024, 12, 2302791. [Google Scholar] [CrossRef]
- Tan, H.-J.; Yang, G.-X.; Deng, Y.-L.; Cao, C.; Tan, J.-H.; Zhu, Z.-L.; Chen, W.-C.; Xiong, Y.; Jian, J.-X.; Lee, C.-S.; et al. Deep-Blue OLEDs with Rec.2020 Blue Gamut Compliance and EQE Over 22% Achieved by Conformation Engineering. Adv. Mater. 2022, 34, 2200537. [Google Scholar] [CrossRef]
- Park, D.; Kang, S.; Ryoo, C.H.; Jhun, B.H.; Jung, S.; Le, T.N.; Suh, M.C.; Lee, J.; Jun, M.E.; Chu, C.; et al. High-performance blue OLED using multiresonance thermally activated delayed fluorescence host materials containing silicon atoms. Nat. Commun. 2023, 14, 5589. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, S.; Jo, U.; Kim, S.C.; Lee, D.R.; Ahn, H.J.; Kim, J.Y.; Baek, J.-H.; Lee, J.Y. Boron-based thermally activated delayed fluorescence host materials as universal hosts for blue phosphorescent organic light-emitting diodes. Mater. Today 2024, 75, 27–36. [Google Scholar] [CrossRef]
- Matsuoka, K.; Albrecht, K.; Yamamoto, K.; Fujita, K. Mulifunctional Dendritic Emitter: Aggregation-Induced Emission Enhanced, Thermally Activated Delayed Fluorescent Material for Solution-Processed Multilayered Organic Light-Emitting Diodes. Sci. Rep. 2017, 7, 41780. [Google Scholar] [CrossRef]
- Kreiza, G.; Banevičius, D.; Jovaišaitė, J.; Juršėnas, S.; Javorskis, T.; Vaitkevičius, V.; Orentas, E.; Kazlauskas, K. Realization of deep-blue TADF in sterically controlled naphthyridines for vacuum- and solution-processed OLEDs. J. Mater. Chem. C 2020, 8, 8560–8566. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Miao, J.; Qiu, Y.; Xie, Z.; Yang, C. Narrowband blue emission with insensitivity to the doping concentration from an oxygen-bridged triarylboron-based TADF emitter: Nondoped OLEDs with a high external quantum efficiency up to 21.4. Chem. Sci. 2022, 13, 3402–3408. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; de Sa Pereira, D.; Oh, C.S.; Kukhta, N.; Lee, H.L.; Lee, J.Y.; Monkman, A.P. Influence of Multiple rISC Channels on the Maximum Efficiency and Roll-Off of TADF OLEDs. J. Phys. Chem. C 2024, 128, 16308–16319. [Google Scholar] [CrossRef]
- Hu, Y.X.; Miao, J.; Hua, T.; Huang, Z.; Qi, Y.; Zou, Y.; Qiu, Y.; Xia, H.; Liu, H.; Cao, X.; et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat. Photonics 2022, 16, 803–810. [Google Scholar] [CrossRef]
- Lim, H.; Cheon, H.J.; Woo, S.-J.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Highly Efficient Deep-Blue OLEDs using a TADF Emitter with a Narrow Emission Spectrum and High Horizontal Emitting Dipole Ratio. Adv. Mater. 2020, 32, 2004083. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, I.R.; Scholz, S.; Hermenau, M.; Tietze, M.L.; Schwab, T.; Hofmann, S.; Gather, M.C.; Leo, K. Impact of temperature on the efficiency of organic light emitting diodes. Org. Electron. 2015, 26, 158–163. [Google Scholar] [CrossRef]
- Braveenth, R.; Lee, H.; Park, J.D.; Yang, K.J.; Hwang, S.J.; Naveen, K.R.; Lampande, R.; Kwon, J.H. Achieving Narrow FWHM and High EQE Over 38% in Blue OLEDs Using Rigid Heteroatom-Based Deep Blue TADF Sensitized Host. Adv. Funct. Mater. 2021, 31, 2105805. [Google Scholar] [CrossRef]
- Jin, P.; Yang, X.; Su, W.-T.; Zhan, S.-H.; Chen, X.; Sun, H.; Yang, B.; Su, S.-J.; Hu, J.-Y. Narrow emission band ultraviolet/deep-blue thermally activated delayed fluorescence emitters modified with carbazole/carboline as a donor. J. Mater. Chem. C 2024, 12, 19498–19505. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.-S.; Kim, S.; Jeong, H.; Koo, K.-K.; Park, J. New Anthracene Derivative Including t-Butyl Group as Blue Emitter in Solution Process Organic Light-Emitting Diode. J. Nanosci. Nanotechnol. 2016, 16, 10923–10926. [Google Scholar] [CrossRef]
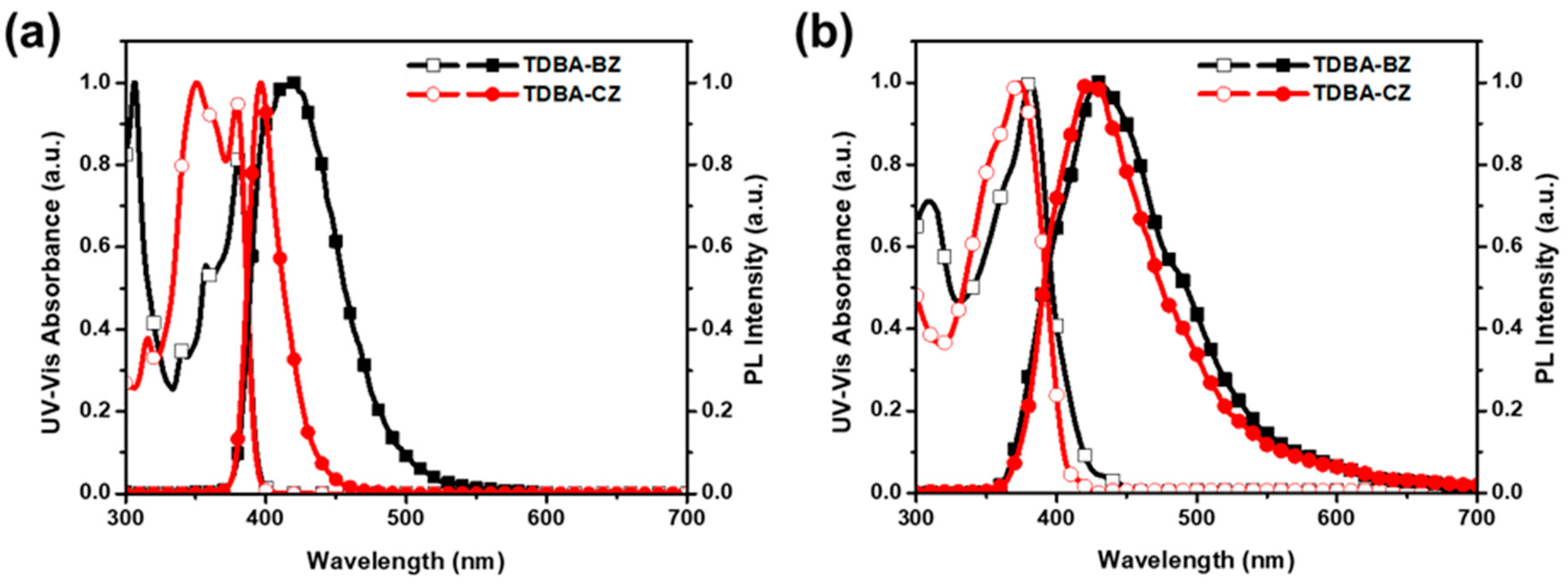
| Compound | Solution a | Film b | PLQY a,b (%) | krad c (107 s−1) | knr c (107 s−1) | krad/knr | ||
|---|---|---|---|---|---|---|---|---|
| UVmax (nm) | PLmax (FWHM) (nm) | UVmax (nm) | PLmax (FWHM) (nm) | |||||
| TDBA-BZ | 306, 340, 357, 381 | 420 (67) | 309, 381 | 429 (100) | 31/12 | 3.78 | 5.37 | 0.70 |
| TDBA-CZ | 315, 350, 379 | 396 (28) | 373 | 422 (84) | 69/11 | 5.45 | 2.77 | 1.98 |
| Compound | ES/ET a (eV) | ΔEST b (eV) | HOMO c (eV) | LUMO (eV) | Band Gap (eV) |
|---|---|---|---|---|---|
| TDBA-BZ | 3.25/3.03 | 0.22 | −5.69 | −2.78 | 2.91 |
| TDBA-CZ | 3.28/3.20 | 0.08 | −5.79 | −2.75 | 3.04 |
| EML | Von a (V) | CE b (cd/A) | EQE c (%) | CIE (x, y) d | ELmax (nm) | FWHM (nm) | ||
|---|---|---|---|---|---|---|---|---|
| Max | 2000 nit | Max | 2000 nit | |||||
| mCP: 30 wt% TDBA-BZ | 6.79 | 2.26 | 1.96 | 1.98 | 1.69 | (0.181, 0.114) | 436 | 55 |
| mCP: 30 wt% TPDBA-CZ | 6.82 | 7.25 | 6.98 | 6.45 | 6.14 | (0.167, 0.086) | 413 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Kwon, H.; Park, S.; Kang, S.; Kim, S.-T.; Lee, K.; Lee, H.; Park, J. High-Efficiency Deep-Blue Solution-Processed OLED Devices Enabled by New Dopant Materials. Materials 2025, 18, 2213. https://doi.org/10.3390/ma18102213
Oh S, Kwon H, Park S, Kang S, Kim S-T, Lee K, Lee H, Park J. High-Efficiency Deep-Blue Solution-Processed OLED Devices Enabled by New Dopant Materials. Materials. 2025; 18(10):2213. https://doi.org/10.3390/ma18102213
Chicago/Turabian StyleOh, Saeyoung, Hyukmin Kwon, Sangwook Park, Seokwoo Kang, Sang-Tae Kim, Kiho Lee, Hayoon Lee, and Jongwook Park. 2025. "High-Efficiency Deep-Blue Solution-Processed OLED Devices Enabled by New Dopant Materials" Materials 18, no. 10: 2213. https://doi.org/10.3390/ma18102213
APA StyleOh, S., Kwon, H., Park, S., Kang, S., Kim, S.-T., Lee, K., Lee, H., & Park, J. (2025). High-Efficiency Deep-Blue Solution-Processed OLED Devices Enabled by New Dopant Materials. Materials, 18(10), 2213. https://doi.org/10.3390/ma18102213







