Selenium-Containing Multi-Resonance Thermally Activated Delayed Fluorescence Host Material for Green and Red Phosphorescent OLEDs
Highlights
- A new selenium-containing MR-TADF host material, TDBA-SePh, was synthesized.
- TDBA-SePh enhanced SOC and accelerated RISC.
- The green and red devices exhibited high EQE and low roll-off values of 2.5% and 4.3% at 1000 cd m−2.
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 1-Bromo-3,5-bis-(4-tert-butyl-phenoxy)-benzene (1)
2.1.2. Synthesis of 2-[3,5-Bis-(4-tert-butyl-phenoxy)-phenyl]-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (2)
2.1.3. Synthesis of 2′-Bromo-3,5-bis-(4-tert-butyl-phenoxy)-biphenyl (3)
2.1.4. Synthesis of (3′,5′-Bis(4-(tert-butyl)phenoxy)-[1,1′-biphenyl]-2-yl)boronic acid (4)
2.1.5. Synthesis of 2,4-Bis-(4-tert-butyl-phenoxy)-dibenzoselenophene (5)
2.1.6. Synthesis of 3,6-di-tert-butyl-9,16-dioxa-15-selena-4b-boraindeno[2,1-a]naphtho[3,2,1-de]anthracene (TDBA-SePh)
3. Results and Discussion
3.1. Molecular Design, Synthesis, and Optical Properties
3.2. Theoretical Calculation
3.3. Thermal Properties
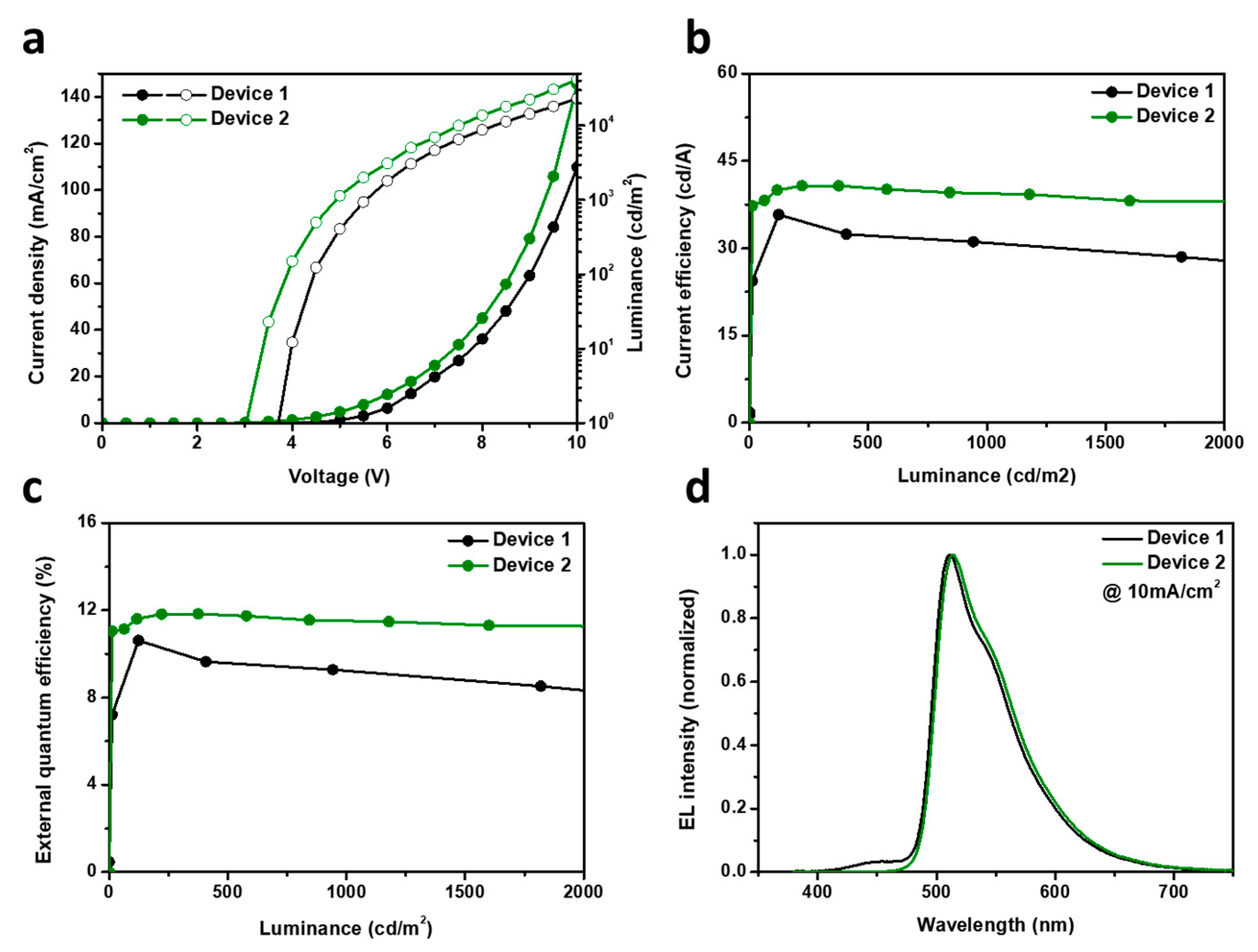
3.4. Electroluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuhrmann, T.; Salbeck, J. Organic Materials for Photonic Devices. MRS Bull. 2003, 28, 354–359. [Google Scholar] [CrossRef]
- Huang, J.; Sua, J.-H.; Tian, H. The development of anthracene derivatives for organic light-emitting diodes. J. Mater. Chem. C 2012, 22, 10977–10989. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Forrest, S.R. White Organic Light-Emitting Devices for Solid-State Lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Guo, S.; Jin, X.; Zhang, D.; Zhou, H.; Wang, G.; Miao, Y.; Huang, J.; Zhang, Z.; Wang, H.; Su, J. Phenanthrene-based deep-blue fluorophores with balanced carrier transport ability for high-performance OLEDs with a CIEy < 0.04. J. Mater. Chem. C 2022, 10, 14711–14721. [Google Scholar]
- Kang, S.; Kwon, H.; Pu, Y.-J.; Park, J. High-efficiency deep-blue emitter consisting of a chrysene core and optimized side groups. Mater. Today Energy 2021, 21, 100706. [Google Scholar] [CrossRef]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-Based Materials for OLED Applications. Molecules 2017, 22, 1522. [Google Scholar] [CrossRef]
- Park, S.; Kwon, H.; Park, S.; Oh, S.; Lee, K.; Lee, H.; Kang, S.; Park, D.; Park, J. New Bipolar Host Materials Based on Indolocarbazole for Red Phosphorescent OLEDs. Materials 2024, 17, 4347. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.L.; Thilagavathy, S. Phosphorescent organic light-emitting devices: Iridium-based emitter materials—An overview. Coord. Chem. Rev. 2023, 483, 215100. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Symalla, F.; Heidrich, S.; Friederich, P.; Strunk, T.; Neumann, T.; Minami, D.; Jeong, D.; Wenzel, W. Multiscale Simulation of Photoluminescence Quenching in Phosphorescent OLED Materials. Adv. Theory Simul. 2020, 3, 1900222. [Google Scholar] [CrossRef]
- Reineke, S.; Walzer, K.; Leo, K. Triplet-exciton quenching in organic phosphorescent light-emitting diodes with Ir-based emitters. Phys. Rev. B 2007, 75, 125328. [Google Scholar] [CrossRef]
- Zhang, S.; Forrest, S.R. Triplet diffusion leads to triplet–triplet annihilation in organic phosphorescent emitters. Chem. Phys. Lett. 2012, 590, 106–110. [Google Scholar] [CrossRef]
- Schwartz, G.; Reineke, S.; Rosenow, T.C.; Walzer, K.; Leo, K. Triplet annihilation in highly efficient organic light-emitting diodes based on iridium complexes. Adv. Funct. Mater. 2009, 19, 1319–1333. [Google Scholar] [CrossRef]
- Wang, J.; Yin, J.; Li, X.; Wang, Z.; Wu, R.; Zhou, L. Highly Efficient Solution-Processed Blue Phosphorescent Organic Light-Emitting Diodes Based on Co-Dopant and Co-Host System. Molecules 2022, 27, 6882. [Google Scholar] [CrossRef]
- Park, J.; Jeon, W.; Yu, J.; Pode, R.; Kwon, J. Efficiency optimization of green phosphorescent organic light-emitting device. Thin Solid Films 2011, 519, 3259–3263. [Google Scholar] [CrossRef]
- Liu, D.; Du, M.; Chen, D.; Ye, K.; Zhang, Z.; Liu, Y.; Wang, Y. A novel tetraphenylsilane–phenanthroimidazole hybrid host material for highly efficient blue fluorescent, green and red phosphorescent OLEDs. J. Mater. Chem. C 2015, 3, 4394–4401. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure blue thermally activated delayed fluorescence molecules: Efficient HOMO-LUMO separation by the multiple resonance effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Oda, S.; Ricci, G.; Gotoh, H.; Tabata, K.; Kawasumi, R.; Beljonne, D.; Olivier, Y.; Hatakeyama, T. Hypsochromic shift of multiple-resonance-induced thermally activated delayed fluorescence by oxygen atom incorporation. Angew. Chem. Int. Ed. 2021, 60, 17910–17914. [Google Scholar] [CrossRef]
- Murawski, C.; Leo, K.; Gather, M.C. Efficiency roll-off in organic light-emitting diodes. Adv. Mater. 2013, 25, 6801–6827. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, G.; Muruganantham, S.; Kim, H.; Oh, J.; Ham, J.; Yadav, S.B.; Lee, J.; Chae, M.; Kim, Y.-H.; et al. Modified t-butyl in tetradentate platinum (II) complexes enables exceptional lifetime for blue-phosphorescent organic light emitting diodes. Nat. Commun. 2024, 8, 15566. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, C.; Batagoda, T.; Coburn, C.; Thompson, M.E.; Forrest, S.R. Hot excited state management for long-lived blue phosphorescent organic light-emitting diodes. Nat. Commun. 2017, 8, 15566. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Zhang, Z.; Zhao, C.; He, J.; Yan, C.; Meng, H. Narrowband Deep-Blue Multi-Resonance Induced Thermally Activated Delayed Fluorescence: Insights from the Theoretical Molecular Design. Molecules 2022, 27, 348. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, Y.-C.; Wu, H.; Xiong, X.; Yu, J.; Fan, X.-C.; Wang, K.; Zhang, X.-H. Hanging heavy atom-containing chains onto a multiple resonance framework: Influence on the TADF properties and device performances. Chem. Eng. J. 2023, 465, 142900. [Google Scholar] [CrossRef]
- Güven, Z.; Dolati, H.; Wessel, L.; Frank, R. Facile Synthetic Access Towards Sulfur- and Selenium-Functionalized Boron-Based Multiresonance TADF Emitters. Molecules 2024, 29, 5819. [Google Scholar] [CrossRef]
- Wang, F.; Ye, J.; Liu, J.; Chen, X.-K.; Yang, Y.; Bin, Z.; You, J. Synthesis of Selenium-Doped Heteroacenes: Unveiling the External Heavy-Atom Effect in Host Materials. Angew. Chem. Int. Ed. 2025, e202502380. [Google Scholar] [CrossRef]
- Park, I.; Min, H.; Yasuda, T. Ultrafast Triplet–Singlet Exciton Interconversion in Narrowband Blue Organoboron Emitters Doped with Heavy Chalcogens. Angew. Chem. Int. Ed. 2022, 61, e202205684. [Google Scholar] [CrossRef]
- Jin, J.; Wang, S.; Jiang, H.; Wang, L.; Wong, W.-Y. Peripheral Selenium Modification of Multi-Resonance Thermally Activated Delayed Fluorescence Molecules for High-Performance Blue Organic Light-Emitting Diodes. Adv. Opt. Mater. 2024, 12, 7354. [Google Scholar] [CrossRef]
- Li, M.; Li, R.; Li, Z.; Chen, Z.; Liu, D.; Yang, Z.; Xie, H.; Liu, K.; Su, S.-J. Design of a Novel Selenium-Containing Spiro Donor for Narrowband Blue Thermally Activated Delayed Fluorescence Emitters in OLEDs. Adv. Opt. Mater. 2024, 2402822. [Google Scholar] [CrossRef]
- Park, D.; Kang, S.; Ryoo, C.; Jhun, B.; Jung, S.; Le, T.; Suh, M.; Lee, J.; Jun, M.; Chu, C.; et al. High-performance blue OLED using multiresonance thermally activated delayed fluorescence host materials containing silicon atoms. Nat. Commun. 2023, 14, 5589. [Google Scholar] [CrossRef]
- Park, J.; Han, S.; Jo, U.; Kim, S.C.; Lee, D.R.; Ahn, H.J.; Kim, J.Y.; Baek, J.-H.; Lee, J.Y. Boron-based thermally activated delayed fluorescence host materials as universal hosts for blue phosphorescent organic light-emitting diodes. Mater. Today 2024, 75, 27–36. [Google Scholar] [CrossRef]
- Jin, J.; Chen, M.; Jiang, H.; Zhang, B.; Xie, Z.; Wong, W.-Y. Fusion of Selenium-Embedded Multi-Resonance Units Toward Narrowband Emission and Fast Triplet-Singlet Exciton Conversion. Adv. Opt. Mater. 2024, 12, 2400794. [Google Scholar] [CrossRef]
- Einzinger, M.; Zhu, T.; de Silva, P.; Belger, C.; Swager, T.M.; Voorhis, T.V.; Baldo, M.A. Shorter Exciton Lifetimes via an External Heavy-Atom Effect: Alleviating the Effects of Bimolecular Processes in Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1701987. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Cai, M.; Zhang, D.; Duan, L. Improving reverse intersystem crossing in exciplex-forming hosts by introducing heavy atom effect. Mater. Today Energy 2021, 21, 100705. [Google Scholar] [CrossRef]
- Park, S.; Kwon, H.; Lee, H.; Lee, K.; Kang, S.; Kim, K.J.; Kim, T.; Park, J. Novel blue multiresonance thermally activated delayed fluorescence host materials, including Ge-based bulky groups. J. Mater. Chem. C 2024, 12, 4384–4391. [Google Scholar] [CrossRef]
- Alam, M.I.; Nagar, M.R.; Barman, D.; Iyer, P.K.; Jou, J.-H.; Vaidyanathan, S. Tailoring structural rigidity utilizing a lock/unlock donor strategy for highly efficient solution processed blue and green HLCT OLEDs. J. Mater. Chem. C 2024, 12, 13585–13595. [Google Scholar] [CrossRef]
- Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Solution-Processable Pure Green Thermally Activated Delayed Fluorescence Emitter Based on the Multiple Resonance Effect. Adv. Mater. 2020, 32, 2004072. [Google Scholar] [CrossRef]
- Kondo, Y.; Yoshiura, K.; Kitera, S.; Nishi, H.; Oda, S.; Gotoh, H.; Sasada, Y.; Yanai, M.; Hatakeyama, T. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonics 2019, 13, 678–682. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, F.; Guo, K.; Wang, H.; Wei, B.; Xu, B. Carrier transfer and luminescence characteristics of concentration-dependent phosphorescent Ir(ppy)3 doped CBP film. Opt. Laser Technol. 2014, 45, 20–24. [Google Scholar] [CrossRef]
- Hirai, H.; Nakajima, K.; Nakatsuka, S.; Shiren, K.; Ni, J.; Nomura, S.; Ikuta, T.; Hatakeyama, T. One-Step Borylation of 1,3-Diaryloxybenzenes Towards Efficient Materials for Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. Engl. 2015, 5491, 13581–13585. [Google Scholar] [CrossRef]
- Park, H.; Moon, J.; Jo, U.; Han, S.; Lee, D.R.; Ahn, H.J.; Kim, Y.J.; Baek, J.-H.; Lee, J.Y. Boron- and Silane-Based Electron Transport–Type Host Materials for Long-Lifetime Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Opt. Mater. 2024, 12, 2302791. [Google Scholar] [CrossRef]

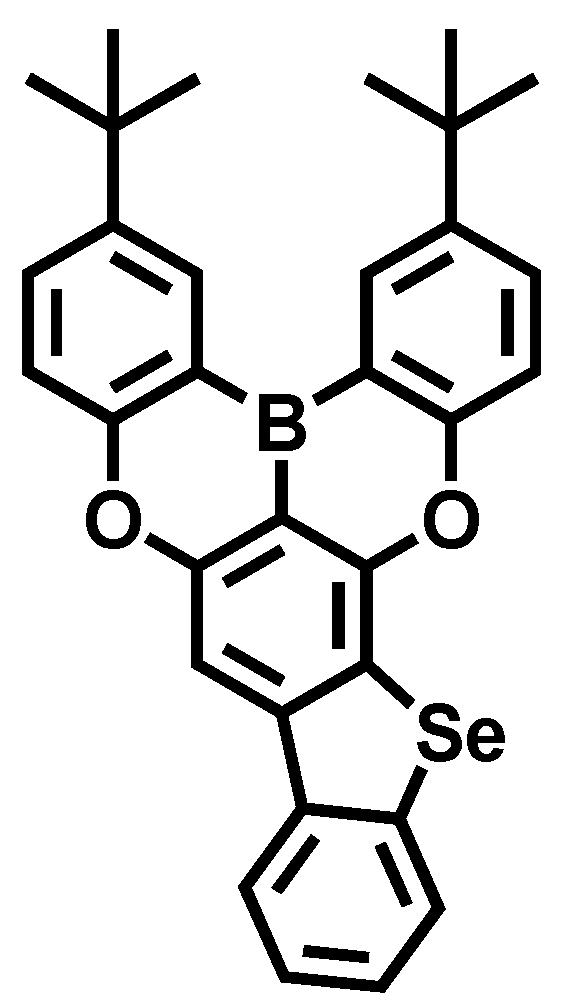



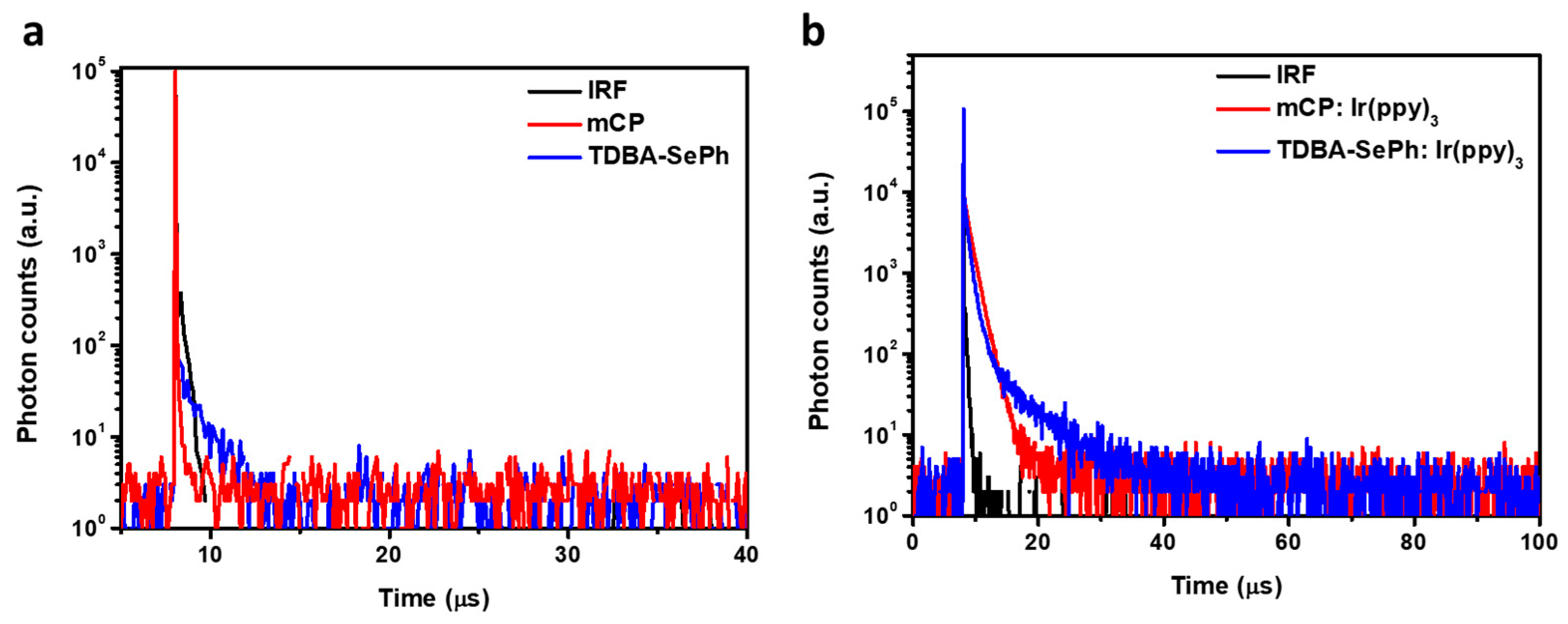

| Solution a | Film b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λAbs (nm) | λPL (nm) | FWHM c (nm) | ϕF (%) | λAbs (nm) | λPL (nm) | FWHM (nm) | ϕF (%) | ES/ET/ΔEST (eV) | Τd (μs) | HOMO d (eV) | LUMO (eV) | Band Gap (eV) | |
| TDBA-SePh | 420 | 447 | 35 | 14.0 | 430 | 468 | 78 | 8.6 | 2.90/2.66/0.24 | 1.42 | −5.38 | −2.64 | 2.74 |
| Devices | Von a (V) | LE (cd/A) | Luminance (cd/m2) | EQE (%) | CIE (x, y) | ELmax (nm) | FWHM (nm) |
|---|---|---|---|---|---|---|---|
| Device 1 | 3.71 | 25.9 | 2495 | 7.8 | (0.293, 0.601) | 511 | 64 |
| Device 2 | 3.05 | 33.0 | 3100 | 9.3 | (0.309, 0.621) | 513 | 66 |
| Device 3 | 3.86 | 5.5 | 538 | 7.8 | (0.682, 0.317) | 629 | 85 |
| Device 4 | 3.01 | 7.4 | 759 | 11.5 | (0.687, 0.313) | 631 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Kang, S.; Park, S.; Oh, S.; Kim, S.-T.; Lee, K.; Lee, H.; Park, J. Selenium-Containing Multi-Resonance Thermally Activated Delayed Fluorescence Host Material for Green and Red Phosphorescent OLEDs. Materials 2025, 18, 2040. https://doi.org/10.3390/ma18092040
Kwon H, Kang S, Park S, Oh S, Kim S-T, Lee K, Lee H, Park J. Selenium-Containing Multi-Resonance Thermally Activated Delayed Fluorescence Host Material for Green and Red Phosphorescent OLEDs. Materials. 2025; 18(9):2040. https://doi.org/10.3390/ma18092040
Chicago/Turabian StyleKwon, Hyukmin, Seokwoo Kang, Sangwook Park, Saeyoung Oh, Sang-Tae Kim, Kiho Lee, Hayoon Lee, and Jongwook Park. 2025. "Selenium-Containing Multi-Resonance Thermally Activated Delayed Fluorescence Host Material for Green and Red Phosphorescent OLEDs" Materials 18, no. 9: 2040. https://doi.org/10.3390/ma18092040
APA StyleKwon, H., Kang, S., Park, S., Oh, S., Kim, S.-T., Lee, K., Lee, H., & Park, J. (2025). Selenium-Containing Multi-Resonance Thermally Activated Delayed Fluorescence Host Material for Green and Red Phosphorescent OLEDs. Materials, 18(9), 2040. https://doi.org/10.3390/ma18092040







