Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology
Abstract
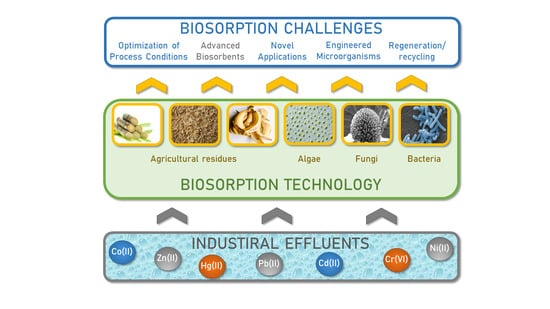
1. Introduction
2. The Negative Impact of Heavy Metals on Human Health
3. Development of Advanced/Modified Biosorbents
4. Optimization of Process Conditions
5. Exploration of Novel Applications
6. Engineered Microorganisms and Nanomaterials
7. Deactivation and Regeneration of Biosorbents
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Methodology of the Bibliometric Analysis
- PlanningA.1 Initial idea formulationBiosorption for the removal of heavy metals: addressing challenges, exploring opportunities, examining perspectives, and charting research directions.A.2 PICOCPopulation: Biosorption.Intervention: Use of biosorption for removal of heavy metals from wastewater.Comparison: The development of biosorbents for use in real sludge.Outcomes: Treatment support, separation efficiency, and real conditions.Context: Challenges and opportunities associated with the applications of biosorbents in heavy metals removal.
- Research questionsWhat biosorbents can be used to remove heavy metals from wastewater?What is the efficiency of the biosorption process?For which real wastewaters can biosorption be used?What modifications and improvements are proposed?What research directions are being pursued?What challenges are linked to the application of biosorption processes for the removal of heavy metals?
- Digital library sourcesGoogle Scholar, Scopus, and PubMed.
- Inclusion and exclusion criteriaInclusion: Results obtained by searching using biosorption with heavy metals, cobalt, nickel, lead, chromium cadmium, zinc, wastewater, and industrial influents.Exclusion: Articles published before 2019 and those categorized as reviews, with a few exceptions made for studies providing fundamental insights into the issues raised.
- Quality Assessment (QA) checklistE.1. Ensure that:The search strategy was accurately delineated. The inclusion and exclusion criteria were suitable and clearly defined. The studies chosen aligned well with the research question.The selected papers demonstrated high quality, characterized by robust design and execution.The data were pertinent and thoroughly described. The methods used for paper inclusion were fitting and well documented. The consistency of the results was sufficiently assessed and presented.E.2. Data extraction formBy metals: Cobalt, copper, nickel, lead, chromium cadmium, zinc, and arsenic.By wastewater: Metal industries: mining, electroplating, and metallurgical processes; textile industries; and the leather industry.E.3. ConductingStudies were gathered using the Mendeley reference management tool as a database resource.E.4. Study selection and refinement
References
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A Comprehensive Review on Conventional and Biological-Driven Heavy Metals Removal from Industrial Wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.-C.; Hursthouse, A.; van Hullebusch, E.D. Sorption vs Adsorption: The Words They Are a-Changin’, Not the Phenomena. Sci. Total Environ. 2022, 838, 156545. [Google Scholar] [CrossRef]
- AlKaabi, N.; Al-Ghouti, M.A.; Jaoua, S.; Zouari, N. Potential for Native Hydrocarbon-Degrading Bacteria to Remediate Highly Weathered Oil-Polluted Soils in Qatar through Self-Purification and Bioaugmentation in Biopiles. Biotechnol. Rep. 2020, 28, e00543. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Zhao, X.; Gao, X.; Zheng, Y.; Zuo, H.; Wei, Z. Roles of Different Humin and Heavy-Metal Resistant Bacteria from Composting on Heavy Metal Removal. Bioresour. Technol. 2020, 296, 122375. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Biosorption of Heavy Metals by Dry Biomass of Metal Tolerant Bacterial Biosorbents: An Efficient Metal Clean-up Strategy. Environ. Monit. Assess. 2020, 192, 801. [Google Scholar] [CrossRef]
- Yadav, A.; Rene, E.R.; Kanti Mandal, M.; Kumar Dubey, K. Biodegradation of Cyclophosphamide and Etoposide by White Rot Fungi and Their Degradation Kinetics. Bioresour. Technol. 2022, 346, 126355. [Google Scholar] [CrossRef]
- Mahdy, S.; Suttinun, O. Decolorization of Remazol Brilliant Blue R by White-Rot Fungus Trametes Hirsuta AK04 Immobilized on Lignocellulosic Oil Palm Fibers. Appl. Biochem. Microbiol. 2023, 59, 867–880. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and Mechanics of Fungal Mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef] [PubMed]
- El-Sabbagh, S.M.; Mira, H.I.; Desouky, O.A.; Hussien, S.S.; Elgohary, D.M.; Ali, A.O.; El Naggar, A.M.A. Synthesis of Fungal Chitosan–Polystyrene Modified by Nanoparticles of Binary Metals for the Removal of Heavy Metals from Waste Aqueous Media. RSC Adv. 2023, 13, 29735–29748. [Google Scholar] [CrossRef] [PubMed]
- Zvulunov, Y.; Ben-Barak-Zelas, Z.; Fishman, A.; Radian, A. A Self-Regenerating Clay-Polymer-Bacteria Composite for Formaldehyde Removal from Water. Chem. Eng. J. 2019, 374, 1275–1285. [Google Scholar] [CrossRef]
- Chong, Z.T.; Soh, L.S.; Yong, W.F. Valorization of Agriculture Wastes as Biosorbents for Adsorption of Emerging Pollutants: Modification, Remediation and Industry Application. Results Eng. 2023, 17, 100960. [Google Scholar] [CrossRef]
- Tokay, B.; Akpınar, I. A Comparative Study of Heavy Metals Removal Using Agricultural Waste Biosorbents. Bioresour. Technol. Rep. 2021, 15, 100719. [Google Scholar] [CrossRef]
- Arthi, D.; Michael Ahitha Jose, J.; Edinsha Gladis, E.H.; Shajin Shinu, P.M.; Joseph, J. Removal of Heavy Metal Ions from Water Using Adsorbents from Agro Waste Materials. Mater. Today Proc. 2021, 45, 1794–1798. [Google Scholar] [CrossRef]
- Wierzba, S.; Kłos, A. Heavy Metal Sorption in Biosorbents—Using Spent Grain from the Brewing Industry. J. Clean. Prod. 2019, 225, 112–120. [Google Scholar] [CrossRef]
- Blázquez, G.; Martín-Lara, M.Á.; Iáñez-Rodríguez, I.; Morales, I.; Pérez, A.; Calero, M. Cobalt Biosorption in Fixed-Bed Column Using Greenhouse Crop Residue as Natural Sorbent. Separations 2022, 9, 316. [Google Scholar] [CrossRef]
- Diehl, M.; Silva, L.F.O.; Schnorr, C.; Netto, M.S.; Bruckmann, F.S.; Dotto, G.L. Cassava Bagasse as an Alternative Biosorbent to Uptake Methylene Blue Environmental Pollutant from Water. Environ. Sci. Pollut. Res. 2023, 30, 51920–51931. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Yaseen, B.; Gangwar, C.; Nayak, R.; Kumar, I.; Pandey, P.K.; Sarkar, J.; Naik, R.M. Chapter 12—Optimized Conditions for Removal of Ni(II) Using Sugarcane Bagasse Biosorbent as an Adsorbent. In Advances in Pollution Research; Srivastav, A.L., Grewal, A.S., Tiwari, M., Pham, T.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 115–123. ISBN 978-0-443-15291-7. [Google Scholar]
- Irto, A.; Raccuia, S.G.M.; Lando, G.; De Stefano, C.; Arena, K.; Salerno, T.M.G.; Pettignano, A.; Cacciola, F.; Mondello, L.; Cardiano, P. Valorization of Citrus Waste for Circular Economy: A Case Study on Bergamot Pomace as Sorbent for Cd2+ Removal and Source of Added Value Compounds. Microchem. J. 2023, 193, 109183. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, F.; Kamal, M.; Khan, S.; Aghar, M.A. Effectiveness of Citrus Fruit Peel as a Biosorbent for the Mitigation of Aflatoxins in Vitro. Food Addit. Contam. Part A 2022, 39, 1987–2001. [Google Scholar] [CrossRef]
- Šabanović, E.; Memić, M.; Sulejmanović, J.; Selović, A. Simultaneous Adsorption of Heavy Metals from Water by Novel Lemon-Peel Based Biomaterial. Polish J. Chem. Technol. 2020, 22, 46–53. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Priyantha, N.; Lu, Y.; Zaidi, N.A.H.M. Adsorption of Heavy Metal Lead Using Citrus Grandis (Pomelo) Leaves as Low-Cost Adsorbent. Desalin. WATER Treat. 2019, 166, 44–52. [Google Scholar] [CrossRef]
- Farias, K.C.S.; Guimarães, R.C.A.; Oliveira, K.R.W.; Nazário, C.E.D.; Ferencz, J.A.P.; Wender, H. Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater. Toxics 2023, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Fabre, E.; Vale, C.; Pereira, E.; Silva, C.M. Sustainable Water Treatment: Use of Agricultural and Industrial Wastes to Remove Mercury by Biosorption. Water Air Soil Pollut. 2021, 232, 284. [Google Scholar] [CrossRef]
- Jain, H.; Yadav, V.; Rajput, V.D.; Minkina, T.; Agarwal, S.; Garg, M.C. An Eco-Sustainable Green Approach for Biosorption of Methylene Blue Dye from Textile Industry Wastewater by Sugarcane Bagasse, Peanut Hull, and Orange Peel: A Comparative Study Through Response Surface Methodology, Isotherms, Kinetic, and Thermodynamics. Water Air Soil Pollut. 2022, 233, 187. [Google Scholar] [CrossRef]
- Kumari, S.; Verma, A.; Sharma, P.; Agarwal, S.; Rajput, V.D.; Minkina, T.; Rajput, P.; Singh, S.P.; Garg, M.C. Introducing Machine Learning Model to Response Surface Methodology for Biosorption of Methylene Blue Dye Using Triticum Aestivum Biomass. Sci. Rep. 2023, 13, 8574. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Muthulingam, S.; Kumar, M.; Rajasekar, N.; Mohanraj, S.; Malar, C.G. Biosorption of Methylene Blue Dye Using a Novel Chitosan Pectinase Blend. Environ. Sci. Pollut. Res. 2023, 30, 48948–48961. [Google Scholar] [CrossRef]
- Rápó, E.; Aradi, L.E.; Szabó, Á.; Posta, K.; Szép, R.; Tonk, S. Adsorption of Remazol Brilliant Violet-5R Textile Dye from Aqueous Solutions by Using Eggshell Waste Biosorbent. Sci. Rep. 2020, 10, 8385. [Google Scholar] [CrossRef] [PubMed]
- Figueirôa, J.A.; Menezes Novaes, G.U.; de Souza Gomes, H.; de Morais Silva, V.L.M.; de Moraes Lucena, D.; Lima, L.M.R.; de Souza, S.A.; Viana, L.G.F.C.; Rolim, L.A.; da Silva Almeida, J.R.G.; et al. Opuntia Ficus-Indica Is an Excellent Eco-Friendly Biosorbent for the Removal of Chromium in Leather Industry Effluents. Heliyon 2021, 7, e07292. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al-Dhabi, N.; Arasu, M.V. Biosorption of Hazardous Waste from the Municipal Wastewater by Marine Algal Biomass. Environ. Res. 2022, 204, 112115. [Google Scholar] [CrossRef]
- Ahiahonu, E.K.; Anku, W.W.; Roopnarain, A.; Green, E.; Serepa-Dlamini, M.H.; Govender, P.P. Exploring Indigenous Freshwater Chlorophytes in Integrated Biophotovoltaic System for Simultaneous Wastewater Treatment, Heavy Metal Biosorption, CO2 Biofixation and Biodiesel Generation. Bioelectrochemistry 2022, 147, 108208. [Google Scholar] [CrossRef]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of Diclofenac in Wastewater Using Biosorption and Advanced Oxidation Techniques: Comparative Results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Dey, S.; Veerendra, G.T.N.; Phani Manoj, A.V.; Anjaneya Babu, P.S.S. Performances of Plant Leaf Biosorbents for Biosorption of Phosphorous from Synthetic Water. Clean. Mater. 2023, 8, 100191. [Google Scholar] [CrossRef]
- Dall’Agnol, P.; Libardi, N.; da Silva, E.C.; da Costa, R.H.R. Biosorption of Phosphorus Using Alginate-Like Exopolymers: Investigation of Removal Mechanism, Kinetic and Thermodynamic Properties. J. Polym. Environ. 2022, 30, 695–706. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Boddu, S.; Chandra, A.; Ali Khan, A. Biosorption of Cu(II), Pb(II) from Electroplating Industry Effluents by Treated Shrimp Shell. Mater. Today Proc. 2022, 57, 1520–1527. [Google Scholar] [CrossRef]
- Ume, O.L.; Ekeoma, B.C.; Yusuf, M.; Al-Kahtani, A.A.; Ubaidullah, M.; Sillanpää, M. Batch Studies of Hexavalent Chromium Biosorption from Mining Wastewater Using Aspergillus Niger. Results Chem. 2022, 4, 100490. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hamouda, R.A.; Abuelmagd, M.A.; Abdelgalil, S.A. Bioprocess Development for Biosorption of Cobalt Ions and Congo Red from Aquatic Mixture Using Enteromorpha Intestinalis Biomass as Sustainable Biosorbent. Sci. Rep. 2021, 11, 14953. [Google Scholar] [CrossRef] [PubMed]
- Dvoynenko, O.; Lo, S.-L.; Chen, Y.-J.; Chen, G.W.; Tsai, H.-M.; Wang, Y.-L.; Wang, J.-K. Speciation Analysis of Cr(VI) and Cr(III) in Water with Surface-Enhanced Raman Spectroscopy. ACS Omega 2021, 6, 2052–2059. [Google Scholar] [CrossRef]
- Terlecka, E. Arsenic Speciation Analysis in Water Samples: A Review of The Hyphenated Techniques. Environ. Monit. Assess. 2005, 107, 259–284. [Google Scholar] [CrossRef]
- Campbell, K.M.; Nordstrom, D.K. Arsenic Speciation and Sorption in Natural Environments. Rev. Mineral. Geochem. 2014, 79, 185–216. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. What Are Heavy Metals? Long-Standing Controversy over the Scientific Use of the Term ‘Heavy Metals’—Proposal of a Comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253. [Google Scholar] [CrossRef]
- Tanvi, D.A.; Pratam, K.M.; Lohit, R.T.; Vijayalakshmi, B.K.; Devaraja, T.N.; Vasudha, M.; Ramesh, A.; Chakra, P.S.; Gayathri, D. Biosorption of Heavy Metal Arsenic from Industrial Sewage of Davangere District, Karnataka, India, Using Indigenous Fungal Isolates. SN Appl. Sci. 2020, 2, 1860. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Baszuk, P.; Marciniak, W.; Wojtyś, M.; Dębniak, T.; Cybulski, C.; Gronwald, J.; Alchimowicz, J.; Masojć, B.; et al. Influence of the Levels of Arsenic, Cadmium, Mercury and Lead on Overall Survival in Lung Cancer. Biomolecules 2021, 11, 1160. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Verma, N.; Rachamalla, M.; Kumar, P.S.; Dua, K. Assessment and Impact of Metal Toxicity on Wildlife and Human Health. In Metals in Water; Shukla, S.K., Kumar, S., Madhav, S., Mishra, P.K., Hashmi, M.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 93–110. ISBN 978-0-323-95919-3. [Google Scholar]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Sharma, P.; Gaur, P.; Varjani, S.; Ngo, H.H.; Guo, W.; Chaturvedi, P.; Singhania, R.R. Sustainable Mitigation of Heavy Metals from Effluents: Toxicity and Fate with Recent Technological Advancements. Bioengineered 2021, 12, 7297–7313. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Novo, D.L.R.; Druzian, G.T.; Landero, J.A.; Caruso, J.; Mesko, M.F.; Flores, E.M.M. Influence of Culinary Treatment on the Concentration and on the Bioavailability of Cadmium, Chromium, Copper, and Lead in Seafood. J. Trace Elem. Med. Biol. 2021, 65, 126717. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Sao, K.; Pandey, M.; Pandey, P.K.; Khan, F. Highly Efficient Biosorptive Removal of Lead from Industrial Effluent. Environ. Sci. Pollut. Res. 2017, 24, 18410–18420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; M.M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, M.E.; Pardal, M.A. Mercury Accumulation in Fish Species along the Portuguese Coast: Are There Potential Risks to Human Health? Mar. Pollut. Bull. 2020, 150, 110740. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Jagaba, A.H.; Kutty, S.R.M.; Khaw, S.G.; Lai, C.L.; Isa, M.H.; Baloo, L.; Lawal, I.M.; Abubakar, S.; Umaru, I.; Zango, Z.U. Derived Hybrid Biosorbent for Zinc(II) Removal from Aqueous Solution by Continuous-Flow Activated Sludge System. J. Water Process Eng. 2020, 34, 101152. [Google Scholar] [CrossRef]
- Rani, S.; Sharma, S.; Bansal, M.; Garg, R.; Garg, R. Enhanced Zn(II) Adsorption by Chemically Modified Sawdust Based Biosorbents. Environ. Sci. Pollut. Res. 2022, 30, 99046–99061. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Biosorbents for Heavy Metal Removal from Dilute Aqueous Solution. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–132. ISBN 9780128197868. [Google Scholar]
- Jayakumar, V.; Govindaradjane, S.; Rajamohan, N.; Rajasimman, M. Biosorption Potential of Brown Algae, Sargassum Polycystum, for the Removal of Toxic Metals, Cadmium and Zinc. Environ. Sci. Pollut. Res. 2022, 29, 41909–41922. [Google Scholar] [CrossRef] [PubMed]
- Bibak, M.; Sattari, M.; Tahmasebi, S. Investigation of Biosorption Capacity of Algae: Selection of Most Efficient Biosorbent for Metal Removal. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 217–226. [Google Scholar] [CrossRef]
- Richards, S.; Dawson, J.; Stutter, M. The Potential Use of Natural vs Commercial Biosorbent Material to Remediate Stream Waters by Removing Heavy Metal Contaminants. J. Environ. Manag. 2019, 231, 275–281. [Google Scholar] [CrossRef]
- Cheng, Z.; Feng, K.; Su, Y.; Ye, J.; Chen, D.; Zhang, S.; Zhang, X.; Dionysiou, D.D. Novel Biosorbents Synthesized from Fungal and Bacterial Biomass and Their Applications in the Adsorption of Volatile Organic Compounds. Bioresour. Technol. 2020, 300, 122705. [Google Scholar] [CrossRef]
- Manna, M.C.; Sahu, A.; De, N.; Thakur, J.K.; Mandal, A.; Bhattacharjya, S.; Ghosh, A.; Rahman, M.M.; Naidu, R.; Singh, U.B.; et al. Novel Bio-Filtration Method for the Removal of Heavy Metals from Municipal Solid Waste. Environ. Technol. Innov. 2020, 17, 100619. [Google Scholar] [CrossRef]
- Agunwamba, J.C.; Amu, A.M.; Nwonu, D.C. An Efficient Biosorbent for the Removal of Arsenic from a Typical Urban-Generated Wastewater. Environ. Monit. Assess. 2022, 194, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Akar, S.; Lorestani, B.; Sobhanardakani, S.; Cheraghi, M.; Moradi, O. Surveying the Efficiency of Platanus Orientalis Bark as Biosorbent for Ni and Cr(VI) Removal from Plating Wastewater as a Real Sample. Environ. Monit. Assess. 2019, 191, 373. [Google Scholar] [CrossRef] [PubMed]
- Cortés, S.; Soto, E.E.; Ordóñez, J.I. Recovery of Copper from Leached Tailing Solutions by Biosorption. Minerals 2020, 10, 158. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of Heavy Metals Present in Water from the Yautepec River Morelos México, Using Opuntia Ficus-Indica Mucilage. Environ. Adv. 2022, 7, 100160. [Google Scholar] [CrossRef]
- Díaz, A.; Marrero, J.; Cabrera, G.; Coto, O.; Gómez, J.M. Biosorption of Nickel, Cobalt, Zinc and Copper Ions by Serratia Marcescens Strain 16 in Mono and Multimetallic Systems. Biodegradation 2022, 33, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Sajid, M.; Raza, N.; Sohail, Y.; Hayat, M.; Manzoor, S.; Shakeel, N.; Aziz Gill, K.; Ifseisi, A.A.; Zahid Ansari, M. Study of Modified Biomass of Gossypium Hirsutum as Heavy Metal Biosorbent. Arab. J. Chem. 2023, 16, 105332. [Google Scholar] [CrossRef]
- Liu, G.; Geng, W.; Wu, Y.; Zhang, Y.; Chen, H.; Li, M.; Cao, Y. Biosorption of Lead Ion by Lactic Acid Bacteria and the Application in Wastewater. Arch. Microbiol. 2024, 206, 18. [Google Scholar] [CrossRef] [PubMed]
- Randrianarison, G.; Ashraf, M.A.; Zaonarivelo, J.R. The Potentiality of Arthrospira Platensis Microalgal Species for Mining Wastewater Bioremediation by Biosorption Removal of Heavy Metals (Zn2+, Cu2+, Pb2+, Fe2+). Desalin. WATER Treat. 2021, 239, 1–12. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, T.; Kumar, R.; Kumar, R.; Alothman, A.A.; mana AL-Anazy, M.; Alqahtani, K.N.; Umar, A. Multi-Biological Combined System: A Mechanistic Approach for Removal of Multiple Heavy Metals. Chemosphere 2021, 276, 130018. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn(II) from Industrial Effluents Using Sugar Beet Pulp and F. Vesiculosus: From Laboratory Tests to a Pilot Approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef]
- Dai, Q.H.; Bian, X.Y.; Li, R.; Jiang, C.B.; Ge, J.M.; Li, B.L.; Ou, J. Biosorption of Lead(II) from Aqueous Solution by Lactic Acid Bacteria. Water Sci. Technol. 2019, 79, 627–634. [Google Scholar] [CrossRef]
- Chen, Z.; Leng, X.; Zhou, F.; Shen, W.; Zhang, H.; Yu, Q.; Meng, X.; Fan, H.; Qin, M. Screening and Identification of Probiotic Lactobacilli from the Infant Gut Microbiota to Alleviate Lead Toxicity. Probiotics Antimicrob. Proteins 2023, 15, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of Fungi in Bioremediation of Emerging Pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Cárdenas González, J.F.; Rodríguez Pérez, A.S.; Vargas Morales, J.M.; Martínez Juárez, V.M.; Rodríguez, I.A.; Cuello, C.M.; Fonseca, G.G.; Escalera Chávez, M.E.; Muñoz Morales, A. Bioremoval of Cobalt(II) from Aqueous Solution by Three Different and Resistant Fungal Biomasses. Bioinorg. Chem. Appl. 2019, 2019, 8757149. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Matter, I.A.; Gharieb, M.M.; Darwesh, O.M. Biosorption Performance of the Multi-Metal Tolerant Fungus Aspergillus Sp. for Removal of Some Metallic Nanoparticles from Aqueous Solutions. Heliyon 2023, 9, e16125. [Google Scholar] [CrossRef]
- Rozumová, L.; Životský, O.; Seidlerová, J.; Motyka, O.; Šafařík, I.; Šafaříková, M. Magnetically Modified Peanut Husks as an Effective Sorbent of Heavy Metals. J. Environ. Chem. Eng. 2016, 4, 549–555. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Bagheri, F. Optimization of the Lead Removal from Aqueous Solution Using Two Starch Based Adsorbents: Design of Experiments Using Response Surface Methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 102793. [Google Scholar] [CrossRef]
- Mendoza, D.V.M.; Sebastian, F.M.C.; Gabriel, J.P.; Hipolito, M. Succinylated Rice Bran Starch as Adsorbent of Heavy Metals in Aqueous Solution. J. Res. Ecol. 2018, 6, 2365–2372. [Google Scholar]
- Liu, G.; Liao, L.; Dai, Z.; Qi, Q.; Wu, J.; Ma, L.Q.; Tang, C.; Xu, J. Organic Adsorbents Modified with Citric Acid and Fe3O4 Enhance the Removal of Cd and Pb in Contaminated Solutions. Chem. Eng. J. 2020, 395, 125108. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Y.; Wu, M.; Zhang, L.; Wang, L.; Ni, H. Surface Functionalization of Cellulose with Hyperbranched Polyamide for Efficient Adsorption of Organic Dyes and Heavy Metals. J. Clean. Prod. 2019, 232, 774–783. [Google Scholar] [CrossRef]
- Hamdan, A.M.; Abd-El-Mageed, H.; Ghanem, N. Biological Treatment of Hazardous Heavy Metals by Streptomyces Rochei ANH for Sustainable Water Management in Agriculture. Sci. Rep. 2021, 11, 9314. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.Y.; Lima, E.C.; Abatal, M.; Tariq, R.; Santiago, A.A.; Alfonso, I.; Aguilar, C.; Vazquez-Olmos, A.R. Biosorption of Pb(II) Using Natural and Treated Ardisia Compressa K. Leaves: Simulation Framework Extended through the Application of Artificial Neural Network and Genetic Algorithm. Molecules 2023, 28, 6387. [Google Scholar] [CrossRef]
- Esfandyari, M.; Khodadadi, M.; Nekoo Ghadirli, R.; Jafari, D. Prediction and Optimization of Heavy Metal Ions Removal Efficiency from the Active Sludge Using Intelligent Systems. Desalin. Water Treat. 2022, 252, 167–176. [Google Scholar] [CrossRef]
- Saber, W.I.A.; El-Naggar, N.E.-A.; El-Hersh, M.S.; El-khateeb, A.Y.; Elsayed, A.; Eldadamony, N.M.; Ghoniem, A.A. Rotatable Central Composite Design versus Artificial Neural Network for Modeling Biosorption of Cr6+ by the Immobilized Pseudomonas Alcaliphila NEWG-2. Sci. Rep. 2021, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.M.; Mohammed, A.A.; Kawady, N.A.; Elaasy, I.E.; Soliman, E.R.S. Costly Effective Bioleaching of Valuable Metals from Low Grade Ore Using Aspergillus Nidulans. Int. J. Environ. Sci. Technol. 2024, 21, 5469–5482. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb (II) and Zn (II) from Aqueous Solution by Oceanobacillus Profundus Isolated from an Abandoned Mine. Sci. Rep. 2020, 10, 21189. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, N.; Wei, M.; Wen, T.; Wang, X.; Liu, H.; Zhong, S.; Xu, H. Cadmium Biosorption and Mechanism Investigation Using a Novel Bacillus Subtilis KC6 Isolated from Pyrite Mine. J. Clean. Prod. 2021, 312, 127749. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, B.; Yu, Q. Genetic Engineering-Facilitated Coassembly of Synthetic Bacterial Cells and Magnetic Nanoparticles for Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2020, 12, 22948–22957. [Google Scholar] [CrossRef]
- Dey, S.; Sreenivasulu, A.; Veerendra, G.T.N.; Phani Manoj, A.V.; Haripavan, N. Synthesis and Characterization of Mango Leaves Biosorbents for Removal of Iron and Phosphorous from Contaminated Water. Appl. Surf. Sci. Adv. 2022, 11, 100292. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Zhang, H.; Liu, J.; Yu, J.; Wang, J. A Novel 3D Reticular Anti-Fouling Bio-Adsorbent for Uranium Extraction from Seawater: Polyethylenimine and Guanidyl Functionalized Hemp Fibers. Chem. Eng. J. 2020, 382, 122555. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Shao, X.; Tong, J.; Chen, R.; Yang, Q.; Yang, X.; Li, G.; Zimmerman, A.R.; Gao, B. Preparation of Biosorbent for the Removal of Organic Dyes from Aqueous Solution via One-Step Alkaline Ball Milling of Hickory Wood. Bioresour. Technol. 2022, 348, 126831. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Sharma, E.; Guleria, A.; Sangar, S.; Singh, K. Modification and Management of Lignocellulosic Waste as an Ecofriendly Biosorbent for the Application of Heavy Metal Ions Sorption. Mater. Today Proc. 2020, 32, 608–619. [Google Scholar] [CrossRef]
- Fathollahi, A.; Coupe, S.J.; El-Sheikh, A.H.; Nnadi, E.O. Cu(II) Biosorption by Living Biofilms: Isothermal, Chemical, Physical and Biological Evaluation. J. Environ. Manag. 2021, 282, 111950. [Google Scholar] [CrossRef]
- Tadic, D.C.; Lozano, F.V.; Maza, M.E.Z.; Elicer, P.L.V. Process for the Removal of Metals by Biosorption from Mining or Industrial Effluents. U.S. Patent 7,326,344, 5 February 2008. [Google Scholar]
- Putro, J.N.; Ju, Y.-H.; Soetaredjo, F.E.; Santoso, S.P.; Ismadji, S. Biosorption of Dyes. In Green Chemistry and Water Remediation: Research and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–133. ISBN 9780128177426. [Google Scholar]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial Survival Strategies and Responses under Heavy Metal Stress: A Comprehensive Overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, Q.; Hu, X.; Wang, Y.; Chen, Y.; Zhang, S.; Zheng, H. Adsorption Behavior of Heavy Metal Ions on a Polymer-Immobilized Amphoteric Biosorbent: Surface Interaction Assessment. J. Hazard. Mater. 2021, 403, 123801. [Google Scholar] [CrossRef] [PubMed]
- Ayub, A.; Raza, Z.A.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Development of Sustainable Magnetic Chitosan Biosorbent Beads for Kinetic Remediation of Arsenic Contaminated Water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Metal | Industrial Sources | Negative Effect on Human Health | Ref. |
|---|---|---|---|
| Arsenic | Mining, smelting, pesticide manufacturing, wood preservatives | Skin lesions, cardiovascular diseases, neurotoxicity, developmental effects, diabetes, cancers (skin, lung, bladder, liver, kidney) | [43,44] |
| Cadmium | Metallurgical, electroplating, mining industry, manufacturing of paintings | Lung and kidney diseases; breast, lung, prostate, nasopharynx, pancreas, and kidney cancers; fetal growth restriction; skeletal damage | [45,46,47] |
| Cobalt | Electroplating, metallurgy, mining, superalloys “resistant to corrosion and wear”, manufacturing industries, rechargeable battery electrodes, nuclear power plants, petrochemicals, electronics, paints and pigments, chemical industry | Damage to liver, heart failure, asthma, allergy, bone defects, hair loss, low blood pressure, nervous system disorders, reduced thyroid activity (goiter), vomiting, genotoxicity, risk for cancer | [38,48] |
| Copper | Electroplating, metallurgical industry | Kidney damage, anemia | [49] |
| Chromium (especially Cr(VI)) | Electroplating, tanning and mining industry | Mutagenic and carcinogenic effects, kidney dysfunction, lung cancer, and critical health impacts like diarrhea, ulcers, and damage to blood cells | [47,50,51] |
| Lead | Metallurgical, electroplating, metal finishing industries, manufacturing of paints, storage batteries, petroleum refining and drainage from ore mines | Risk of lung, stomach, and bladder cancer; damage to the kidney, nervous system, reproductive system, liver, and brain; causing sickness during pregnancy, Pb can also hamper fetal growth in the early stage | [45,52,53] |
| Mercury | Metal smelting, coal production, waste disposal, and chemical synthesis | Neurotoxicity and nephrotoxicity, cognitive impairment in children and fetal abnormalities | [45,54] |
| Nickel | Alloy production, electroplating, production of nickel–cadmium batteries | Allergy, cardiovascular and kidney diseases, lung fibrosis, lung and nasal cancer | [47,55] |
| Zinc | Electroplating, hot-dip galvanizing, metallurgy, production of batteries, pigments | Disorders to the immune system, prostate problems, diabetes and macular degeneration | [56,57] |
| Metal/Sorbent | Source | Result | Ref. |
|---|---|---|---|
| As(III) Fungal isolates, APR-1 (Aspergillus niger) and APR-2 (Aspergillus spp.), immobilized on Luffa aegyptiaca (sponge gourd) (an agro-waste as biosorbent) | Industrial sewage from different regions of Davangere, Davangere District, India Conc. in mg/L 17,995.87 (As) | R in %: 53.94 and 52.54 for APR-1 and APR-2 No results: regeneration, the possibility of reuse, or further treatment of the biomass | [44] |
| As(V) Chemically pretreated (NaOH) unshelled Moringa oleifera seeds | Cassava wastewater, Nsukka, India Conc. in mg/L: 1.81–5.42 (As), 0–0.05 (Pb), 0.06–0.78 (Mn), 0.31–0.82 (Ni), 0.35–0.61 (Zn), 0.03–0.06 (Cu), 0.09–0.35 (Cd), 0.06–0.35 (Cr) | R in %: 92.9 for optimal conditions: pH 4.0, contact time of 30 min, and dosage of 2 g | [64] |
| Cr(VI) Aspergillus niger: cleaned with distilled water, boiled with 0.5 M NaOH for 15 min, rinsed with deionized water, dried at 60 °C for 24 h, and ground | Mining wastewater was recovered from a mine pit located in Abaja community, Ebonyi State, Nigeria Conc. in mg/L: 1.445 (Cr(VI)), 0.381 (Cr(III)), 0.537(Fe(III)), 0.840 (PO43−), 0.296 (SO42−), 0.01 (Cl−); pH 6, relative density 1.09, conductivity 18.7∙10−6 S/m, turbidity 3.92 NTU, TSS 0.12 mg/L, TDS 93.5 mg/L | qmax: 0.0574 mg/g Conditions: 5 h contact time, biosorbent dosage 2.8 g, 200 rpm agitation speed No results: regeneration or the possibility of reuse | [37] |
| Cr(III) Cladodes of Oputinia ficus-inida var. ‘Orelha de elefante’: washed with water, cut into pieces with dimensions of 3 cm × 3 cm × 1 cm, then dried at 55 °C for 72 h and ground | Tannery stabilization pond in Brazil Conc. of Cr2O3 in g/L: 1.55–1.72, pH 7.25 | R in %: 74.8 and 84.9 using 2 and 4 g of biomass, respectively qmax: 611.49 mg/g Conditions: 60 min, without correction of pH No results: regeneration or the possibility of reuse | [29] |
| Cr(VI), Ni(II) Platanus orientalis bark: washed with water, dried in sun for 3 days, ground to a fine powder, and dried in oven at 100 °C for 24 h Modification of sorbent: acid activation by 0.4 M HNO3 and ddH2O for 24 h (to increase the surface area and to prevent the elution of tannin compounds) | Plating industry, Tehran, Iran Conc. in mg/L: 556.5 (Ni), 46.7 (Fe), 86.39 (Cr(VI)), 2 (Cu), 0.68 (Ag), 0.48 (Al), 0.47 (Sn), 0.44 (Pb), 0.35 (Ba), 0.18 (Sb), Hg (0.02), <0.01 (As, Bi, Co, Cd, Mo) | R in %: 89.6 and 90.7 (Cr, with non-modified and modified bark), 74.5 and 56.5 (Ni, with non-modified and modified bark) Conditions: for Cr, pH 5, 2 g/L of sorbent dosage, 5 h; for Ni, pH 3, 2 g/L sorbent dosage, 1.5 h q in mg/g: 13.42 and 19.92 (Cr, with non-modified and modified bark), 126.58 and 285.714 (Ni, with non-modified and modified bark) No results: regeneration or the possibility of reuse | [65] |
| Cu(II) and Pb(II) Shrimp shells (without heads): cleaned with water, dried at 70 °C for 12 h, ground, biological pigment and protein removed (mixed with 5 wt. %. NaOH and 1 wt. H2O2 for 3 days at 30 °C), and washed with water | Semiconductor electroplating wastewater (Vizianagaram, Andhra Pradesh, India) Conc. of Cu and Pb = 25–460 mg/L | Maximum efficiency in % (R): 96.4 for Cu and 89.8 for Pb Sorption capacity in mg/g (qmax): 5.78 for Cu and 5.39 for Pb Conditions: pH 5 for Cu, pH 6 for Pb, metal conc. 20 mg/L, biosorbent dosage 0.1 g and temp. 30 °C No results: regeneration or the possibility of reuse | [36] |
| Cu(II) Red alga Gracilaria chilensis Material pretreatment: dried alga suspended in 0.2 M CaCl2 at pH 5 for 4 h, then washed several times with deionized water to remove excess calcium, filtered, and dried for 12 h at 60 °C | A solution obtained after leaching with 1 M H2SO4 of mining tailings from an abandoned deposit in the north of Chile and subsequent Fe precipitation Conc. in mg/L: 200 (Fe ions), 150 (Cu(II)), pH 1.5 | Cu(II) qmax 0.311 mmol/g No data for Fe ion sorption 35% Cu(II) desorption with 0.05 M H2SO4 | [66] |
| Fe, Mn, Cr, As, Cd, Ni, and Pb Opuntia ficus-indica mucilage: washed with fresh water and liquid soap before procedure of mucilage extraction (heated at 40 °C, stirred at 300 rpm for 4 h, filtered and refrigerated at 4 °C for 18 h, freeze-dried under vacuum (0.04 mbar) for 6 days) | Yautepec River, Morelos, México; sources of pollution: livestock, agricultural, recreation, public and industrial activities (automotive, food, cosmetic, pharmaceutical, colorant, textile, chemical, agrochemical, and metallurgical), ashes and gases from Popocatépetl volcano Conc. in µg/L: 4.3–14.7 (Cu), 0.2–9.5 (Cd), 3.7–13.4 (Cr), 0–44.1 (Ni), 9.3–80.8 (Pb), 7.4–19.6 (Zn), 3–405.9 (Mn), 44.5–1546 (Fe), 2–9.1 (As), pH 5.9–8.5, turbidity 0–3.4 NTU, conductivity 239–2628 µS/cm | R in %: 96 (Fe), 91 (Mn), 70 (As), 60 (Cr), 39 (Ni), 32 (Cd), 26 (Pb) No results: regeneration or the possibility of reuse | [67] |
| Co(II), Ni(II), Zn(II), Cu(II) Serratia marcescens strain 16: isolated from serpentine deposits located in Moa (Cuba) | Synthetic solution based on composition from residual liquor WL from the company Moa Nickel S. A. Cuba Conc. in mg/L: 2 (Co(II)), 25 (Ni(II)), 15 (Zn(II)), (Cu(II)) | R in % after four cycles in monometallic systems: 60.9 (Co), 53.6 (Ni), 43.1 (Cu), 78.8% (Zn) R in % after four cycles in multimetallic systems: 39.7 (Co), 40.2% (Ni), 42.8% (Cu), 44.7 (Zn) The monometallic system exhibited a sorption capacity two to three times greater compared to the presence of bi-metallic and multimetallic solutions, q for monometallic solution in mg/g: 2.3 (Co), 11.4 (Ni), 8.6 (Cu), 11.9 (Zn) Conditions: contact time 2 h, biomass 0.6 g/L Desorption of metals and reuse: 0.1 M HCl for desorption (90% after 10 min), several times reduction in sorption capacity after 4 cycles | [68] |
| Pb(II), Cu(II), Zn(II), Ni(II) Gossypium hirsutum stems from the fields of Khanewal, Pakistan: dried for 15 days, washed with water, dried at 50 °C, then ground, washed with water, and dried in an oven at 60 °C. Modification in the solution of 0.2 M HCl and 0.2 M NaOH separately | Industrial effluents from discharge points (including textile and electric cable manufacturers; tanneries; and pesticide, pharmaceutical, and fertilizer plants) in Multan, Pakistan Conc. in mg/L: 3.7 (Pb), 3.9 (Cu), 6.3 (Zn), 2.5 (Ni) | R in %: 78.5 (Pb), 80.3 (Cu), 81.4 (Zn) and 82.6 (Ni) q in mg/g: 121.2 (Pb), 117.09 (Cu), 130.6 (Zn), 111.09 (Ni) Conditions: 0.5 g sorbent, 30 °C, pH 5.5, 30 min Regeneration using 0.1 M HNO3, only from model solutions, 5 cycles, with desorption efficiency in % (92.9–85.4 (Pb), 93.2–86.1 (Cu), 92.5–85.9 (Zn), 93.8–84.8 (Ni)) | [69] |
| Pb(II) Lactic acid bacteria: Limosilactobacillus fermentum CN-005, Lactobacillus fermentum CN-011 | Simulated wastewater collected from Taihu Lake, China, and spiked with Pb(II) standard solution at five levels: 12.92, 16.17, 17.70, 20.22, and 23.94 mg/L | Average sorption efficiency of Pb(II): 73.38% with CN-011, 74.15% with CN-005 No results: the possibility of reuse or further treatment of the biomass | [70] |
| Zn(II), Fe(II), Pb(II), Cu(II) Arthrospira platensis microalgae cultivated in mining wastewater | Mining wastewater from surface and underground water in Huangshaping, Hunan Province, China | Biosorption efficiency at pH > 7.1: 93% SO42−, 99% Fe(II), 95% Pb(II), 89% Zn(II), 94% Cu(II). No results: regeneration, the possibility of reuse, or further treatment of the biomass | [71] |
| Pb(II), Ni(II) 24 heavy metal-resistant fungi isolated from different industrial wastes from India (near areas of different metal-fed industries) | Sewage, sludge, and effluents collected from 23 different industrial units located at different locations in India | R in %: 93 for Pb using resistant fungi, Aspergillus terreus and Talaromyces islandicus; 91 for Ni using Neurospora crassa and Aspergillus flavus; and 95 for Pb and Ni using the fungal consortia | [72] |
| Zn(II) Sawdust of Indian rosewood from a timber industry, Bathinda (Punjab), India Sawdust-derived biosorbents: after boiling (SDB), chemical modification with formaldehyde (SDF) and sulfuric acid (SDS) | A real electroplating industrial effluent (Ludhiana, Punjab, India) Conc. in mg/L: 26–46 (Zn), 0.14–1.86 (Cu), 0.05–1.76 (Ni); 37–540 ppm of sulfates, pH 1.65–5.36 | Zn(II) qeq: 35.72 mg/g (SDB), 43.74 mg/g (SDF), 45.87 mg/g (SDS) at pH 6 Pore size of biosorbents in m2/g: 232.928 (SDB), 291.102 (SDF), 498.873 (SDS) | [57] |
| Zn(II) Sugarbeet pulp and brown alga Fucus vesiculosus from the northern Atlantic coast of Spain in small glass columns (2.5 cm inner diameter and 40 cm length) or F. vesiculosus in glass columns (7.5 cm inner diameter and 100 cm length)—a pilot plant | Continuous biosorption tests with real effluents from Industrial Goñabe (Valladolid, Spain) Conc. in mg/L: 546 (Zn), 22.9 (Fe), 10.8 (Cr), 0.129 (Cu), 0.050 (Ni), 116 (sulfate), 2520 (chloride), pH 1.45 | At F. vesiculosus qmax = 0.94 mmol Zn(II)/g at pH adjusted to 5 3 consecutive cycles of continuous sorption–desorption (with 1 N HNO3) and regeneration with deionized water | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staszak, K.; Regel-Rosocka, M. Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology. Materials 2024, 17, 1155. https://doi.org/10.3390/ma17051155
Staszak K, Regel-Rosocka M. Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology. Materials. 2024; 17(5):1155. https://doi.org/10.3390/ma17051155
Chicago/Turabian StyleStaszak, Katarzyna, and Magdalena Regel-Rosocka. 2024. "Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology" Materials 17, no. 5: 1155. https://doi.org/10.3390/ma17051155
APA StyleStaszak, K., & Regel-Rosocka, M. (2024). Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology. Materials, 17(5), 1155. https://doi.org/10.3390/ma17051155