The Selective Removal of Bisphenol A Using a Magnetic Adsorbent Fused with Bisphenol A-Binding Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
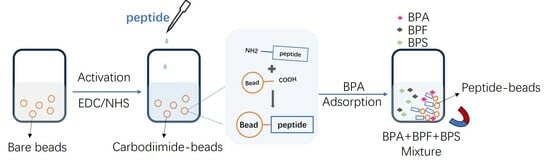
2.2. Construction of Peptide Bead
2.3. Bisphenol A Adsorption
2.4. Analysis
3. Results
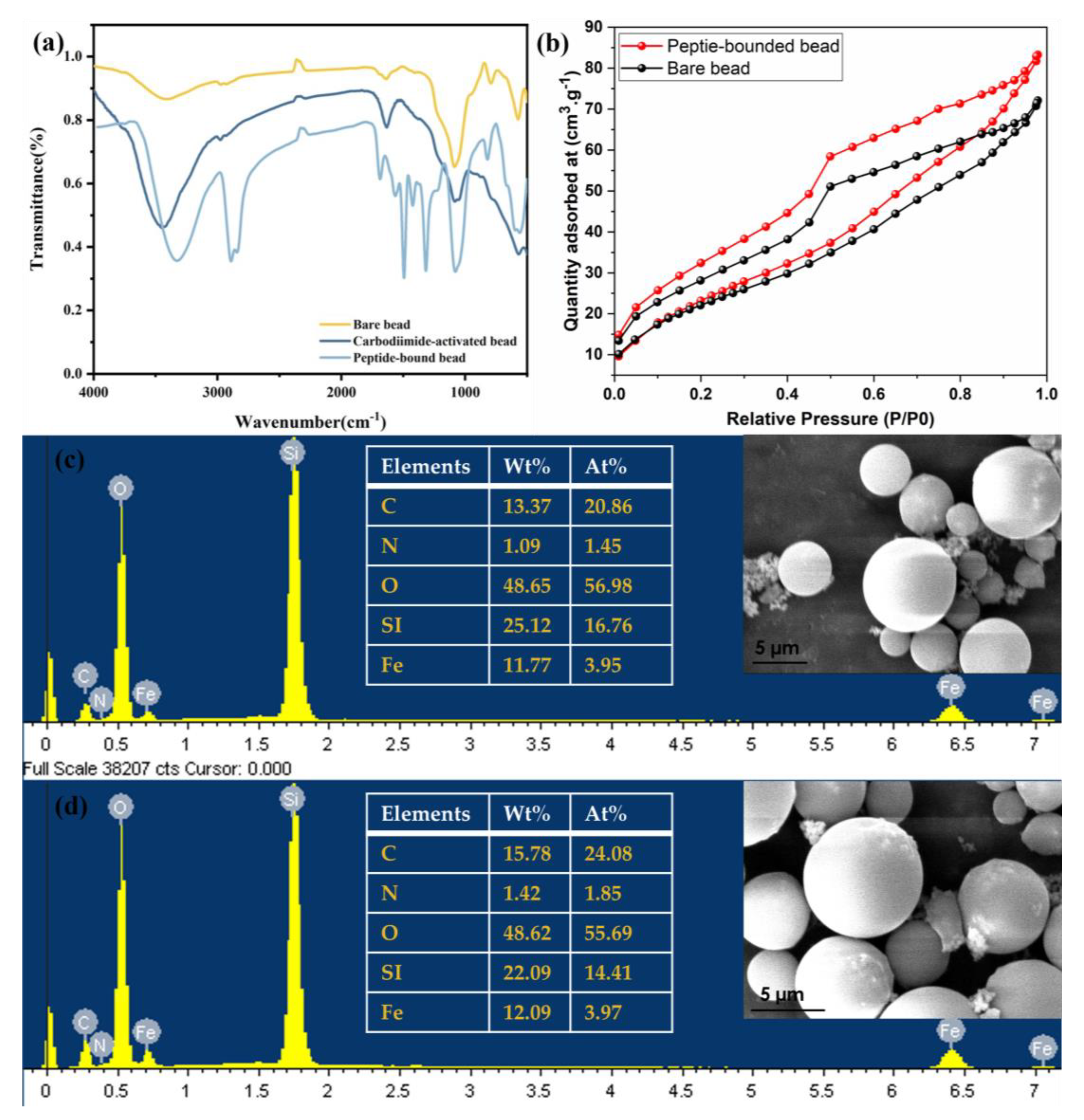
3.1. Preparation and Characterization of Peptide Beads
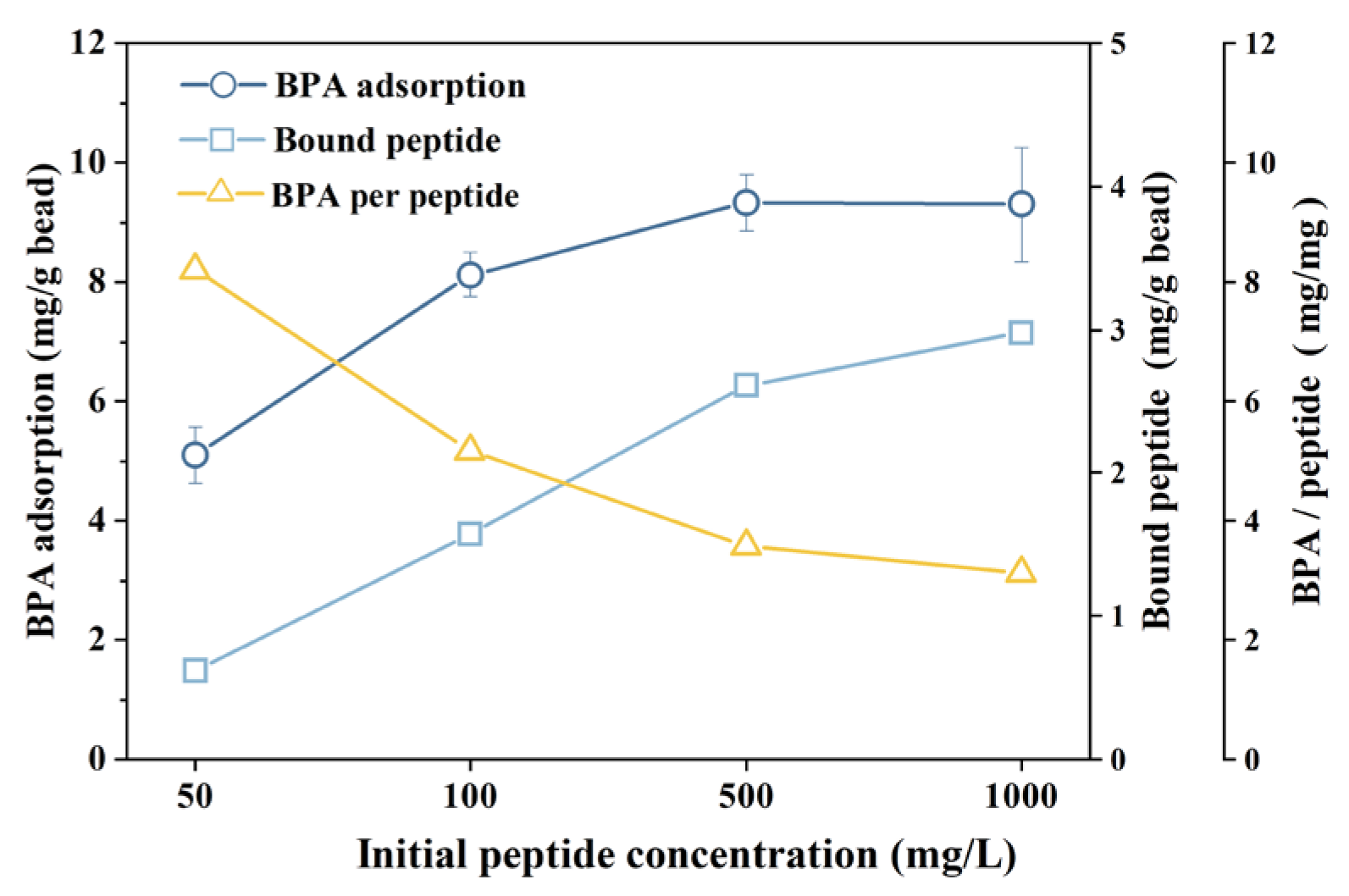
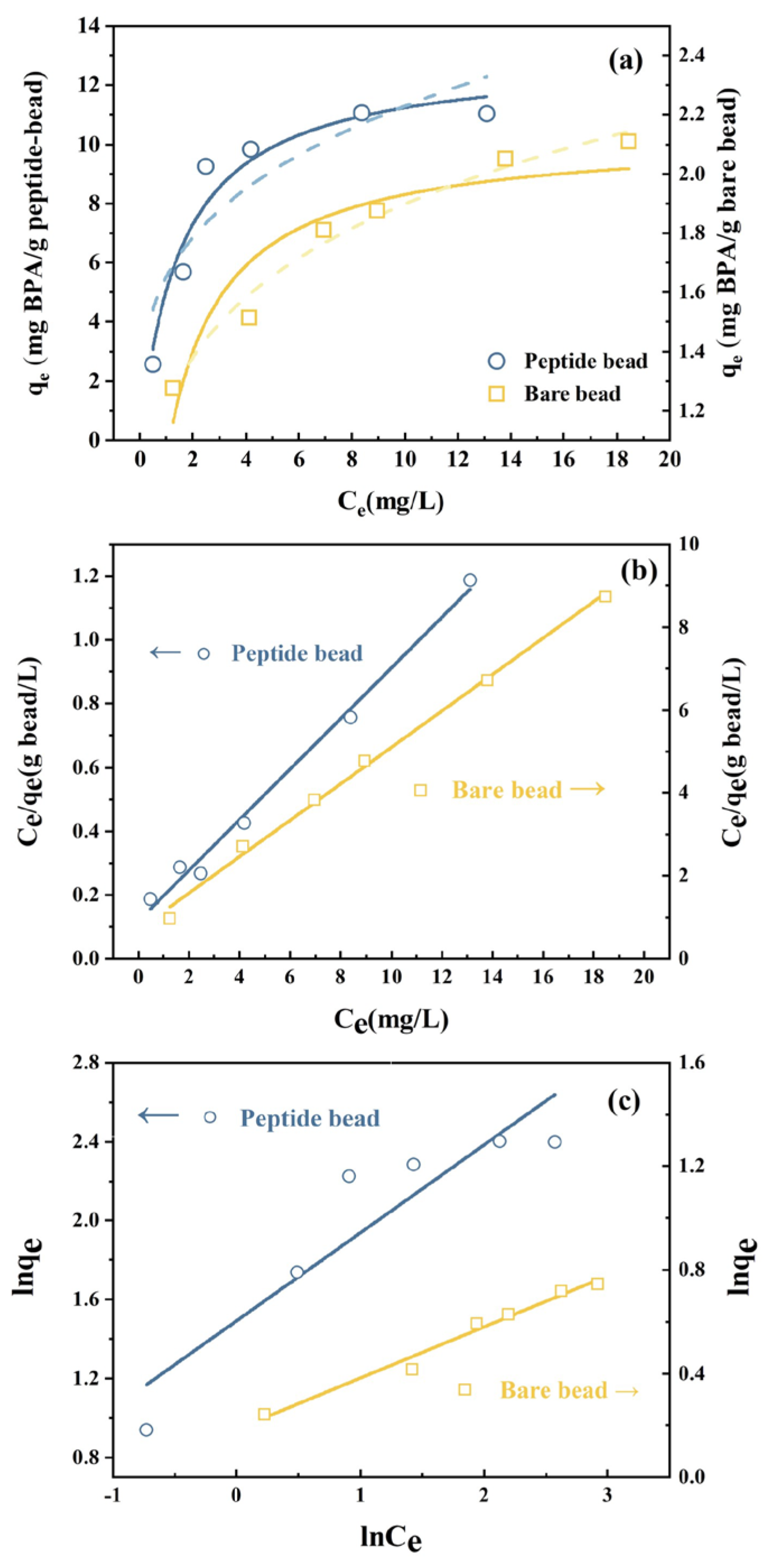
3.2. Bisphenol A Adsorption on Peptide Beads
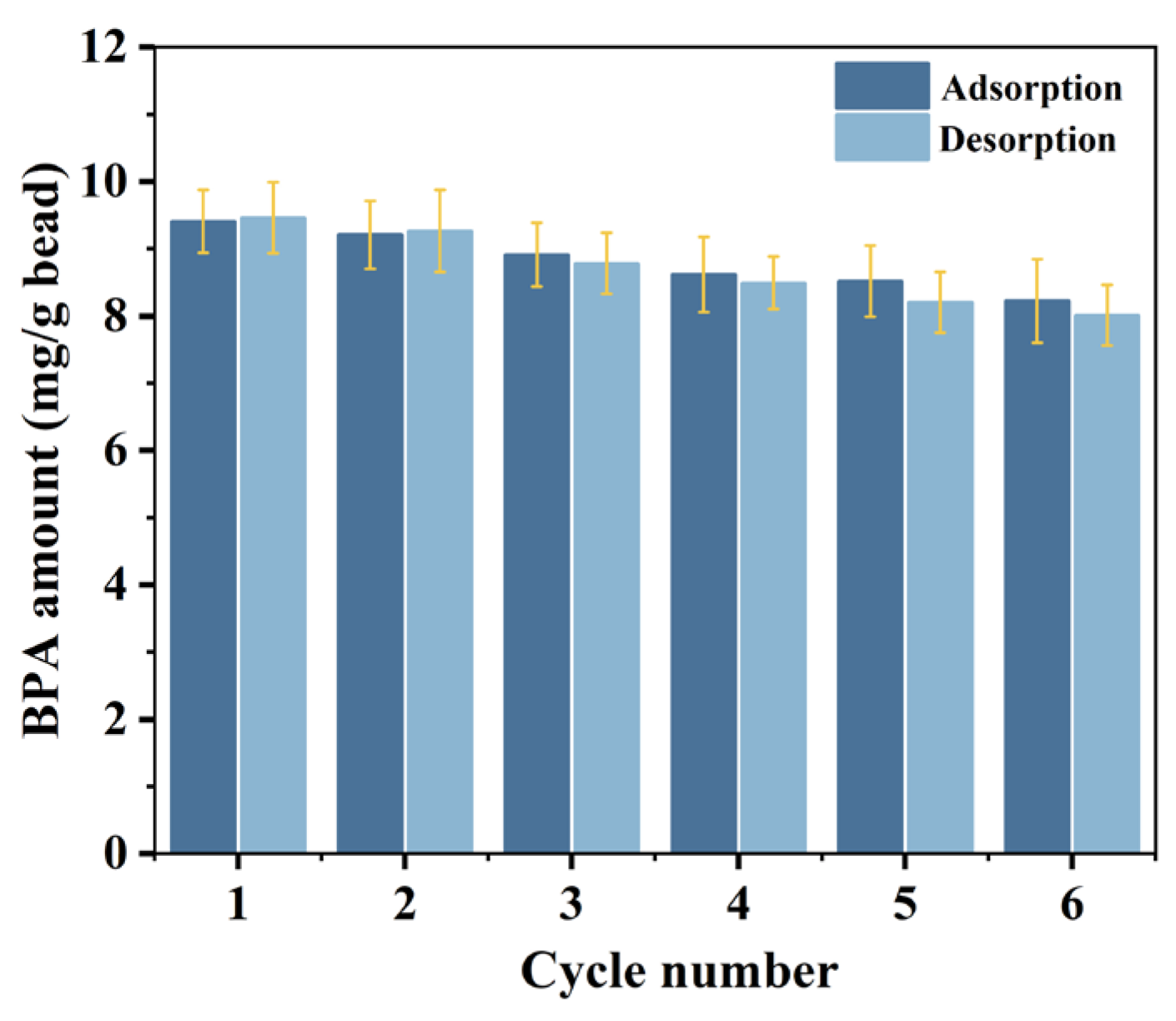
3.3. Reusability of Peptide Bead
3.4. Selective Bisphenol A Adsorption
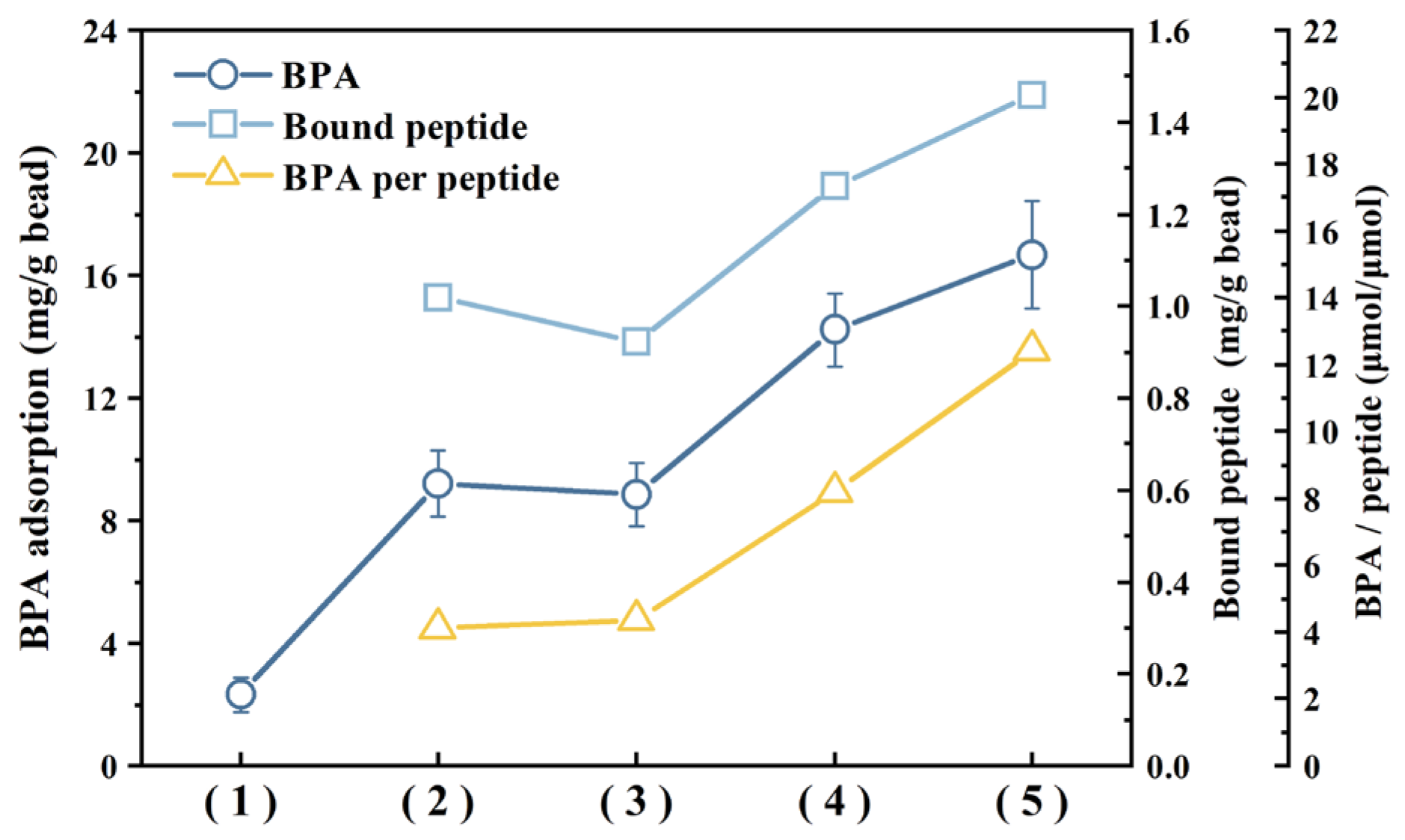
3.5. Bisphenol A Adsorption Using Different Lengths of Peptide Repeats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Anastopoulos, L. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Kim, H.J.; Choi, Y.K.; Lee, H.J.; Ham, S.; Cho, J.Y.; Kim, S.H.; Lee, S.H.; et al. Adsorptive removal of synthetic plastic components bisphenol-A and solvent black-3 dye from single and binary solutions using pristine pinecone biochar. Chemosphere 2022, 296, 134034. [Google Scholar] [CrossRef] [PubMed]
- Orimolade, B.O.; Adekola, F.A.; Adebayo, G.B. Adsorptive removal of bisphenol A using synthesized magnetite nanoparticles. Appl. Water Sci. 2018, 8, 46. [Google Scholar] [CrossRef]
- Orozco, A.F.; Lobo, C.; Contreras, E.; Zaritzky, N. Biodegradation of bisphenol-A (BPA) in activated sludge batch reactors: Analysis of the acclimation process. Int. Biodeterior. Biodegrad. 2013, 85, 392–399. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, H.; Zhang, Y.; Deng, Q.; Zhang, J. Degradation of bisphenol A using ozone/persulfate process: Kinetics and mechanism. Water Air Soil Pollut. 2016, 227, 53. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Cao, S.; Duan, X. Investigation on enhanced photocatalytic degradation of bisphenol A with bismuth oxyiodide catalyst using response surface methodology. RSC Adv. 2018, 8, 5967–5975. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ryu, K.; Kim, E.J.; Choe, W.S.; Cha, G.C.; Yoo, I.-K. Degradation of bisphenol A and nonylphenol by nitrifying activated sludge. Process. Biochem. 2007, 42, 1470–1474. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.-H.; Zhang, J.; Huang, R.-P.; Yin, H.; Dang, Z.; Wu, P.-X.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Ghadermazi, M.; Bhatnagar, A.; Sadighara, P.; Jahed-Khaniki, G.; Heibati, B.; McKay, G. Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. J. Environ. Chem. Eng. 2016, 4, 2647–2655. [Google Scholar] [CrossRef]
- Xiang, Y.; Yan, H.; Zheng, B.; Faheem, A.; Chen, W.; Hu, Y.G.E. coli@ UiO-67 composites as a recyclable adsorbent for bisphenol A removal. Chemosphere 2021, 270, 128672. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; Saadoun, I.; Kumar, K.; Helmy, M.; Dhankher, O.P. Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front. Plant Sci. 2016, 7, 303. [Google Scholar] [CrossRef]
- Park, G.Y.; Lee, J.Y.; Himes, R.A.; Thomas, G.S.; Blackburn, N.J.; Karlin, K.D. Copper–peptide complex structure and reactivity when found in conserved his-xaa-his sequences. J. Am. Chem. Soc. 2014, 136, 12532–12535. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, S.-E.; Cho, M.; Yoo, I.-K.; Choe, W.-S.; Lee, Y. Highly sensitive and selective determination of bisphenol-A using peptide-modified gold electrode. Biosens. Bioelectron. 2014, 61, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of Palladium (II) with a Magnetic Sorbent Based on Polyvinyl Alcohol Gel, Metallic Iron, and an Environmentally Friendly Polydentate Phosphazene-Containing Extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, Y.; Gao, X.; Zheng, H.; Niu, Y.; Liu, J. Adsorption Performance of Magnetic Covalent Organic Framework Composites for Bisphenol A and Ibuprofen. Molecules 2023, 28, 5214. [Google Scholar] [CrossRef]
- Rekos, K.; Kampouraki, Z.-C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene oxide based magnetic nanocomposites with polymers as effective bisphenol–A nanoadsorbents. Materials 2019, 12, 1987. [Google Scholar] [CrossRef]
- Wang, R.-Z.; Huang, D.-L.; Liu, Y.-G.; Peng, Z.-W.; Zeng, G.-M.; Lai, C.; Xu, P.; Huang, C.; Zhang, C.; Gong, X.-M. Selective removal of BPA from aqueous solution using molecularly imprinted polymers based on magnetic graphene oxide. RSC Adv. 2016, 6, 106201–106210. [Google Scholar] [CrossRef]
- Fischer, M.J.E. Amine coupling through EDC/NHS: A practical approach. In Surface Plasmon Resonance; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 627, pp. 55–73. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef]
- Yoo, I.-K.; Choe, W.-S. Screening of peptide sequences with affinity to bisphenol A by biopanning. Korean J. Microbiol. 2013, 49, 211–214. [Google Scholar] [CrossRef]
- Wang, C.K.; Swedberg, J.E.; Northfield, S.E.; Craik, D.J. Effects of cyclization on peptide backbone dynamics. J. Phys. Chem. B 2015, 119, 15821–15830. [Google Scholar] [CrossRef]
- Bhargawa, B.; Sharma, V.; Ganesh, M.-R.; Cavalieri, F.; Ashokkumar, M.; Neppolian, B.; Sundaramurthy, A. Lysozyme microspheres incorporated with anisotropic gold nanorods for ultrasound activated drug delivery. Ultrason. Sonochem. 2022, 86, 106016. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Choo, K.-H.; Lee, B.; Choi, S.-J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Guo, W.; Hu, W.; Pan, J.; Zhou, H.; Guan, W.; Wang, X.; Dai, J.; Xu, L. Selective adsorption and separation of BPA from aqueous solution using novel molecularly imprinted polymers based on kaolinite/Fe3O4 composites. Chem. Eng. J. 2011, 171, 603–611. [Google Scholar] [CrossRef]
- Bhargawa, B.; Hong, S.H.; Yoo, I.-K. Adsorptive lithium recovery by magnetic beads harboring lithium-binding peptide. Desalination 2024, 577, 117412. [Google Scholar] [CrossRef]
- Bautista-Toledo, I.; Ferro-García, M.A.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Fernández, F.J.V. Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA; London, UK, 1999. [Google Scholar]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Fu, L.; Li, A. Enhanced adsorption of bisphenol A from water by acetylaniline modified hyper-cross-linked polymeric adsorbent: Effect of the cross-linked bridge. Chem. Eng. J. 2012, 191, 171–176. [Google Scholar] [CrossRef]
- Chen, H.; Su, X.; Neoh, K.-G.; Choe, W.-S. QCM-D Analysis of binding mechanism of phage particles displaying a constrained heptapeptide with specific affinity to SiO2 and TiO2. Anal. Chem. 2006, 78, 4872–4879. [Google Scholar] [CrossRef]
- Chen, H.; Su, X.; Neoh, K.-G.; Choe, W.-S. Context-dependent adsorption behavior of cyclic and linear peptides on metal oxide surfaces. Langmuir 2008, 25, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Thai, C.K.; Dai, H.; Sastry, M.; Sarikaya, M.; Schwartz, D.T.; Baneyx, F. Identification and characterization of Cu2O-and ZnO-binding polypeptides by Escherichia coli cell surface display: Toward an understanding of metal oxide binding. Biotechnol. Bioeng. 2004, 87, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R.; Örnek, D.; Wood, T.K. Aluminum- and mild steel-binding peptides from phage display. Appl. Microbiol. Biotechnol. 2005, 68, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Chatla, A.; Almanassra, I.W.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Efficient removal of eriochrome black T (EBT) dye and chromium (Cr) by hydrotalcite-derived Mg-Ca-Al mixed metal oxide composite. Catalysts 2022, 12, 1247. [Google Scholar] [CrossRef]
- de Lima, H.H.C.; Llop, M.E.G.; Maniezzo, R.d.S.; Moisés, M.P.; Janeiro, V.; Arroyo, P.A.; Guilherme, M.R.; Rinaldi, A.W. Enhanced removal of bisphenol A using pine-fruit shell-derived hydrochars: Adsorption mechanisms and reusability. J. Hazard. Mater. 2021, 416, 126167. [Google Scholar] [CrossRef] [PubMed]
- Gaber, D.; Abu Haija, M.; Eskhan, A.; Banat, F. Graphene as an efficient and reusable adsorbent compared to activated carbons for the removal of phenol from aqueous solutions. Water Air Soil Pollut. 2017, 228, 320. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y.; Liman, G.; Celikbicak, O.; Salih, B. Removal of bisphenol A from aqueous medium using molecularly surface imprinted microbeads. Chemosphere 2016, 150, 275–284. [Google Scholar] [CrossRef]
- Maruthamuthu, M.K.; Hong, J.; Arulsamy, K.; Somasundaram, S.; Hong, S.; Choe, W.-S.; Yoo, I.-K. Development of bisphenol A-removing recombinant Escherichia coli by monomeric and dimeric surface display of bisphenol A-binding peptide. Bioprocess Biosyst. Eng. 2017, 41, 479–487. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, B.; Choo, K.-H.; Choi, S.-J. Selective adsorption of bisphenol A by organic–inorganic hybrid mesoporous silicas. Microporous Mesoporous Mater. 2011, 138, 184–190. [Google Scholar] [CrossRef]
- Le Noir, M.; Lepeuple, A.-S.; Guieysse, B.; Mattiasson, B. Selective removal of 17β-estradiol at trace concentration using a molecularly imprinted polymer. Water Res. 2007, 41, 2825–2831. [Google Scholar] [CrossRef]
- Fan, X.; Tu, B.; Ma, H.; Wang, X. Adsorption behavior of environmental hormone Bisphenol A onto mesoporous silicon dioxide. Bull. Korean Chem. Soc. 2011, 32, 2560–2564. [Google Scholar] [CrossRef][Green Version]
- Duan, F.; Chen, C.; Zhao, X.; Yang, Y.; Liu, X.; Qin, Y. Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol A from aqueous solution. Environ. Sci. Nano 2016, 3, 213–222. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Huang, J.; Wei, C. Detailed characteristics of adsorption of bisphenol A by highly hydrophobic MCM-41 mesoporous molecular sieves. Res. Chem. Intermed. 2016, 42, 7169–7183. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, D.; Zhang, B.S. Novel molecularly imprinted polymer prepared by palygorskite as support for selective adsorption of bisphenol A in aqueous solution. Desalin. Water Treat. 2016, 57, 12433–12442. [Google Scholar] [CrossRef]
- Maruthamuthu, M.K.; Selvamani, V.; Eom, G.T.; Hong, S.H. Development of recA promoter based bisphenol-A sensing and adsorption system by recombinant Escherichia coli. Biochem. Eng. J. 2017, 122, 31–37. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wu, Y.; Bhargawa, B.; Hong, S.H.; Yoo, I.-K. The Selective Removal of Bisphenol A Using a Magnetic Adsorbent Fused with Bisphenol A-Binding Peptides. Materials 2024, 17, 1651. https://doi.org/10.3390/ma17071651
Xu Y, Wu Y, Bhargawa B, Hong SH, Yoo I-K. The Selective Removal of Bisphenol A Using a Magnetic Adsorbent Fused with Bisphenol A-Binding Peptides. Materials. 2024; 17(7):1651. https://doi.org/10.3390/ma17071651
Chicago/Turabian StyleXu, Yue, Yujie Wu, Bharat Bhargawa, Soon Ho Hong, and Ik-Keun Yoo. 2024. "The Selective Removal of Bisphenol A Using a Magnetic Adsorbent Fused with Bisphenol A-Binding Peptides" Materials 17, no. 7: 1651. https://doi.org/10.3390/ma17071651
APA StyleXu, Y., Wu, Y., Bhargawa, B., Hong, S. H., & Yoo, I.-K. (2024). The Selective Removal of Bisphenol A Using a Magnetic Adsorbent Fused with Bisphenol A-Binding Peptides. Materials, 17(7), 1651. https://doi.org/10.3390/ma17071651