Polysaccharide-Based Composite Systems in Bone Tissue Engineering: A Review
Abstract
1. Introduction
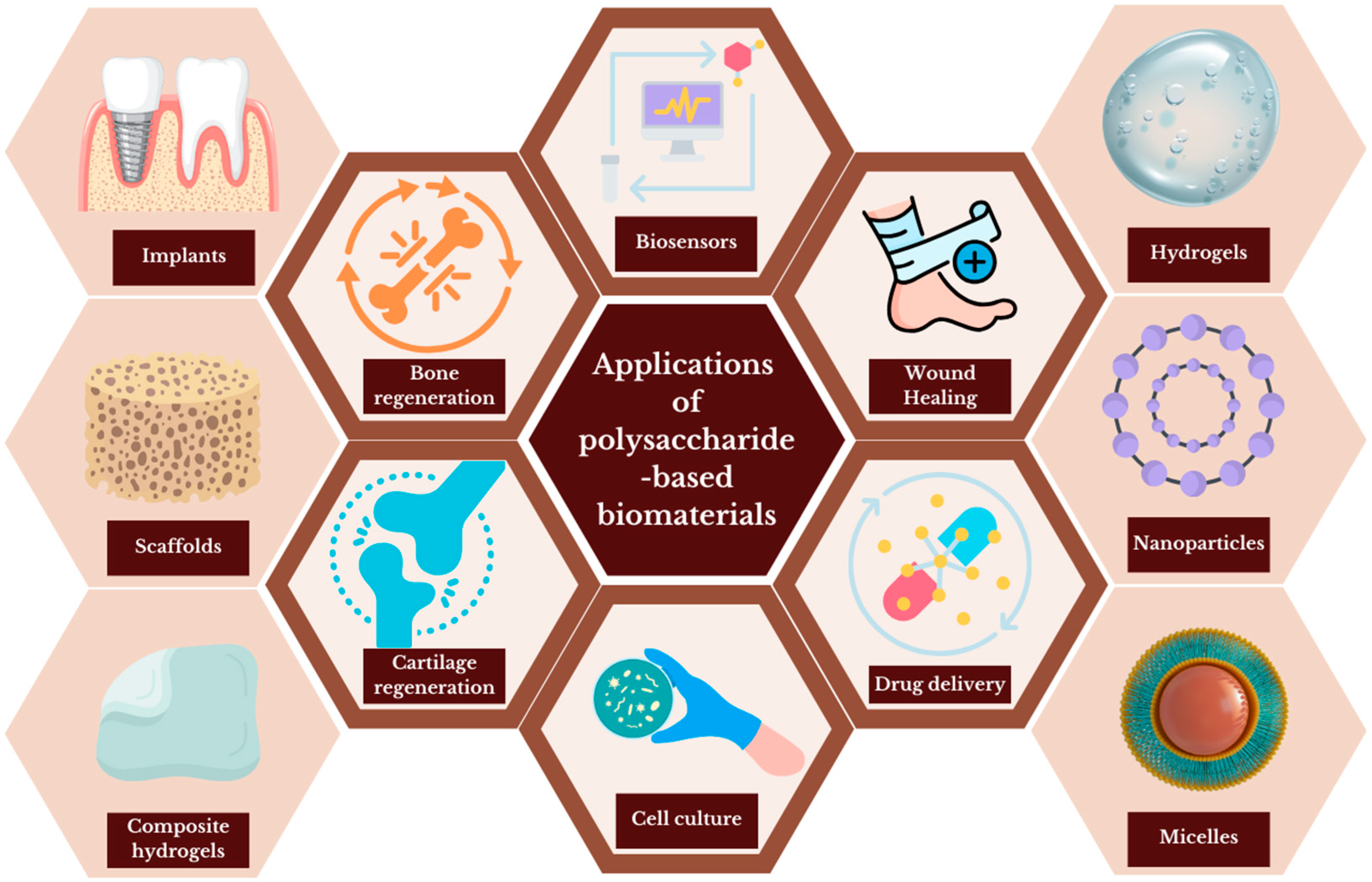
2. Biomedical Applications of Polysaccharide-Based Biomaterials
| Polysaccharide | Advantages | Disadvantages | References |
|---|---|---|---|
| Chitosan |
|
| [17,18] |
| Hyaluronic acid |
|
| [19,20] |
| Pullulan |
|
| [21,22] |
| Arabinoxylan |
|
| [23,24] |
| Inulin |
|
| [25] |
| Dextran |
|
| [26] |
| Chondroitin sulfate |
|
| [27] |
| Heparin |
|
| [28] |
3. Selected Polysaccharides in Bone Tissue Regeneration
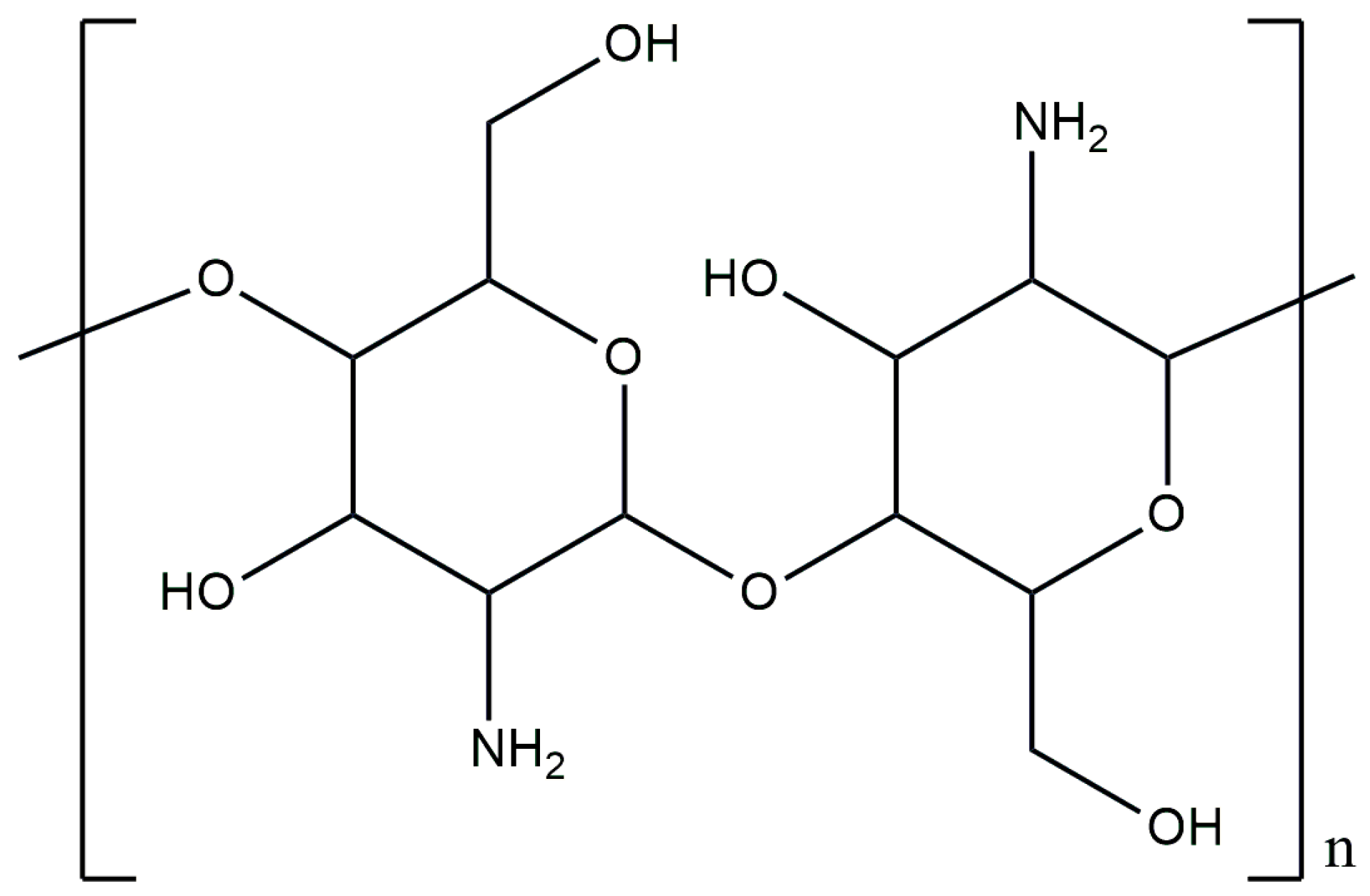
3.1. Chitosan
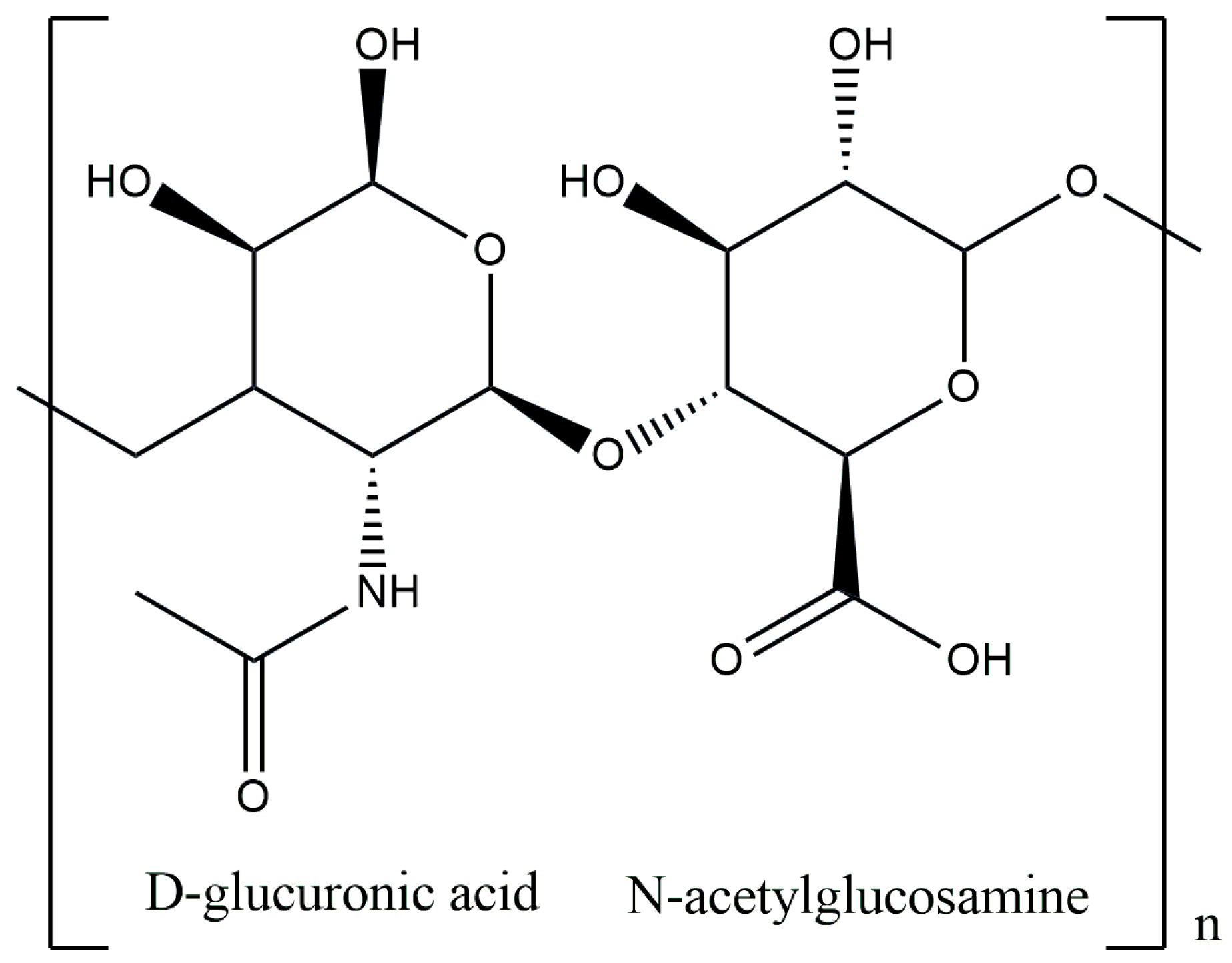
3.2. Hyaluronic Acid
3.3. Pullulan
3.4. Arabinoxylan
3.5. Inulin
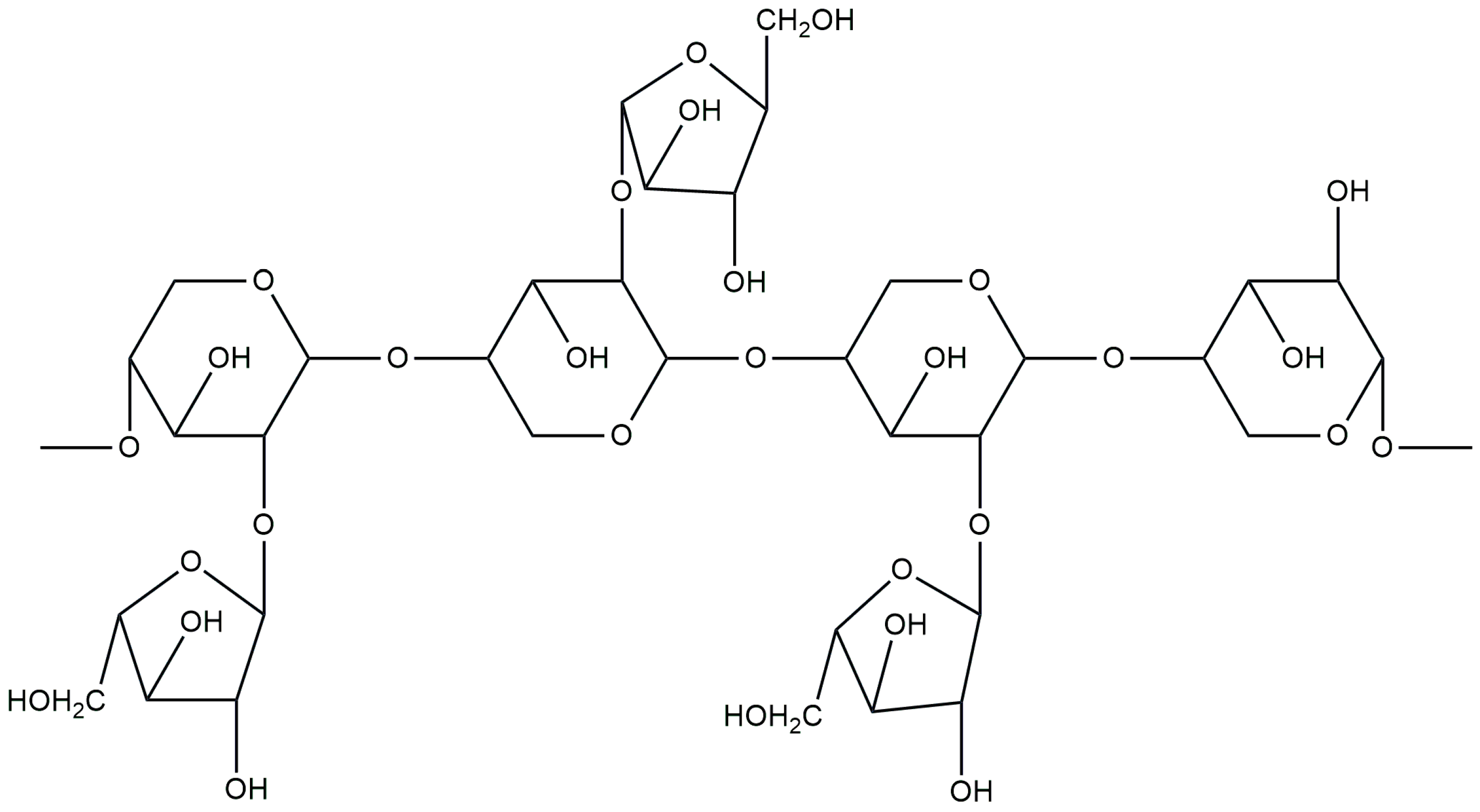
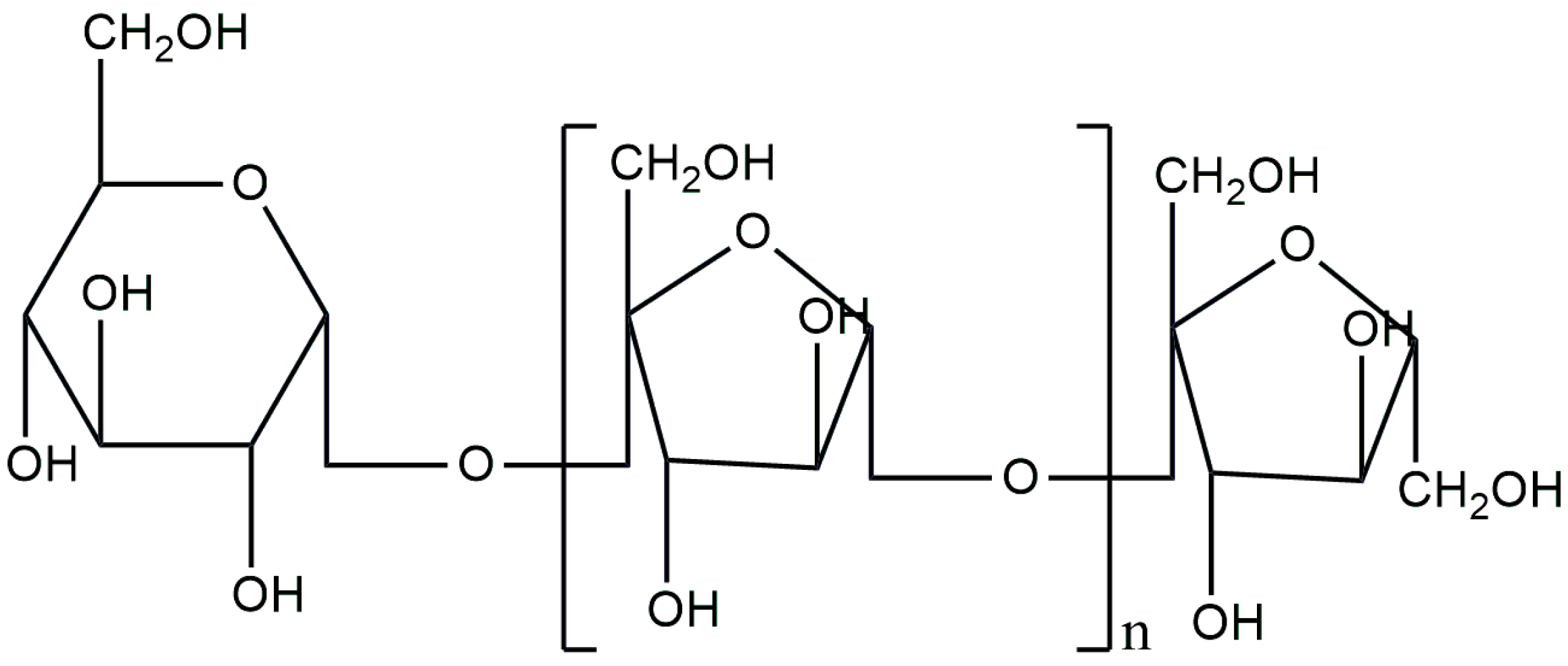
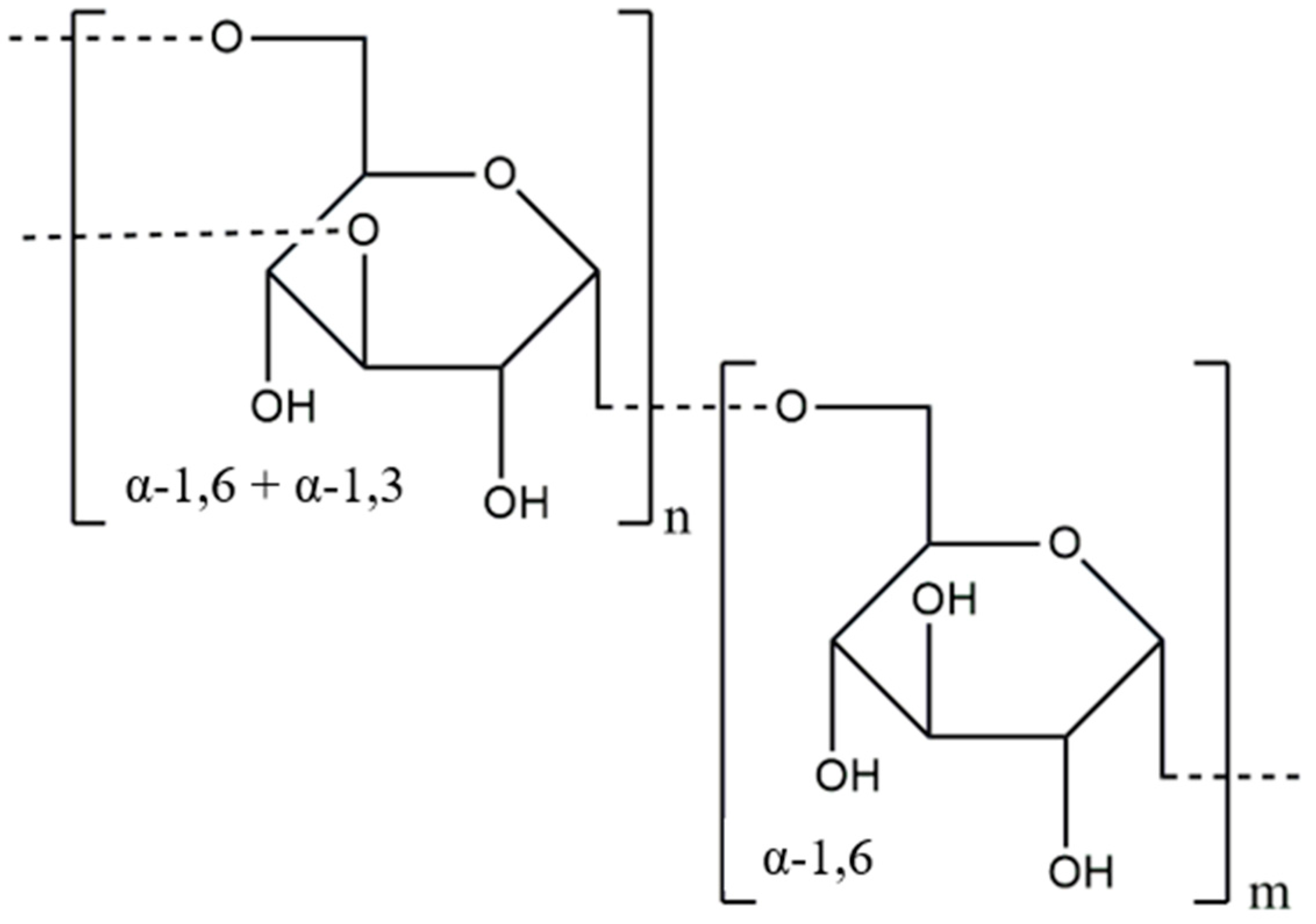
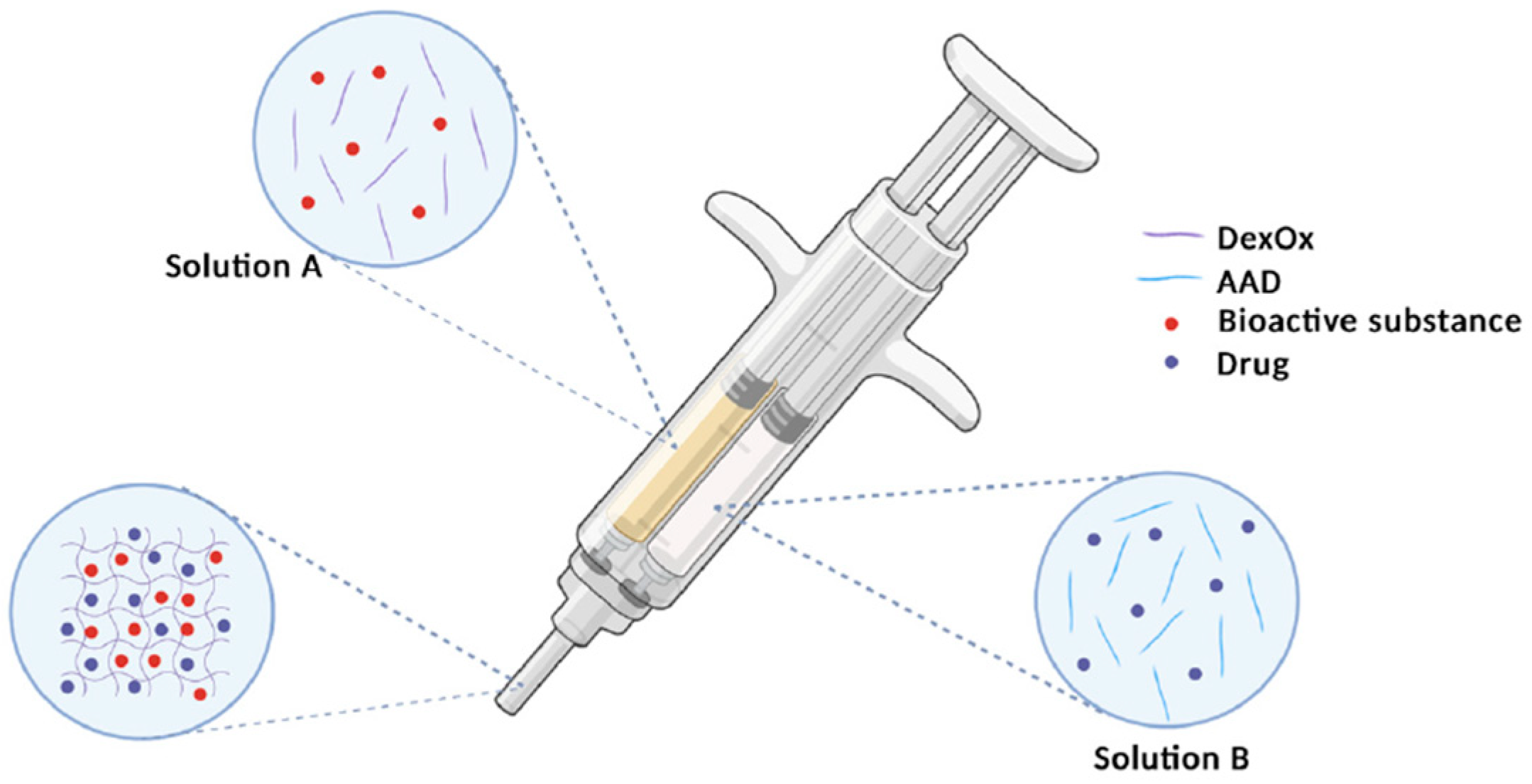
3.6. Dextran
3.7. Chondroitin Sulfate
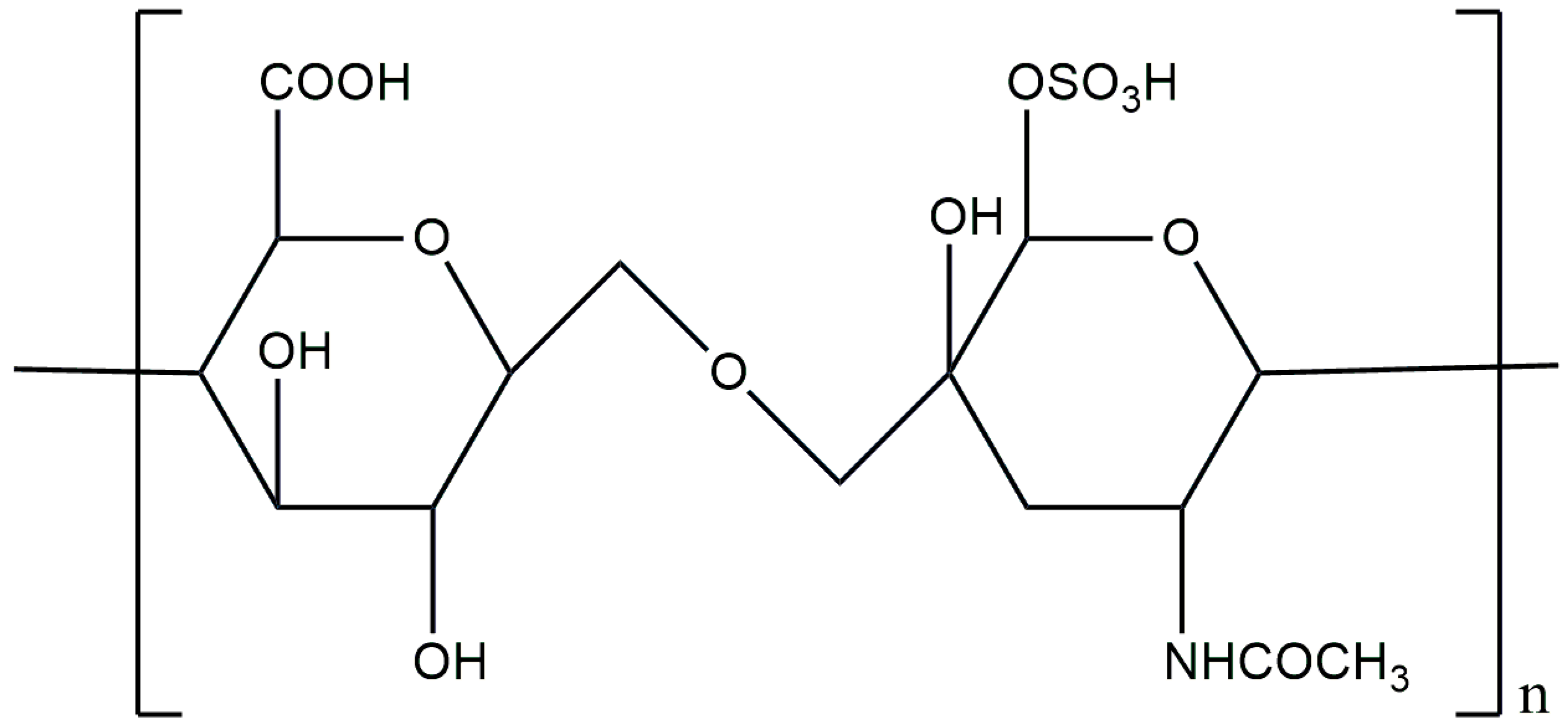
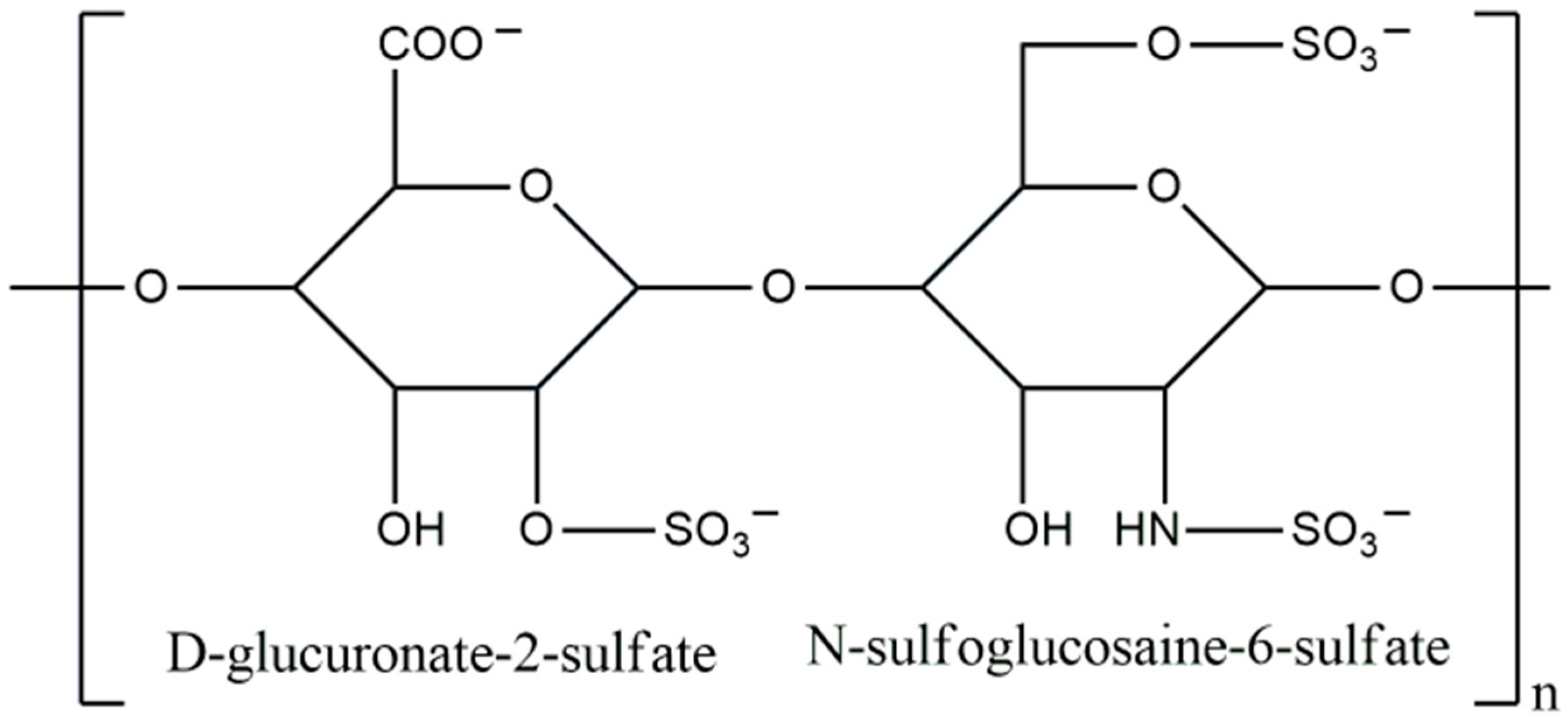
3.8. Heparin
4. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AX | arabinoxylan |
| BHa | biomimetic hydroxyapatite |
| BMP | bone morphogenic protein |
| BTE | bone tissue engineering |
| CAGR | compound annual growth rate |
| CaP | calcium phosphate ceramic |
| ChS | chondroitin sulfate |
| COL | collagen |
| CS | chitosan |
| DCPD | dicalcium phosphate dihydrate |
| DD | degree of deacetylation |
| DDS | drug delivery system |
| DEX | dextran |
| DS | diatom shell |
| ECM | extracellular matrix |
| FDA | Food and Drug Administration |
| GAG | glycosaminoglycans |
| GE | gelatin |
| GF | growth factors |
| GO | graphene oxide |
| GRAS | generally recognized as safe |
| HA | hyaluronic acid |
| HAp | hydroxyapatite |
| HE | heparin |
| HIT | heparin-induced thrombocytopenia |
| hMSCs | human mesenchymal stem cells |
| HMW-HA | high-molecular-weight hyaluronic acid |
| INL | inulin |
| LMW-HA | low-molecular-weight hyaluronic acid |
| nHAp | nanohydroxyapatite |
| PCL | poly(ε-caprolactone) |
| PDGF | platelet-derived growth factor |
| PHB | poly(3-hydroxybutyrate) |
| PHBV | poly(hydroxybutyrate-co-hydroxyvalerate) |
| PLA | polylactic acid |
| PSAs | polysaccharides |
| PUL | pullulan |
| PVA | polyvinyl alcohol |
| SBF | simulated body fluid |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
| β-TCP | tricalcium phosphate |
References
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Fattore, A. Del Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Biomaterials Market by Type (Metallic (Gold, Magnesium), Ceramic (Aluminum Oxide, Carbon), Polymer (Polyethylene, Polyester), Natural (Hyaluronic Acid, Collagen, Gelatin)), Application (Orthopedic, Dental, CVD, Ophthalmology) & Region—Global Forecast to 20. Available online: https://www.marketsandmarkets.com/Market-Reports/biomaterials-393.html (accessed on 8 July 2024).
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021, 27, 604–626. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Novel Approaches and Biomaterials for Bone Tissue Engineering: A Focus on Silk Fibroin. Materials 2022, 15, 6952. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Safaei, M.; Zarei, K.; Moradi, M.; Yazdani Nezhad, H. A Review on Biomedical Application of Polysaccharide-Based Hydrogels with a Focus on Drug Delivery Systems. Polymers 2022, 14, 5432. [Google Scholar] [CrossRef]
- Jabeen, N.; Atif, M. Polysaccharides Based Biopolymers for Biomedical Applications: A Review. Polym. Adv. Technol. 2024, 35, e6203. [Google Scholar] [CrossRef]
- Verma, A.; Tiwari, A.; Panda, P.K.; Saraf, S.; Jain, A.; Jain, S.K. Locust Bean Gum in Drug Delivery Application. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128170557. [Google Scholar]
- Wen, Y.; Oh, J.K. Recent Strategies to Develop Polysaccharide-Based Nanomaterials for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J.Y. Advancing the Stimuli Response of Polymer-Based Drug Delivery Systems for Ocular Disease Treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Abbasi, Y.F.; Bera, H.; Cun, D.; Yang, M. Recent Advances in PH/Enzyme-Responsive Polysaccharide-Small-Molecule Drug Conjugates as Nanotherapeutics. Carbohydr. Polym. 2023, 312, 120797. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Sajadi, S.M.; Khodadadi Yazdi, M.; Seidi, F.; Jouyandeh, M.; Zarrintaj, P.; Kar, S.; Kim, S.J.; Kuang, T.; et al. Polysaccharide-Based Nanocomposites for Biomedical Applications: A Critical Review. Nanoscale Horiz. 2022, 7, 1136–1160. [Google Scholar] [CrossRef]
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural Polymers Used in the Dressing Materials for Wound Healing: Past, Present and Future. J. Polym. Sci. 2023, 61, 1389–1414. [Google Scholar] [CrossRef]
- Hawthorne, B.; Simmons, J.K.; Stuart, B.; Tung, R.; Zamierowski, D.S.; Mellott, A.J. Enhancing Wound Healing Dressing Development through Interdisciplinary Collaboration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1967–1985. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Wang, K.; Zhao, S.; Yu, P.; Wei, W.; Wang, D.; Han, W.; Jiang, K.; Shen, G. Biocompatible and Biodegradable Functional Polysaccharides for Flexible Humidity Sensors. Research 2020, 2020, 8716847. [Google Scholar] [CrossRef] [PubMed]
- Azeman, N.H.; Arsad, N.; Bakar, A.A.A. Polysaccharides as the Sensing Material for Metal Ion Detection-Based Optical Sensor Applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef] [PubMed]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide Matrices Used in 3D In Vitro Cell Culture Systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Rodríguez-Núñez, J.R. An Overview of the Chemical Modifications of Chitosan and Their Advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, T.V.; Muhamed, J.; Jose, A.; Jyothi, A.; Mohanan, P.V.; Krishnan, L.K. Advantages of Hyaluronic Acid as a Component of Fibrin Sheet for Care of Acute Wound. Biologicals 2011, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.O.; Marinho, E.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Pullulan Hydrogels as Drug Release Platforms in Biomedicine. J. Drug Deliv. Sci. Technol. 2023, 89, 105066. [Google Scholar] [CrossRef]
- Manivannan, M.; Nathan, S.S.; Sasikumar, P.; Ramkumar, L.; Navaneethan, D.; Prabu, P.; Anjalin, F.M.; Dharamarj, N.; Alqahtani, M.S.; Abbas, M. Review on Applications of Pullulan in Bone Tissue Engineering: Blends and Composites with Natural and Synthetic Polymers. Polym. Polym. Compos. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Mendez-Encinas, M.A.; Carvajal-Millan, E.; Rascon-Chu, A.; Astiazaran-Garcia, H.F.; Valencia-Rivera, D.E. Ferulated Arabinoxylans and Their Gels: Functional Properties and Potential Application as Antioxidant and Anticancer Agent. Oxidative Med. Cell. Longev. 2018, 2018, 2314759. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Arabinoxylans from Cereal By-Products: Insights into Structural Features, Recovery, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780081022146. [Google Scholar]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The Physiological Functions and Pharmaceutical Applications of Inulin: A Review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jalili, S. Dextran, as a Biological Macromolecule for the Development of Bioactive Wound Dressing Materials: A Review of Recent Progress and Future Perspectives. Int. J. Biol. Macromol. 2022, 207, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.; Costa, D.; Dias, C.; Cruz, P.; Barros, P. Chondroitin Sulfate Supplements for Osteoarthritis: A Critical Review. Cureus 2023, 15, e40192. [Google Scholar] [CrossRef]
- Hao, C.; Xu, H.; Yu, L.; Zhang, L. Heparin: An Essential Drug for Modern Medicine, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 163, ISBN 9780128177402. [Google Scholar]
- Ahmed Omar, N.; Amédée, J.; Letourneur, D.; Fricain, J.C.; Fenelon, M. Recent Advances of Pullulan and/or Dextran-Based Materials for Bone Tissue Engineering Strategies in Preclinical Studies: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 889481. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Liu, Y.; Li, X.; Wu, S. Application of Chitosan in Fruit Preservation: A Review. Food Chem. X 2024, 23, 101589. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and Challenges in the Use of Chitosan and Its Derivatives in Biomedical Fields: A Review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Ejeromedoghene, O.; Lambo, M.T.; Alowakennu, M.; Alli, Y.A.; Ere-Richard, A.A.; Min, L.; Zhao, J.; Wang, X.; Jiao, H.; et al. Chitosan and Chitosan-based Composites as Beneficial Compounds for Animal Health: Impact on Gastrointestinal Functions and Biocarrier Application. J. Funct. Foods 2023, 104, 105520. [Google Scholar] [CrossRef]
- El-araby, A.; El Ghadraoui, L.; Errachidi, F. Physicochemical Properties and Functional Characteristics of Ecologically Extracted Shrimp Chitosans with Different Organic Acids during Demineralization Step. Molecules 2022, 27, 8285. [Google Scholar] [CrossRef]
- Skołucka-Szary, K.; Piaskowski, S.; Rieske, P. Practical Aspects Related to Application of Chitin and Its Derivatives in Wound Management. Chemik 2016, 70, 89–98. [Google Scholar]
- Al-Rooqi, M.M.; Hassan, M.M.; Moussa, Z.; Obaid, R.J.; Suman, N.H.; Wagner, M.H.; Natto, S.S.A.; Ahmed, S.A. Advancement of Chitin and Chitosan as Promising Biomaterials. J. Saudi Chem. Soc. 2022, 26, 101561. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Misra, S.K.; Sharma, A.; Pathak, K. Exploration of Chitosan and Its Modified Derivatives as Vaccine Adjuvant: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100537. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Antonini, C.; Tagliaro, I.; Radice, V.; Nistic, R. Chitosan Electrolyte Hydrogel with Low Ice Adhesion Properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134695. [Google Scholar] [CrossRef]
- Stefanowska, K.; Bucher, M.; Reichert, C.L.; Sip, A.; Wo, M.; Schmid, M.; Dobrucka, R.; Ratajczak, I. Chitosan-Based Films with Nanocellulose and Propolis as Active Packaging Materials. Ind. Crop. Prod. 2024, 219, 119112. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-Based Nanostructured Biomaterials: Synthesis, Properties, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and Drug Delivery Applications of Chitosan Biopolymer and Its Modified Nanocomposite: A Review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef]
- Wu, M.Y.; Huang, S.W.; Kao, I.F.; Yen, S.K. The Preparation and Characterization of Chitosan/Calcium Phosphate Composite Microspheres for Biomedical Applications. Polymers 2024, 16, 167. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Recent Progress in Preparation and Applications of Chitosan/Calcium Phosphate Composite Materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef]
- Zia, I.; Jolly, R.; Mirza, S.; Rehman, A.; Shakir, M. Nanocomposite Materials Developed from Nano-Hydroxyapatite Impregnated Chitosan/κ-Carrageenan for Bone Tissue Engineering. ChemistrySelect 2022, 7, e202103234. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and Hydroxyapatite Based Biomaterials to Circumvent Periprosthetic Joint Infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Hydroxyapatite-Hybridized Chitosan/Chitin Whisker Bionanocomposite Fibers for Bone Tissue Engineering Applications. Carbohydr. Polym. 2016, 144, 419–427. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/Hydroxyapatite Nanocomposite Scaffolds to Modulate Osteogenic and Inflammatory Response. J. Biomed. Mater. Res. Part A 2022, 110, 266–272. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.; Ren, H.T.; Peng, H.K.; Lou, C.W.; Lin, J.H. Two-Step Strategy for Constructing Hierarchical Pore Structured Chitosan–Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2021, 260, 117765. [Google Scholar] [CrossRef]
- Adamski, R.; Siuta, D. Mechanical, Structural, and Biological Properties of Chitosan/Hydroxyapatite/Silica Composites for Bone Tissue Engineering. Molecules 2021, 26, 1976. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, S.; Li, J.; Li, X.; Yang, P.; Li, G. Chitosan/Calcium Phosphate Flower-like Microparticles as Carriers for Drug Delivery Platform. Int. J. Biol. Macromol. 2020, 155, 174–183. [Google Scholar] [CrossRef]
- Piaia, L.; Silva, S.S.; Gomes, J.M.; Franco, A.R.; Fernandes, E.M.; Lobo, F.C.M.; Rodrigues, L.C.; Leonor, I.B.; Fredel, M.C.; Salmoria, G.V.; et al. Chitosan/β-TCP Composites Scaffolds Coated with Silk Fibroin: A Bone Tissue Engineering Approach. Biomed. Mater. 2022, 17, 015003. [Google Scholar] [CrossRef]
- De la Riva, B.; Sánchez, E.; Hernández, A.; Reyes, R.; Tamimi, F.; López-Cabarcos, E.; Delgado, A.; Évora, C. Local Controlled Release of VEGF and PDGF from a Combined Brushite-Chitosan System Enhances Bone Regeneration. J. Control. Release 2010, 143, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ergul, N.M.; Unal, S.; Kartal, I.; Kalkandelen, C.; Ekren, N.; Kilic, O.; Chi-Chang, L.; Gunduz, O. 3D Printing of Chitosan/Poly(Vinyl Alcohol) Hydrogel Containing Synthesized Hydroxyapatite Scaffolds for Hard-Tissue Engineering. Polym. Test. 2019, 79, 106006. [Google Scholar] [CrossRef]
- Wang, J.Q.; Jiang, B.J.; Guo, W.J.; Zhao, Y.M. Indirect 3D Printing Technology for the Fabrication of Customised β-TCP/Chitosan Scaffold with the Shape of Rabbit Radial Head—An In Vitro Study. J. Orthop. Surg. Res. 2019, 14, 102. [Google Scholar] [CrossRef]
- Ramirez Caballero, S.S.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D Printing of Chitosan-Calcium Phosphate Inks: Rheology, Interactions and Characterization. J. Mater. Sci. Mater. Med. 2019, 30, 6. [Google Scholar] [CrossRef]
- Carton, F.; Malatesta, M. Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3975. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Maruoka, H.; Haraguchi-Kitakamae, M.; Nakanishi, K.; Yamamoto, T.; Ishizu, H.; Shimizu, T.; et al. Phosphorylated Pullulan Promotes Calcification during Bone Regeneration in the Bone Defects of Rat Tibiae. Front. Bioeng. Biotechnol. 2023, 11, 1243951. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Hynnekleiv, L.; Magno, M.; Vernhardsdottir, R.R.; Moschowits, E.; Tønseth, K.A.; Dartt, D.A.; Vehof, J.; Utheim, T.P. Hyaluronic Acid in the Treatment of Dry Eye Disease. Acta Ophthalmol. 2022, 100, 844–860. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fang, Y.H.; Sivasubramanian, S.; Lin, F.H.; Lin, C. Hydroxyapatite-Calcium Sulfate-Hyaluronic Acid Composite Encapsulated with Collagenase as Bone Substitute for Alveolar Bone Regeneration. Biomaterials 2016, 74, 99–108. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B. Preparation and Characterization of Composites Based on the Blends of Collagen, Chitosan and Hyaluronic Acid with Nano-Hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, M.G.; Demina, T.S.; Dregval, O.A.; Gaidar, A.I.; Andreeva, E.R.; Zelenetskii, A.N.; Akopova, T.A.; Markvicheva, E. Macroporous Hyaluronic Acid/Chitosan Polyelectrolyte Complex-Based Hydrogels Loaded with Hydroxyapatite Nanoparticles: Preparation, Characterization and In Vitro Evaluation. Polysaccharides 2022, 3, 745–760. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A.M. In Vivo Study on Scaffolds Based on Chitosan, Collagen, and Hyaluronic Acid with Hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Wu, A.; Xu, H. An Injectable Nano-Hydroxyapatite (n-HA)/Glycol Chitosan (G-CS)/Hyaluronic Acid (HyA) Composite Hydrogel for Bone Tissue Engineering. RSC Adv. 2016, 6, 33529–33536. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Zhao, Y.; Bhagia, S.; Meng, X.; Yong, Q.; Ragauskas, A.J. Bio-Inspired Nanocomposite by Layer-by-Layer Coating of Chitosan/Hyaluronic Acid Multilayers on a Hard Nanocellulose-Hydroxyapatite Matrix. Carbohydr. Polym. 2019, 222, 115036. [Google Scholar] [CrossRef] [PubMed]
- Taşdelen, B.; Erdoǧan, S.; Bekar, B.I. Radiation Synthesis and Characterization of Chitosan/Hyraluronic Acid/Hydroxyapatite Hydrogels: Drug Uptake and Drug Delivery Systems. Mater. Today Proc. 2018, 5, 15990–15997. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery Gels: Gelling Behavior and Gel Properties of Gelatin in Concentrated Sugar Solutions. Food Hydrocoll. 2022, 124, 107132. [Google Scholar] [CrossRef]
- Hachinohe, Y.; Taira, M.; Hoshi, M.; Yoshida, D.; Hatakeyama, W.; Sawada, T.; Kondo, H. Self-Prepared Hyaluronic Acid/Alkaline Gelatin Composite with Nano-Hydroxyapatite and Bone Morphogenetic Protein for Cranial Bone Formation. Int. J. Mol. Sci. 2023, 24, 1104. [Google Scholar] [CrossRef]
- Kim, J.W.; Han, Y.S.; Lee, H.M.; Kim, J.K.; Kim, Y.J. Effect of Morphological Characteristics and Biomineralization of 3d-Printed Gelatin/Hyaluronic Acid/Hydroxyapatite Composite Scaffolds on Bone Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 6794. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Wang, Y.; Duan, R.; Liu, L. Gelatin/Hyaluronic Acid Photocrosslinked Double Network Hydrogel with Nano-Hydroxyapatite Composite for Potential Application in Bone Repair. Gels 2023, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Dobra, J.K.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B.; et al. Hyaluronic Acid Facilitates Bone Repair Effects of Calcium Phosphate Cement by Accelerating Osteogenic Expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef]
- Aguado, E.; Pascaretti-Grizon, F.; Gaudin-Audrain, C.; Goyenvalle, E.; Chappard, D. β-TCP Granules Mixed with Reticulated Hyaluronic Acid Induce an Increase in Bone Apposition. Biomed. Mater. 2014, 9, 015001. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic Acid/Corn Silk Extract Based Injectable Nanocomposite: A Biomimetic Antibacterial Scaffold for Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 107, 110195. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, Y.H.; Liu, C.J.; Chung, R.J. Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics 2018, 10, 37. [Google Scholar] [CrossRef]
- Lis, K.; Szechyńska, J.; Träger, D.; Sadlik, J.; Niziołek, K.; Słota, D.; Jampilek, J.; Sobczak-Kupiec, A. Hybrid Polymer–Inorganic Materials with Hyaluronic Acid as Controlled Antibiotic Release Systems. Materials 2024, 17, 58. [Google Scholar] [CrossRef]
- Han, S.H.; Jung, S.H.; Lee, J.H. Preparation of Beta-Tricalcium Phosphate Microsphere-Hyaluronic Acid-Based Powder Gel Composite as a Carrier for RhBMP-2 Injection and Evaluation Using Long Bone Segmental Defect Model. J. Biomater. Sci. Polym. Ed. 2019, 30, 679–693. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in Biomedical Research and Development—A Review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M.V. Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef]
- Agrawal, S.; Budhwani, D.; Gurjar, P.; Telange, D.; Lambole, V. Pullulan Based Derivatives: Synthesis, Enhanced Physicochemical Properties, and Applications. Drug Deliv. 2022, 29, 3328–3339. [Google Scholar] [CrossRef]
- Al-Mamoori, Z.Z.; Embaby, A.M.; Hussein, A.; Mahmoud, H.E. Correction: A Molecular Study on Recombinant Pullulanase Type I from Metabacillus indicus. AMB Express 2023, 13, 40, Erratum in AMB Express 2023, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Le, N.M.N.; Le-Vinh, B.; Friedl, J.D.; Jalil, A.; Kali, G.; Bernkop-Schnürch, A. Polyaminated Pullulan, a New Biodegradable and Cationic Pullulan Derivative for Mucosal Drug Delivery. Carbohydr. Polym. 2022, 282, 119143. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan-Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Farris, S.; Unalan, I.U.; Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A. Pullulan-Based Films and Coatings for Food Packaging: Present Applications, Emerging Opportunities, and Future Challenges. J. Appl. Polym. Sci. 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Golińska, P. Emerging Trends in Pullulan-Based Antimicrobial Systems for Various Applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, W.; Liu, Y.; Li, W.; Fan, D.; Zhu, C.; Wang, Y. HLC/Pullulan and Pullulan Hydrogels: Their Microstructure, Engineering Process and Biocompatibility. Mater. Sci. Eng. C 2016, 58, 1046–1057. [Google Scholar] [CrossRef]
- Shaat, F.; Pavaloiu, R.; Hlevca, C. Current status of the applications of pullulan and its derivatives in biomedical field. Sci. Bull. 2022, XXVI, 125–132. [Google Scholar]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Daliri Joupari, M. Bioactive and Biostable Hyaluronic Acid-Pullulan Dermal Hydrogels Incorporated with Biomimetic Hydroxyapatite Spheres. Mater. Sci. Eng. C 2020, 112, 110906. [Google Scholar] [CrossRef]
- Amrita; Arora, A.; Sharma, P.; Katti, D.S. Pullulan-Based Composite Scaffolds for Bone Tissue Engineering: Improved Osteoconductivity by Pore Wall Mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar] [CrossRef]
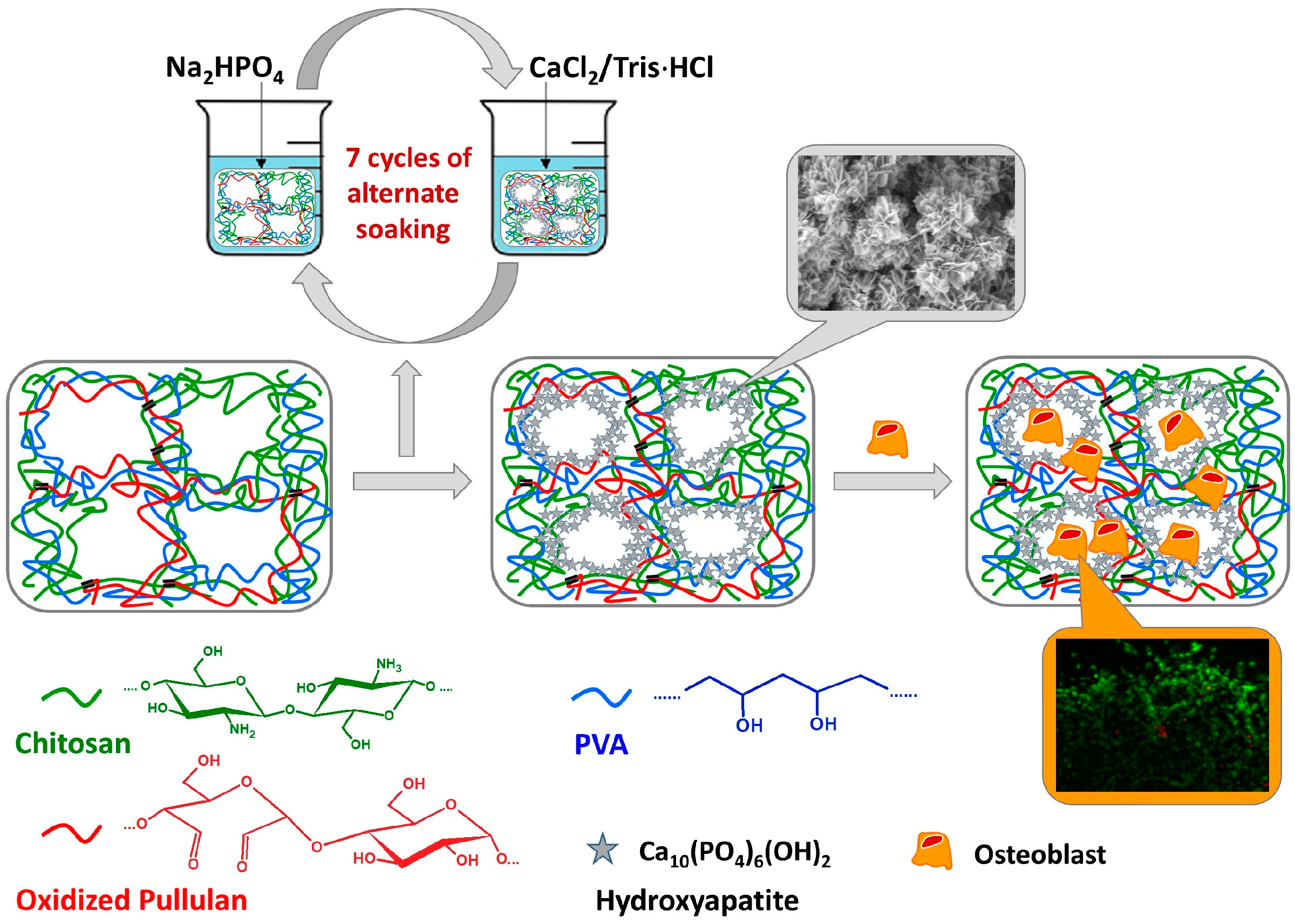
- Pelin, I.M.; Popescu, I.; Calin, M.; Rebleanu, D.; Voicu, G.; Ionita, D.; Zaharia, M.M.; Constantin, M.; Fundueanu, G. Tri-Component Hydrogel as Template for Nanocrystalline Hydroxyapatite Deposition Using Alternate Soaking Method for Bone Tissue Engineering Applications. Gels 2023, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Ding, Z.; Ren, H.; Chen, H.; Yan, Y.; Zhang, Q. Effects of Pullulan on the Biomechanical and Anti-Collapse Properties of Dicalcium Phosphate Dihydrate Bone Cement. J. Biomater. Appl. 2021, 36, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Li, Y.; Wu, S. Fabrication and Characterisation of Collagen/Pullulan Ultra-Thin Fibers by Electrospinning. Food Chem. X 2024, 21, 101138. [Google Scholar] [CrossRef]
- Nosoudi, N.; Jacob, A.O.; Stultz, S.; Jordan, M.; Aldabel, S.; Hohne, C.; Mosser, J.; Archacki, B.; Turner, A.; Turner, P. Electrospinning Live Cells Using Gelatin and Pullulan. Bioengineering 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Ruggeri, M.; Vigani, B.; Totaro Fila, C.; Icaro Cornaglia, A.; Boselli, C.; Viseras, C.; Rossi, S.; Sandri, G. Gas Foamed Scaffolds as Smart 3D Structures in Skin Tissue Engineering. J. Drug Deliv. Sci. Technol. 2024, 95, 105541. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Crosslinked Pullulan/Cellulose Acetate Fibrous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 69, 1103–1115. [Google Scholar]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom Shell Incorporated PHBV/PCL-Pullulan Co-Electrospun Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef]
- Popescu, R.A.; Tăbăran, F.A.; Bogdan, S.; Fărcăṣanu, A.; Purdoiu, R.; Magyari, K.; Vulpoi, A.; Dreancă, A.; Sevastre, B.; Simon, S.; et al. Bone Regeneration Response in an Experimental Long Bone Defect Orthotopically Implanted with Alginate-Pullulan-Glass-Ceramic Composite Scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1129–1140. [Google Scholar] [CrossRef]
- Pérez-Flores, J.G.; García-Curiel, L.; Pérez-Escalante, E.; Contreras-López, E.; Olloqui, E.J. Arabinoxylans Matrixes as a Potential Material for Drug Delivery Systems Development—A Bibliometric Analysis and Literature Review. Heliyon 2024, 10, e25445. [Google Scholar] [CrossRef]
- Grootaert, C.; Verstraete, W.; Van de Wiele, T. Microbial Metabolism and Prebiotic Potency of Arabinoxylan Oligosaccharides in the Human Intestine. Trends Food Sci. Technol. 2007, 18, 64–71. [Google Scholar] [CrossRef]
- Bhatia, M.; Ahuja, M. Psyllium Arabinoxylan: Carboxymethylation, Characterization and Evaluation for Nanoparticulate Drug Delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef]
- Jabeen, N.; Iram, F.; Atif, M.; Fatimah, N.E. Functionalization of Arabinoxylan in Chitosan Based Blends by Periodate Oxidation for Drug Release Study. ChemistrySelect 2024, 9, e202303496. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and PH-Sensitive RGO/Arabinoxylan/Chitosan Composite for Wound Dressing: In-Vitro Drug Delivery, Antibacterial Activity, and Biological Activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Al-Arjan, W.S.; Khan, M.U.A.; Nazir, S.; Razak, S.I.A.; Kadir, M.R.A. Development of Arabinoxylan-Reinforced Apple Pectin/Graphene Oxide/Nano-Hydroxyapatite Based Nanocomposite Scaffolds with Controlled Release of Drug for Bone Tissue Engineering: In-Vitro Evaluation of Biocompatibility and Cytotoxicity against MC3T3-E1. Coatings 2020, 10, 1120. [Google Scholar] [CrossRef]
- Aslam Khan, M.U.; Haider, A.; Abd Razak, S.I.; Abdul Kadir, M.R.; Haider, S.; Shah, S.A.; Hasan, A.; Khan, R.; Khan, S.-u.d.; Shakir, I. Arabinoxylan/Graphene-Oxide/NHAp-NPs/PVA Bionano Composite Scaffolds for Fractured Bone Healing. J. Tissue Eng. Regen. Med. 2021, 15, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Abd Razak, S.I.; Mehboob, H.; Abdul Kadir, M.R.; Anand, T.J.S.; Inam, F.; Shah, S.A.; Abdel-Haliem, M.E.F.; Amin, R. Synthesis and Characterization of Silver-Coated Polymeric Scaffolds for Bone Tissue Engineering: Antibacterial and In Vitro Evaluation of Cytotoxicity and Biocompatibility. ACS Omega 2021, 6, 4335–4346. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Shah, S.A.; Razak, S.I.A.; Hassan, S.A.; Kadir, M.R.A.; Haider, A. Arabinoxylan-Co-AA/HAp/TiO2 Nanocomposite Scaffold a Potential Material for Bone Tissue Engineering: An In Vitro Study. Int. J. Biol. Macromol. 2020, 151, 584–594. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Kim, K.J.; Hwang, M.J.; Yun, Y.H.; Yoon, S. Do Synthesis and Drug Release Behavior of Functional Montelukast Imprinted Inulin-Based Biomaterials as Asthma Treatment. J. Ind. Eng. Chem. 2022, 109, 221–229. [Google Scholar] [CrossRef]
- Youn, H.G.; Je, J.Y.; Lee, C.M.; Yoon, S. Do Inulin/PVA Biomaterials Using Thiamine as an Alternative Plasticizer. Carbohydr. Polym. 2019, 220, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.K.; Noh, K.M.; Kim, S.; Kim, K. Engineered Inulin-Based Hybrid Biomaterials for Augmented Immunomodulatory Responses. Carbohydr. Polym. 2024, 340, 122311. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Van Der Voort Maarschalk, K.; Hinrichs, W.L.J. Inulin, a Flexible Oligosaccharide I: Review of Its Physicochemical Characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Gillis, R.B.; Kok, M.S.; Harding, S.E.; Adams, G.G. Application and Use of Inulin as a Tool for Therapeutic Drug Delivery. Biotechnol. Genet. Eng. Rev. 2012, 28, 33–46. [Google Scholar] [CrossRef]
- Öner, M.; Uysal, U. Synthesis of Hydroxyapatite Crystals Using Carboxymethyl Inulin for Use as a Delivery of Ibuprofen. Mater. Sci. Eng. C 2013, 33, 482–489. [Google Scholar] [CrossRef]
- Tommasino, C.; Auriemma, G.; Sardo, C.; Alvarez-Lorenzo, C.; Garofalo, E.; Morello, S.; Falcone, G.; Aquino, R.P. 3D Printed Macroporous Scaffolds of PCL and Inulin-g-P(D,L)LA for Bone Tissue Engineering Applications. Int. J. Pharm. 2023, 641, 123093. [Google Scholar] [CrossRef] [PubMed]
- Albu, M.G.; Trandafir, V.; Suflet, D.M.; Chitanu, G.C.; Budrugeac, P.; Titorencu, I. Biocomposites Based on Collagen and Phosphorylated Dextran for Bone Regeneration. J. Mater. Res. 2012, 27, 1086–1096. [Google Scholar] [CrossRef]
- Wang, Y.; Tuccillo, F.; Niklander, K.; Livi, G.; Siitonen, A.; Pöri, P.; Edelmann, M.; Sawadogo-Lingani, H.; Maina, N.H.; Jouppila, K.; et al. Masking Off-Flavors of Faba Bean Protein Concentrate and Extrudate: The Role of In Situ and In Vitro Produced Dextran. Food Hydrocoll. 2024, 150, 109692. [Google Scholar] [CrossRef]
- Paul, M.; Pramanik, S.D.; Sahoo, R.N.; Dey, Y.N.; Nayak, A.K. Dental Delivery Systems of Antimicrobial Drugs Using Chitosan, Alginate, Dextran, Cellulose and Other Polysaccharides: A Review. Int. J. Biol. Macromol. 2023, 247, 125808. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Z.; Yan, D.; He, H.; Xi, W.; Hu, J.; Zhang, R.; Yan, Y.; Zhang, Q. Double-Network Composites Based on Inorganic Fillers Reinforced Dextran-Based Hydrogel with High Strength. Carbohydr. Polym. 2022, 296, 119900. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Wu, J.; Zheng, Z.; Yu, Z.; She, F.; Lai, L.; Li, H.; Chen, Y.; Wei, L. Dextran: A Multifunctional and Universal Electrolyte Additive for Aqueous Zn Ion Batteries. Adv. Energy Mater. 2023, 13, 2301743. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, D.; Zhang, W.; Stressler, T.; Mu, W. Lactic Acid Bacteria-Derived α-Glucans: From Enzymatic Synthesis to Miscellaneous Applications. Biotechnol. Adv. 2021, 47, 107708. [Google Scholar] [CrossRef]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A Strong, Tough, and Osteoconductive Hydroxyapatite Mineralized Polyacrylamide/Dextran Hydrogel for Bone Tissue Regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, F.; Hu, Y.; Pang, Q.; Shi, L.; Du, T.; Cao, Y.; Song, B.; Yu, X.; Cao, Z.; et al. An Oxidized Dextran-Composite Self-Healing Coated Magnesium Scaffold Reduces Apoptosis to Induce Bone Regeneration. Carbohydr. Polym. 2024, 327, 121666. [Google Scholar] [CrossRef]
- Alves, P.; Simão, A.F.; Graça, M.F.P.; Mariz, M.J.; Correia, I.J.; Ferreira, P. Dextran-Based Injectable Hydrogel Composites for Bone Regeneration. Polymers 2023, 15, 4501. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Wang, B.; Deng, H.; Liu, X.; Peng, S.; Zhang, Q.; Yan, Y. Double Network Composite Scaffolds Based on Oxidized Dextran/Gelatin Hydrogel and Magnesium Calcium Phosphate Cement. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132307. [Google Scholar] [CrossRef]
- El-Meliegy, E.; Mabrouk, M.; Kamal, G.M.; Awad, S.M.; El-Tohamy, A.M.; El Gohary, M.I. Anticancer Drug Carriers Using Dicalcium Phosphate/Dextran/CMCnanocomposite Scaffolds. J. Drug Deliv. Sci. Technol. 2018, 45, 315–322. [Google Scholar] [CrossRef]
- Nikpour, P.; Salimi-Kenari, H.; Rabiee, S.M. Biological and Bioactivity Assessment of Dextran Nanocomposite Hydrogel for Bone Regeneration. Prog. Biomater. 2021, 10, 271–280. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Han, Y. Chondroitin Sulfate-Based Biomaterials for Tissue Engineering. Turkish J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional Chondroitin Sulphate for Cartilage Tissue-Biomaterial Integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin Sulfate: Emerging Biomaterial for Biopharmaceutical Purpose and Tissue Engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Cimini, D.; De Rosa, M. Production of Chondroitin Sulfate and Chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1209–1220. [Google Scholar] [CrossRef]
- Derakhshan, Z.H.; Shaghaghi, B.; Asl, M.P.; Majidi, M.; Ghazizadeh, L.; Chegini, A.; Bonakdar, S. In Situ Forming Hydrogel Based on Chondroitin Sulfate-Hydroxyapatite for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 919–926. [Google Scholar] [CrossRef]
- Xiao, X.; He, D.; Liu, F.; Liu, R. Preparation and Characterization of Hydroxyapatite/Chondroitin Sulfate Composites by Biomimetic Synthesis. Mater. Chem. Phys. 2008, 112, 838–843. [Google Scholar] [CrossRef]
- Watanabe, H.; Ikoma, T.; Sotome, S.; Okawa, A. Local Administration and Enhanced Release of Bone Metabolic Antibodies from Hydroxyapatite/Chondroitin Sulfate Nanocomposite Microparticles Using Zinc Cations. J. Mater. Chem. B 2021, 9, 757–766. [Google Scholar] [CrossRef]
- Rhee, S.H.; Tanaka, J. Synthesis of a Hydroxyapatite/Collagen/Chondroitin Sulfate Nanocomposite by a Novel Precipitation Method. J. Am. Ceram. Soc. 2001, 84, 459–461. [Google Scholar] [CrossRef]
- Moldovan, L.; Craciunescu, O.; Oprita, E.I.; Balan, M.; Zarnescu, O. Collagen-Chondroitin Sulfate-Hydroxyapatite Porous Composites: Preparation, Characterization and In Vitro Biocompatibility Testing. Roum. Biotechnol. Lett. 2009, 14, 4459–4466. [Google Scholar]
- Zarnescu, O.; Craciunescu, O.; Moldovan, L. Collagen-Chondroitin Sulphate-Hydroxyapatite Porous Composites: A Histochemical and Electron Microscopy Approach. Microsc. Microanal. 2010, 16, 137–142. [Google Scholar] [CrossRef]
- Schneiders, W.; Reinstorf, A.; Biewener, A.; Serra, A.; Grass, R.; Kinscher, M.; Heineck, J.; Rehberg, S.; Zwipp, H.; Rammelt, S. In Vivo Effects of Modification of Hydroxyapatite/Collagen Composites with and without Chondroitin Sulphate on Bone Remodeling in the Sheep Tibia. J. Orthop. Res. 2009, 27, 15–21. [Google Scholar] [CrossRef]
- Rammelt, S.; Heck, C.; Bernhardt, R.; Bierbaum, S.; Scharnweber, D.; Goebbels, J.; Ziegler, J.; Biewener, A.; Zwipp, H. In Vivo Effects of Coating Loaded and Unloaded Ti Implants with Collagen, Chondroitin Sulfate, and Hydroxyapatite in the Sheep Tibia. J. Orthop. Res. 2007, 25, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan-Amylopectin/Hydroxyapatite and Chitosan-Chondroitin Sulphate/Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic Mineralized Hierarchical Hybrid Scaffolds Based on In Situ Synthesis of Nano-Hydroxyapatite/Chitosan/Chondroitin Sulfate/Hyaluronic Acid for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Elbert, S.E.S. Incorporation of Heparin into Biomaterials. Acta Biomater. 2015, 10, 1581–1587. [Google Scholar] [CrossRef]
- Liang, Y.; Kiick, K.L. Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef]
- Rönnberg, E.; Melo, F.R.; Pejler, G. Mast Cell Proteoglycans. J. Histochem. Cytochem. 2012, 60, 950–962. [Google Scholar] [CrossRef]
- St Ange, K.; Onishi, A.; Fu, L.; Sun, X.; Lin, L.; Mori, D.; Zhang, F.; Dordick, J.S.; Fareed, J.; Hoppensteadt, D.; et al. Analysis of Heparins Derived from Bovine Tissues and Comparison to Porcine Intestinal Heparins. Clin. Appl. Thromb. 2016, 22, 520–527. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, X.; Liu, X.; Sheng, A.; Jin, L.; Linhardt, R.J.; Chi, L. Comparison of Low-Molecular-Weight Heparins Prepared from Bovine Lung Heparin and Porcine Intestine Heparin. J. Pharm. Sci. 2016, 105, 1843–1850. [Google Scholar] [CrossRef]
- Zhao, D.M.; Wang, Y.X.; Chen, Z.Y.; Xu, R.W.; Wu, G.; Yu, D.S. Preparation and Characterization of Modified Hydroxyapatite Particles by Heparin. Biomed. Mater. 2008, 3, 25016. [Google Scholar] [CrossRef]
- Rajeswari, A.; Kumar, V.G.; Karthick, V.; Dhas, T.S.; Potluri, S.L. Hydrothermal Synthesis of Hydroxyapatite Plates Prepared Using Low Molecular Weight Heparin (LMWH). Colloids Surf. B Biointerfaces 2013, 111, 764–768. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, D.W.; Yun, Y.P.; Shim, K.S.; Jeon, D.I.; Rhee, J.K.; Kim, H.J.; Park, K. Heparin-Immobilized Hydroxyapatite Nanoparticles as a Lactoferrin Delivery System for Improving Osteogenic Differentiation of Adipose-Derived Stem Cells. Biomed. Mater. 2016, 11, 25004. [Google Scholar] [CrossRef]
- Teixeira, S.; Yang, L.; Dijkstra, P.J.; Ferraz, M.P.; Monteiro, F.J. Heparinized Hydroxyapatite/Collagen Three-Dimensional Scaffolds for Tissue Engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Nájera-Romero, G.V.; Yar, M.; Rehman, I.U. Heparinized Chitosan/Hydroxyapatite Scaffolds Stimulate Angiogenesis. Funct. Compos. Mater. 2020, 1, 9. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin-and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Pang, X.; Zhitomirsky, I. Electrophoretic Deposition of Composite Hydroxyapatite-Chitosan-Heparin Coatings. J. Mater. Process. Technol. 2009, 209, 1597–1606. [Google Scholar] [CrossRef]
- Bozzini, B.; Barca, A.; Bogani, F.; Boniardi, M.; Carlino, P.; Mele, C.; Verri, T.; Romano, A. Electrodeposition of Nanostructured Bioactive Hydroxyapatite-Heparin Composite Coatings on Titanium for Dental Implant Applications. J. Mater. Sci. Mater. Med. 2014, 25, 1425–1434. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niziołek, K.; Słota, D.; Sobczak-Kupiec, A. Polysaccharide-Based Composite Systems in Bone Tissue Engineering: A Review. Materials 2024, 17, 4220. https://doi.org/10.3390/ma17174220
Niziołek K, Słota D, Sobczak-Kupiec A. Polysaccharide-Based Composite Systems in Bone Tissue Engineering: A Review. Materials. 2024; 17(17):4220. https://doi.org/10.3390/ma17174220
Chicago/Turabian StyleNiziołek, Karina, Dagmara Słota, and Agnieszka Sobczak-Kupiec. 2024. "Polysaccharide-Based Composite Systems in Bone Tissue Engineering: A Review" Materials 17, no. 17: 4220. https://doi.org/10.3390/ma17174220
APA StyleNiziołek, K., Słota, D., & Sobczak-Kupiec, A. (2024). Polysaccharide-Based Composite Systems in Bone Tissue Engineering: A Review. Materials, 17(17), 4220. https://doi.org/10.3390/ma17174220







