Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BiOI Precursor Film
2.2. Preparation of BiVO4 Electrode
2.3. Photo-Assisted Electrodeposition of CoPi Cocatalyst
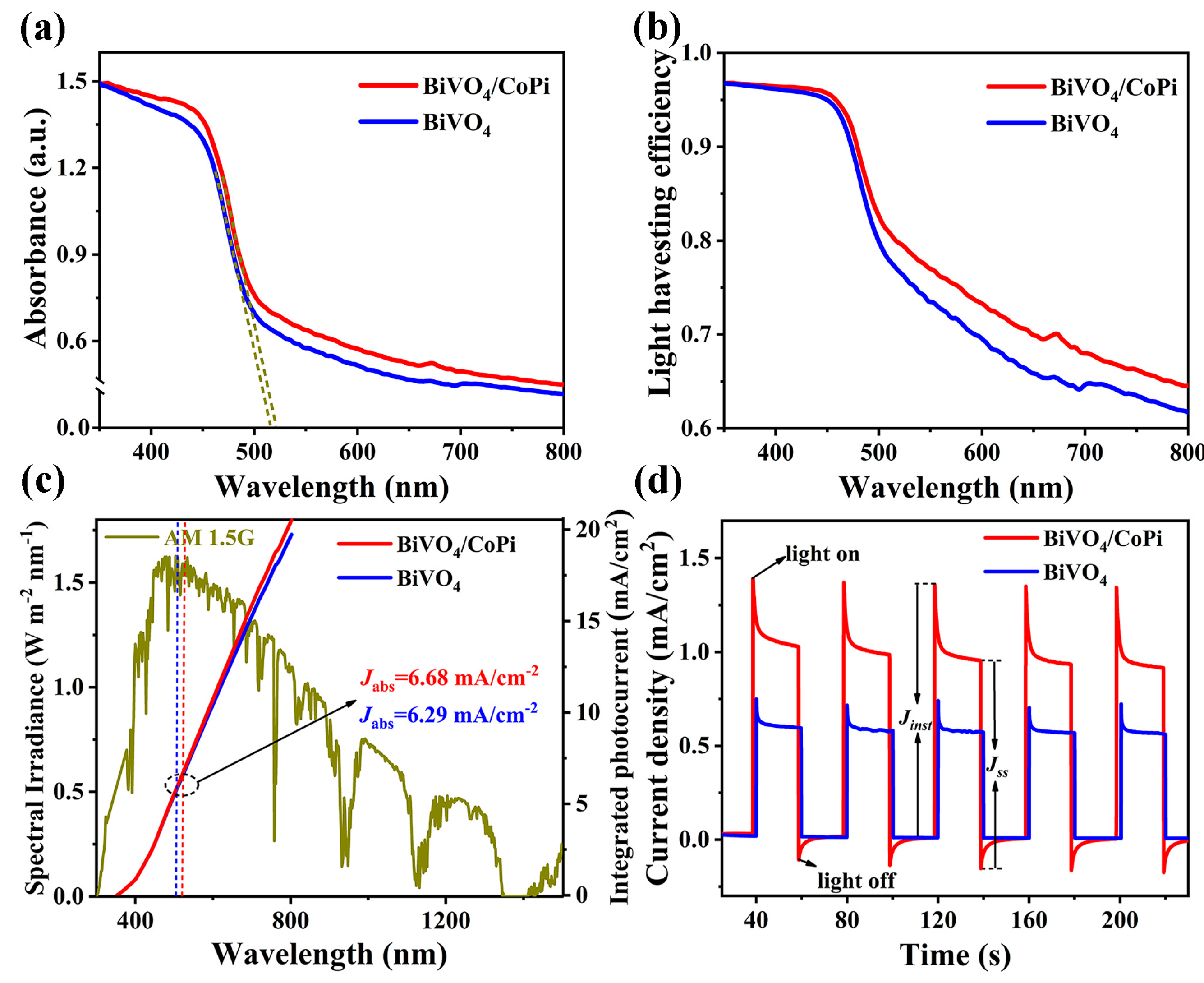
2.4. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, T.; Chen, S.; Wang, J.; Zhang, Y.; Li, J.; Bai, J.; Zhou, B. Dramatically enhanced solar-driven water splitting of BiVO4 photoanode via strengthening hole transfer and light harvesting by co-modification of CQDs and ultrathin β-FeOOH layers. Chem. Eng. J. 2021, 403, 126350. [Google Scholar] [CrossRef]
- Xiao, J.; Hou, X.; Zhao, L.; Li, Y. A carbon-quantum-dot-sensitized ZnO:Ga/ZnO multijunction composite photoanode for photoelectrochemical water splitting under visible light irradiation. J. Catal. 2017, 346, 70. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Benedoue, S.; Benedet, M.; Gasparotto, A.; Gauquelin, N.; Orekhov, A.; Verbeeck, J.; Seraglia, R.; Pagot, G.; Rizzi, G.A.; Balzano, V.; et al. Insights into the Photoelectrocatalytic Behavior of gCN-Based Anode Materials Supported on Ni Foams. Nanomaterials 2023, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, Z.; Wang, T.; Gong, J. Surface, Bulk, and Interface: Rational Design of Hematite Architecture toward Efficient Photo-Electrochemical Water Splitting. Adv. Mater. 2018, 30, 1707502. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Hu, K.; Humayun, M.; Chen, S.; Jing, L. Improved photoelectrocatalytic activities of BiOCl with high stability for water oxidation and MO degradation by coupling RGO and modifying phosphate groups to prolong carrier lifetime. Appl. Catal. B 2017, 203, 355. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ni, D.; Chen, Z.; Wang, X.; Bu, Y.; Ao, J.P. Improvement of BiVO4 Photoanode Performance During Water Photo-Oxidation Using Rh-Doped SrTiO3 Perovskite as a Co-Catalyst. Adv. Funct. Mater. 2019, 29, 1902101. [Google Scholar] [CrossRef]
- Duan, L.; Tong, L.; Xu, Y.; Sun, L. Visible light-driven water oxidation—From molecular catalysts to photoelectrochemical cells. Energy Environ. Sci. 2011, 4, 3296. [Google Scholar] [CrossRef]
- Li, Z.; Luo, L.; Li, M.; Chen, W.; Liu, Y.; Yang, J.; Xu, S.M.; Zhou, H.; Ma, L.; Xu, M.; et al. Photoelectrocatalytic C–H halogenation over an oxygen vacancy-rich TiO2 photoanode. Nat. Commun. 2021, 12, 6698. [Google Scholar] [CrossRef]
- Cho, I.S.; Logar, M.; Lee, C.H.; Cai, L.; Prinz, F.B.; Zheng, X. Rapid and Controllable Flame Reduction of TiO2 Nanowires for Enhanced Solar Water-Splitting. Nano Lett. 2014, 14, 24. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744. [Google Scholar] [CrossRef]
- Ronconi, F.; Syrgiannis, Z.; Bonasera, A.; Prato, M.; Argazzi, R.; Caramori, S.; Cristino, V.; Bignozzi, C.A. Modification of Nanocrystalline WO3 with a Dicationic Perylene Bisimide: Applications to Molecular Level Solar Water Splitting. J. Am. Chem. Soc. 2015, 137, 4630. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, P.; Bai, Y.; Yun, J.H.; Liu, G.; Wang, L. New BiVO4 Dual Photoanodes with Enriched Oxygen Vacancies for Efficient Solar-Driven Water Splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.T.; Nguyen, N.T.; Nakajima, T.; He, J.; Liu, X.; Zhang, X.; Wang, L.; Wu, L. Dynamic semiconductor-electrolyte interface for sustainable solar water splitting over 600 hours under neutral conditions. Sci. Adv. 2023, 9, eade4589. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Jia, Q.; Nishiyama, H.; Yamada, T.; Kudo, A.; Domen, K. A Front-Illuminated Nanostructured Transparent BiVO4 Photoanode for >2% Efficient Water Splitting. Adv. Energy Mater. 2016, 6, 1501645. [Google Scholar] [CrossRef]
- Hanway, P.J.; Winter, A.H. Heteroaryl Oxenium Ions Have Diverse and Unusual Low-Energy Electronic States. J. Phys. Chem. A 2016, 116, 9398–9403. [Google Scholar] [CrossRef]
- Ma, Y.; Kafizas, A.; Pendlebury, S.R.; Le Formal, F.; Durrant, J.R. Photoinduced Absorption Spectroscopy of CoPi on BiVO4: The Function of CoPi during Water Oxidation. Adv. Funct. Mater. 2016, 26, 4951–4960. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Fan, X.; Li, Y.; Zhou, D.; Cai, B.; Wang, L.; Fan, K.; Zhang, K. Boosting Charge Transport in BiVO4 Photoanode for Solar Water Oxidation. Adv. Mater. 2022, 34, 2108178. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, S.; Kim, D.H.; Park, I.J.; Kim, J.S.; Huang, P.-S.; Lee, J.K.; Cho, I.S.; Zheng, X. Boosting the solar water oxidation performance of a BiVO4 photoanode by crystallographic orientation control. Energy Environ. Sci. 2018, 11, 1299–1306. [Google Scholar] [CrossRef]
- Rettie, A.J.E.; Lee, H.C.; Marshall, L.G.; Lin, J.F.; Capan, C.; Lindemuth, J.; McCloy, J.S.; Zhou, J.; Bard, A.J.; Mullins, C.B. Combined Charge Carrier Transport and Photoelectrochemical Characterization of BiVO4 Single Crystals: Intrinsic Behavior of a Complex Metal Oxide. J. Am. Chem. Soc. 2013, 135, 11389–11396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, B.; Cao, K.; Brillet, J.; Chen, J.; Wang, M.; Shen, Y. A perovskite solar cell-TiO2/BiVO4 photoelectrochemical system for direct solar water splitting. J. Mater. Chem. A 2015, 3, 21630–21636. [Google Scholar] [CrossRef]
- McDonald, K.J.; Choi, K.-S. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ. Sci. 2012, 5, 8553–8557. [Google Scholar] [CrossRef]
- Vo, T.G.; Tai, Y.; Chiang, C.Y. Novel hierarchical ferric phosphate/bismuth vanadate nanocactus for highly efficient and stable solar water splitting. Appl. Catal. B 2019, 243, 657–666. [Google Scholar] [CrossRef]
- Zhang, B.; Chou, L.; Bi, Y. Tuning surface electronegativity of BiVO4 photoanodes toward high-performance water splitting. Appl. Catal. B 2020, 262, 118267. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Chu, J.; Niu, S.; Wang, J.; Du, Y.; Li, Z.; Han, X.; Xu, P. Understanding the Phase-Induced Electrocatalytic Oxygen Evolution Reaction Activity on FeOOH Nanostructures. ACS Catal. 2019, 9, 10705–10711. [Google Scholar] [CrossRef]
- Li, G.; Hou, S.; Gui, L.; Feng, F.; Zhang, D.; He, B.; Zhao, L. Carbon quantum dots decorated Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite nanofibers for boosting oxygen evolution reaction. Appl. Catal. B 2019, 257, 117919. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 2008, 321, 1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, T.; Chen, M.; Ishaq, M.; Tang, R.; Zheng, Z.; Su, Z.; Li, X.; Qiao, X.; Fan, P.; et al. Crystal growth promotion and interface optimization enable highly efficient Sb2Se3 photocathodes for solar hydrogen evolution. Nano Energy 2022, 99, 107417. [Google Scholar] [CrossRef]
- Joshi, G.P.; Saxena, N.S.; Mangal, R.; Mishra, A.; Sharma, T.P. Band gap determination of Ni-Zn ferrites. Bull. Mater. Sci. 2003, 26, 387–389. [Google Scholar] [CrossRef]
- Chen, S.; Fu, Y.; Ishaq, M.; Li, C.; Ren, D.; Su, Z.; Qiao, X.; Fan, P.; Liang, G.; Tang, J. Carrier recombination suppression and transport enhancement enable high-performance self-powered broadband Sb2Se3 photodetectors. InfoMat 2023, e12400. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Ishaq, M.; Chen, Z.; Tang, R.; Zheng, Z.; Su, Z.; Fan, P.; Zhang, X.; Chen, S. Heterojunction interface engineering enabling high onset potential in Sb2Se3/CdS photocathodes for efficient solar hydrogen production. Chem. Eng. J. 2022, 431, 133359. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Li, L.; Feng, K.; An, N.; Ikeda, S.; Kuang, Y.; Ng, Y.; Jiang, F. 3.17% efficient Cu2ZnSnS4–BiVO4 integrated tandem cell for standalone overall solar water splitting. Energy Environ. Sci. 2021, 14, 1480–1489. [Google Scholar] [CrossRef]
- Tan, J.; Yang, W.; Oh, Y.; Lee, H.; Park, J.; Boppella, R.; Kim, J.; Moon, J. Fullerene as a Photoelectron Transfer Promoter Enabling Stable TiO2-Protected Sb2Se3 Photocathodes for Photo-Electrochemical Water Splitting. Adv. Energy Mater. 2019, 9, 1900179. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Edu. 2007, 84, 685. [Google Scholar] [CrossRef]
- Liang, G.; Li, Z.; Ishaq, M.; Zheng, Z.; Su, Z.; Ma, H.; Zhang, X.; Fan, P.; Chen, S. Charge Separation Enhancement Enables Record Photocurrent Density in Cu2ZnSn(S,Se)4 Photocathodes for Efficient Solar Hydrogen Production. Adv. Energy Mater. 2023, 2300215. [Google Scholar] [CrossRef]
- Beranek, R. Photoelectrochemical methods for the determination of the band edge positions of TiO2-based nanomaterials. Adv. Phys. Chem. 2011, 2011, 786759. [Google Scholar] [CrossRef]
- Trasatti, S. The absolute electrode potential: An explanatory note. Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Chen, S.; Zheng, Z.; Fan, P.; Tan, M.; Yan, C.; Zhang, X.; Su, Z.; Liang, G. Unveiling the Selenization Reaction Mechanisms in Ambient Air-Processed Highly Efficient Kesterite Solar Cells. Adv. Energy Mater. 2023, 2300521. [Google Scholar] [CrossRef]
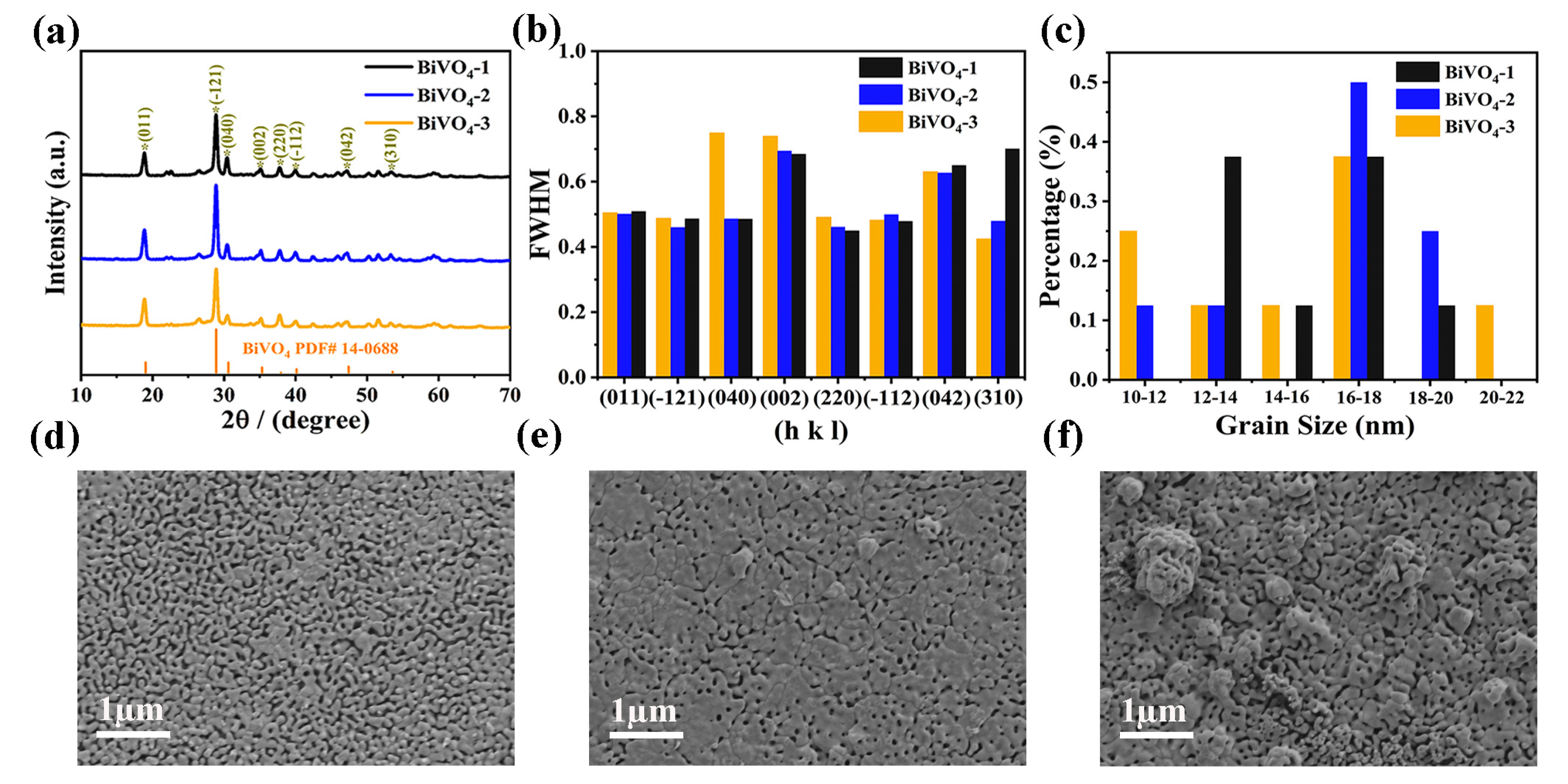
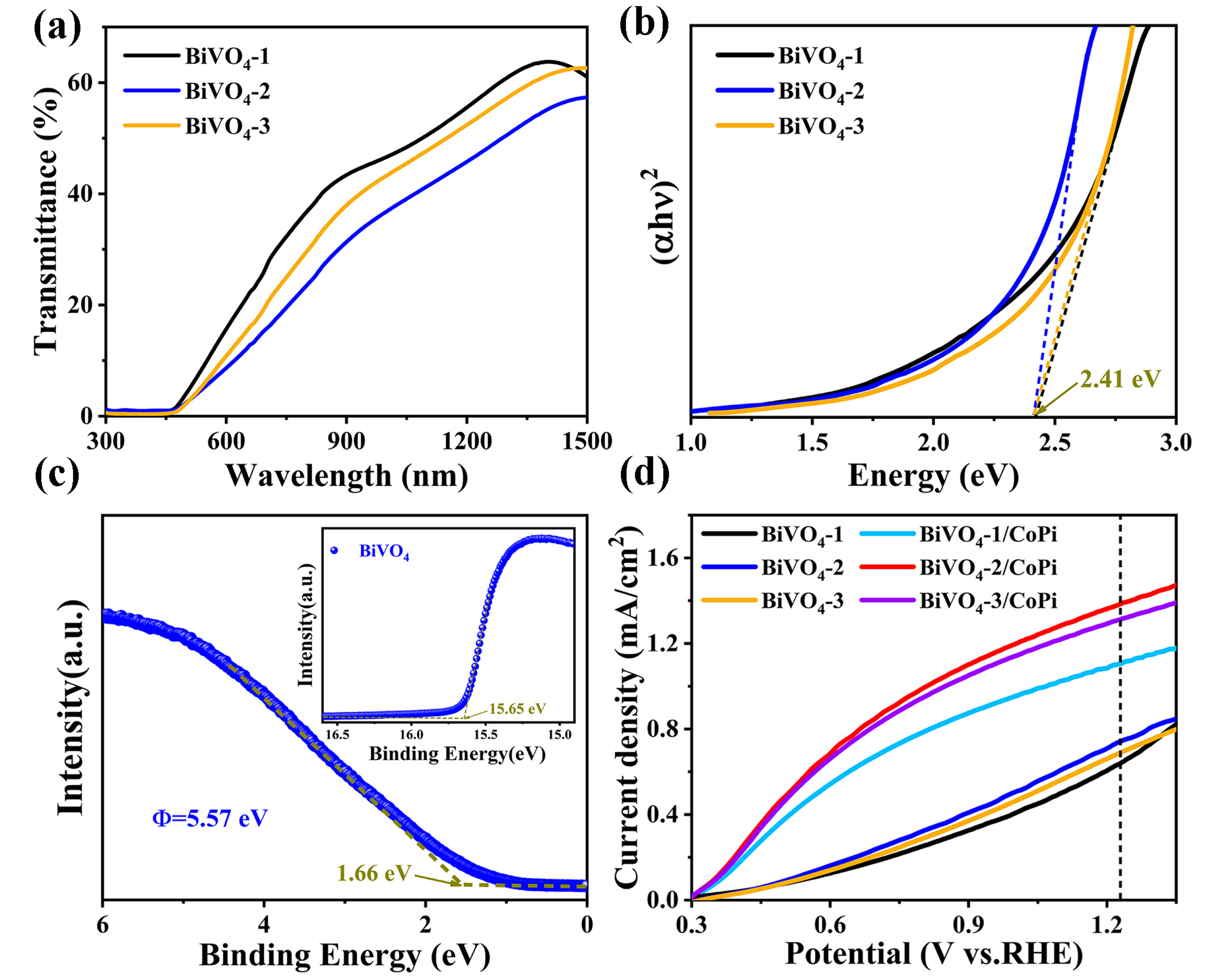
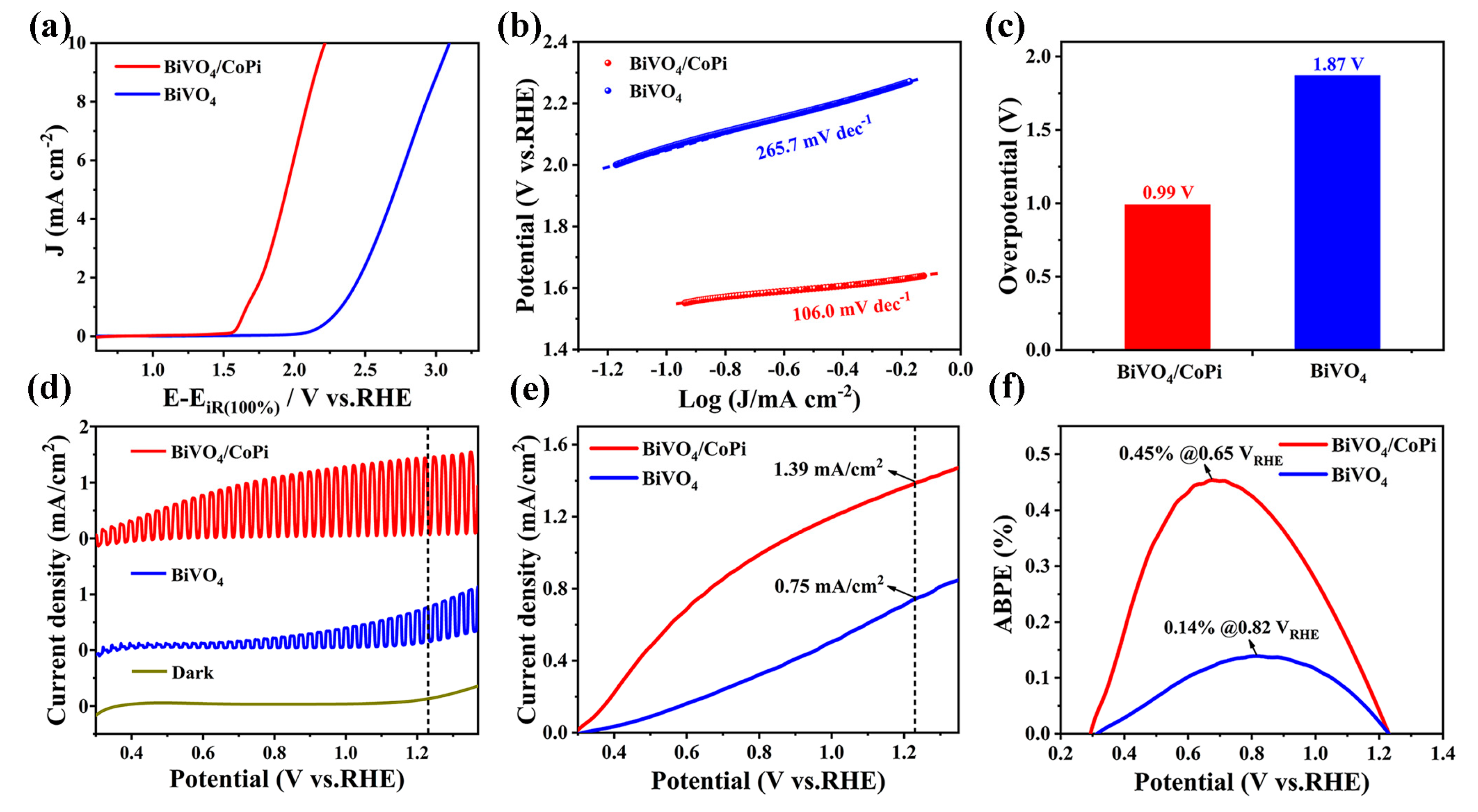
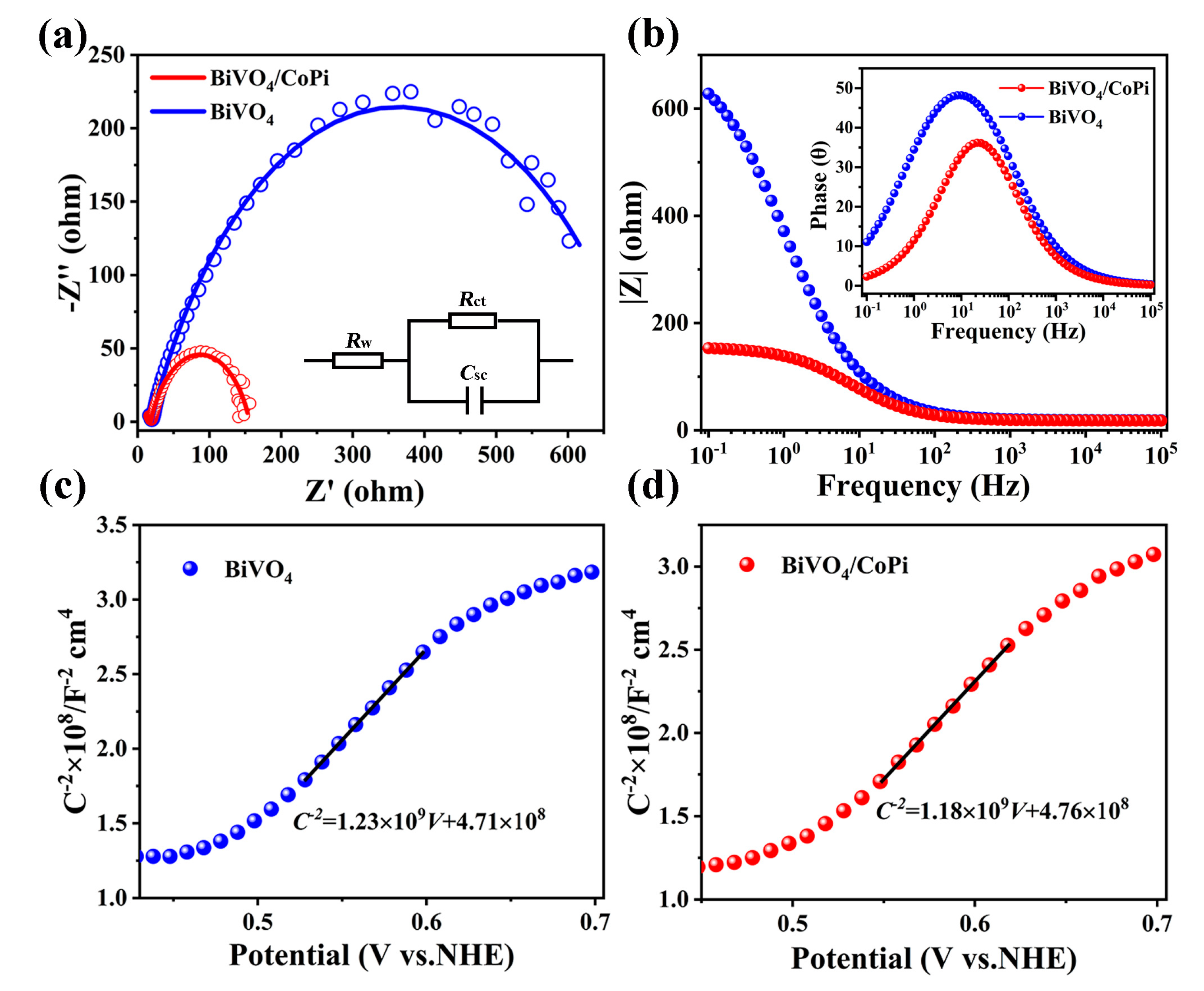
| Photoanodes | RW (Ω) | Rct (Ω) | Csc (F) |
| FTO/BiVO4 | 18.8 | 690.3 | 5.03 × 10−4 |
| FTO/BiVO4/CoPi | 18.22 | 137.9 | 5.20 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xie, Z.; Li, W.; Aziz, H.S.; Abbas, M.; Zheng, Z.; Su, Z.; Fan, P.; Chen, S.; Liang, G. Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation. Materials 2023, 16, 3414. https://doi.org/10.3390/ma16093414
Li Z, Xie Z, Li W, Aziz HS, Abbas M, Zheng Z, Su Z, Fan P, Chen S, Liang G. Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation. Materials. 2023; 16(9):3414. https://doi.org/10.3390/ma16093414
Chicago/Turabian StyleLi, Zhidong, Zhibin Xie, Weibang Li, Hafiz Sartaj Aziz, Muhammad Abbas, Zhuanghao Zheng, Zhenghua Su, Ping Fan, Shuo Chen, and Guangxing Liang. 2023. "Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation" Materials 16, no. 9: 3414. https://doi.org/10.3390/ma16093414
APA StyleLi, Z., Xie, Z., Li, W., Aziz, H. S., Abbas, M., Zheng, Z., Su, Z., Fan, P., Chen, S., & Liang, G. (2023). Charge Transport Enhancement in BiVO4 Photoanode for Efficient Solar Water Oxidation. Materials, 16(9), 3414. https://doi.org/10.3390/ma16093414









