Plasmonic Nanodomains Decorated on Two-Dimensional Oxide Semiconductors for Photonic-Assisted CO2 Conversion
Abstract
1. Introduction
2. Materials and Methods
3. Results
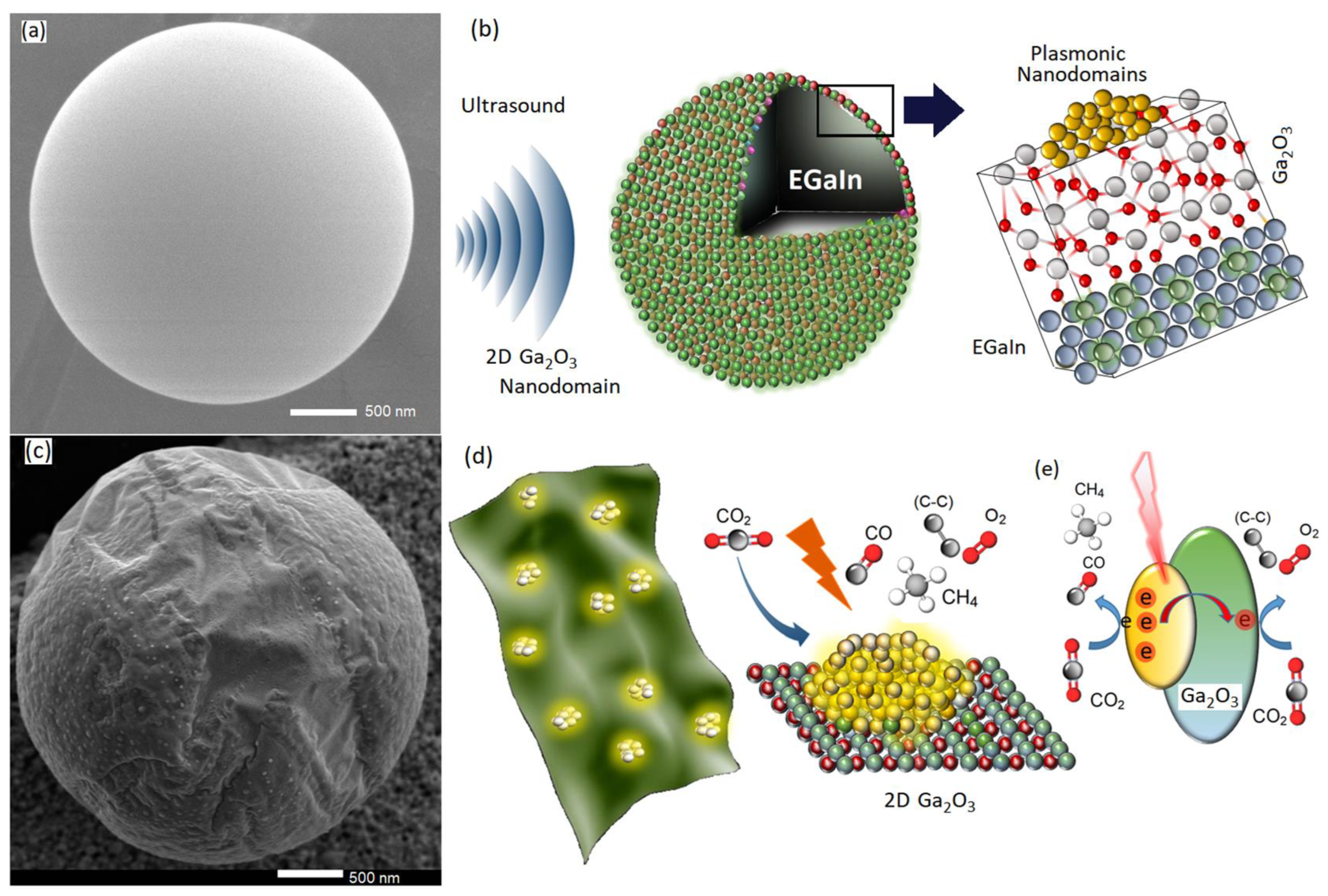
3.1. Synthesis of 2D Ga2O3 Nanosheets with Plasmonic Nanodomains
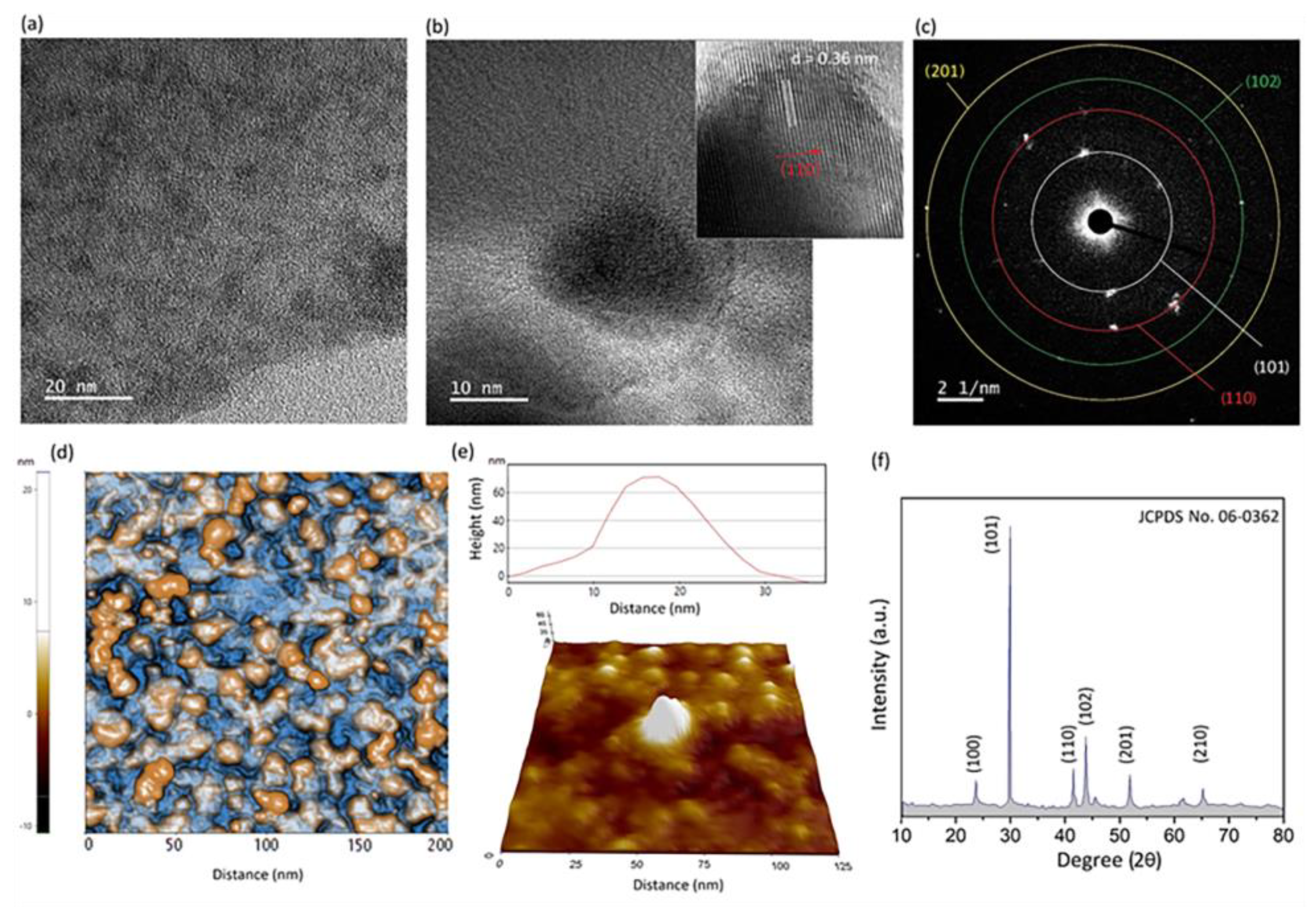
3.2. Characterization of 2D Ga2O3 Nanosheets
3.3. Solar-Powered Acoustic-Activated CO2 Conversion
| Materials | Source of Energy | Conversion Product & Rate | Ref. |
|---|---|---|---|
| Ga2O3-Ag | 250 W Xenon-lamp + ultrasonic (20 Hz, 380 W) | Solid carbon; ~360 μmol g−1h−1 | Present |
| TiO2 nanosheets | 300 W Hg lamp | HCOOH; 1.9 μmol g−1h−1 | [77] |
| TiO2 nanosheets/graphene | 300 W Xe lamp | CO; 52.3 μmol g−1h−1 | [78] |
| SnS2/TiO2 nanosheets | 300 W Xe lamp | CH4; 23.0 μmol g−1h−1 | [79] |
| Cu2O octahedrons/WO3 nanoflakes composite | 300 W Xe lamp | CO; 3.45 μmol g−1h−1 | [80] |
| ZnV2O6 nanosheet/RGO nanosheet | 35 W HID Xe lamp | CH3OH; 515.4 μmol g−1h−1 | [81] |
| Graphene bridged ZnV2O6/pCN nanosheets | 35 W Xe lamp | CH3OH; 542.92 μmol g−1h−1 | [82] |
| BiOBr nanosheets | 300 W Xe lamp | CO; 4.45 μmol g−1h−1 | [83] |
| Bi4O5Br2 nanosheet | 300 W Xe lamp | CO; 31.57 μmol g−1h−1 | [84] |
| BiOBr nanosheets with surface Bi vacancies | 300 W Xe lamp | CO; 20.1 μmol g−1h−1 | [85] |
| MoS2-nanosheets/TiO2-nanosheets | 300 W Xe lamp | CH3OH 10.6 μmol g−1h−1 | [86] |
| Cs2SnI6/SnS2 nanosheet | 100 W Xe lamp | CH4; 6.09 μmol g−1h−1 | [87] |
| Ni metaleorganic framework monolayers | 5 W white LED light | CO; 12,500 μmol g−1h−1 | [88] |
| CeO2/Ti3C2 | 350 W Xe lamp | CO; 40.2 μmol g−1h−1 | [89] |
| CsPbBr3/Ti3C2Tx | 300 W Xe lamp | CO; 26.32 μmol g−1h−1 CH4 7.25 μmol g−1h−1 | [90] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linic, S.; Chavez, S.; Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 2021, 20, 916–924. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Ojambati, O.S.; Duffin, T.J.; Kang, K.; Lee, T.; Scheer, E.; Xiang, D.; Nijhuis, C.A. Plasmonic phenomena in molecular junctions: Principles and applications. Nat. Rev. Chem. 2022, 6, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Arya, G.; Tao, A. Self-orienting nanocubes for the assembly of plasmonic nanojunctions. Nat. Nanotechnol. 2012, 7, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.Y. Plasmon-enhanced light–matter interactions and applications. NPJ Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Zaccaria, R.P.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized surface plasmon resonance in semiconductor nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef]
- Wallace, J.; Soham, S.; Vladimir, S.M.; Alexandra, B.; Marcello, F. Transparent conducting oxides: From all-dielectric plasmonics to a new paradigm in integrated photonics. Adv. Opt. Photonics 2022, 14, 148. [Google Scholar]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Detavernier, C.; Solano, E.; Verpoort, F.; Zhuiykov, S. ALD-Developed plasmonic two-dimensional Au–WO3–TiO2 heterojunction architectonics for design of photovoltaic devices. ACS Appl. Mater. Interfaces 2018, 10, 10304–10314. [Google Scholar] [CrossRef]
- Xu, H.; Karbalaei Akbari, M.; Verpoort, F.; Zhuiykov, S. Nano-engineering and functionalization of hybrid Au–MexOy–TiO2 (Me = W, Ga) hetero-interfaces for optoelectronic receptors and nociceptors. Nanoscale 2020, 12, 20177–20188. [Google Scholar] [CrossRef]
- Stanford, M.G.; Rack, P.D.; Jariwala, D. Emerging nanofabrication and quantum confinement techniques for 2D materials beyond graphene. NPJ 2D Mater. Appl. 2018, 2, 20. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Zhuiykov, S. Photonic and plasmonic devices based on two-dimensional semiconductors. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 145–169. [Google Scholar]
- Turunen, M.; Brotons-Gisbert, M.; Dai, Y.; Wang, Y.; Scerri, E.; Bonato, C.; Jöns, K.L.; Sun, Z.; Gerardot, B.D. Quantum photonics with layered 2D materials. Nat. Rev. Phys. 2022, 4, 219–236. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Karbalaei Akbari, M. Metal/semiconductor hetero-interface engineering for photocurrent controlling in plasmonic photodetectors. In Proceedings of the SMSI 2021-Sensors and Instrumentation, Nuremberg, Germany, 3 May 2021; pp. 165–166. [Google Scholar]
- Xu, H.; Karbalaei Akbari, M.; Zhuiykov, S. 2D semiconductor nanomaterials and heterostructures: Controlled synthesis and functional applications. Nanoscale Res. Lett. 2021, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Vitiello, M.S.; Viti, L.; Cupolillo, A.; Politano, A. Plasmonics with two-dimensional semiconductors: From basic research to technological applications. Nanoscale 2018, 10, 8938–8946. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.P.; Erhart, P.; Kuisma, M. Hot-carrier generation in plasmonic nanoparticles: The importance of atomic structure. ACS Nano 2020, 14, 9963–9971. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Akbari, M.; Verpoort, F.; Zhuiykov, S. Bioinspired patterned photonic junctions for plasmon-enhanced metal photoluminescence and fluorescence: Design of optical cavities for near-infrared electronics. Mater. Today Energy 2022, 26, 101003. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Wang, Q.; Hui, R.; Chen, Z.; Maroni, V.A.; Wu, J. Plasmonic graphene transparent conductors. Adv. Mater. 2012, 24, OP71–OP76. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Karbalaei Akbari, M.; Wang, S.; Chen, S.; Kats, E.; Verpoort, F.; Hue, J.; Zhuiyko, S. Tunability of near infrared opto-synaptic properties of thin MoO3 films fabricated by atomic layer deposition. Appl. Surf. Sci. 2022, 539, 153399. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Ramachandran, R.K.; Detavernier, C.; Hu, J.; Kim, J.; Verpoort, F.; Zhuiykov, S. Heterostructured plasmonic memristors with tunable optosynaptic functionalities. J. Mater. Chem. C 2021, 9, 2539–2549. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hu, J.; Verpoort, F.; Lu, H.; Zhuiykov, S. Nanoscale all-oxide-heterostructured bio-inspired optoresponsive nociceptor. Nano-Micro Lett. 2020, 12, 83. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Zhuiykov, S. Optoelectronic nociceptive sensors based on heterostructured semiconductor films. In Proceedings of the SMSI 2021-Sensors and Instrumentation, Nuremberg, Germany, 3 May 2021; pp. 159–160. [Google Scholar]
- Oh, S.H.; Altug, H.; Jin, X.; Low, T.; Koester, S.J.; Ivanov, A.P.; Edel, J.B.; Avouris, P.; Strano, M.S. Nanophotonic biosensors harnessing van der Waals materials. Nat. Commun. 2021, 12, 3824. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Siraj Lopa, N.; Shahriari, M.; Najafzadehkhoee, A.; Galusek, D.; Zhuiykov, S. Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing. J. Funct. Biomater. 2023, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Akbari, M.; Zhuiykov, S. A bioinspired optoelectronically engineered artificial neurorobotics device with sensorimotor functionalities. Nat. Commun. 2019, 10, 3873. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, L.; Tan, W.-C.; Feng, X.; Chen, L.; Huang, X.; Ang, K.-W. 2D photovoltaic devices: Progress and prospects. Small Methods 2018, 2, 1700294. [Google Scholar] [CrossRef]
- Berquist, Z.J.; Turaczy, K.K.; Lenert, A. Plasmon-enhanced greenhouse selectivity for high-temperature solar thermal energy conversion. ACS Nano 2020, 14, 12605–12613. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Cuenya, B.R. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Ackermann, S.; Sauvin, L.R.; Castiglioni, R.; Rupp, J.L.M.; Scheffe, J.R.; Steinfeld, A. Kinetics of CO2 reduction over nonstoichiometric ceria. J. Phys. Chem. C 2015, 119, 16452–16461. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Verpoor, F.; Zhuiykov, S. State-of-the-art surface oxide semiconductors of liquid metals: An emerging platform for development of multifunctional two-dimensional materials. J. Mater. Chem. A 2021, 9, 34–73. [Google Scholar] [CrossRef]
- Verma, R.; Belgamwar, R.; Polshettiwar, V. Plasmonic photocatalysis for CO2 conversion to chemicals and fuels. ACS Mater. Lett. 2021, 3, 574–598. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hai, Z.; Zhuiykov, S. Wafer-scale two-dimensional Au-TiO2 bilayer films for photocatalytic degradation of Palmitic acid under UV and visible light illumination. Mater. Res. Bull. 2017, 95, 380–391. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.H.; Yang, R.; Bao, L.H.; Meng, L.; Luo, H.L.; Cai, Y.Q.; Liu, G.D.; Zhao, W.J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Zhuiykov, S. Chemical vapor deposition of two-dimensional semiconductors. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 1–33. [Google Scholar]
- Karbalaei Akbari, M.; Zhuiykov, S. Atomic layer deposition of two-dimensional semiconductors. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 43–73. [Google Scholar]
- Karbalaei Akbari, M.; Zhuiykov, S. Self-limiting two-dimensional surface oxides of liquid metals. In Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications; CRC Press Tylor and Francis: Boca Raton, FL, USA, 2020; Volume 1, pp. 70–107. [Google Scholar]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Doktycz, S.; Suslick, K. Interparticle collisions driven by ultrasound. Science 1990, 247, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Gopi, K.R.; Nagarajan, R. Advances in nanoalumina ceramic particle fabrication using sonofragmentation. IEEE Trans. Nanotechnol. 2008, 7, 532–537. [Google Scholar] [CrossRef]
- Pérez-Maqueda, L.A.; Duran, A.; Pérez-Rodríguez, J.L. Preparation of submicron talc particles by sonication. Appl. Clay Sci. 2005, 28, 245–255. [Google Scholar] [CrossRef]
- Zeiger, B.W.; Suslick, K.S. Sonofragmentation of molecular crystals. J. Am. Chem. Soc. 2011, 137, 14530–14533. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Hai, Z.; Wei, Z.; Ramachandran, R.K.; Detavernier, C.; Patel, M. Sonochemical functionalization of the low-dimensional surface oxide of galinstan for heterostructured optoelectronic applications. J. Mater. Chem. C 2019, 7, 5584–5595. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Suslick, K.S. Plasma formation and temperature measurement during single-bubble cavitation. Nature 2005, 434, 52–55. [Google Scholar] [CrossRef]
- Didenko, Y.T.; McNamara, W.B.; Suslick, K.S. Molecular emission from single-bubble sonoluminescence. Nature 2000, 407, 877–879. [Google Scholar] [CrossRef]
- Taleyarkhan, R.P.; West, C.D.; Cho, J.S.; Lahey, R.T.; Nigmatulin, R.I.; Block, R.C. Evidence for nuclear emissions during acoustic cavitation. Science 2002, 295, 1868–1873. [Google Scholar] [CrossRef]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Martínez, R.F.; Cravotto, G.; Cintas, P. Organic Sonochemistry: A Chemist’s timely perspective on mechanisms and reactivity. J. Org. Chem. 2021, 86, 13833–13856. [Google Scholar] [CrossRef] [PubMed]
- Kis-Csitári, J.; Kónya, Z.; Kiricsi, I. Sonochemical Synthesis of Inorganic Nanoparticles. In Functionalized Nanoscale Materials, Devices and Systems. NATO Science for Peace and Security Series B: Physics and Biophysics; Vaseashta, A., Mihailescu, I.N., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Suslick, K.S.; Hyeon, T.; Fang, M. Nanostructured materials generated by high-intensity ultrasound: Sonochemical synthesis and catalytic studies. Chem. Mater. 1996, 8, 2172–2179. [Google Scholar] [CrossRef]
- Kranert, C.; Sturm, C.; Schmidt-Grund, R.; Grundmann, M. Raman tensor elements of β-Ga2O3. Sci. Rep. 2016, 6, 35964. [Google Scholar] [CrossRef] [PubMed]
- Remple, C.; Huso, J.; McCluskey, M.D. Photoluminescence and Raman mapping of β-Ga2O3. AIP Adv. 2021, 11, 105006. [Google Scholar] [CrossRef]
- Mi, W.; Luan, C.; Li, Z.; Zhao, C.; Feng, X.; Ma, J. Ultraviolet–green photoluminescence of ß-Ga2O3 films deposited on MgAl6O10 (100) substrate. Opt. Mater. 2013, 35, 2624–2628. [Google Scholar] [CrossRef]
- Hao, J.H.; Cocivera, M. Optical and luminescent properties of undoped and rare-earth-doped Ga2O3 thin films deposited by spray pyrolysis. J. Phys. D Appl. Phys. 2002, 35, 433. [Google Scholar] [CrossRef]
- Lorenz, M.R.; Woods, J.F.; Gambino, R.J. Some electrical properties of the semiconductor β-Ga2O3. J. Phys. Chem. Solids 1967, 28, 403. [Google Scholar] [CrossRef]
- Hoshyargar, F.; Crawford, J.; O’Mullane, A.P. Galvanic Replacement of the Liquid Metal Galinstan. J. Am. Chem. Soc. 2017, 139, 1464–1471. [Google Scholar] [CrossRef]
- Echeverria, C.A.; Tang, J.; Cao, Z.; Esrafilzadeh, D.; Kalantar-Zadeh, K. Ag-Ga bimetallic nanostructures ultrasonically prepared from silver–liquid gallium core-shell systems engineered for catalytic applications. ACS Appl. Nano Mater. 2022, 5, 6820–6831. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, J.Y.; Lee, Y.H.; Park, J.Y.; Song, H. Formation of metal selenide and metal–selenium nanoparticles using distinct reactivity between selenium and noble metals. Asian J. Chem. 2015, 10, 1452–1456. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Healthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.; Mayers, B.; Cattle, B.; Xia, Y. Synthesis and characterization of uniform nanowires of trigonal selenium. Adv. Funct. Mater. 2002, 12, 219–227. [Google Scholar] [CrossRef]
- Yin, H.; Xu, Z.; Bao, H.; Bai, J.; Zheng, Y. Single crystal trigonal selenium nanoplates converted from selenium nanoparticles. Chem. Lett. 2005, 34, 22–23. [Google Scholar] [CrossRef]
- Goldan, A.H.; Li, C.; Pennycook, S.J.; Schneider, J.; Blom, A.; Zhao, W. Molecular structure of vapor-deposited amorphous selenium. J. Appl. Phys. 2016, 120, 135101. [Google Scholar] [CrossRef]
- Gates, B.; Brian, M.; Andrew, G.; Younan, X. A sonochemical approach to the synthesis of crystalline selenium nanowires in solutions and on solid supports. Adv. Matter. 2002, 14, 1749–1752. [Google Scholar] [CrossRef]
- Kumar, H.; Rani, R. Structural characterization of silver nanoparticles synthesized by micro emulsion route. Inter J Eng. Innov. Technol. 2013, 3, 344–348. [Google Scholar]
- Alim-Al-Razy, M.; Asik Bayazid, G.M.; Ur Rahman, R.; Bosu, R.; Shamma, S.S. Silver nanoparticle synthesis, UV-Vis spectroscopy to find particle size and measure resistance of colloidal solution. J. Phys. Conf. Ser. 2020, 1706, 012020. [Google Scholar] [CrossRef]
- Zakaria, R.; Hamdan, K.S.; Che Noh, S.M.; Supangat, A.; Sookhakian, M. Surface plasmon resonance and photoluminescence studies of Au and Ag micro-flowers. Opt. Mater. 2015, 5, 943–950. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Sun, X.; Li, H.; Song, H.; Jiang, H.; Chen, Y. Tunable dipole surface plasmon resonances of silver nanoparticles by cladding dielectric layers. Sci. Rep. 2015, 5, 12555. [Google Scholar] [CrossRef]
- Balzarotti, A.; Piacentrini, M.; Burattirii, E.; Piacentini, P. Electroreflectance and band structure of gallium selenide. J. Phys. C Solid State Phys. 1971, 4, L273. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Bondarchuk, I.S.; Losytskyy, M.Y.; Alexeenko, A.A. Temperature dependence of photoluminescence from silver nanoparticles. Plasmonics 2014, 9, 93–101. [Google Scholar] [CrossRef]
- Senthil kumaran, C.K.; Agilan, S.; Velauthapillai, D.; Muthukumarasamy, N.; Thambidurai, M.; Senthil, T.S.; Balasundaraprabhu, R. Synthesis and characterization of selenium nanowires. ISRN Nanotechnol. 2011, 4, 589073. [Google Scholar] [CrossRef]
- Jangir, R.; Porwal, S.; Tiwari, P.; Mondal, P.; Rai, S.K.; Srivastava, A.K.; Bhaumik, I.; Ganguli, T. Correlation between surface modification and photoluminescence properties of β-Ga2O3 nanostructures. AIP Adv. 2016, 6, 035120. [Google Scholar] [CrossRef]
- Köck, E.-M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In Situ FT-IR spectroscopic study of CO2 and CO adsorption on Y2O3. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.E.; Baltanas, M.A.; Bonivardi, A.L. Infrared spectroscopic study of the carbon dioxide adsorption on the Surface of Ga2O3 polymorphs. J. Phys. Chem. B 2006, 110, 5498–5507. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.E.; Baltanas, M.A.; Bonivardi, A.L. An infrared study of the intermediates of methanol synthesis from carbon dioxide over Pd/β-Ga2O3. J. Catal. 2004, 226, 410–421. [Google Scholar] [CrossRef]
- Tang, J.; Mayyas, M.; Ghasemian, M.B.; Kalantar-Zade, K. Liquid-metal-enabled mechanical-energy-induced CO2 conversion. Adv. Matter. 2022, 34, 2105789. [Google Scholar] [CrossRef] [PubMed]
- Cui1, C.; Xue, F.; Hu, W.J.; Li, L.J. Two-dimensional materials with piezoelectric and ferroelectric functionalities. NPJ 2D Mater. 2018, 2, 18. [Google Scholar] [CrossRef]
- Qamar, S.; Lei, F.; Liang, L.; Gao, S.; Liu, K.; Sun, Y. Ultrathin TiO2 flakes optimizing solar light driven CO2 reduction. Nano Energy 2016, 26, 692–698. [Google Scholar] [CrossRef]
- Yang, J.; Wen, Z.; Shen, X.; Dai, J.; Li, Y. A comparative study on the photocatalytic behavior of graphene-TiO2 nanostructures: Effect of TiO2 dimensionality on interfacial charge transfer. Chem. Eng. J. 2018, 334, 907–921. [Google Scholar] [CrossRef]
- She, H.; Zhou, H.; Li, L.; Zhao, Z.; Jiang, M.; Huang, J. Construction of a two dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain. Chem. Eng. 2018, 7, 650–659. [Google Scholar] [CrossRef]
- Shi, W.; Guo, X.; Wang, J.C.; Li, Y.; Liu, L.; Hou, Y. Enhanced photocatalytic 3D/2D architecture for CO2 reduction over cuprous oxide octahedrons supported on hexagonal phase tungsten oxide nanoflakes. J. Alloys Compd. 2020, 830, 154683. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M.; Amin, N.A.S. Synergistic effects of 2D/2D ZnV2O6/RGO nanosheets heterojunction for stable and high performance photo-induced CO2 reduction to solar fuels. Chem. Eng. J. 2018, 334, 2142–2153. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M.; Ali Khan, A.; Saidina Amin, N.A. Indirect Z-scheme assembly of 2D ZnV2O6/RGO/g-C3N4 nanosheets with RGO/pCN as solid-state electron mediators toward visible-light-enhanced CO2 reduction. Ind. Eng. Chem. Res. 2019, 58, 8612–8624. [Google Scholar] [CrossRef]
- Wu, D.; Ye, L.; Yip, H.Y.; Wong, P.K. Organic-free synthesis of {001} facet dominated BiOBr nanosheets for selective photoreduction of CO2 to CO. Catal. Sci. Technol. 2017, 7, 265–271. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, P.; Wang, L.; Yang, B.; Xie, H.; Zhou, Y. Ultrathin Bi4O5Br2 nanosheets for selective photocatalytic CO2 conversion into CO. Chem. Eng. J. 2019, 360, 473–482. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M. Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2019, 11, 30786–30792. [Google Scholar] [CrossRef]
- Tu, W.; Li, Y.; Kuai, L.; Zhou, Y.; Xu, Q.; Li, H. Construction of unique two dimensional MoS2-TiO2 hybrid nanojunctions: MoS2 as a promising cost effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 2017, 9, 9065–9070. [Google Scholar] [CrossRef]
- Wang, X.D.; Huang, Y.H.; Liao, J.F.; Jiang, Y.; Zhou, L.; Zhang, X.Y. In situ construction of a Cs2SnI6 perovskite nanocrystal/SnS2 nanosheet heterojunction with boosted interfacial charge transfer. J. Am. Chem. Soc. 2019, 141, 13434–13441. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H. Nickel metal-organic framework monolayers for photoreduction of diluted CO2: Metal-nodedependent activity and selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shen, J.; Zhang, W.; Yu, X.; Tang, H.; Zhang, M. Built-in electric field induced CeO2/Ti3C2-MXene Schottky-junction for coupled photocatalytic tetracycline degradation and CO2 reduction. Ceram. Int. 2019, 45, 24146–24153. [Google Scholar] [CrossRef]
- Pan, A.; Ma, X.; Huang, S.; Wu, Y.; Jia, M.; Shi, Y. CsPbBr3 perovskite nanocrystal grown on MXene nanosheets for enhanced photoelectric detection and photocatalytic CO2 reduction. J. Phys. Chem. Lett. 2019, 10, 6590–6597. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbalaei Akbari, M.; Siraj Lopa, N.; Park, J.; Zhuiykov, S. Plasmonic Nanodomains Decorated on Two-Dimensional Oxide Semiconductors for Photonic-Assisted CO2 Conversion. Materials 2023, 16, 3675. https://doi.org/10.3390/ma16103675
Karbalaei Akbari M, Siraj Lopa N, Park J, Zhuiykov S. Plasmonic Nanodomains Decorated on Two-Dimensional Oxide Semiconductors for Photonic-Assisted CO2 Conversion. Materials. 2023; 16(10):3675. https://doi.org/10.3390/ma16103675
Chicago/Turabian StyleKarbalaei Akbari, Mohammad, Nasrin Siraj Lopa, Jihae Park, and Serge Zhuiykov. 2023. "Plasmonic Nanodomains Decorated on Two-Dimensional Oxide Semiconductors for Photonic-Assisted CO2 Conversion" Materials 16, no. 10: 3675. https://doi.org/10.3390/ma16103675
APA StyleKarbalaei Akbari, M., Siraj Lopa, N., Park, J., & Zhuiykov, S. (2023). Plasmonic Nanodomains Decorated on Two-Dimensional Oxide Semiconductors for Photonic-Assisted CO2 Conversion. Materials, 16(10), 3675. https://doi.org/10.3390/ma16103675







