Performance Improvement of Quantum Dot Light-Emitting Diodes Using a ZnMgO Electron Transport Layer with a Core/Shell Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Syntheses of Materials
2.2. Device Fabrication
2.3. Characterizations
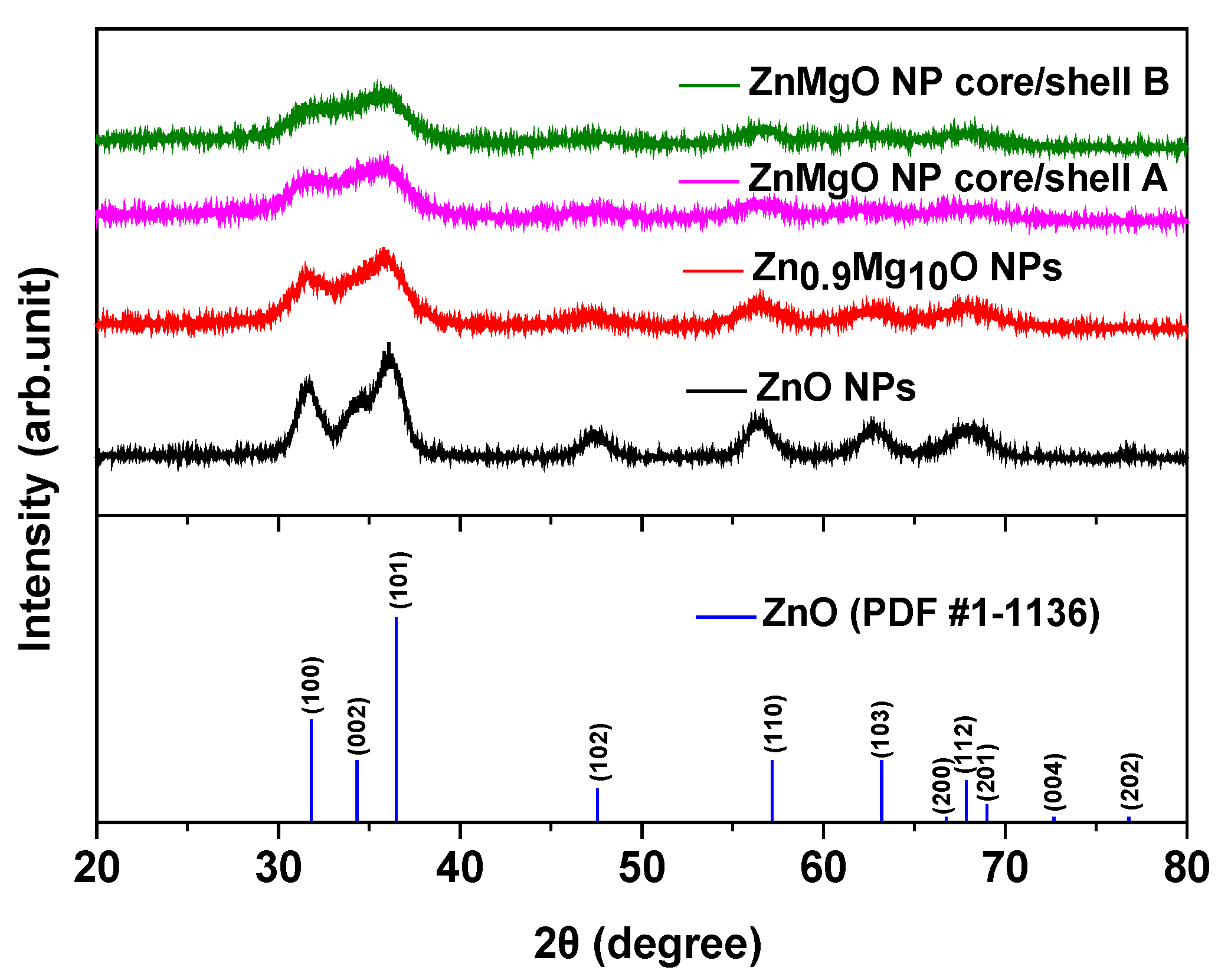
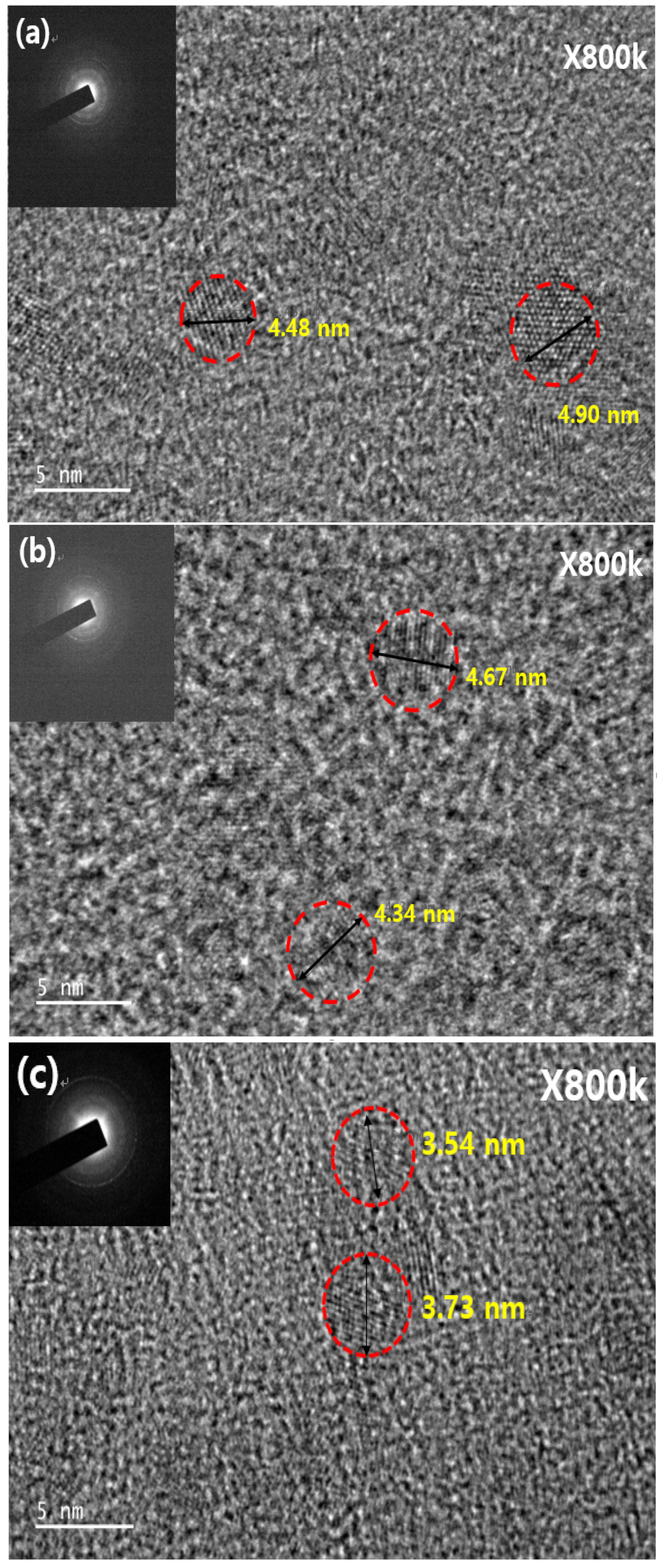
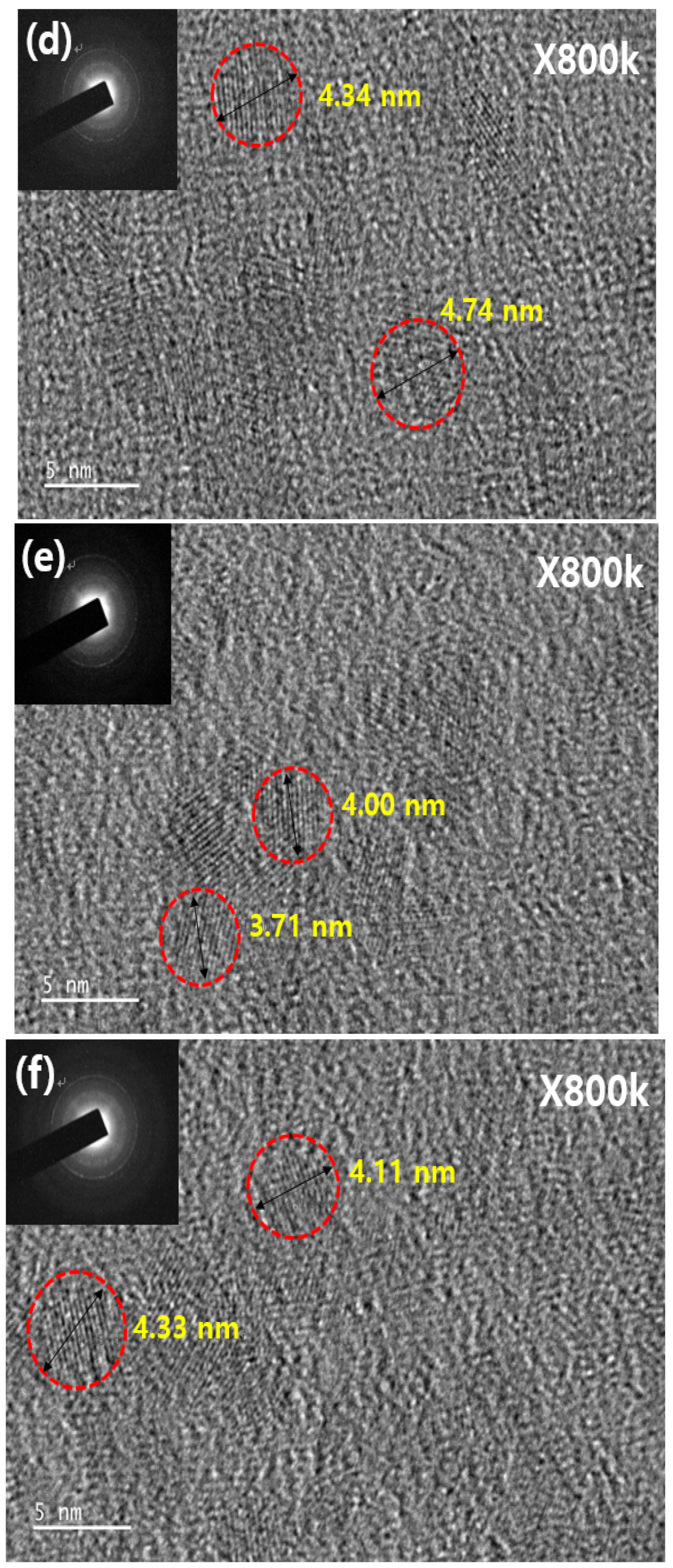
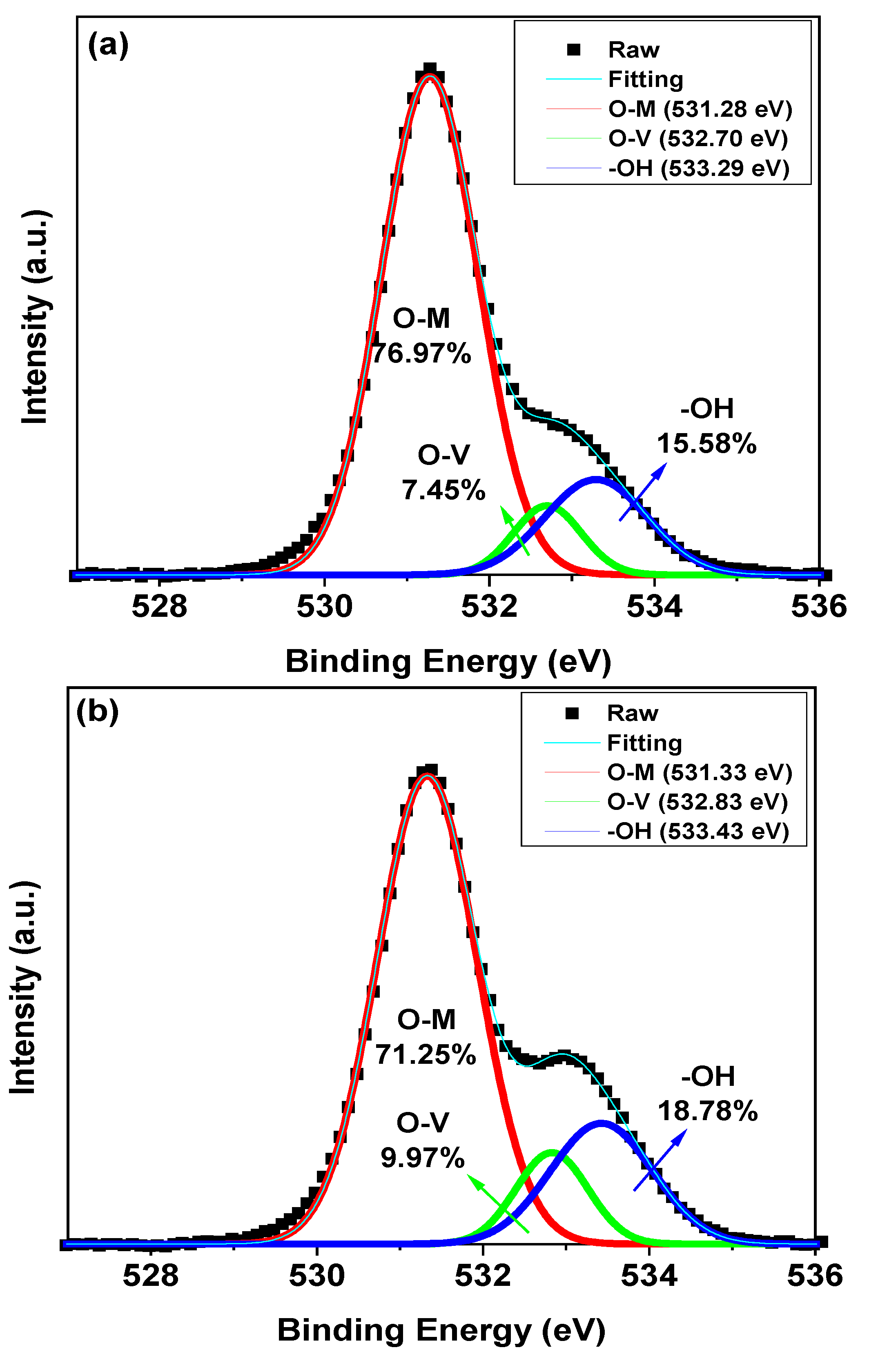
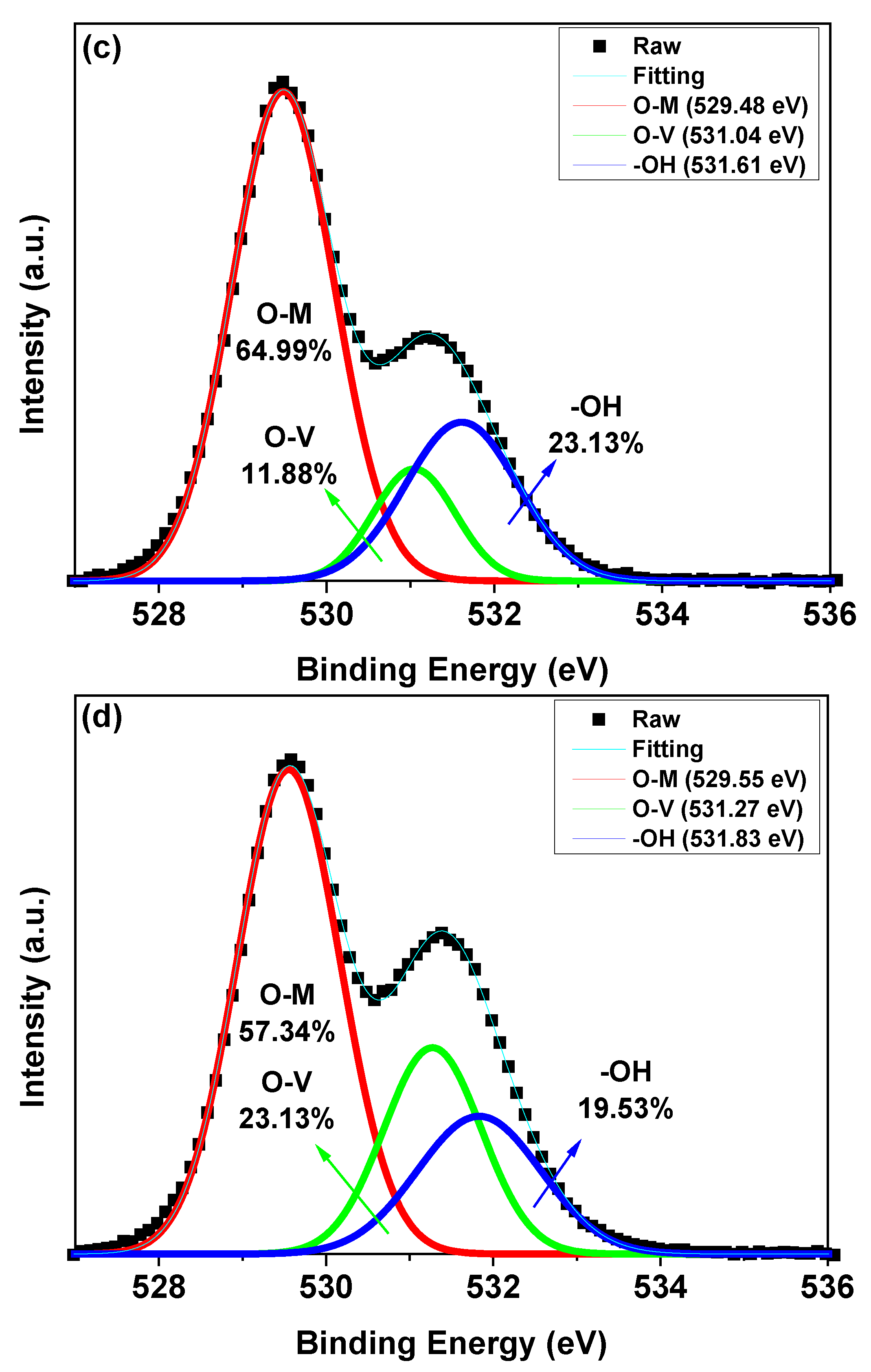
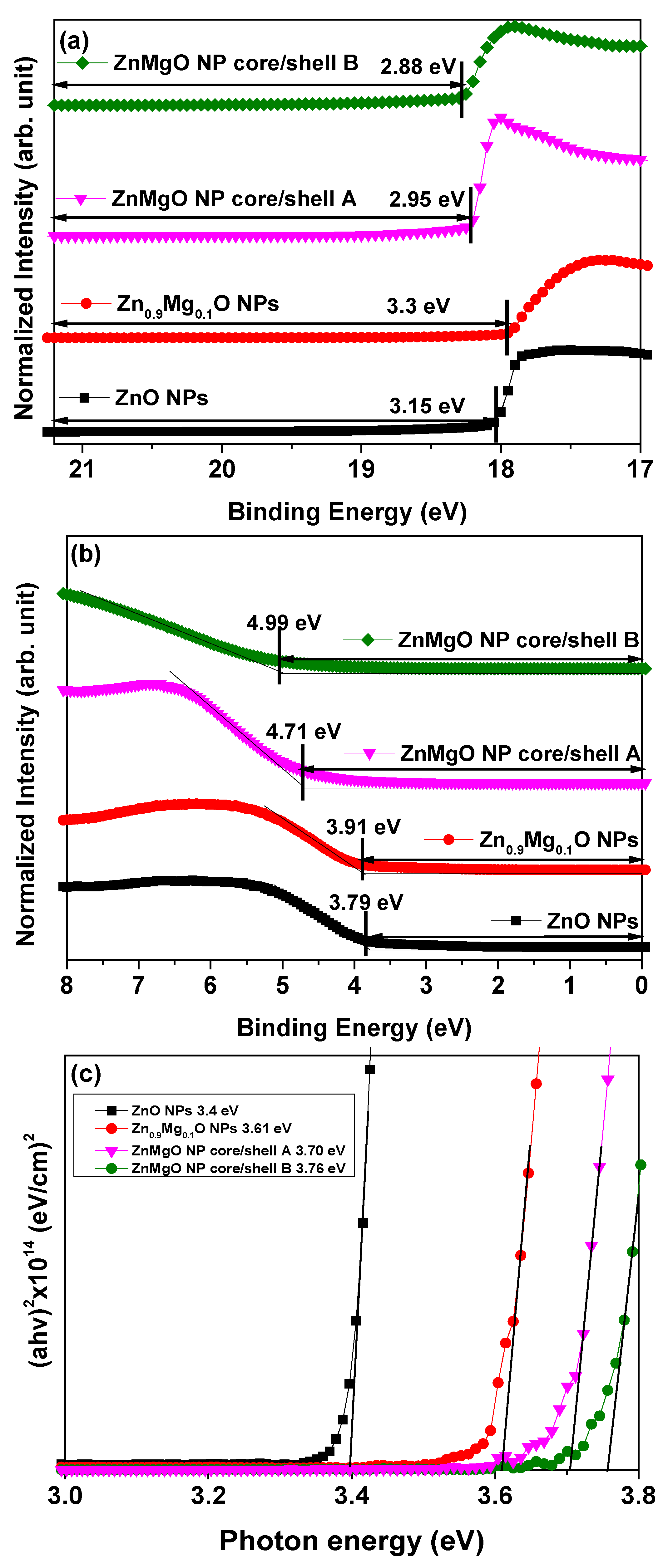
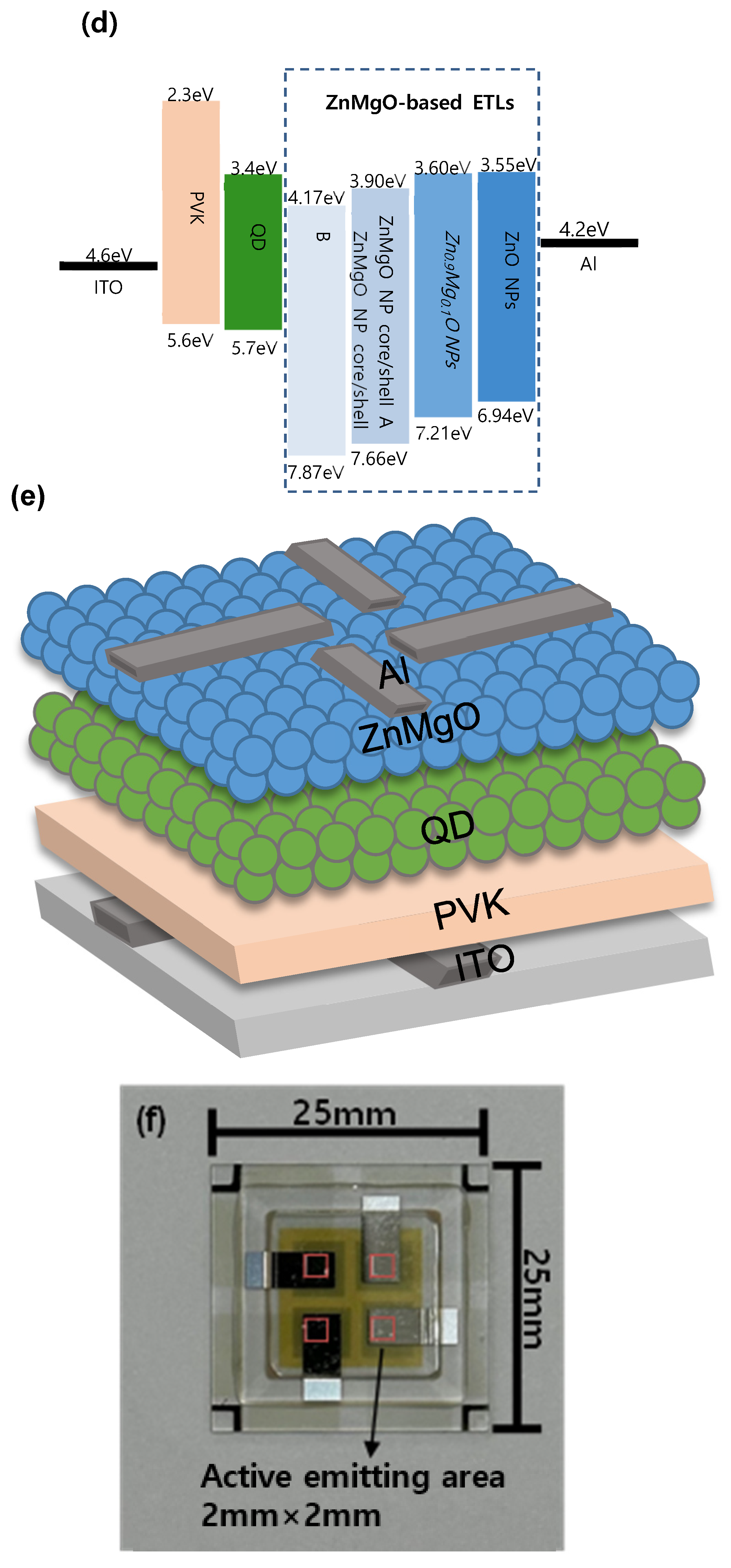
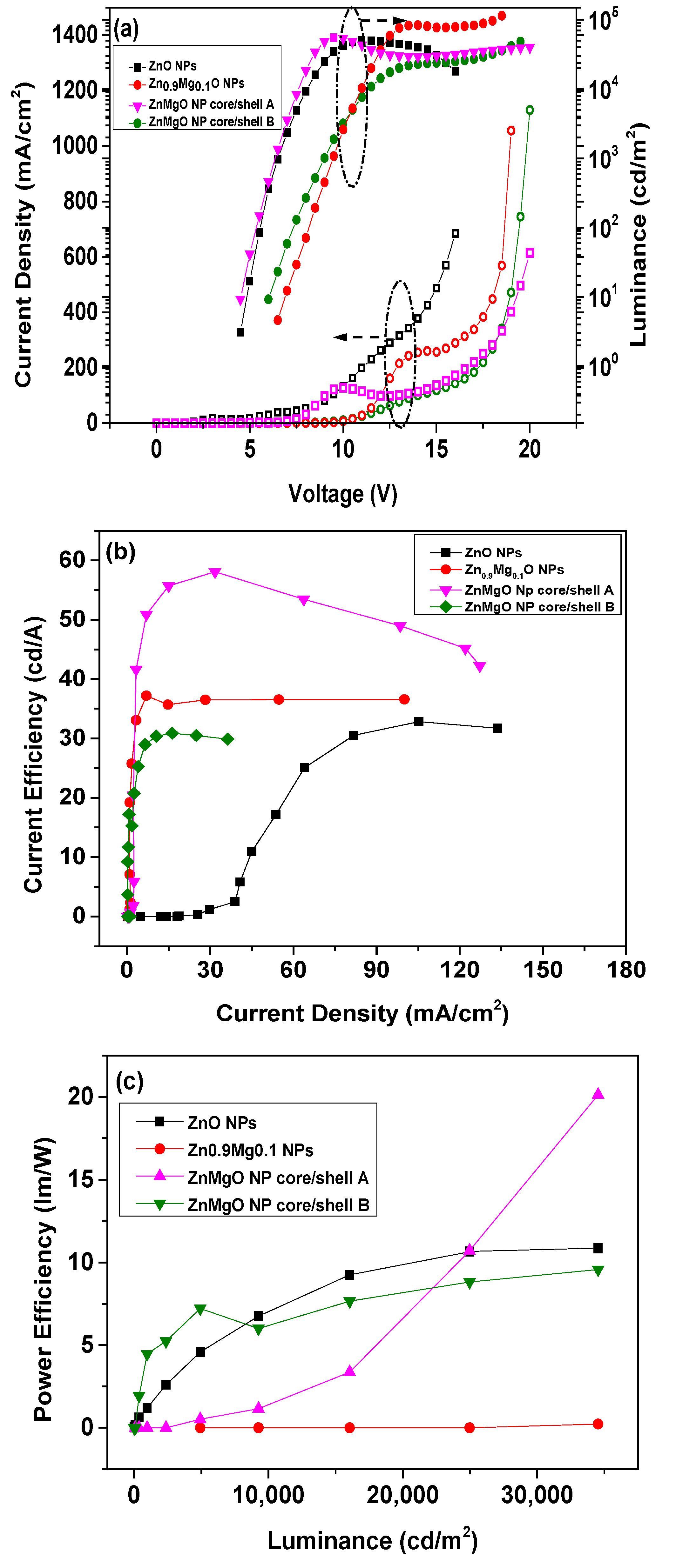
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Colvin, V.L.; Schlamp, M.C.; Alivisators, A.P. Light-Emitting Diodes Made from Cadmium Selenide Nanocrystals and a Semiconducting Polymers. Nature 1994, 370, 354–357 . [Google Scholar] [CrossRef]
- Kim, B.H.; Nam, S.; Oh, N.; Cho, S.Y.; Yu, K.J.; Lee, C.H.; Zhang, J.; Deshpande, K.; Trefonas, P.; Kim, J.H.; et al. Multilayer Transfer Printing for Pixelated, Multicolor Quantum Dot Light-Emitting Diodes. ACS Nano 2016, 10, 4920–4925 . [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size Tunable ZnO Nanoparticles To Enhance Electron Injection in Solution Processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Pan, J.Y.; Chen, J.; Huang, Q.Q.; Khan, Q.; Liu, X.; Tao, Z.; Lei, W.; Xu, F.; Zhang, Z.C. Flexible Quantum Dot Light Emitting Diodes Based on ZnO Nanoparticles. RSC Adv. 2015, 5, 82192–82198. [Google Scholar] [CrossRef]
- Pan, J.; Chen, J.; Zhao, D.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W. Surface Plasmon-enhanced Quantum Dot Light-emitting Diodes by Incorporating Gold Nanoparticles. Opt. Express 2016, 24, A33–A43 . [Google Scholar] [CrossRef]
- Chang, S.; Zhang, X.; Wang, Z.W.; Han, D.B.; Tang, J.L.; Bai, Z.L.; Zhong, H.Z. Alcohol-Soluble Quantum Dots: Enhanced Solution Processability and Charge Injection for Electroluminescence Devices. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1900708. [Google Scholar] [CrossRef]
- Caruge, J.M.; Halpert, J.E.; Wood, V.; Bulovic, V.; Bawendi, M.G. Colloidal quantum-dot light-emitting diodes with metal-oxide charge transport layers. Nat. Photonics 2008, 2, 247–250 . [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient quantum-dot light-emitting diodes based on solution-processed multilayer structures. Nat. Photonics 2011, 5, 543–548 . [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23 . [Google Scholar] [CrossRef]
- Kwak, J.; Bae, W.K.; Lee, D.; Park, I.; Lim, J.; Park, M.; Cho, H.; Woo, H.; Yoon, D.Y.; Char, K.; et al. Bright and efficient full-color colloidal quantum dot light-emitting diodes using an inverted device structure. Nano Lett. 2012, 12, 2362–2366 . [Google Scholar] [CrossRef]
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S.; et al. High-efficiency quantum-dot light-emitting devices with enhanced charge injection. Nat. Photonics 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed. high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, W.; Chen, S. Efficient quantum-dot light-emitting diodes with 4,4,4-tris(N-carbazolyl)-triphenylamine (TcTa) electron-blocking layer. IEEE Electron. Device Lett. 2015, 36, 369–371. [Google Scholar] [CrossRef]
- Son, S.-R.; Yang, K.P.; Park, J.; Lee, J.H.; Lee, K. Highly Efficient and Eco-Friendly InP-Based Quantum Dot Light-Emitting Diodes with a Synergetic Combination of a Liquid Metal Cathode and Size-Controlled ZnO Nanoparticles. Mater. Chem. Phys. 2022, 3, 26322. [Google Scholar] [CrossRef]
- Yu, P.; Cao, S.; Shan, Y.; Bi, Y.; Hu, Y.; Zeng, R.; Zou, B.; Wang, Y.; Zhao, J. Highly Efficient Green InP-Based Quantum Dot Light-Emitting Diodes Regulated by Inner Alloyed Component. Light Sci. Appl. 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, C.; Song, C.; Wang, J.; Mu, L.; He, Z.; Zhong, Z.; Cun, Y.; Mai, C.; Wang, J.; et al. Highly Efficient All-Solution Processed Inverted Quantum Dots Based Light Emitting Diodes. ACS Nano 2016, 12, 1564–1570 . [Google Scholar] [CrossRef]
- Song, J.; Wang, O.; Shen, H.; Lin, Q.; Li, Z.; Wang, L.; Zhang, X.; Li, L.S. Over 30% External Quantum Efficiency Light-Emitting Diodes by Engineering Quantum Dot-Assisted Energy Level Match for Hole Transport Layer. Adv. Funt. Mater. 2019, 29, 1808377. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Sun, L.; Xu, Y.; Li, M.; Hu, G.; Tang, T.; Wen, J.; Li, X.; Zhang, J.; et al. High fluorescent sulfur regulating graphene quantum dots with tunable photoluminescence properties. J. Colloid Interface Sci. 2018, 529, 205–213 . [Google Scholar] [CrossRef]
- Liu, Z.; Li, F.; Luo, Y.; Li, M.; Hu, G.; Pu, X.; Tang, T.; Wen, J.; Li, X.; Li, W. Size Effect of Graphene Quantum Dots on Photoluminescence. Molecules 2021, 26, 3922. [Google Scholar] [CrossRef]
- Kim, H.-M.; Cho, S.; Kim, J.; Shin, H.; Jang, J. Li and Mg co-doped zinc oxide electron transporting layer for highly efficient quantum dot light-emitting diodes. ACS Appl. Mater. Interfaces. 2018, 16, 24028–24036 . [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.-S.; Lim, J.; Lee, D.; Padiha, I.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, M.; Klimov, V.I. Controlling the Influence of Auger Recombination on the performance of Quantum-Dot Light-Emitting Diodes. Nat. Commun. 2013, 4, 2661. [Google Scholar] [CrossRef]
- Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic Insights into the Performance of Quantum Dot Light-Emitting Diodes. Nat. Commun. 2013, 38, 721–730 . [Google Scholar] [CrossRef]
- Davidson-Hall, T.; Aziz, H. The Role of Polyethylenimine in Enhancing the Efficiency of Quantum Dot Light-Emitting Devices. Nanoscale 2018, 10, 2623–2631 . [Google Scholar] [CrossRef] [PubMed]
- Heo, S.B.; Shin, J.S.; Kim, T.Y.; Park, S.; Jung, W.H.; Kim, H.; Hong, J.-A.; Kim, B.-S.; Park, Y.; Chin, B.D.; et al. Highly Efficient and Low Turn-on Voltage Quantum-Dot Light-Emitting Diodes Using a ZnMgO/ZnO double Electron Transport Layer. Curr. Appl. Phys. 2021, 29, 107–113 . [Google Scholar] [CrossRef]
- Zhang, B.; Mai, C.; Mu, L.; Li, M.; Wang, J.; Xu, W.; Peng, J. Effects of ZnMgO Electron Transport Layer on the Performance of InP-Based Inverted Quantum Dot Light-Emitting Diodes. Nanomaterials 2021, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Lu, M.; Sun, C.; Zhang, T.; Yu, W.W. Enhanced Stability and Performance in Perovskite Nanocrystal Light-Emitting Devices Using a ZnMgO Interfacial Layer. Adv. Opt. Mater. 2017, 5, 1700377. [Google Scholar] [CrossRef]
- Kim, B.; Lee, D.; Hwang, B.; Eun, Y.; Ha, M.-Y.; Kim, C.K. High performance top-emission quantum dot light-emitting diodes with Mg-doped ZnO nanoparticles used as an electron transport layer. J. Nanosci. Nanotechnol. 2021, 21, 3747–3752. [Google Scholar] [CrossRef]
- Li, J.; Guo, Q.; Jin, H.; Wang, K.; Xu, D.; Xu, Y.; Xu, G.; Xu, X. Improved performance of quantum dot light emitting diode by modulating electron injection with yttrium-doped ZnO nanoparticles. J. Appl. Physic. 2017, 122, 135501. [Google Scholar] [CrossRef]
- Hwang, B.; Eun, Y.; Jang, G.-P.; Yang, J.-H.; Ha, M.-Y.; Moon, D.-G.; Kim, C.K. Highly efficient and bright quantum-dot light-emitting diodes with enhanced charge balance by adjusting the thickness of Zn0.9Mg0.1O electron transport layer. Phys. Status Solidi A 2022, 2100856. [Google Scholar] [CrossRef]
- Liu, B.; Lan, L.; Tao, H.; Li, H.; Xu, H.; Zou, J.; Xu, M.; Wang, L.; Wang, L.; Peng, J.; et al. Improved performance of quantum dot light-emitting diodes by hybrid electron transport layer comprised of ZnO nanoparticles doped organic small molecule. Organ. Electron. 2019, 74, 144–151 . [Google Scholar] [CrossRef]
- Chrzanowski, M.; Kuchowicz, M.; Szukiewicz, R.; Misiewicz, J. Enhnaced efficiency of quantum dot light-emitting diode by sol-gel derived Zn1-xMgxO electron transport layer. Organ. Electron. 2020, 80, 105656. [Google Scholar] [CrossRef]
- Rim, Y.S.; Kim, D.L.; Jeong, W.H.; Kim, H.J. Effect of Zr addition on ZnSnO thin-film transistors using a solution process. Appl. Phys. Lett. 2010, 97, 233502. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Jiang, Q.; Lian, J.S. Synthesis and optical properties of flower-like ZnO nanorods by thermal evaporation method. Appl. Surf. Sci. 2011, 257, 5083. [Google Scholar] [CrossRef]
- Han, X.G.; He, H.Z.; Kuang, Q.; Zhou, X.; Zhang, X.H.; Xu, T.; Xie, Z.X.; Zheng, L.S. Controlling Morphologies and Tuning the Related Properties of Nano/Microstructured ZnO Crystallites. J. Phys. Chem. 2009, 113, 584. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, J.; Zhao, J.; Yang, Z.; Li, C.; Guan, X.; Yang, W.; Shang, M.; Wu, T. Enhancing the Performance of Quantum Dot Light-Emitting Diodes Using Room-Temperature-Processed Ga-Doped ZnO Nanoparticles as the Electron Transport Layer. ACS Appl. Mater. Interfaces 2017, 9, 15605–15614 . [Google Scholar] [CrossRef]
- Park, Y.R.; Doh, J.H.; Shin, K.; Seo, Y.S.; Kim, Y.S.; Kim, S.Y.; Choi, W.K.; Hong, Y.J. Solution-processed quantum dot light-emitting diodes with PANI:PSS hole-transport interlayer. Org. Electron. 2015, 19, 131–139 . [Google Scholar] [CrossRef]
- Znao, J.; Bardecker, J.A.; Munro, A.M.; Liu, M.S.; Niu, Y.; Ding, I.-K.; Luo, J.; Chen, B.; Jen, A.K.-Y.; Ginger, D.S. Efficient CdSe/CdS quantum dot light-emitting diodes using a thermally polymerized hole transport layer. Nano Lett. 2006, 3, 463–467 . [Google Scholar] [CrossRef]
- Lee, K.-H.; Lee, J.-H.; Kang, H.-D.; Park, B.; Kwon, Y.; Ko, H.; Lee, C.; Lee, J.; Yang, H. Over 40 cd/A efficient green quantum dot electroluminescent device comprising uniquely large-sized quantum dots. ACS Nano 2014, 8, 4893–4901. [Google Scholar] [CrossRef] [PubMed]
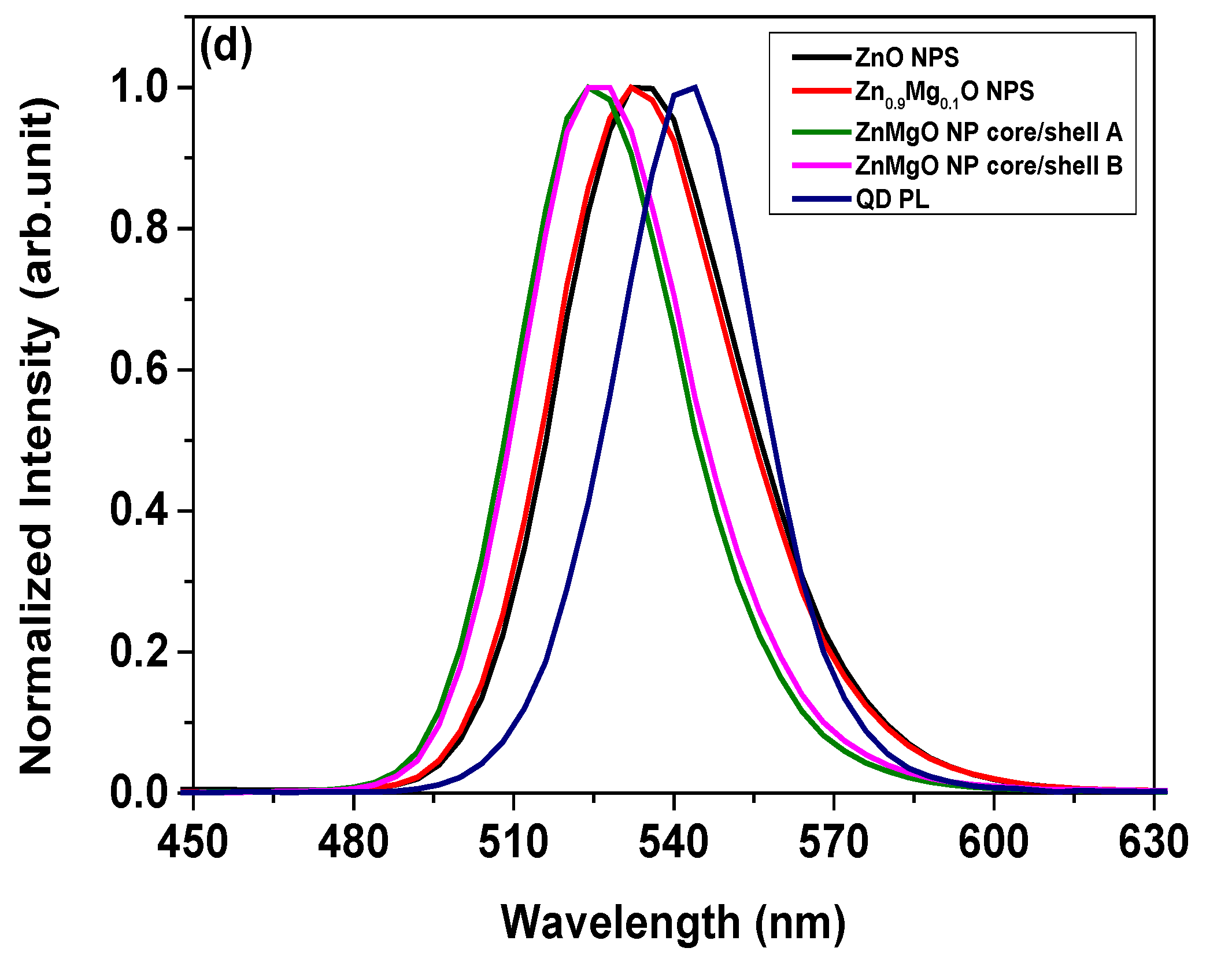
| Sample | Peak (nm) | FWHM (nm) |
|---|---|---|
| PL of QD | 542.4 | 33.3 |
| EL of QLED with ZnO NPs | 536.1 | 40.6 |
| EL of QLED with Zn0.9Mg0.1O NPs | 535.0 | 40.9 |
| EL of QLED with ZnMgO NP core/shell A | 527.0 | 37.3 |
| EL of QLED with ZnMgO NP core/shell B | 527.8 | 37.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eun, Y.-B.; Jang, G.-P.; Yang, J.-H.; Kim, S.-Y.; Chae, Y.-B.; Ha, M.-Y.; Moon, D.-G.; Kim, C.-K. Performance Improvement of Quantum Dot Light-Emitting Diodes Using a ZnMgO Electron Transport Layer with a Core/Shell Structure. Materials 2023, 16, 600. https://doi.org/10.3390/ma16020600
Eun Y-B, Jang G-P, Yang J-H, Kim S-Y, Chae Y-B, Ha M-Y, Moon D-G, Kim C-K. Performance Improvement of Quantum Dot Light-Emitting Diodes Using a ZnMgO Electron Transport Layer with a Core/Shell Structure. Materials. 2023; 16(2):600. https://doi.org/10.3390/ma16020600
Chicago/Turabian StyleEun, Ye-Bin, Gyeong-Pil Jang, Ji-Hun Yang, Su-Young Kim, Young-Bin Chae, Mi-Young Ha, Dae-Gyu Moon, and Chang-Kyo Kim. 2023. "Performance Improvement of Quantum Dot Light-Emitting Diodes Using a ZnMgO Electron Transport Layer with a Core/Shell Structure" Materials 16, no. 2: 600. https://doi.org/10.3390/ma16020600
APA StyleEun, Y.-B., Jang, G.-P., Yang, J.-H., Kim, S.-Y., Chae, Y.-B., Ha, M.-Y., Moon, D.-G., & Kim, C.-K. (2023). Performance Improvement of Quantum Dot Light-Emitting Diodes Using a ZnMgO Electron Transport Layer with a Core/Shell Structure. Materials, 16(2), 600. https://doi.org/10.3390/ma16020600





