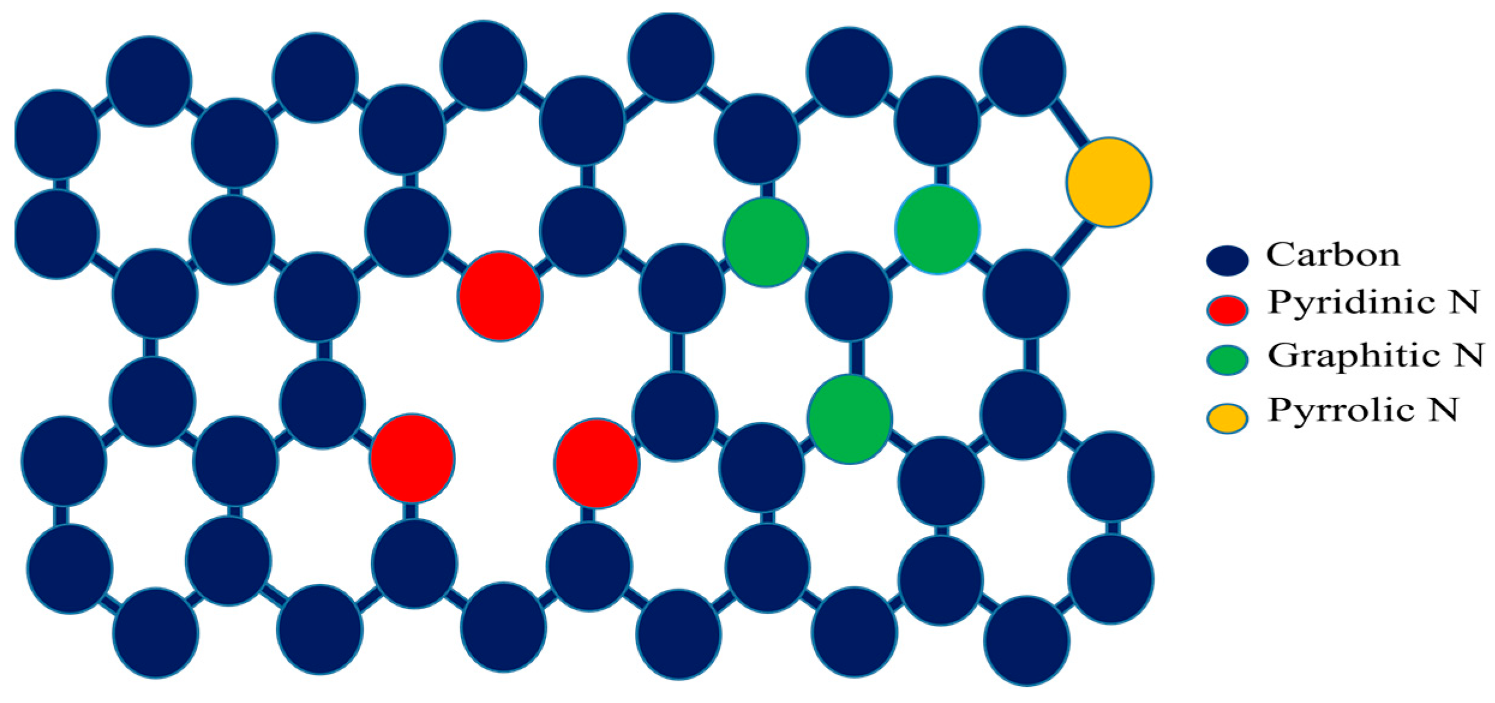
Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Procedure
2.2. Characterization Methods
3. Results and Discussion
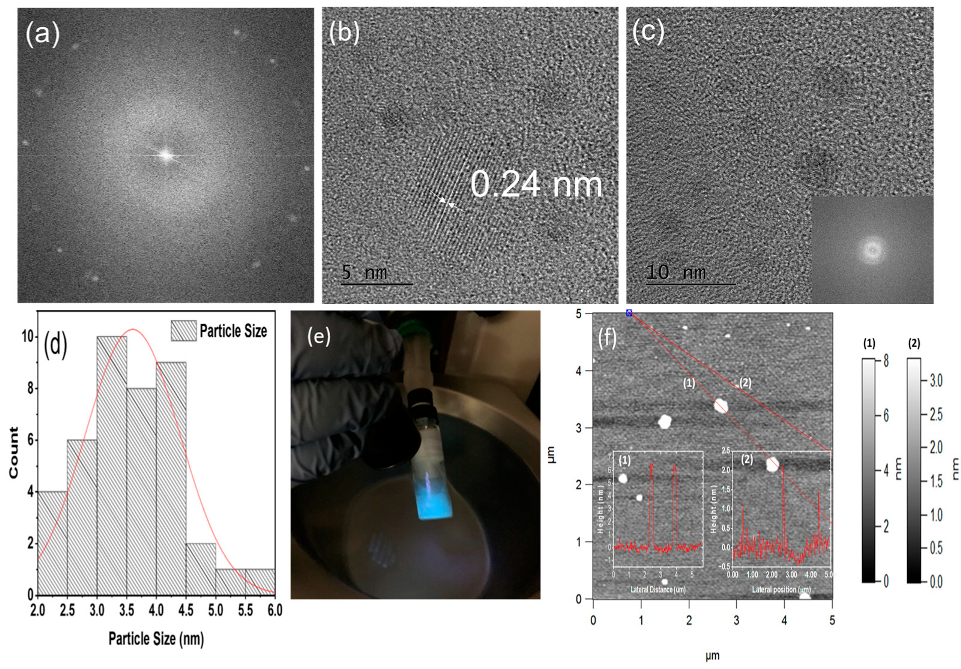
3.1. Morphology
3.2. Elemental Analysis
3.3. Optical Properties
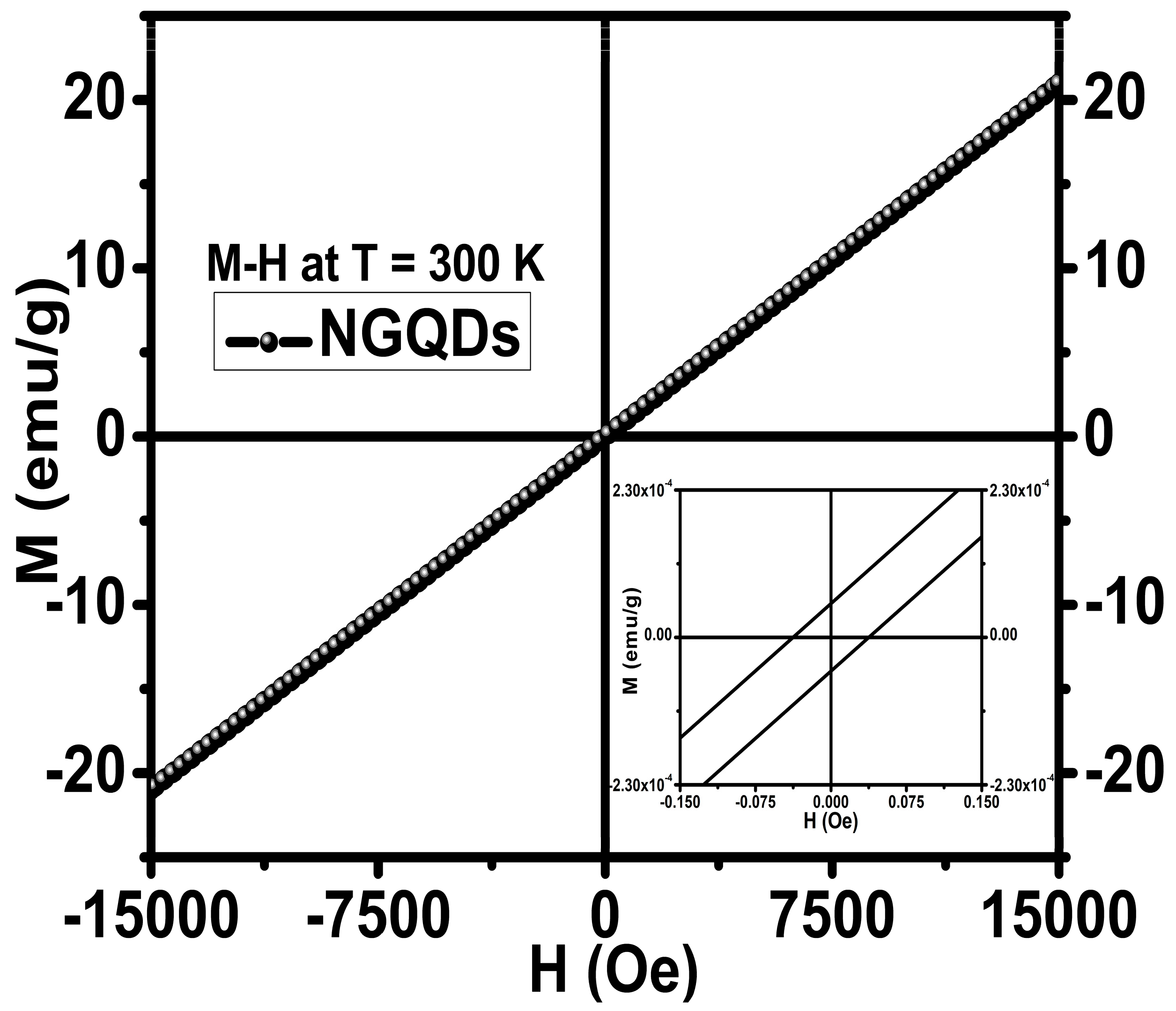
3.4. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Girit, C.O.; Meyer, J.C.; Erni, R.; Rossell, M.D.; Kisielowski, C.; Yang, L.; Park, C.-H.; Crommie, M.F.; Cohen, M.L.; Louie, S.G.; et al. Graphene at the Edge: Stability and Dynamics. Science 2009, 323, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.A.; Lyding, J.W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 2009, 8, 235–242. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Ji, X.; Liu, W.; Wan, W.; Wang, Y.; Pan, X.; Lu, Z. Graphene quantum dots as a highly efficient electrocatalyst for lithium–oxygen batteries. J. Mater. Chem. A 2020, 8, 22356–22368. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminescent graphene quantum dots for organic photovoltaic devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.; Dong, H.; He, W.; Dong, L.; Yu, L. High-Performance Supercapacitor of Graphene Quantum Dots with Uniform Sizes. ACS Appl. Mater. Interfaces 2018, 10, 12983–12991. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Pan, H.; Chen, J.; Zhang, W.; Fu, L.; Zhang, K.; Tang, N.; Du, Y. Magnetism of graphene quantum dots. NPJ Quantum Mater. 2017, 2, 5. [Google Scholar] [CrossRef]
- Hong, J.; Niyogi, S.; Bekyarova, E.; Itkis, M.E.; Ramesh, P.; Amos, N.; Litvinov, D.; Berger, C.; de Heer, W.A.; Khizroev, S.; et al. Effect of Nitrophenyl Functionalization on the Magnetic Properties of Epitaxial Graphene. Small 2011, 7, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Jia, R.X.; Wang, Y.T.; Dong, L.P.; Hu, J.C.; Zhang, Y.M. Concentration of point defects in 4H-SiC characterized by a magnetic measurement. AIP Adv. 2016, 6, 095201. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Chen, J.; Zhang, W.; Tang, N.; Du, Y. Intrinsic magnetism of monolayer graphene oxide quantum dots. Appl. Phys. Lett. 2016, 108, 033105. [Google Scholar] [CrossRef]
- Voznyy, O.; Güçlü, A.D.; Potasz, P.; Hawrylak, P. Effect of edge reconstruction and passivation on zero-energy states and magnetism in triangular graphene quantum dots with zigzag edges. Phys. Rev. B 2011, 83, 165417. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Helm, L. Defect-induced magnetism in graphene. Phys. Rev. B 2007, 75, 125408. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Matte, H.S.S.R.; Subrahmanyam, K.S.; Maitra, U. Unusual magnetic properties of graphene and related materials. Chem. Sci. 2011, 3, 45–52. [Google Scholar] [CrossRef]
- Sajjad, M.; Makarov, V.; Sultan, M.S.; Jadwisienczak, W.M.; Weiner, B.R.; Morell, G. Synthesis, Optical, and Magnetic Properties of Graphene Quantum Dots and Iron Oxide Nanocomposites. Adv. Mater. Sci. Eng. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Espinosa-Ortega, T.; Luk’yanchuk, I.A.; Rubo, Y.G. Magnetic properties of graphene quantum dots. Phys. Rev. B 2013, 87, 205434. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, F.; Xue, Y.; Xiao, D.; Guo, Y. Effects of Edge Oxidation on the Stability and Half-Metallicity of Graphene Quantum Dots. ChemPhysChem 2013, 15, 157–164. [Google Scholar] [CrossRef]
- Sepioni, M.; Nair, R.R.; Rablen, S.; Narayanan, J.; Tuna, F.; Winpenny, R.; Geim, A.K.; Grigorieva, I.V. Limits on Intrinsic Magnetism in Graphene. Phys. Rev. Lett. 2010, 105, 207205. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Tang, N.; Zheng, Y.; Wan, X.; Liu, Y.; Liu, F.; Xu, Q.; Du, Y. Robust magnetic moments on the basal plane of the graphene sheet effectively induced by OH groups. Sci. Rep. 2015, 5, 8448. [Google Scholar] [CrossRef]
- Wang, H.; Revia, R.; Wang, K.; Kant, R.J.; Mu, Q.; Gai, Z.; Hong, K.; Zhang, M. Paramagnetic Properties of Metal-Free Boron-Doped Graphene Quantum Dots and Their Application for Safe Magnetic Resonance Imaging. Adv. Mater. 2016, 29, 1605416. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiao, W.; Liu, Y.; Zheng, Y.; Lin, Y.; Li, J.; Ye, Q.; Huang, Z. Novel Synthesis of Slightly Fluorinated Graphene Quantum Dots with Luminescent and Paramagnetic Properties through Thermal Cutting of Fluorinated Graphene. Materials 2018, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Christodoulou, C.; Nardi, M.V.; Koch, N.; Kläui, M.; Sachdev, H.; Müllen, K. Tuning the Magnetic Properties of Carbon by Nitrogen Doping of Its Graphene Domains. J. Am. Chem. Soc. 2015, 137, 7678–7685. [Google Scholar] [CrossRef]
- Błoński, P.; Tuček, J.; Sofer, Z.; Mazánek, V.; Petr, M.; Pumera, M.; Otyepka, M.; Zbořil, R. Doping with Graphitic Nitrogen Triggers Ferromagnetism in Graphene. J. Am. Chem. Soc. 2017, 139, 3171–3180. [Google Scholar] [CrossRef]
- Li, X.-F.; Lian, K.-Y.; Liu, L.; Wu, Y.; Qiu, Q.; Jiang, J.; Deng, M.; Luo, Y. Unraveling the formation mechanism of graphitic nitrogen-doping in thermally treated graphene with ammonia. Sci. Rep. 2016, 6, 23495. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Chen, T.; Hu, L.; Du, Y.; Yao, Y.; Goh, M.C. Simply synthesized nitrogen-doped graphene quantum dot (NGQD)-modified electrode for the ultrasensitive photoelectrochemical detection of dopamine. Nanophotonics 2019, 9, 3831–3839. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Tong, X.; Wang, X.; Yang, P.; Yao, F.; Guo, R.; Yuan, C. Preparation of graphene quantum dots with high quantum yield by a facile one-step method and applications for cell imaging. Mater. Lett. 2020, 271, 127806. [Google Scholar] [CrossRef]
- Kumar, S.; Aziz, S.T.; Girshevitz, O.; Nessim, G.D. One-Step Synthesis of N-Doped Graphene Quantum Dots from Chitosan as a Sole Precursor Using Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 2343–2349. [Google Scholar] [CrossRef]
- León, I.N.-D.; Johny, J.; Vázquez-Rodríguez, S.; García-Gómez, N.; Bernal, S.Y.C.; Mendivil, I.; Shaji, S.; Sepúlveda-Guzmán, S. Tuning the luminescence of nitrogen-doped graphene quantum dots synthesized by pulsed laser ablation in liquid and their use as a selective photoluminescence on–off–on probe for ascorbic acid detection. Carbon 2019, 150, 455–464. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, Y.; Li, P.; Wang, Z.; Cao, B.; Qi, F.; Liu, J.; Qiu, Z.; Zhang, W. Ultrafast ammonia-driven, microwave-assisted synthesis of nitrogen-doped graphene quantum dots and their optical properties. Nanophotonics 2017, 6, 259–267. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef]
- Gupta, D.; Jamwal, D.; Rana, D.; Katoch, A. Microwave synthesized nanocomposites for enhancing oral bioavailability of drugs. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A.M.A., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 619–632. [Google Scholar] [CrossRef]
- Rodríguez, A.M.; Prieto, P.; de la Hoz, A.; Díaz-Ortiz, Á.; Martín, D.R.; García, J.I. Influence of Polarity and Activation Energy in Microwave-Assisted Organic Synthesis (MAOS). Chemistryopen 2015, 4, 308–317. [Google Scholar] [CrossRef]
- Granzier-Nakajima, T.; Fujisawa, K.; Anil, V.; Terrones, M.; Yeh, Y.-T. Controlling Nitrogen Doping in Graphene with Atomic Precision: Synthesis and Characterization. Nanomaterials 2019, 9, 425. [Google Scholar] [CrossRef] [PubMed]
- Yutomo, E.B.; Noor, F.A.; Winata, T. Effect of the number of nitrogen dopants on the electronic and magnetic properties of graphitic and pyridinic N-doped graphene—A density-functional study. RSC Adv. 2021, 11, 18371–18380. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.; Anjali, B.A.; Setiawan, O.; Ostrikov, K.K.; Chung, Y.G.; Chiang, W.-H. Microplasma Band Structure Engineering in Graphene Quantum Dots for Sensitive and Wide-Range pH Sensing. ACS Appl. Mater. Interfaces 2021, 14, 1670–1683. [Google Scholar] [CrossRef]
- Calabro, R.L.; Yang, D.-S.; Kim, D.Y. Controlled Nitrogen Doping of Graphene Quantum Dots through Laser Ablation in Aqueous Solutions for Photoluminescence and Electrocatalytic Applications. ACS Appl. Nano Mater. 2019, 2, 6948–6959. [Google Scholar] [CrossRef]
- Pillar-Little, T.; Kim, D.Y. Differentiating the impact of nitrogen chemical states on optical properties of nitrogen-doped graphene quantum dots. RSC Adv. 2017, 7, 48263–48267. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H.; Fan, L.; Han, M.; et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Hasan, T.; Gonzalez-Rodriguez, R.; Ryan, C.; Coffer, J.L.; Naumov, A.V. Variation of Optical Properties of Nitrogen-doped Graphene Quantum Dots with Short/Mid/Long-wave Ultraviolet for the Development of the UV Photodetector. ACS Appl. Mater. Interfaces 2019, 11, 39035–39045. [Google Scholar] [CrossRef]
- Osman, W.; Abdelsalam, H.; Ali, M.; Teleb, N.; Yahia, I.; Ibrahim, M.; Zhang, Q. Electronic and magnetic properties of graphene quantum dots doped with alkali metals. J. Mater. Res. Technol. 2021, 11, 1517–1533. [Google Scholar] [CrossRef]
- Sultan, M.S.; Makarov, V.I.; Mendoza, F.; Sajjad, M.; Barrionuevo, D.; Inbanathan, F.P.N.; Premadasa, U.I.; Cimatu, K.L.A.; Jadwisienczak, W.M.; Habiba, K.; et al. Superparamagnetic Properties of Metal-Free Nitrogen-Doped Graphene Quantum Dots. J. Appl. Phys. 2023, in press. [Google Scholar]
- Gómez, I.J.; Sulleiro, M.V.; Dolečková, A.; Pizúrová, N.; Medalová, J.; Roy, R.; Nečas, D.; Zajíčková, L. Exploring the Emission Pathways in Nitrogen-Doped Graphene Quantum Dots for Bioimaging. J. Phys. Chem. C 2021, 125, 21044–21054. [Google Scholar] [CrossRef]
- Muthamma, K.; Sunil, D.; Shetty, P. Carbon dots as emerging luminophores in security inks for anti-counterfeit applications-An up-to-date review. Appl. Mater. Today 2021, 23, 101050. [Google Scholar] [CrossRef]
- Selvakumar, T.; Rajaram, M.; Natarajan, A.; Harikrishnan, L.; Alwar, K.; Rajaram, A. Highly Efficient Sulfur and Nitrogen Codoped Graphene Quantum Dots as a Metal-Free Green Photocatalyst for Photocatalysis and Fluorescent Ink Applications. ACS Omega 2022, 7, 12825–12834. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Z.; Zhou, W.; Liu, Y.; Fan, L.; Li, Y.; Li, X. Graphene quantum dots as smart probes for biosensing. Anal. Methods 2016, 8, 4001–4016. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Bi, S. Two-Dimensional Quantum Dot-Based Electrochemical Biosensors. Biosensors 2022, 12, 254. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inbanathan, F.P.N.; Cimatu, K.L.A.; Ingram, D.C.; Erasquin, U.J.; Dasari, K.; Sultan, M.S.; Sajjad, M.; Makarov, V.; Weiner, B.R.; Morell, G.; et al. Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots. Materials 2023, 16, 3410. https://doi.org/10.3390/ma16093410
Inbanathan FPN, Cimatu KLA, Ingram DC, Erasquin UJ, Dasari K, Sultan MS, Sajjad M, Makarov V, Weiner BR, Morell G, et al. Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots. Materials. 2023; 16(9):3410. https://doi.org/10.3390/ma16093410
Chicago/Turabian StyleInbanathan, Flavia P. N., Katherine Leslee A. Cimatu, David C. Ingram, Uriel Joseph Erasquin, Kiran Dasari, Muhammad Shehzad Sultan, Muhammad Sajjad, Vladimir Makarov, Brad R. Weiner, Gerardo Morell, and et al. 2023. "Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots" Materials 16, no. 9: 3410. https://doi.org/10.3390/ma16093410
APA StyleInbanathan, F. P. N., Cimatu, K. L. A., Ingram, D. C., Erasquin, U. J., Dasari, K., Sultan, M. S., Sajjad, M., Makarov, V., Weiner, B. R., Morell, G., Sharifi Abdar, P., & Jadwisienczak, W. M. (2023). Paramagnetism in Microwave-Synthesized Metal-Free Nitrogen-Doped Graphene Quantum Dots. Materials, 16(9), 3410. https://doi.org/10.3390/ma16093410